Abstract
A large body of studies has suggested that peroxisome proliferator-activated receptor γ (PPARγ) ligands, such as thiazolidinedione, are potent candidates for chemopreventive agents. MCC-555 is a PPARγ/α dual agonist and has been previously shown to induce apoptosis in vitro; however, the molecular mechanisms by which MCC-555 affects anti-tumorigenesis in vivo are poorly understood. In this study, we explored the anti-tumorigenic effects of MCC-555 both in cell culture and in Apc-deficient mice, an animal model for human familial adenomatous polyposis. MCC-555 increased MUC2 expression in colorectal and lung cancer cells, and treatment with the PPARγ antagonist GW9662 revealed that MUC2 induction by MCC-555 was mediated in a PPARγ-dependent manner. Moreover, MCC-555 increased transcriptional activity of human and mouse MUC2 promoters. Subsequently, treatment with MCC-555 (30 mg/kg/day) for 4 weeks reduced the number of small intestinal polyps to 54.8% of that in control mice. In agreement with in vitro studies, enhanced Muc2 expression was observed in the small intestinal tumors of Min mice treated with MCC-555, suggesting that MUC2 expression may be associated at least in part with the anti-tumorigenic action of MCC-555. In addition, highly phosphorylated extracellular signal-regulated kinase (ERK) was found in the intestinal tumors of MCC-555-treated Min mice, and inhibition of the ERK pathway by a specific inhibitor markedly suppressed MCC-555-induced Muc2 expression in vitro. Overall, these results indicate that MCC-555 has a potent tumor suppressor activity in intestinal tumorigenesis, likely involving MUC2 up-regulation by ERK and PPARγ pathways.
Keywords: MCC-555, colorectal cancer, ApcMin/+ mice, MUC2, PPARs, ERK pathway
Introduction
Thiazolidinediones, synthetic peroxisome proliferator-activated receptorγ (PPARγ) ligands, are a novel class of antidiabetic drugs for patients with type 2 diabetes, and two of these, rosiglitazone and pioglitazone, are currently available for clinical use (1). In addition, thiazolidinediones have recently been found to have anti-tumorigenic activity in a wide variety of cancer cells. As a transcription factor, PPARγ targets genes associated with the cell cycle, differentiation, and apoptosis (2), implying that PPARγ ligands can be potent candidates for cancer prevention and/or therapy. Some of the most extensive studies have been done in the colon, where PPARγ is highly expressed in both adenocarcinomas and normal colonic mucosa (3, 4). For example, PPARγ ligands alter the expression of apoptotic and cell proliferation genes, thereby enhancing their anti-tumorigenic activity through a PPARγ-dependent mechanism in colorectal cancer cells (2, 5). Somatic PPARγ mutations were also found in sporadic colon cancers, and these mutations cause deletion of the entire ligand binding domain and loss of transactivation ability, resulting in incomplete function of the protein (6). In addition, several reports showed new PPARγ-dependent and -independent target genes of thiazolidinediones, resulting in modulation of cell proliferation and apoptosis in cancer cells (7-11). For example, the thiazolidinedione troglitazone was found to induce not only cell death in colon cancer cells (12, 13), but also to reduce the clonogenic capacity of all human colorectal cancer cells tested (3). Subsequently, the cDNA microarray analysis from colon cancer cells treated by PPARγ agonists identified many target genes linked to the cell growth regulatory pathway (14, 15).
Several animal models for human colorectal cancer have been used to study whether thiazolidinediones possess these anti-tumorigenic activities in vivo. Adenomatous polyposis coli (APC) mutations, occurring early in the transformation process, are found in the majority of sporadic colorectal tumors as well as in familial adenomatous polyposis (FAP). A number of promising chemopreventive agents, such as non-steroidal anti-inflammatory drugs, have been reported to strongly suppress tumor formation or growth in the small intestine of multiple intestinal neoplasia (Min) mice (16, 17). In fact, treatment of Apc1309 mice with pioglitazone (100 and 200 ppm) for 6 weeks significantly reduced the total number of intestinal polyps to 67% of control (18). Results from another animal model, using the colonic carcinogen azoxymethane, support the anti-tumorigenic activity of troglitazone and pioglitazone with significant suppression of azoxymethane-induced aberrant crypt foci (precursor lesions for colon carcinoma) formation (19). While most studies indicate that thiazolidinediones suppress tumors in animal models, troglitazone has also been reported to enhance polyp formation in the intestinal track of Min mice (20). In addition, it has been recently revealed that troglitazone is hepato-toxic (21). This variety of actions of thiazolidinedione might be due to a multi-target property of PPARγ ligands that remains to be elucidated. Therefore, better thiazolidinedione compounds are needed to achieve anti-tumorigenic activity with less liver toxicity.
Mucus in the gastrointestinal tract plays an important role as a physiological barrier between the intestinal contents and underlying epithelial cells. Alteration of the expression of mucins, the major glycoprotein constituents in mucus, is a common feature of colonic neoplasia (22). Mucin 2 (MUC2), secreted by goblet cells of the small and large intestines, is the major structural component of the mucus gel. Levels of MUC2 mRNA expression are often decreased in colon cancer, although that expression depends on the type of colon cancer and its progression (23-25). Furthermore, Muc2-deficient mice developed adenomas in the small intestine, along with increased proliferation, decreased apoptosis, and increased migration of intestinal adenocarcinoma cells, suggesting MUC2 is linked to suppression of colorectal cancer (26).
In this study, the novel synthetic PPAR ligand MCC-555 was investigated to determine the effect on MUC2 expression in vitro and in vivo. MCC-555, a novel thiazolidinedione (also known as netoglitazone), was found to have a great effect on decreasing blood glucose levels in animal models of type 2 diabetes, and to possess characteristic binding to PPARs (27). We previously reported that MCC-555 induces apoptosis in human colorectal cancer cells (28). In this study, we demonstrated that MCC-555 increased MUC2 expression and suppressed intestinal polyposis in Min mice. In addition, we showed a possible mechanism of MUC2 up-regulation via the extracellular signal-regulated kinase (ERK) pathway. This is the first report suggesting that MUC2 is a novel PPARγ target gene and that its expression plays a role in colorectal tumorigenesis.
