Abstract
The present study examined EEG gamma (35-70 Hz) spectral power distributions during worry inductions in participants suffering from generalized anxiety disorder (GAD) and in control participants without a history of psychiatric illness. As hypothesized, the EEG gamma band was useful for differentiating worry from baseline and relaxation. During worry induction, GAD patients showed higher levels of gamma activity than control participants in posterior electrode sites that have been previously associated with negative emotion. Gamma fluctuations in these electrode sites were correlated with subjective emotional experience ratings lending additional support to interpretations of negative affect. Following 14 weeks of psychotherapy, the GAD group reported less negative affect with worry inductions and the corresponding gamma sites that previously differentiated the clinical from control groups changed for the GAD patients in the direction of control participants. These findings suggest converging evidence that patients suffering from GAD experience more negative emotion during worry and that the EEG gamma band is useful for monitoring fluctuations in pathological worry expected to follow successful treatment.
Keywords: Generalized Anxiety Disorder, Electroencephalography, Gamma band, Psychotherapy, Emotion
Introduction
EEG gamma band and emotion
The gamma rhythm (30-100 Hz) is widespread in the central nervous system including in areas associated with emotional processing such as the amygdala and perirhinal cortex (Collins et al., 2001). Recent research continues to suggest connections between EEG gamma activity and emotion with special emphasis on negative emotional processing (e.g. Luo et al., 2007; Matsumoto et al., 2006). Intracranial field potentials recorded from the amygdala confirm that gamma power is highest for aversive stimulus presentations as compared with neutral or pleasant stimuli (Oya et al., 2002).
Spectral power in the gamma band has been associated with emotional processing when both EEG alpha frequency and beta frequency activity have not shown sensitivity to emotional stimulus variations (Müller et al., 1999). Another attribute of gamma as opposed to other indexes of emotional perception is that gamma induced by emotional stimuli is typically not phase-locked to the onset of visual stimulus presentations (Oya et al., 2002). Instead, induced gamma is usually measured over periods of several seconds as with successive presentations of visual stimuli (Müller et al., 1999). This suggests that induced gamma reflects a more integrative or reflective aspect of processing emotional material.
Consistent with the idea that induced gamma fluctuates with extended periods of emotional processing, experimental tasks thought to induce emotional experience have been shown to increase gamma activity. When asked to imagine a phobic object, individuals suffering from a specific phobia show increases in gamma band activation as well as increases in heart rate and respiration (Gemignani et al., 2000). Also, gamma has been shown to decrease during periods of relaxation and to increase during periods of imagining negative emotional material (Sebastiani et al., 2003). Thus, the present study records periods of several minutes during which emotional experiences were thought to be induced.
Distributions of gamma activation recorded from the scalp surface may be important for discovering links between specific emotional experiences and physiological recordings. For example, relatively more gamma power in the right temporal area is associated with positively valenced stimulus presentations and relatively more gamma in the left temporal area is associated with negative stimulus presentations (Müller et al., 1999). The present study sought to contribute to a growing literature linking distributions of induced EEG gamma spectral power to pathological and non-pathological experiences of emotion. We utilized EEG spectral power as well as ratings of subjective experience to assess differences between GAD and non-psychiatric control groups; between baseline, relaxation and worry tasks; and between pre- and post-treatment assessments in our GAD group. Based on a literature linking GAD and worry to negative emotion, we expected gamma during worry to differentiate our patients from controls and to be sensitive to changes in the GAD group expected to follow treatment.
Worry, GAD and reports of negative affect
The present study focused on worry as a central negative emotional experience for chronic worriers suffering from GAD. Generalized anxiety disorder (GAD) is characterized by excessive anxiety and uncontrollable worry about a variety of topics (DSM-IV-TR; American Psychiatric Association, 2000). The process of worry in itself is a negative emotional experience whether or not the worrier suffers from GAD (Borkovec & Inz, 1999; Andrews & Borkovec, 1998). Though worry increases reports of negative affect and anxiety, particular physiological systems may not register emotional arousal during worry. When asked to worry, research participants report increases in anxiety while cardiovascular measures do not consistently reflect the change (Borkovec & Hu, 1990; Borkovec et al., 1993). Also, chronic worry does not increase fear-potentiated startle eyeblink EMG amplitudes to emotional stimuli (Nitschke et al., 2002) or muscle activity recorded using EMG (Oathes, Bruce, & Nitschke, in press; though see that paper for evidence of worry influences on motor preparation). Thus, it is important to identify a psychophysiological measure which not only characterizes worry experiences but also differentiates individuals suffering from pathological anxiety (GAD) from non-anxious individuals. The present study suggests that the EEG gamma band might function as such an index.
