Abstract
Treatment with nontoxic monophosphoryl lipid A increased the magnitude of the immunoglobulin M (IgM) antibody response to type III pneumococcal polysaccharide in young (2- to 4-week-old) mice. This was accompanied by the appearance of significant numbers of IgG1- and IgG3- secreting antibody-forming cells in 4-week-old mice. These findings indicate that monophosphoryl lipid A can be used as an adjuvant to improve the immunogenicity of poorly immunogenic antigens in young, immunologically immature animals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
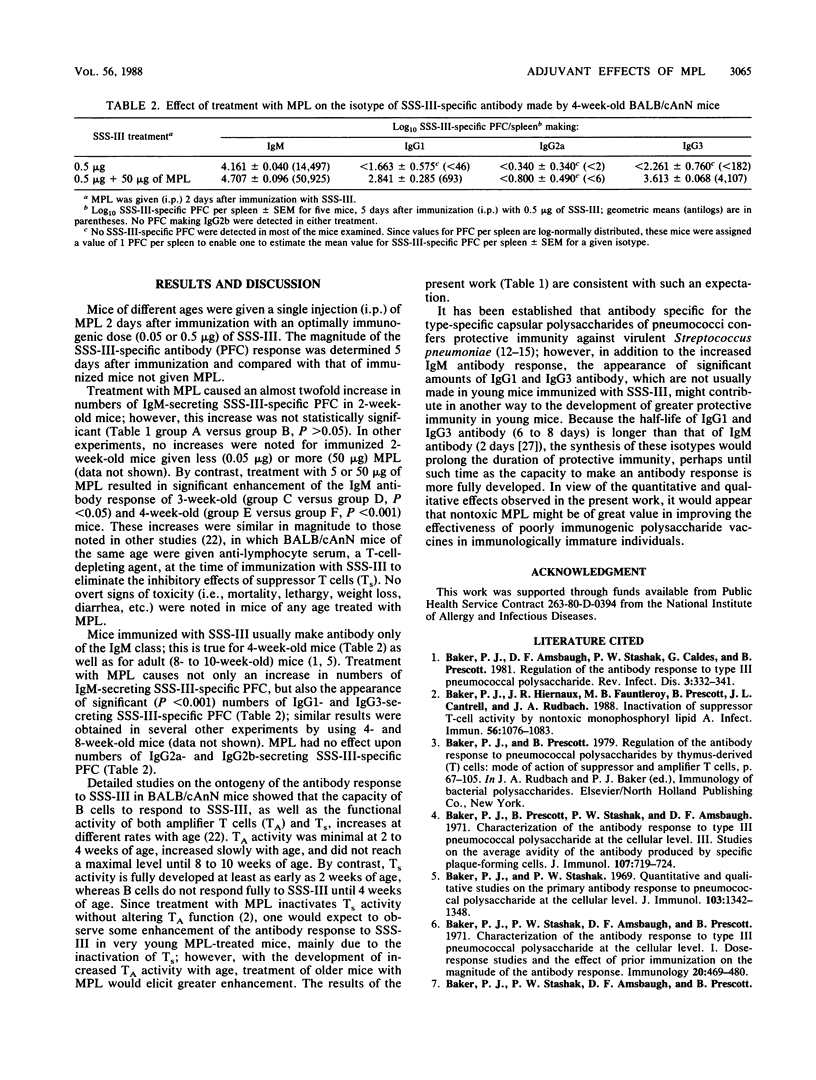
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Regulation of the antibody response to pneumococcal polysaccharide by thymus-derived cells. Rev Infect Dis. 1981 Mar-Apr;3(2):332–341. doi: 10.1093/clinids/3.2.332. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Prescott B., Cantrell J. L., Rudbach J. A. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun. 1988 May;56(5):1076–1083. doi: 10.1128/iai.56.5.1076-1083.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Prescott B., Stashak P. W., Amsbaugh D. F. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. 3. Studies on the average avidity of the antibody produced by specific plaque-forming cells. J Immunol. 1971 Sep;107(3):719–724. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates K. L. Serum opsonic activity for Haemophilus influenzae type b in infants immunized with polysaccharide-protein conjugate vaccines. J Infect Dis. 1985 Nov;152(5):1076–1077. doi: 10.1093/infdis/152.5.1076. [DOI] [PubMed] [Google Scholar]
- Cowan M. J., Ammann A. J., Wara D. W., Howie V. M., Schultz L., Doyle N., Kaplan M. Pneumococcal polysaccharide immunization in infants and children. Pediatrics. 1978 Nov;62(5):721–727. [PubMed] [Google Scholar]
- Einhorn M. S., Weinberg G. A., Anderson E. L., Granoff P. D., Granoff D. M. Immunogenicity in infants of Haemophilus influenzae type B polysaccharide in a conjugate vaccine with Neisseria meningitidis outer-membrane protein. Lancet. 1986 Aug 9;2(8502):299–302. doi: 10.1016/s0140-6736(86)90001-2. [DOI] [PubMed] [Google Scholar]
- FELTON L. D., KAUFFMANN G., PRESCOTT B., OTTINGER B. Studies on the mechanism of the immunological paralysis induced in mice by pneumococcal polysaccharides. J Immunol. 1955 Jan;74(1):17–26. [PubMed] [Google Scholar]
- FELTON L. D., PRESCOTT B., KAUFFMANN G., OTTINGER B. Pneumococcal antigenic polysaccharide substances from animal tissues. J Immunol. 1955 Mar;74(3):205–213. [PubMed] [Google Scholar]
- FELTON L. D. The significance of antigen in animal tissues. J Immunol. 1949 Jan;61(1):107–117. [PubMed] [Google Scholar]
- Gold R., Lepow M. L., Goldschneider I., Gotschlich E. C. Immune Response of human infants of polysaccharide vaccines of group A and C Neisseria meningitidis. J Infect Dis. 1977 Aug;136 (Suppl):S31–S35. doi: 10.1093/infdis/136.supplement.s31. [DOI] [PubMed] [Google Scholar]