Materials and Methods
Cell Lines and Reagents
Human colorectal cancer SW480 cells, mouse rectal cancer CMT-93 cells, and human lung cancer NCI-H292 cells were purchased from American Type Culture Collection (Manassas, VA). MCC-555 (Fig. 1A) was obtained from Mitsubishi Pharma Corporation (Tokyo, Japan). Ciglitazone, rosiglitazone, prostaglandin 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2), and GW9662 were purchased from Cayman Chemical Company (Ann Arbor, MI). Troglitazone was obtained from Calbiochem (La Jolla, CA). Anti-MUC2 and anti-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), whereas anti-phospho-Erk1/2 (Thr202/Tyr204) and anti-Erk1/2 MAP kinases were obtained from Cell Signaling Technology (Beverly, MA).
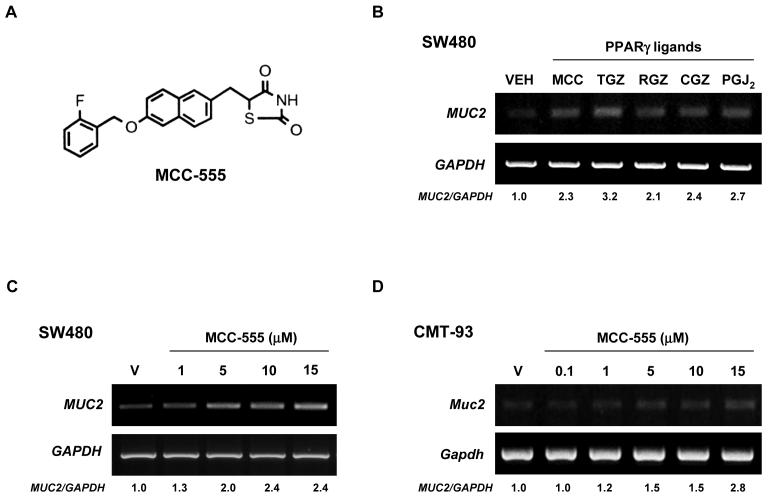
Figure 1. Increased expression of MUC2 by different PPARγ ligands in colorectal cancer cells.
A, Molecular structure of MCC-555. B, Human colorectal SW480 cancer cells treated with DMSO (VEH), MCC-555 (MCC, 10 μM), troglitazone (TGZ, 10 μM), rosiglitazone (RGZ, 10 μM), ciglitazone (CGZ, 10 μM), and PGJ2 (PGJ2, 1 μM). C, SW480 cells treated with different doses of MCC-555. D, Mouse rectal CMT-93 cancer cells treated with different doses of MCC-555. After 24 h of treatment, total RNAs were extracted, and semi-quantitative RT-PCRs were performed as described under “Material and Methods.” GAPDH served as the internal control. Fold inductions over the vehicle-treated sample are shown at the bottom.
RNA Purification and RT-PCR
Normal and tumor tissues isolated from the small and large intestine, and liver tissues were kept in RNAlater solution (Ambion, Austin, TX) and stored at −80°C. Cells were treated with different PPARγ ligands at the indicated doses and time points. Total RNA was extracted from these tissues and cells using Perfect RNA Eukaryotic Mini (Eppendorf, Westbury, NY) or TRIzol (Invitrogen, Carlsbad, CA), and then cDNA was synthesized from 1 μg of total RNA using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to the manufacturer's protocol. PCR was performed with specific primers, for human: mucin 2 (MUC2, S: 5′-GACCTCCAGCACAGTTTTATCAACA-3′, AS: 5′- GCCAGCAACAATTGACACGTATCT-3′), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, S: 5′-GACCACAGTCCATGCCATCACT-3′, AS: 5′-TCCACCACCCTGTTGCTGTAG-3′), beta 2-microglobulin (β2MG, S: 5′- CTCGCGCTACTCTCTCTTTCTGG-3′, AS: 5′-GCTTACATGTCTCGATCCCACTTAA-3′); for mouse: Muc2 (S: 5′-TGTGGCCTGTGTGGGAACTTT-3′, AS: 5′-CATAGAGGGCCTGTCCTCAGG-3′), glucose transporter type 2 (Glut2, S: 5′-TGGGATGAAGAGGAGACTGAA-3′, AS: 5′-TGAAAAATGCTGGTTGAATAG-3′), liver fatty acid binding protein 2 (I-Fabp, S: 5′-AACTTCTCCGGCAAGTACCA-3′, AS: 5′-CACCTTCCAGCTTGACGACT-3′), adipocyte protein 2 (aP2, S: 5′-AAGAAGTGGGAGTGGGCTTT-3′, AS: 5′-CTTGTGGAAGTCACGCCTTT-3′), cytochrome P450, family 4, subfamily a, polypeptide 10 (Cyp4a10, S: 5′- ACCACAATGTGCATCAAGGA-3′, AS: 5′-CTGAGAAGGGCAGGAATGAG-3′), lipoprotein lipase (Lpl, S: 5′-GGATCCGTGGCCGCAGCAGACGCAGGAAGA-3′, AS: 5′-GAATTCCATCCAGTTGATGAATCTGGCCAC-3′), and Gapdh (S: 5′-CAGGAGCGAGACCCCACTAACAT-3′, AS: 5′- GTCAGATCCACGACGGACACATT-3′). The signal contour length on images was measured using Scion Image software (Scion Corp., Frederick, MD).
Luciferase Assay
Transient transfections were performed using the Lipofectamine or Lipofectamine 2000 transfection reagents (Invitrogen) according to the manufacturer's instructions. The cells were plated in 12-well plates at the concentration of 2×105 cells/well. After overnight growth, human SW480 and NCI-H292 cells were transfected with reporter plasmid containing human MUC2 promoter (phMUC2−2096/+27LUC) using Lipofectamine 2000, and mouse CMT-93 cells were transfected with plasmid containing mouse Muc2 promoter (pmMuc2−1001/+29LUC) using Lipofectamine as described previously (29, 30). After 24 h transfection, the cells were treated with vehicle or MCC-555 for 24 h, and luciferase activity was measured as described previously (31).