Hypotheses
Our initial manipulation check to test the hypothesis that gamma might be sensitive to experimentally induced emotional intensity was based on a prediction that gamma spectral power would be increasingly present in the order from least to greatest beginning with our relaxation task followed by baseline recordings and that worry would facilitate the most gamma activity across our two groups. The relaxation task served as a comparison to worry in that relaxation was also a cognitive induction (which may influence gamma activity; cf. Jensen et al., 2007) but was expected to differ from worry according to the degree of negative affect induced by the experimental instructions. Based on relationships between negative emotion and GAD (e.g. Borkovec & Inz, 1990; Borkovec & Ruscio, 2001) and between negative emotion and a particular distribution of scalp recorded induced EEG gamma activity (Müller et al., 1999), we expected greater left posterior gamma activity for the GAD group compared to control participants. It was expected that this difference would be especially pronounced during the worry task, as this was the task thought to be most relevant to the GAD diagnosis and its associated negative emotionality. Worry is especially relevant to studying GAD in that chronic uncontrollable worry is the essential feature common to all individuals diagnosed with GAD. Though the Penn State Worry Questionnaire or another measure of trait anxiety might be relevant to chronic worry and GAD, the present study sought to examine a less often assessed aspect of worry: negative affect. To support interpretations related to negative emotionality for group differences in EEG gamma during worry, we assessed ratings of subjective emotional experience during our physiological recording sessions that we expected to correlate with EEG gamma activity.
Method
Participants
Anxious participants were drawn from newspaper advertisements or from outside agency referrals. Advertisements also invited control participants with a request for individuals between 18-65 years of age “without current or past anxiety or depression difficulties.” Fifteen clients and 15 control participants were used from the first wave recruited for a therapy outcome study (see Newman et al., 2004 for details). The study was approved by the Office for Research Protections (IRB) at the Pennsylvania State University. All participants gave signed informed consent (in accord with Helsinki Declaration) to participate in the therapy outcome portion of this study and signed a separate consent for the psychophysiological assessments. For GAD clients, clinical assessors conducted phone screens in order to initially assess a GAD diagnosis. The assessor then met eligible clients to administer the Anxiety Disorder Interview Schedule-IV (ADIS-IV; DiNardo, Brown, & Barlow, 1994) as well as a variety of psychiatric symptom scales including the Beck Depression Inventory (BDI-II; Beck et al., 1996) and Penn State Worry Questionnaire (PSWQ; Meyer et al., 1990). Within 2 weeks of the first interview, a clinical psychologist conducted a second ADIS-IV interview to ensure the reliable presence of a GAD diagnosis. Criteria for inclusion of GAD clients were: Formal principal diagnosis of GAD according to DSM-IV criteria by either both assessors or consensus of the assessors in case of discrepancy; lack of psychosis, substance abuse and/or medical or physical conditions linked to anxiety; a global severity of 4 or more (moderate anxiety) on an 0-8 point clinician determined severity scale; and between ages 18-65. Psychoactive medication use was permitted if the client agreed to maintain constant dosage levels for the duration of treatment and assessment. Among the fifteen participants used for analysis, three were taking a selective serotonin reuptake inhibitor (SSRI; two to treat major depressive disorder and the third for panic disorder). All but two of our GAD sample had additional diagnoses including six diagnoses of social phobia, four of major depressive disorder, two of panic disorder, two of specific phobias, and one of obsessive compulsive disorder. The final sample of 15 GAD clients consisted of 13 female and 2 male participants. All were Caucasian by self-identified ethnicity and ranged in age from 22 to 45 years (M= 36.4, SD= 8.21). Control participants were also given the ADIS-IV and were included based on lack of current or past diagnosable psychiatric disorder, as well as lack of current or past alcohol or substance dependence or any prior psychological or pharmacological treatment for a psychological problem. Control participants were asked for medical histories and excluded if medical problems frequently linked to anxiety were discovered (e.g., thyroid problems). The 15 members of the control group consisted of 12 females and 3 males. The control group was also Caucasian with age ranges from 22 to 45 years (M= 36.6, SD= 7.84). The two groups were not significantly different in age, gender or education (i.e. category of educational attainment such as high school diploma, 2 year degree, etc.).
Procedure
One week before physiological sessions, GAD clients were given a tour of the laboratory including a description of recording methods and tasks to be administered. The remaining procedures of the experimental session for GAD clients and control participants were identical. As electrodes were being affixed, he/she was told, “At one point during the experiment, you will be asked about a worry topic. Can you have something in mind for later use?” Participants were encouraged to consider a topic of “current concern” that they would be able to “worry intensely about” for several minutes. Participants were then left alone in the room with the door closed. An audiotaped instruction sequence announced the tasks, while the experimenters monitored the participant by videocamera and via intercom in an adjacent room.