- Gottlieb C. F. Application of transformations to normalize the distribution of plaque-forming cells. J Immunol. 1974 Jul;113(1):51–57. [PubMed] [Google Scholar]
- Hilgers L. A., Snippe H., Jansze M., Willers J. M. Immunomodulating properties of two synthetic adjuvants: dependence upon type of antigen, dose, and time of administration. Cell Immunol. 1984 Jul;86(2):393–401. doi: 10.1016/0008-8749(84)90394-0. [DOI] [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Stashak P. W., Prescott B., Baker P. J., Alling D. W. Kinetics of the antibody response to type III pneumococcal polysaccharide. I. Evidence that suppressor cells function by inhibiting the recruitment and proliferation of antibody-producing cells. J Immunol. 1976 Mar;116(3):647–656. [PubMed] [Google Scholar]
- Klein J. O. The epidemiology of pneumococcal disease in infants and children. Rev Infect Dis. 1981 Mar-Apr;3(2):246–253. doi: 10.1093/clinids/3.2.246. [DOI] [PubMed] [Google Scholar]
- Lee C. J., Takaoka Y., Saito T. Maternal immunization and the immune response of neonates to pneumococcal polysaccharides. Rev Infect Dis. 1987 May-Jun;9(3):494–510. doi: 10.1093/clinids/9.3.494. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Prescott B., Cross S. S., Stashak P. W., Baker P. J. Regulation of the antibody response to type III pneumococcal polysaccharide. V. Ontogeny of factors influencing the magnitude of the plaque-forming cell response. J Immunol. 1976 Feb;116(2):279–287. [PubMed] [Google Scholar]
- Pasanen V. J., Asofsky R., Baker P. J. Synthesis of two classes of antibody, gammaM and gammaG or gammaM and gammaA, by identical cells. Amplification of the antibody response to pneumococcal polysaccharide type III. J Exp Med. 1979 May 1;149(5):1227–1237. doi: 10.1084/jem.149.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Virtanen M., Mäkelä P. H. Prevention of Hemophilus influenzae type b bacteremic infections with the capsular polysaccharide vaccine. N Engl J Med. 1984 Jun 14;310(24):1561–1566. doi: 10.1056/NEJM198406143102404. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. Responses of children immunized with the capsular polysaccharide of Hemophilus influenzae, type b. Pediatrics. 1973 Nov;52(5):637–644. [PubMed] [Google Scholar]
- Vieira P., Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988 Feb;18(2):313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- Zigterman G. J., Snippe H., Jansze M., Ernste E. B., De Reuver M. J., Willers J. M. Nonionic block polymer surfactants enhance immunogenicity of pneumococcal hexasaccharide-protein vaccines. Infect Immun. 1988 May;56(5):1391–1393. doi: 10.1128/iai.56.5.1391-1393.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