Immunohistochemistry
Small intestine and colon tissues were formalin-fixed, embedded in paraffin, and sectioned at 4 μm thickness. Tissue sections were then heated, deparaffinized in xylene, rehydrated in graded alcohol to PBS, and pretreated with 10 mM citrate buffer, pH 6.0, for 10 min at just below boiling. Endogenous peroxidase activity was quenched by incubation in 3% H2O2 in PBS for 15 min at room temperature, and tissues were incubated with protein block (Biogenex USA, San Ramon, CA) for 30 min at room temperature. Slides were then incubated for 1 h with anti-MUC2 antibody (1:200), followed by biotinylated anti-rabbit IgG (30 min at room temperature) and streptavidin/biotin-horseradish peroxidase complex (20 min at room temperature), which was visualized by 3,3′-diaminobenzidine tetra-hydrochloride solution (DAB, 0.7 g/L; Biogenex) for 10 min. Slides were lightly counterstained with Mayer's hematoxylin.
Animals and Experimental Design
All animal research procedures were approved by the University of Tennessee Animal Care and Use Committee and were in accordance with NIH guidelines. C57BL/6J ApcMin/+ mice (The Jackson Laboratory, Bar Harbor, ME) were randomly assigned to their respective experimental groups (n=7 per each group). Min mice were maintained at 22 ± 2°C on a 12 h light/dark cycle and with free access to standard rodent chow and water. MCC-555 was suspended in 1.5% carboxymethylcellulose with 0.2% Tween 20. At 10 weeks of age, the experimental group (3 males and 4 females) received the MCC-555 suspension (30 mg/kg/day, 5 days a week) for 4 weeks by gavage. The control group (4 males and 3 females) was gavaged with the suspending vehicle solution alone. Twenty four hours after final treatment, the mice were euthanized, and the intestinal tract was isolated and washed with phosphate-buffered saline. Tumor numbers and sizes in the small intestine and colon were assessed with a stereoscopic microscope as previously described (32).
Statistical Analysis
Statistical analyses were performed with the Mann-Whitney's U-test or Student t test. Results were considered statistically significant at P<0.05.
Results
Enhanced MUC2 mRNA Expression by PPARγ Ligands in Colorectal Cancer Cells
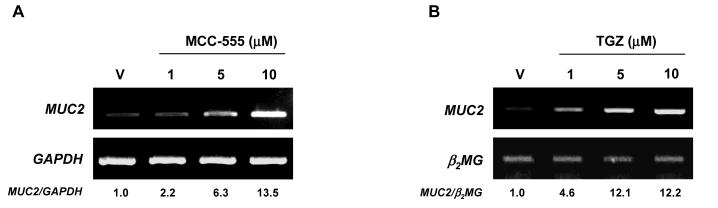
We and others have reported that PPARγ ligands display anti-tumorigenic activity in colorectal cancer (7, 28, 33), and MUC2 is known to have a tumor suppressor function in colorectal cancer (26). Hence, we examined whether PPARγ ligands increase MUC2 expression in SW480 human colorectal cancer cells. As shown in Fig. 1B, all the tested PPARγ ligands increased MUC2 expression 2-3 fold. Upon further examination of MCC-555, we found that MUC2 was increased in a dose-dependent manner (Fig. 1C). In addition, in CMT-93 mouse colorectal cancer cells, MCC-555 increased Muc2 expression in a dose-dependent manner (Fig. 1D). Since MUC2 and PPARγ ligands play an important role in lung tumorigenesis (34, 35), human lung cancer cells NCI-H292 were treated with different PPARγ ligands, and MUC2 expression was measured. As shown in Fig. 2, MCC-555 and troglitazone (TGZ) also increased MUC2 expression in lung cancer cells in a dose-dependent manner.
Figure 2. Increased expression of MUC2 in response to PPARγ ligands in NCI-H292 human lung cancer cells.
A, NCI-H292 cells treated with different doses of MCC-555. B, NCI-H292 cells treated with different doses of troglitazone (TGZ). After 24 h of treatment, total RNAs were extracted, and semi-quantitative RT-PCRs were performed as described under “Material and Methods.” GAPDH and β2MG served as the internal control. Fold inductions over the vehicle-treated sample are shown at the bottom.
MUC2 Expression Is Mediated in a PPARγ-dependent Manner
Since PPARγ ligands can alter target gene expression in both PPARγ-dependent and -independent manners (7, 11, 36), we examined whether PPARγ activation is responsible for MUC2 induction. As we previously reported, 5 μM of the PPARγ antagonist GW9662 substantially inhibited PPARγ transactivation by MCC-555 (28). SW480 cells were treated with GW9662 and/or PPARγ ligands, and MUC2 expression was measured. As shown in Fig. 3A, pre-treatment with GW9662 completely blocked induction of MUC2 expression by MCC-555 and TGZ in SW480 cells, suggesting that MUC2 induction is probably mediated through a PPARγ-dependent pathway. Since MUC2 was increased in human and mouse cell lines in the presence of MCC-555, and PPARγ mediates its expression, we examined the transcriptional regulation of MUC2 by MCC-555. The reporter constructs containing human MUC2 promoter (phMUC2-2096/+27LUC) and mouse Muc2 promoter (pmMuc2-1001/+29LUC) were transfected into SW480, NCI-H292, and CMT-93 cells. After MCC-555 treatment for 24 h, luciferase activity was measured. As shown in Fig. 3B and C, MCC-555-treated samples showed increased luciferase activity, compared to vehicle-treated samples, indicating that MCC-555 increases MUC2 expression at the transcriptional level.
Figure 3. Transcriptional regulation of MUC2 by PPARγ ligands.