For the duration of the experiment, participants sat in a noise-controlled room. A 17-channel EEG cap (ElectroCap International) was used to obtain signals from 15 channels referenced to linked ears: left and right frontal (F3, F4), midline frontal (FZ), midline central (CZ), midline parietal (PZ), left and right temporal (T3, T4, T5, T6), left and right parietal (P3, P4, P5, P6), and occipital regions (O1, O2). Electrode placement was based on the International 10-20 System (Jasper, 1958). Signals were amplified using a Nihon-Kohden 21-channel electroencephalograph and digitized by a Neuroscan (Neurosoft) system. Data were collected at a sampling rate of 256 Hz/channel with a 60 Hz notch filter. All impedance levels were 5K ohms or lower. Small Ag/AgCl electrodes were placed above and below the right eye and on the lateral sides (outer canthus) of each eye to record vertical and horizontal electro-oculogram (EOG) activity. Electro-oculogram activity was used to perform off-line correction of eye artifact in EEG channels resulting from eye movement by a semi-automated procedure bundled with Neuroscan software. An average eye blink waveform was created automatically, visually inspected for viability, and then manual blink by blink decisions for rejection or inclusion were made for detected perturbations that resembled the average. Though movement artifact was unusual given the mental nature of our tasks, any significant periods of movement artifact were manually cut from epochs of EEG data before further processing was instantiated. First, participants were asked to: “Please sit still for the next several minutes while we calibrate the recordings” while two minutes of baseline data were collected. The next task involved asking participants to “deeply relax for the next several minutes” and to “please focus your attention on your own breath” and on “each inhalation and exhalation” while also trying to “with the inhale, gather the tension in and with the exhale, let it all go.” This relaxation task lasted for five minutes. After this, participants were asked to engage in a worry task also for five minutes. Each participant was requested to worry “as intensely as you can, in the way that you usually worry” about the topic self-selected during the preparation stage of the experiment. Participants generally reported worry topics related to upcoming potentially negative events, concerns about relationships, and concerns about performance/competence at work/school across both groups. For all three tasks, participants were asked to keep movement to a minimum and to sit with eyes closed during recording. A modified Osgood's Semantic Differential Scale (Osgood & Tannenbaum, 1957) was administered to participants before the baseline and after the relaxation and worry inductions. Participants were asked to rate their current emotional state on each of fifteen items by placing a vertical line along a horizontal continuum of opposing descriptors. For example, the first item of the scale asked the participants to place a line on the scale with “anxious” as the anchor on the left side of the continuum and “relaxed” as the anchor on the right side of the continuum. For the present study, we focused on a subset of the most relevant scales including: relaxed vs. anxious, fearful vs. courageous, sad vs. happy, negative vs. positive, and liked vs. didn't like this part of the experiment. This laboratory session was conducted in an identical manner for the GAD clients after they completed 14 psychotherapy sessions. Psychotherapy consisted primarily of cognitive behavioral psychotherapy shown to be effective for treating GAD (Borkovec & Ruscio, 2001). Cognitive behavioral therapy included cognitive therapy, applied relaxation, and imagery rehearsal of cognitive and relaxation coping strategies in a self-control desensitization technique (see Borkovec & Sharpless, 2004) during the first hour of each session; 11 patients received supportive listening and 4 received interpersonal therapy during the second hour (see Newman et al., 2004).
Analyses
The initial Baseline, Relax and Worry tasks were analyzed for the 30 participants. EEG data (eye artifact corrected) were fast Fourier transformed (cosine, 10% taper), converted to amplitudes, averaged, then squared so that spectral power could be calculated for the gamma (35-70 Hz) frequency band with averages taken according to the individual tasks. These averages were then log-transformed to normalize distributions for subsequent analysis.
Based on prior gamma findings in temporal and parietal electrode sites, a Group (pre-therapy GAD and control participants) x Task (Baseline, Relax, and Worry) x Electrode Site (T3, T4, P3, P4, T5, T6) analysis of variance (ANOVA) was conducted. Worry induction was the most relevant task for understanding group differences between GAD patients and control participants. This task was expected to differentiate the groups based on findings of negative emotionality associated with GAD (Borkovec & Inz, 1990; Borkovec & Ruscio, 2001) and associated with the gamma frequency band (Müller et al., 1999; Gemignani et al., 2000; Sebastiani et al., 2003; Luo et al., 2007; Matsumoto et al., 2006). Thus, we conducted a separate ANOVA with one between-subjects factor (group) and one within-subjects factor (electrode site) for worry, alone. Uncorrected t-scale difference maps confirmed these electrode sites as reflecting primary task differences and group differences (GAD pre-therapy vs. controls; GAD pre- vs. post-treatment) during the worry task. Individual electrode site effects are not reported when not part of an interaction with task or group status or within the worry task because specific hypotheses concerning EEG spectral power distributions independent of these factors were not part of the conceptualization of this study. Effect sizes were calculated for primary contrasts between GAD and control participants and between pre and post-therapy GAD participants (Cohen, 1998). Corrections for small sample size were employed (Hedges & Olkin, 1985) and 95% confidence intervals for effect sizes are reported using separate asymptotic standard effects for independent or dependent measures (Kline, 2004). F-ratio degrees of freedom were calculated using Greenhouse-Geisser epsilon corrections, where appropriate, to counteract heterogeneity of variance violations. Significant main effects from ANOVAs were followed up with independent and paired samples t-tests for group and task contrasts, respectively. Pearson's 2-tailed correlations with subjective experience ratings focused on EEG indicators of group and pre- to post-therapy differences.