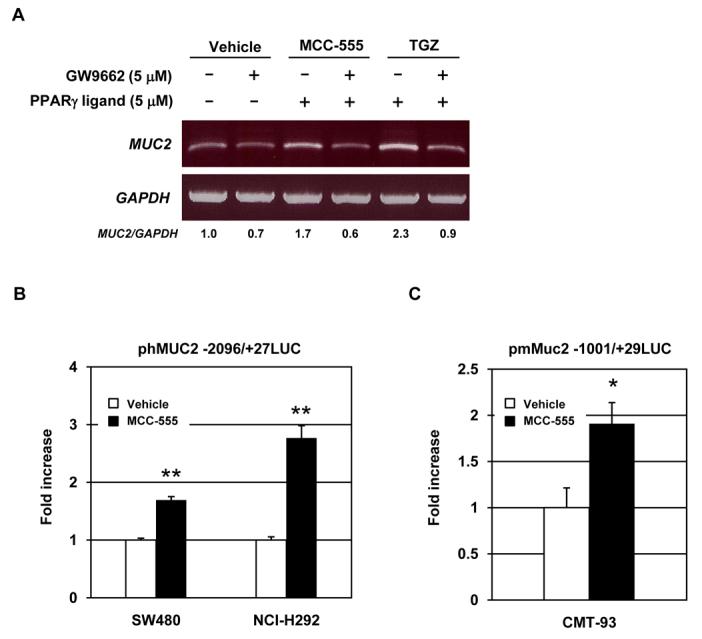
A, SW480 cells were pretreated with PPARγ antagonist GW9662 (5 μM) for 30 min prior to the addition of vehicle, MCC-555 (5 μM), or troglitazone (TGZ, 5 μM). After 24 h, total RNA were isolated for RT-PCR analysis. GAPDH served as the internal control. The data represent two independent experiments. B, SW480 and NCI-H292 cells transfected with phMUC2 −2096/+27LUC construct were treated with 10 μM of MCC-555 for 24 h. C, CMT-93 cells transfected with pmMUC2 −1001/+29LUC construct were treated with 10 μM of MCC-555 for 24 h, and luciferase activity was measured as described under “Material and Methods”. The y-axis indicates fold increase of relative luciferase unit (RLU) compared to RLU of vehicle-treated samples. The data represent mean ± SD from 3 replicates. *P<0.05, **P<0.01 from vehicle-treated samples.
Suppression of Intestinal Polyp Formation by MCC-555
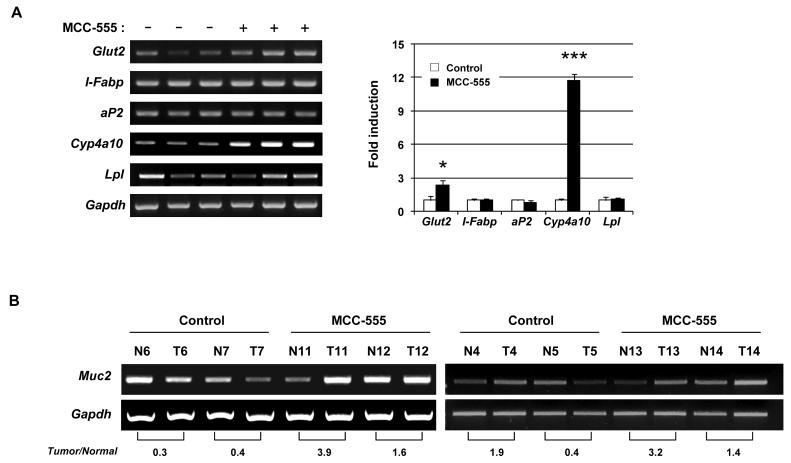
Since anti-tumorigenic effects of MCC-555 on intestinal cancer had not been previously determined, we treated Min mice with MCC-555 (30 mg/kg/day) for 4 weeks and evaluated tumor formation in the small intestine and colon. MCC-555 reduced the total numbers of small intestinal polyps to 54.8% of those in control mice (control: 125.1 ± 22.6 vs MCC-555: 68.6 ± 9.0). The size distribution of intestinal polyps in control and MCC-555-treated groups is shown in Fig. 4A. Significant reductions of polyp numbers by MCC-555 were observed in polyps measuring 1.0 - 1.5 mm and 1.5 - 2.0 mm in diameter. Tumor load analysis (polyp number X polyp size) demonstrates that MCC-555 significantly suppressed intestinal polyp formation (Fig. 4B). We also examined polyps in the colon and found no statistical significance in polyp number (control: 2.3 ± 0.6 vs MCC-555: 1.4 ± 0.5). Since MCC-555 dramatically reduced the number of small intestinal polyps in Min mice and induced MUC2 expression in human and mouse cancer cells (Fig. 1), the effects of MCC-555 on intestinal Muc2 expression were examined in vivo. The histology of both the small and large intestine was typical of Min adenomas, and there was no evidence of deep invasion (Fig. 4C). To examine the distribution of Muc2, immunostaining was performed using small intestine and colonic tissues from Min mice containing tumors. As shown in Fig. 4C, Muc2 was positively stained in goblet cells in the small intestine and colon as previously reported (26). Interestingly, Muc2 was highly expressed in normal tissue compared to adjacent tumor tissue, supporting the previous report that MUC2 is a tumor suppressor protein.
Figure 4. Treatment with MCC-555 suppresses tumorigenesis in Min mice.
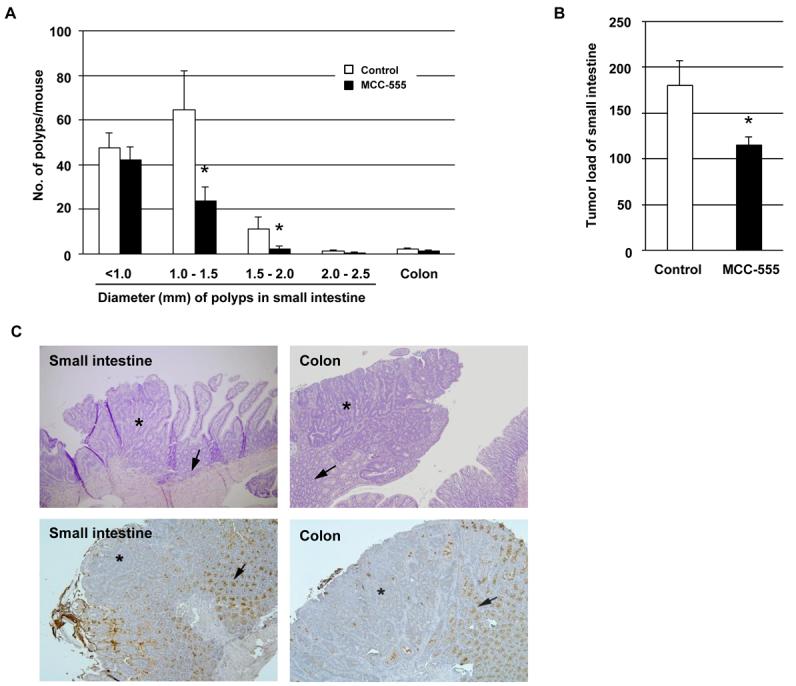
A, Number of tumors in the small intestine and colon from control and MCC-555-treated mice. Small intestinal tumors were grouped at intervals of 0.5 mm, according to their diameter. Each value represents mean ± SE from 7 mice. *P<0.05 from control mice. B, Tumor load analysis shows a significant reduction of tumors in MCC-555-treated mice. Each value represents mean ± SE from 7 mice. *P<0.05 from control mice. C, Localization of Muc2 in the small intestine and colon of Min mice. On top, H&E stained sections of tumors arising in the small intestine and colon of Min mice. On the bottom of the panel, figures show lower expressions of Muc2 in tumors compared to normal tissue in the small intestine and colon. Asterisks indicate areas of neoplasia, and arrows show normal tissue of the small intestine and colon. Magnification: 100X.