Results
Worry and EEG gamma
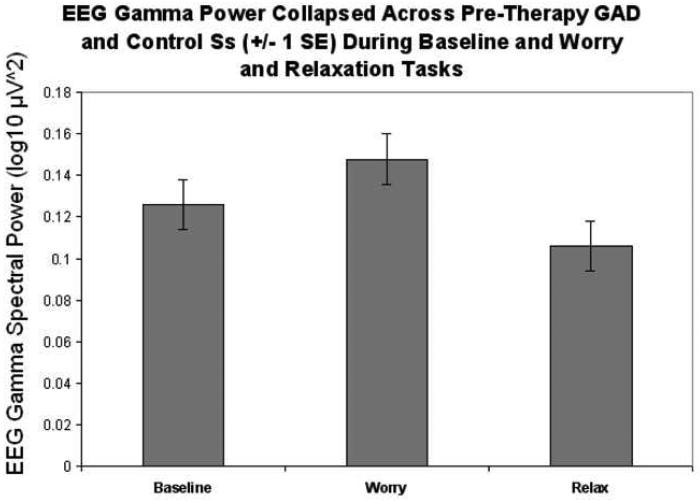
In the gamma frequency band (35-70 Hz), there was a main effect for task, F(2, 56)= 20.25, ε= 0.74, p<.005 (see Figure 1; though also see worry specific results below and Figure 2). A main effect of group status (GAD vs. control) across all tasks was not substantiated F(1, 28)= 3.13, p<.90. There was no group by task interaction. Post-hoc analysis of task differences indicated less gamma power during the relaxation task (M= −0.991, SE= 0.020) compared to the worry task (M= −0.858, SE= 0.026, p<.001), less gamma for relaxation compared to the baseline task (M= −0.913, SE= 0.019, p <.001), and a trend which approached significance indicating more gamma activity for the worry compared to the baseline task (p= .059). These results indicated that gamma activity differentiated the three mental tasks in the predicted direction. A significant interaction between task and electrode site, F(10, 280)= 4.70, ε= 0.23, p<.001, indicated that the distribution of EEG gamma activity reflected a task difference. There was more gamma power for the worry task and this was especially true in the left temporal area, particularly in the T3 electrode site. For the baseline and relaxation tasks, there was bilateral gamma activity especially in the T3 and T6 electrodes. These relationships were consistent across GAD and control participants. Collapsing across electrode sites for each hemisphere, there was no main effect of hemisphere and no interactions between hemisphere and task or group.
Figure 1.
EEG gamma spectral power for baseline, worry and relaxation tasks collapsed across groups (GAD clients before treatment and control participants) and electrode sites of interest (temporal and parietal).
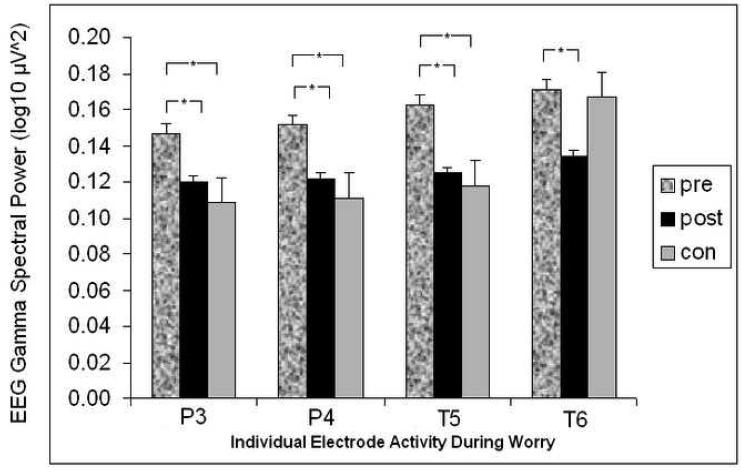
Figure 2.
For worry task alone, EEG gamma power according to participant group (GAD pre-therapy, GAD post-therapy, and control) and according to electrode site (“T” for temporal sites, “P” for parietal; odd numbers for left side electrodes, even numbers for right side). * indicates significant difference at p<.05.
For the worry task, there was a main effect of electrode site F(5, 140)= 3.87, ε= 0.57, p<.01 and a group main effect F(1, 28)= 4.46, p<.05. Specifically, there was more left posterior activity for worry and more overall gamma for the GAD compared to control participants. There was no interaction between group status and electrode site. There were no effects involving hemisphere. Based on the significant group effect for the worry task and based on a subset of electrodes defined a priori (temporal and parietal sites) hypothesized to reflect emotional state effects during worry, the following independent samples (GAD pre-therapy vs. control) and paired sample t-test (GAD pre-therapy vs. GAD post-therapy) comparisons were conducted.