Increased Muc2 Expression in MCC-555-treated Min Mice
To investigate whether MCC-555 increases known PPARγ (Glut2, aP2, and Lpl) and/or PPARα target genes (l-Fabp and Cyp4a10) (37-39), the expression of Glut2, I-Fabp, aP2, Cyp4a10, or Lpl in liver tissue was examined by RT-PCR. Treatment with MCC-555 increased Glut2 and Cyp4a10 mRNA expression, whereas it did not increase other PPAR target genes, I-Fabp, Lpl, and aP2 (Fig. 5A). The RT-PCR analysis showed that the level of Muc2 expression in the normal intestine was higher than that in the corresponding tumor tissue from control mice (mouse numbers 6, 7, and 5 in Fig. 5B), consistent with the Muc2 immunohistochemical staining shown in Fig. 4C. Furthermore, Muc2 mRNA expression was significantly enhanced in MCC-555-treated mouse tumors, compared to tumors from control mice (mouse numbers 11, 12, 13, 14 in Fig. 5B). These results indicate that MUC2 expression induced by MCC-555 may play an important role in the tumors of intestinal tract.
Figure 5. Expression of PPARγ and/or PPARα target genes from control and MCC-555-treated mice.
A, Representative RT-PCR results of mouse liver tissue samples detecting Glut2, I-Fabp, aP2, Cyp4a10, and Lpl gene expressions. The graph on the right indicates the normalized expression of target genes. The values obtained from control mice were defined as 1.0, and each value represents mean ± SE from 3 mice. Gapdh served as the internal control. *P<0.05, ***P<0.001 from control. B, Increased Muc2 expression in small intestinal tumors in mice treated with MCC-555. Total RNAs were isolated from normal (N) and tumor (T) tissues of the small intestine in mice treated with vehicle and MCC-555. Muc2 mRNA expression was analyzed by RT-PCR. The ratio of intensity (tumor/normal) in adjacent pairs shows at the bottom. Gapdh served as the internal control.
Contribution of the ERK Pathway to MCC-555-induced MUC2 Expression in vivo and in vitro
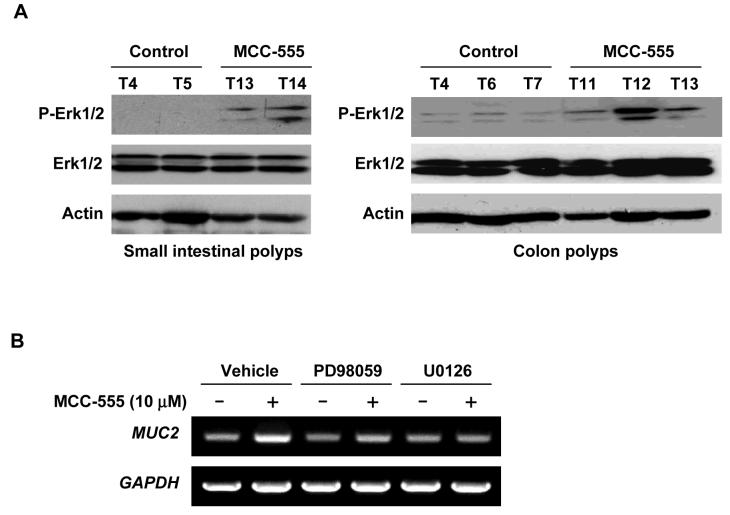
It has been shown that MUC2 is regulated by MAPK pathways involving ERK1/2 (40), and we have reported that PPARγ ligands cause phosphorylation of ERK1/2 in human colorectal cancer cells (7, 28). To elucidate the molecular mechanism by which MCC-555 increases MUC2 expression, we examined the phosphorylation status of Erk1/2 in the small intestine and colon polyps. Enhanced phosphorylation of Erk1/2 was observed in intestinal tumors of Min mice treated with MCC-555. On the other hand, expression of total Erk1/2 was not affected by MCC-555 treatment (Fig. 6A). To confirm the role of the ERK1/2 pathway in the regulation of MUC2 expression, SW480 human colorectal cancer cells were pre-treated with ERK pathway inhibitors, PD98059 and U0126. As shown in Fig. 6B, U0126 completely inhibited MUC2 induction by MCC-555 in SW480 cells. Another ERK pathway inhibitor, PD98059, also markedly reduced it. Taken together with in vivo and in vitro data, these results show that MCC-555 increases ERK phosphorylation in vivo and in vitro, thereby enhancing MUC2 expression.
Figure 6. The ERK pathway regulates MUC2 expression.
A, Tissue samples were prepared using polyps isolated from small intestine and colons. Western analysis was performed using anti-phospho-Erk1/2 (P-Erk1/2), anti-Erk1/2, and anti-actin antibodies and actin expression served as internal control. B, SW480 cells were pretreated with ERK1/2 pathway inhibitors PD98059 (20 μM) or U0126 (2 μM) for 30 min prior to the addition of MCC-555 (10 μM). After 24 h, expression of MUC2 was analyzed by RT-PCR. GAPDH served as the internal control.
Discussion
Promising agents for cancer prevention have been shown to consistently suppress tumorigenesis or the rate of tumor growth in Min mice, which served as a model for FAP. Our results demonstrated that treatment of Min mice with MCC-555 significantly suppressed polyp formation in the small intestine and slightly decreased colonic tumorigenesis (Fig. 4). Although a growing body of evidence from in vitro studies suggests that thiazolidinediones have an anti-proliferative effect (41) and induce apoptosis (7, 12, 28) and differentiation (3, 5) in colorectal cancer cells, conflicting studies showing that these agents can either increase or reduce colonic tumors in mice, raising concerns about the role of PPARγ in colon cancer. These inconsistent results from in vivo studies might be explained by the dose of PPARγ ligand used and/or properties of the various PPARs. For instance, it has been reported that PPARγ ligands show either tumor suppressing or promoting actions in breast cancer cells, depending on the doses used (42). Moreover, Niho et al. demonstrated that both PPARγ and PPARα ligands, pioglitazone and bezafibrate, respectively, suppress polyp formation in Apc1309 mice (18). Indeed, pioglitazone is also a weak PPARα agonist (43). We have reported that MCC-555 showed higher PPARα transactivation activity than any other synthetic PPARγ ligand (9-fold vs troglitazone, 7-fold vs ciglitazone, and 5-fold vs rosiglitazone), although PPARγ activation by MCC-555 was less than that produced by troglitazone, rosiglitazone, and ciglitazone (28). In this study, we also found that MCC-555 increased not only a PPARγ target gene but also a PPARα target gene. This evidence strongly suggests that MCC-555 is a dual agonist for PPARγ and PPARα. The dual agonist function should be considered to be involved in the anti-tumorigenic activity of MCC-555 and other PPARγ ligands.
Min mice were treated with MCC-555 at a dose of 30 mg/kg, a dose used in previous studies (44). This dose could increase PPARγ-responsive genes in adipose tissue of mice, and also could suppress growth of prostate cancer xenografts without lowering body weight in nude mice (44). In this study, treatment with MCC-555 increased both PPARγ- and PPARα-responsive genes including Glut2 and Cyp4a10, respectively, supporting a dual agonist. On the other hand, MCC-555 failed to induce PPAR-target genes I-Fabp and aP2 in liver tissue (Fig. 5A). A recent study suggested that ectopic induction of aP2 by PPAR activation is tissue specific in the mouse (39). Thus, expression of aP2 in the small intestine was investigated; however, the expression was not affected by MCC-555 treatment (data not shown). We also analyzed expression of Lpl in the liver because treatment with pioglitazone could increase its expression in Min mice (18). However, MCC-555 did not affect Lpl expression in the liver. Since MCC-555 has a weak PPARγ binding affinity and transactivation, compared to other PPARγ ligands (27, 28), the concentration of MCC-555 used may not be enough to induce all PPARγ-responsive genes.
In this study, we found that the tumor suppressor MUC2 is commonly induced by PPARγ ligands in several human cancer cells (Fig. 1 and 2). Muc2 was also found to be consistently up-regulated in intestinal tumors of Min mice following MCC-555 treatment. Since all tested PPARγ ligands increased MUC2 expression in SW480 cells, they likely work via a PPARγ-dependent pathway. Indeed, usage of GW9662, a PPARγ inhibitor, demonstrated that MUC2 expression occurs in a PPARγ-dependent manner (Fig. 3A). Interestingly, PPARγ ligands did not induce MUC2 expression in other colorectal cancer cells such as HT-29 and Caco-2 (data not shown). Recently, it was shown that MUC2 expression in HT-29 and Caco-2 cells was repressed epigenetically by DNA methylation and repressive histone code (45). This may explain why we were unable to induce MUC2 expression by PPARγ ligands in these two cell types, although these cells have been shown to express active PPARγ (3).
It has been suggested that alteration in mucin gene expression is likely associated with both the early steps of colon cancer development and later tumor progression. Inactivation of Muc2 causes tumor formation, accompanied by reduced apoptosis and increased proliferation and migration of intestinal adenocarcinoma cells (26). Oncogenic SOX9 or tumor suppressor p53 regulates MUC2 transcriptional activity negatively and positively (46, 47). These studies on transcriptional regulation of MUC2 imply that MUC2 can be associated with tumor suppression. In agreement with its tumor suppressing function, MUC2 expression is reduced in tumors compared to the corresponding sections of the small intestine and colon. Our results clearly showed that MCC-555 increased MUC2 expression in human cancer cells and in Min mice. Thus, MUC2 may be, in part, responsible for the anti-tumorigenic action of MCC-555.
To find the molecular mechanism underlying MUC2 induction by MCC-555, the ERK pathway was analyzed, since MCC-555 promotes phosphorylation of ERK1/2, but not p38 MAPK and the c-Jun N-terminal kinase, in human colorectal cancer cells (28). It is likely that the ERK signaling pathway is a major determinant in the control of diverse cellular processes, such as cell survival, proliferation, differentiation, and motility. However, the ERK pathway contributes to apoptosis induced by some genotoxic agents, such as diallyl disulfide and L-ascorbic acid (48, 49). In fact, ERK pathway inhibition by U0126 and PD98059 reduced MUC2 induction by MCC-555, suggesting that the ERK pathway regulates MUC2 expression (Fig. 6). Recently, Li et al. reported that troglitazone-induced apoptosis is in part ERK1/2 dependent, supporting our observation (50). In addition to this study, we demonstrated, for the first time, that MCC-555 promotes phosphorylation of Erk1/2 in vivo. These results strongly suggest that activation of the ERK signaling pathway by a synthetic PPARγ ligand is linked to tumor suppression. An understanding of the ERK pathway may be thus an important step toward utilizing agents including PPARγ ligands for cancer prevention. Although the use of MCC-555 is not striking when compared with results of various NSAIDs or EGFR inhibitors, our data provide a novel finding that the tumor suppressor MUC2 is a target of PPARγ and the possibility of combinational use of MCC-555 with NSAIDs or EGFR inhibitors in colorectal cancer prevention studies.
Acknowledgements
We thank Ms. Misty R. Bailey (University of Tennessee) for her critical reading of the manuscript, and Dr. Seong-Ho Lee, Dr. Yoichi Furukawa, Jason Liggett, and Mugdha Sukhthankar for providing valuable techniques and comments. We also thank Nancy Neilson for helping with the animal studies.
Grants: This work was supported by grants from the National Institutes of Health (RO1CA108975 to SJB) and from The François Aupetit Association (France) (to IVS).