Group differences in EEG gamma during worry
GAD pre-therapy vs. controls
To determine the specific sites where pre-treatment GAD and control groups differed, independent samples were run and showed significant differences at left parietal (P3), t(28)= 3.13, p<.01, d= 1.15 (95% CI= 0.38-1.92), right parietal (P4), t(28)= 3.24, p<.01, d= 1.15 (95% CI= 0.38-1.92) and left temporal (T5), t(28)= 2.90, p<.01, d= .94 (95% CI= 0.19-1.70) sites (see Table 1). There were no other significant differences between groups in the sites of interest.
Table 1.
Group Differences in Regional EEG Gamma Band Activity During Worry Induction
| Pre-Treatment GAD | Post-Treatment GAD | Controls | |
|---|---|---|---|
| Pre-Treatment GAD | P3*, P4*, T5*, T6* | ||
| Post-Treatment GAD | NS | ||
| Controls | P3**, P4**, T5** |
Note. “Treatment” consisted of Cognitive Behavioral Psychotherapy.
indicates significant difference at p<.05
indicates significant difference at p<.01
NS indicates no significant differences. ‘P’ and ‘T’ denote parietal and temporal lobe electrode sites. Even numbers (i.e. 4 and 6) indicate right hemisphere electrodes; Odd numbers (i.e. 3 and 5) indicate the left hemisphere.
GAD pre- vs. post-therapy
A graphical representation of GAD differences from preto post-therapy show changes in the gamma band during the worry task mostly in posterior areas and more consistently in the left than right hemisphere (see Figure 2). The pre to post treatment main effect across all electrode sites was not significant F(1, 14)=2.64, p>.05 but the electrode main effect F(5,70=7.30, p<.001 and treatment effect by electrode site interaction F(5,70)=3.00, p<.05 were both significant. Driving this interaction, significant differences between the pre- and post-therapy assessments were present in left parietal (P3), t(14)= 2.25, p<.05, d= 0.68 (95% CI= 0.26-1.10), right parietal (P4), t(14)= 2.33, p<.05, d= 0.72 (95% CI= 0.20-1.23), left temporal (T5), t(14)= 2.94, p<.05, d= 0.79 (95% CI= 0.29-1.28) and right temporal (T6), t(14)= 2.56, p<.05, d= 0.61 (95% CI= 0.26-0.95) sites. Thus, the P3, P4 and T5 electrode sites during the worry task not only differentiated GAD clients from control participants but also were sites of change in gamma activity for the GAD clients following treatment (see Table 1).
GAD post-therapy vs. controls
Statistical analyses showed normalization of gamma in the GAD clients. Tests of differences between post-therapy GAD clients and controls during the worry task revealed no significant differences in any of the four temporal or two parietal electrode sites assessed (all with p>.05; see Table 1) despite a non-significant residual difference at T6 indicated by the graph (Figure 2).
Subjective experience
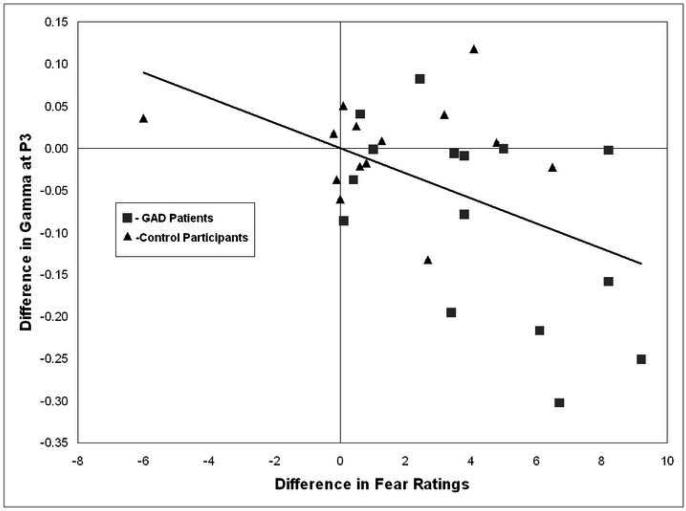
As expected, GAD participants at pre-therapy were more anxious than control participants before baseline recordings and following worry inductions at which time they reported also feeling also more sad, fearful, and negative (all with p<.05 after Bonferonni correction). In the relevant set of electrodes which differentiated GAD from control groups, there were no significant correlations between subjective experience ratings immediately following the worry task and EEG gamma power during the worry task (uncorrected or Bonferonni corrected; all with p>.05). However, an analysis of baseline predictors of worry activation in relevant electrodes indicated that more reported fear following baseline (just before worry induction) predicted more left temporal (T3) gamma during worry, r= .53, p<.05. The T3 site did not differentiate the groups at pre-treatment though it was contiguous with sites of significant difference and there was a trend suggesting that GAD patients had higher levels of gamma activity during worry compared to controls at this site (GAD M=−0.78509; Controls M=−0.8255). To further explore relationships between pre-worry induction subjective experience (following baseline) and subsequent worry responses, difference scores were calculated in gamma activation and subjective experience between pre- and post-worry to test for correlations between them. To minimize effects of multiple comparisons, we considered relationships between subjective report and gamma as significant only when physiology and subjective experience scores differentiated GAD from control groups, separately, and when the differences were also correlated with each other. One electrode site and subjective experience measure met these criteria: The difference between baseline and worry in the P3 electrode (which also differentiated the groups for the worry task alone) was greater for GAD than for control participants, t(28)= 2.37, p<.05, d= 0.87 (95% CI= 0.11-1.62). The difference between pre- (following baseline) and post-worry ratings of fearfulness was greater for GAD participants, t(28)= 2.76, p= .01, and these difference measures were significantly correlated, r=−.49, p<.01 across the GAD patients and control participants (see Figure 3). In this measure of fearfulness at post-therapy, experiences of fear decreased for the GAD patients following worry induction, t(12)= 3.33, p<.01, d= 1.38 (95% CI= −0.04-2.80) as well as before worry induction, t(12)= 2.60, p<.05, d= 0.91 (95% CI= 0.17-1.65).