Abbreviations
- PPAR
peroxisome proliferator-activated receptor
- PGJ2
prostaglandin 15-deoxy-Δ12,14-prostaglandin J2
- Min
multiple intestinal neoplasia
- APC
adenomatous polyposis coli
- l-FABP
liver fatty acid binding protein
- ERK
extracellular signal-regulated kinase
- FAP
familial adenomatous polyposis
Footnotes
No conflict of interest
REFERENCES
- 1.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 2.Koeffler HP. Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res. 2003;9:1–9. [PubMed] [Google Scholar]
- 3.Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998;4:1046–52. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 4.Nixon JB, Kamitani H, Baek SJ, Eling TE. Evaluation of eicosanoids and NSAIDs as PPARgamma ligands in colorectal carcinoma cells. Prostaglandins Leukot Essent Fatty Acids. 2003;68:323–30. doi: 10.1016/s0952-3278(03)00023-1. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura S, Miyazaki Y, Shinomura Y, Kondo S, Kanayama S, Matsuzawa Y. Peroxisome proliferator-activated receptor gamma induces growth arrest and differentiation markers of human colon cancer cells. Jpn J Cancer Res. 1999;90:75–80. doi: 10.1111/j.1349-7006.1999.tb00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarraf P, Mueller E, Smith WM, et al. Loss-of-function mutations in PPAR gamma associated with human colon cancer. Mol Cell. 1999;3:799–804. doi: 10.1016/s1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- 7.Baek SJ, Wilson LC, Hsi LC, Eling TE. Troglitazone, a peroxisome proliferator-activated receptor gamma (PPAR gamma) ligand, selectively induces the early growth response-1 gene independently of PPAR gamma. A novel mechanism for its anti-tumorigenic activity. J Biol Chem. 2003;278:5845–53. doi: 10.1074/jbc.M208394200. [DOI] [PubMed] [Google Scholar]
- 8.Lee KS, Park JH, Lee S, Lim HJ, Jang Y, Park HY. Troglitazone inhibits endothelial cell proliferation through suppression of casein kinase 2 activity. Biochem Biophys Res Commun. 2006;346:83–8. doi: 10.1016/j.bbrc.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 9.Chintharlapalli S, Papineni S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl) methanes inhibit colon cancer cell and tumor growth through PPARgamma-dependent and PPARgamma-independent pathways. Mol Cancer Ther. 2006;5:1362–70. doi: 10.1158/1535-7163.MCT-06-0002. [DOI] [PubMed] [Google Scholar]
- 10.Chintharlapalli S, Papineni S, Baek SJ, Liu S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl) methanes are peroxisome proliferator-activated receptor gamma agonists but decrease HCT-116 colon cancer cell survival through receptor-independent activation of early growth response-1 and nonsteroidal anti-inflammatory drug-activated gene-1. Mol Pharmacol. 2005;68:1782–92. doi: 10.1124/mol.105.017046. [DOI] [PubMed] [Google Scholar]
- 11.Shiau CW, Yang CC, Kulp SK, et al. Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARgamma. Cancer Res. 2005;65:1561–9. doi: 10.1158/0008-5472.CAN-04-1677. [DOI] [PubMed] [Google Scholar]
- 12.Shimada T, Kojima K, Yoshiura K, Hiraishi H, Terano A. Characteristics of the peroxisome proliferator activated receptor gamma (PPARgamma) ligand induced apoptosis in colon cancer cells. Gut. 2002;50:658–64. doi: 10.1136/gut.50.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baek SJ, Kim JS, Nixon JB, DiAugustine RP, Eling TE. Expression of NAG-1, a transforming growth factor-beta superfamily member, by troglitazone requires the early growth response gene EGR-1. J Biol Chem. 2004;279:6883–92. doi: 10.1074/jbc.M305295200. [DOI] [PubMed] [Google Scholar]
- 14.Cekanova M, Yuan JS, Li X, Kim K, Baek SJ. Gene alterations by peroxisome proliferator-activated receptor gamma agonists in human colorectal cancer cells. Int J Oncol. 2008;32:809–19. [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta RA, Brockman JA, Sarraf P, Willson TM, DuBois RN. Target genes of peroxisome proliferator-activated receptor gamma in colorectal cancer cells. J Biol Chem. 2001;276:29681–7. doi: 10.1074/jbc.M103779200. [DOI] [PubMed] [Google Scholar]
- 16.Chiu CH, McEntee MF, Whelan J. Sulindac causes rapid regression of preexisting tumors in Min/+ mice independent of prostaglandin biosynthesis. Cancer Res. 1997;57:4267–73. [PubMed] [Google Scholar]
- 17.Corpet DE, Pierre F. Point: From animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 18.Niho N, Takahashi M, Kitamura T, et al. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Res. 2003;63:6090–5. [PubMed] [Google Scholar]
- 19.Osawa E, Nakajima A, Wada K, et al. Peroxisome proliferator-activated receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology. 2003;124:361–7. doi: 10.1053/gast.2003.50067. [DOI] [PubMed] [Google Scholar]
- 20.Lefebvre AM, Chen I, Desreumaux P, et al. Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med. 1998;4:1053–7. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 21.Masubuchi Y. Metabolic and non-metabolic factors determining troglitazone hepatotoxicity: a review. Drug Metab Pharmacokinet. 2006;21:347–56. doi: 10.2133/dmpk.21.347. [DOI] [PubMed] [Google Scholar]
- 22.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 23.Ajioka Y, Watanabe H, Jass JR. MUC1 and MUC2 mucins in flat and polypoid colorectal adenomas. J Clin Pathol. 1997;50:417–21. doi: 10.1136/jcp.50.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogata S, Uehara H, Chen A, Itzkowitz SH. Mucin gene expression in colonic tissues and cell lines. Cancer Res. 1992;52:5971–8. [PubMed] [Google Scholar]
- 25.Weiss AA, Babyatsky MW, Ogata S, Chen A, Itzkowitz SH. Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J Histochem Cytochem. 1996;44:1161–6. doi: 10.1177/44.10.8813081. [DOI] [PubMed] [Google Scholar]
- 26.Velcich A, Yang W, Heyer J, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–9. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 27.Reginato MJ, Bailey ST, Krakow SL, et al. A potent antidiabetic thiazolidinedione with unique peroxisome proliferator-activated receptor gamma-activating properties. J Biol Chem. 1998;273:32679–84. doi: 10.1074/jbc.273.49.32679. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi K, Lee SH, Eling TE, Baek SJ. A novel peroxisome proliferator-activated receptor gamma ligand, MCC-555, induces apoptosis via posttranscriptional regulation of NAG-1 in colorectal cancer cells. Mol Cancer Ther. 2006;5:1352–61. doi: 10.1158/1535-7163.MCT-05-0528. [DOI] [PubMed] [Google Scholar]
- 29.Mesquita P, Jonckheere N, Almeida R, et al. Human MUC2 Mucin Gene Is Transcriptionally Regulated by Cdx Homeodomain Proteins in Gastrointestinal Carcinoma Cell Lines. J Biol Chem. 2003;278:51549–56. doi: 10.1074/jbc.M309019200. [DOI] [PubMed] [Google Scholar]
- 30.van der Sluis M, Melis MHM, Jonckheere N, et al. The murine Muc2 mucin gene is transcriptionally regulated by the zinc-finger GATA-4 transcription factor in intestinal cells. Biochem Biophys Res Communi. 2004;325:952–60. doi: 10.1016/j.bbrc.2004.10.108. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi K, Lee SH, Kim JS, Wimalasena J, Kitajima S, Baek SJ. Activating transcription factor 3 and early growth response 1 are the novel targets of LY294002 in a phosphatidylinositol 3-kinase-independent pathway. Cancer Res. 2006;66:2376–84. doi: 10.1158/0008-5472.CAN-05-1987. [DOI] [PubMed] [Google Scholar]
- 32.McEntee MF, Chiu CH, Whelan J. Relationship of beta-catenin and Bcl-2 expression to sulindac-induced regression of intestinal tumors in Min mice. Carcinogenesis. 1999;20:635–40. doi: 10.1093/carcin/20.4.635. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki K, Shimizu M, Okuno M, et al. Synergistic effects of RXR alpha and PPAR gamma ligands to inhibit growth in human colon cancer cells--phosphorylated RXR alpha is a critical target for colon cancer management. Gut. 2007;56:1557–63. doi: 10.1136/gut.2007.129858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrais M, Pigny P, Copin M-C, Aubert J-P, Van Seuningen I. Induction of MUC2 and MUC5AC Mucins by Factors of the Epidermal Growth Factor (EGF) Family Is Mediated by EGF Receptor/Ras/Raf/Extracellular Signal-regulated Kinase Cascade and Sp1. J Biol Chem. 2002;277:32258–67. doi: 10.1074/jbc.M204862200. [DOI] [PubMed] [Google Scholar]
- 35.Bren-Mattison Y, Meyer AM, Van Putten V, et al. Antitumorigenic Effects of Peroxisome Proliferator-Activated Receptor-{gamma} (PPAR{gamma}) in Non-small Cell Lung Cancer Cells (NSCLC) are Mediated by Suppression of COX-2 via Inhibition of NF-{kappa}B. Mol Pharmacol. 2008;73:709–17. doi: 10.1124/mol.107.042002. [DOI] [PubMed] [Google Scholar]
- 36.Rumi MA, Ishihara S, Kadowaki Y, et al. Peroxisome proliferator-activated receptor gamma-dependent and -independent growth inhibition of gastrointestinal tumour cells. Genes Cells. 2004;9:1113–23. doi: 10.1111/j.1365-2443.2004.00793.x. [DOI] [PubMed] [Google Scholar]
- 37.Fujimura T, Kimura C, Oe T, et al. A selective peroxisome proliferator-activated receptor gamma modulator with distinct fat cell regulation properties. J Pharmacol Exp Ther. 2006;318:863–71. doi: 10.1124/jpet.106.102459. [DOI] [PubMed] [Google Scholar]
- 38.Im SS, Kim JW, Kim TH, et al. Identification and characterization of peroxisome proliferator response element in the mouse GLUT2 promoter. Exp Mol Med. 2005;37:101–10. doi: 10.1038/emm.2005.14. [DOI] [PubMed] [Google Scholar]
- 39.Motojima K. Differential effects of PPARalpha activators on induction of ectopic expression of tissue-specific fatty acid binding protein genes in the mouse liver. Int J Biochem Cell Biol. 2000;32:1085–92. doi: 10.1016/s1357-2725(00)00046-7. [DOI] [PubMed] [Google Scholar]
- 40.Lee HW, Ahn DH, Crawley SC, et al. Phorbol 12-myristate 13-acetate up-regulates the transcription of MUC2 intestinal mucin via Ras, ERK, and NF-kappa B. J Biol Chem. 2002;277:32624–31. doi: 10.1074/jbc.M200353200. [DOI] [PubMed] [Google Scholar]
- 41.Brockman JA, Gupta RA, Dubois RN. Activation of PPARgamma leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology. 1998;115:1049–55. doi: 10.1016/s0016-5085(98)70072-1. [DOI] [PubMed] [Google Scholar]
- 42.Clay CE, Namen AM, Atsumi G, et al. Magnitude of peroxisome proliferator-activated receptor-gamma activation is associated with important and seemingly opposite biological responses in breast cancer cells. J Investig Med. 2001;49:413–20. doi: 10.2310/6650.2001.33786. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto J, Kimura H, Moriyama S, et al. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun. 2000;278:704–11. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- 44.Kumagai T, Ikezoe T, Gui D, et al. RWJ-241947 (MCC-555), a unique peroxisome proliferator-activated receptor-gamma ligand with antitumor activity against human prostate cancer in vitro and in beige/nude/ X-linked immunodeficient mice and enhancement of apoptosis in myeloma cells induced by arsenic trioxide. Clin Cancer Res. 2004;10:1508–20. doi: 10.1158/1078-0432.ccr-0476-03. [DOI] [PubMed] [Google Scholar]
- 45.Vincent A, Perrais M, Desseyn JL, Aubert JP, Pigny P, Van Seuningen I. Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (MUC2, MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene. 2007;26:6566–76. doi: 10.1038/sj.onc.1210479. [DOI] [PubMed] [Google Scholar]
- 46.Blache P, van de Wetering M, Duluc I, et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ookawa K, Kudo T, Aizawa S, Saito H, Tsuchida S. Transcriptional activation of the MUC2 gene by p53. J Biol Chem. 2002;277:48270–5. doi: 10.1074/jbc.M207986200. [DOI] [PubMed] [Google Scholar]
- 48.Park S, Park CH, Hahm ER, et al. Activation of Raf1 and the ERK pathway in response to l-ascorbic acid in acute myeloid leukemia cells. Cell Signal. 2005;17:111–9. doi: 10.1016/j.cellsig.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Xiao D, Choi S, Johnson DE, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 50.Li M, Lee TW, Yim AP, Mok TS, Chen GG. Apoptosis induced by troglitazone is both peroxisome proliferator-activated receptor-gamma- and ERK-dependent in human non-small lung cancer cells. J Cell Physiol. 2006;209:428–38. doi: 10.1002/jcp.20738. [DOI] [PubMed] [Google Scholar]