Figure 3.
Difference in gamma activity from baseline to worry (baseline-worry) at the P3 electrode (left parietal) as a function of differences from baseline to worry in fear ratings (baseline-worry) across GAD patients (square markers) and control participants (triangle markers). The direction of the correlation suggests that increased fear ratings from baseline to worry were associated with increased gamma activity in the left parietal region (r=−0.49, p<.01, 2-tailed).
Symptom improvement
On the PSWQ, 12 of 15 participants showed improvement following treatment by an average of 20 points (SD= 12) as opposed to declines on average of three points (SD= 3) for the few who did not improve. The BDI results were similar: 13 of 15 improved by an average of 11 points (SD= 7) with the two non-responders to the treatment declining by three and four points, respectively (SD=0.7). The clinical criterion of 50% reduction in symptom scales was reached for one patient according to the PSWQ (7% of patients) and eight patients according to the BDI (53% of patients). Thus, the typically assessed clinical symptom improvements are consistent with improvements in subjective emotional experience and in posterior gamma activation during worry inductions.
Exploratory alpha and beta
Though the gamma band was of primary relevance in the present study, exploratory analyses were conducted for the alpha and beta frequency bands to explore depressive patterns in EEG activity (Henriques & Davdison, 1991) as well as patterns consistent with anxious arousal or fear (Heller et al., 1997; Nitschke et al., 1999; 2000). Alpha (7.5-13.5 Hz) as well as low (14-20 Hz) and high beta (21-30 Hz) spectral power were used to test for an anterior laterality ([F3−F4]/[F3+F4]) asymmetry and an anterior vs. posterior caudality (similar formula) group difference. None of the group main effects were significant (ps>.05) suggesting that despite the presence of depression and arousal type anxiety in some of our clinical group members, the patients did not show previously reported asymmetries associated with depression or anxious arousal.
Gender and medication
We separated groups based on medication status and gender to compare their means and standard deviations. In the electrodes which showed a group difference at pre-therapy during the worry task (P3, P4, T5), GAD subjects on medication were within one SD of the group average for non-medicated GAD patients. Also, GAD patients at post-therapy who were on medications were within one SD of non-medicated patients in the overlapping electrodes which showed a pre- to post-therapy difference. In the same sites, males were within one SD of females for both comparisons. Thus, despite lack of power to do a comprehensive evaluation of medication and gender effects, the EEG results highlighted in the present study do not show evidence of confound by medication or gender.
Discussion
Consistent with predictions based on EEG gamma ties to emotionality (e.g. Sebastiani et al., 2003; Müller et al., 1999), gamma differentiated the worry task from both a relaxation induction period and the resting baseline period (marginal effect). Worry induced a particular pattern of gamma activation which was highly similar for GAD and control participants (no group by electrode site interaction). Instead, the amount of gamma in the relevant sites distinguished our anxious from non-anxious control group during worry. The results are consistent with taxometric analyses of worry which suggest that pathological and normal worry differ primarily by dimension rather than by category (Ruscio et al., 2001). The study also sought to determine a psychophysiological index linked to negative emotionality that could distinguish individuals suffering from GAD from non-anxious individuals as well as reflect normalizing changes following treatment. As predicted, gamma band activity during the worry task differentiated GAD from control participants at pre-therapy and reflected reduction of differences following psychotherapy. Relationships between EEG gamma and subjective experience measures suggest that the GAD and control groups differed with respect to their emotional experiences during worry states. The distribution of these differences, in light of findings from Müller et al. (1999) and as related to subjective reports of emotion during our recordings, support the conclusion that those suffering from GAD experience more negative emotion when worrying compared to control participants.
The rationale for adding a psychophysiological assessment of negative emotionality again at post-treatment was based on the contention that in addition to improvements in clinical scales of depressive and anxious symptoms following therapy, successful intervention should also attenuate negative emotion during worry for individuals suffering from GAD. Gamma changes followed symptom changes following treatment in the direction of control participants. The changes occurred in the same electrode sites that differentiated the GAD from the control group at pre-therapy. The data thus suggest that gamma activity is sensitive to reductions of group differences between GAD and non-anxious individuals that are expected to follow successful treatment.
Caveats to the present results include that the present sample consisted entirely of Caucasian participants. Future research should seek to replicate the present findings with individuals of different ethnic backgrounds. Also, in the interest of studying a representative sample of typical GAD patients, the present study allowed for co-morbid anxiety and depressive disorders in our sample. The exploratory laterality/caudality analyses suggest that despite co-morbid depression and other forms of anxiety in our GAD group, they did not show EEG profiles characteristic of either of these symptom clusters. However, future research with larger groups of participants endorsing a variety of symptoms and disorders will be important to assess potential influences of these factors on EEG gamma. Though muscle activity frequencies overlap with the gamma band sampled in the present study, we observed muscle activity contamination across electrode sites very infrequently during our mental tasks (see Procedures section on how these portions of data were removed). Also, worry inductions using an identical induction procedure do not cause overt muscle activity as recorded in several sites of muscle recordings (Oathes, Bruce, & Nitschke, in press). Thus, we view muscle activity explanations of our data to be unlikely. In terms of therapy outcome, the present results are confirmatory of self-report and assessor reports of symptom improvements using this protocol to treat GAD (Borkovec & Ruscio, 2001). However, for a number of reasons, the data do not stand alone as indicating the specific effectiveness of this treatment. First, we did not assess maturation effects in a group of GAD patients not undergoing psychotherapy or undergoing a more benign treatment. Second, we did not assess the control participants at multiple time points. The patients thus may have naturally experienced some decline in the measures which differentiated the groups at pre-treatment. The post-treatment data in the present study offer converging evidence that EEG gamma activity reflects important indicators of negative affect in GAD patients during worry by the fact that gamma is sensitive to fluctuations in these experiences. It is expected that changes such as this would follow from successful treatment. However, the causal link between these changes and the treatment must be further established. The changes from pre- to post-therapy in the region of the T6 electrode (see Figure 2) were unexpected especially in light of there being no group difference at this site at the pre-treatment assessment. It may be the case that GAD patients have learned to dampen emotional experiences during worry in a global way, as suggested by the bilateral changes in posterior parietal gamma. Future research evaluating changes in the experience of worry and evaluating ways in which GAD patients have learned to cope with the emotional repercussions of worry might suggest an explanation for these findings. Since worry induction was the only emotional task assessed in the present study, it is unclear whether similar group and treatment differences might have also been found using other emotion inducing manipulations (e.g. passively viewing affect laden stimuli). Future research with a wider variety of tasks will be useful to highlight the specificity of the present findings to the worry experience.
These results support continued research to link findings in affective neuroscience, psychopathology, therapy outcome monitoring and studies of emotional processing. The worry induction and its successful impact on subjective emotional reports differentiated anxious from non-psychiatric participants as well as characterized the pre- to post-treatment differences in our anxious participants. Consistent with the directional influence of worry on negative emotion, path analysis supports a stronger influence of worry on anxious feelings rather than the reverse direction (Gana et al., 2000). The findings support the importance of physiological data, and especially EEG gamma power, for studying affective symptoms in psychiatric patients and also to monitor fluctuations in affective symptoms expected to follow successful treatment. Analyses relating physiological measures to prototypical assessor and self-report symptom measures of anxiety and depression are planned for the future.
Acknowledgements
The authors wish to thank Jack Nitschke and Andreas Keil for helpful comments as well as Alison Staples for editing assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: 2000. Text revision. [Google Scholar]
- Andrews VH, Borkovec TD. The differential effects of induction of worry, somatic anxiety, and depression on emotional experience. Journal of Behavior Therapy and Experimental Psychiatry. 1998;19:21–26. doi: 10.1016/0005-7916(88)90006-7. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories –IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Hu S. The effect of worry on cardiovascular response to phobic imagery. Behaviour Research and Therapy. 1990;28:69–73. doi: 10.1016/0005-7967(90)90056-o. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Inz J. The nature of worry in generalized anxiety disorder: A predominance of thought activity. Behaviour Research and Therapy. 1999;28:153–58. doi: 10.1016/0005-7967(90)90027-g. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Lyonfields JD, Wiser SL, Deihl L. Behaviour Fesearch and Therapy. 1993;31:321–324. doi: 10.1016/0005-7967(93)90031-o. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Rucio A. Psychotherapy for generalized anxiety disorder. Journal of Clinical Psychology. 2001;62:37–45. [PubMed] [Google Scholar]
- Borkovec TD, Sharpless B. Generalized anxiety disorder: Bringing cognitive behavioral therapy into the valued present. In: Hayes S, Follette V, Linehan M, editors. New directions in behavior therapy. Guilford Press; New York: 2004. pp. 209–42. [Google Scholar]
- Cohen J. Statistical power analyses for the behavioral sciences. 2nd ed. Erlbaum; Hillsdale, NJ: 1998. [Google Scholar]
- Collins DR, Pelletier JG, Paré D. Slow and fast (gamma) neuronal oscillations in the perirhinal cortex and lateral amygdala. Journal of Neurophysiology. 2001;85:1661–72. doi: 10.1152/jn.2001.85.4.1661. [DOI] [PubMed] [Google Scholar]
- DiNardo PA, Brown TA, Barlow DH. Anxiety disorders interview schedule for DSM-IV: Clinician's manual. Graywind; New York: 1994. [Google Scholar]
- Gana K, Martin B, Canouet MD. Worry and anxiety: Is there a causal relationship? Psychopathology. 2000;34:221–29. doi: 10.1159/000049314. [DOI] [PubMed] [Google Scholar]
- Gemignani A, Santarcangelo E, Sebastiani L, Marchese C, Mammoliti R, Simoni A, Ghelarducci B. Changes in autonomic and EEG patterns induced by hypnotic imagination of aversive stimuli in man. Brain Research Bulletin. 2000;53:105–11. doi: 10.1016/s0361-9230(00)00314-2. [DOI] [PubMed] [Google Scholar]
- Hedges L, Olkin I. Statistical methods for meta-analysis. Academic Press; New York: 1985. [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106:376–85. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clinical Neurophysiology. 2005;116:2719–33. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–75. [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends in Neurosciences. 2007;30:317–24. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Keil A, Müller MM, Ray WJ, Gruber T, Elbert T. Human gamma band activity and perception of a gestalt. Journal of Neuroscience. 1999;19:7152–61. doi: 10.1523/JNEUROSCI.19-16-07152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Stolarova M, Heim S, Gruber T, Müller MM. Temporal stability of high-frequency brain oscillations in the human EEG. Brain Topography. 2003;16:101–10. doi: 10.1023/b:brat.0000006334.15919.2c. [DOI] [PubMed] [Google Scholar]
- Kline RB. Beyond significant testing: Reforming data analysis methods in behavioral research. American Psychological Association; Washington, D.C.: 2004. [Google Scholar]
- Luo Q, Holroyd T, Jones M, Hendler T, Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage. 2007;34:839–47. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Ichikawa Y, Kanayama N, Ohira H, Iidaka T. Gamma band activity and its synchronization reflect the dysfunctional emotional processing in alexithymic persons. Psychophysiology. 2006;43:533–40. doi: 10.1111/j.1469-8986.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–95. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Müller MM, Keil A, Gruber T, Elbert T. Processing of affective pictures modulates right-hemisphere gamma band activity. Clinical Neurophysiology. 1999;110:1913–20. doi: 10.1016/s1388-2457(99)00151-0. [DOI] [PubMed] [Google Scholar]
- Newman MG, Castonguay LG, Borkovec TD, Molnar C. Integrative psychotherapy. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized anxiety disorder. Guilford Press; New York: 2004. pp. 320–50. [Google Scholar]
- Nitschke JB, Heller W, Miller GA. Anxiety, stress, and cortical brain function. In: Borod JC, editor. The neuropsychology of emotion. Oxford University Press; New York: 2000. pp. 298–319. [Google Scholar]
- Nitschke JB, Heller W, Palmieri P, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–37. [PubMed] [Google Scholar]
- Nitschke JB, Larson CL, Smoller MJ, Navin SD, Pederson AJC, Ruffalo D, et al. Startle potentiation in aversive anticipation: Evidence for state but not trait effects. Psychophysiology. 2002;39:254–58. doi: 10.1017/S0048577202010156. [DOI] [PubMed] [Google Scholar]
- Oathes DJ, Bruce JM, Nitschke JB. Worry facilitates corticospinal motor response to transcranial magnetic stimulation. Depression and Anxiety. doi: 10.1002/da.20445. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osgood CE, Suci GJ, Tannenbaum PH. The measurement of meaning. University of Illinois Press; Urbana: 1957. [Google Scholar]
- Oya H, Kawasaki H, Howard MA, Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. Journal of Neuroscience. 2002;22:9502–12. doi: 10.1523/JNEUROSCI.22-21-09502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio AM, Borkovec TD, Ruscio J. A taxometric investigation of the latent structure of worry. Journal of Abnormal Psycholgoy. 2001;110:413–22. doi: 10.1037//0021-843x.110.3.413. [DOI] [PubMed] [Google Scholar]
- Sebastiani L, Simoni A, Gemignani A, Ghelarducci B, Santarcangelo EL. Human hypnosis: autonomic and electroencephalographic correlates of a guided multimodal cognitive-emotional imagery. Neuroscience Letters. 2003;338:41–44. doi: 10.1016/s0304-3940(02)01358-7. [DOI] [PubMed] [Google Scholar]