Abstract
A quantitative method was developed for the determination of fluorinated alkyl substances in municipal wastewater influents and effluents. The method consisted of centrifugation followed by large-volume injection (500 μL) of the supernatant onto a liquid chromatograph with a reverse-phase column and detection by electrospray ionization, and tandem mass spectrometry (LC/MS/MS). The fluorinated analytes studied include perfluoroalkyl sulfonates, fluorotelomer sulfonates, perfluorocarboxylates, and select fluorinated alkyl sulfonamides. Recoveries of the fluorinated analytes from wastewater treatment plant (WWTP) raw influents and final effluent ranged from 77% – 96% and 80% – 99%, respectively. The lower limit of quantitation ranged from 0.5 to 3.0 ng/L depending on the analyte. The method was applied to flow-proportional composites of raw influent and final effluent collected over a 24 hr period from ten WWTPs nationwide. Fluorinated alkyl substances were observed in wastewater at all treatment plants and each plant exhibited unique distributions of fluorinated alkyl substances despite similarities in treatment processes. In nine out of the ten plants sampled, at least one class of fluorinated alkyl substances exhibited increased concentrations in the effluent as compared to the influent concentrations. In some instances, decreases in certain fluorinated analyte concentrations were observed and attributed to sorption to sludge.
Introduction
Fluorinated alkyl substances consist of a diverse class of chemicals that possess unique physical and chemical properties that make them valuable components in many industrial and commercial products, including coatings for furniture, clothing, and carpets and some are active ingredients in cosmetics, household cleaners, firefighting foams, and packaged-food containers (1). Fluorochemicals have ignited widespread interest due to their ubiquitous, worldwide presence in the environment and analytical methods have been developed for their quantitative determination in air (2–4), surface waters (5–11), groundwater (12), biota (13–16), and human serum (17–19). These observations raise concerns about the risks that fluorinated alkyl substances may pose towards humans and other organisms.
The discharge of municipal wastewater effluent is one of the principal routes for introducing organic chemicals that are used in domestic and industrial settings into aquatic environments. The earliest available report is that on a study of six cities; perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) were observed in all sampled wastewater treatment plant (WWTP) effluents (20). More recent peer-reviewed reports indicate the occurrence of additional fluorochemicals in WWTPs (21–23).
At present time, few methods exist for the determination of fluorinated alkyl substances in wastewater matrices (21,22). Wastewater matrices are complex such that wastewater analysis typically requires sample enrichment and reduction of particle load. Solid-phase extraction (SPE) is often used in this capacity with liquid chromatography, tandem mass spectrometry (LC/MS/MS) methods reported for the determination of fluorinated alkyl substances in aqueous matrixes (5,6,8,9,24); however, SPE is time-consuming and can be imprecise. Consequently, there is interest in developing alternatives to SPE for sample concentration and clean-up purposes.
The objective of this study was to develop and demonstrate a simple yet sensitive and quantitative analytical LC/MS/MS method for the determination of the dissolved fraction of a range of fluorinated alkyl substances in municipal wastewater influents and effluents, including some representative of several commercially, industrially, and domestically-used fluorinated alkyl chemicals. The fluorinated alkyl substances studied included perfluoroalkylsulfonates, fluorotelomer sulfonates, and perfluoroalkylcarboxylates, as well as select perfluoroalkylsulfonamides. The methodology was then applied to flow-proportional composites of raw influent and final effluent collected over a 24 hr period from ten municipal WWTPs located nationwide. Comparison of 24 h-composite influent and effluent concentrations provided preliminary insights into the potential behavior of fluorinated alkyl substances during wastewater treatment.
Experimental Section
Standards and Reagents
A standard of 1H,1H,2H,2H-perfluorooctane sulfonate (6:2 FtS, 98%) was purchased from Apollo Scientific Limited (Derbyshire, UK). Standards of potassium perfluorobutane sulfonate (PFBS, 99%), potassium perfluorohexane sulfonate (PFHxS, 99%), potassium perfluorooctane sulfonate (PFOS, 98%), and perfluorooctanesulfonamide (FOSA, 99%) were donated by the 3M Company (St. Paul, MN). Standards of perfluorodecane sulfonate (ammonium form in water/butoxyethanol; PFDS, 25% wt.), perfluoroheptanoic acid (PFHpA, 99%), perfluorooctanoic acid (PFOA, 96%), perfluorononanoic acid (PFNA, 97%), and perfluorodecanoic acid (PFDA, 98%) were acquired from Aldrich Chemical (Milwaukee, WI). Perfluorohexanoic acid (PFHxA, 99%) and the internal standard perfluoro(2-ethoxyethane)sulfonic acid (PFEES, 97%) were obtained from Oakwood Products, Inc. (West Columbia, SC). The secondary internal standard, a dual labeled [1,2-13C2]-perfluorooctanoic acid ([1,2-13C2]PFOA, 97.5%) was acquired from Perkin Elmer (Wellesley, MA) and used as a recovery standard in spike and recovery experiments.
Wastewater Samples
High density polyethylene (HDPE) bottles (125 mL; EaglePicher, Joplin, MO) that had been washed by a proprietary process were sent to ten municipal WWTPs for collection of flow-proportional (e.g., a fixed volume of sample taken every 4×105 L of wastewater) composites of raw influent and final effluent. Characteristics of each WWTP are listed in Table 1. After collection, the samples were shipped on ice overnight and stored at 4 °C until analysis. Samples not analyzed within 48 hrs were stored at −20 °C until analysis. Formalin was not used to inhibit biological activity because it was found to suppress the response of fluorinated alkyl substances in wastewater matrices (data not shown).
Table 1.
Wastewater treatment plant characteristics.a
| WWTP No. | U.S. region | Treatment type | Sample dates | Flow (m3/day) | Populationb | Waste treated |
|---|---|---|---|---|---|---|
| 1 | Pacific Northwest | P + TF + AS | April 2004 | 45,400 | 50,000 | 90% domestic, 10% light industry |
| 2 | Pacific Northwest | P + AS | May 2004 | 211,000 | 600, 000 | 93% domestic, 7% industrial |
| 3 | Pacific Northwest | P + TF + AS | May 2004 | 106,000 | 130,000 | 90% domestic/commercial, 10% industrial |
| 4 | Southeast | P + AS | June 2004 | 53,000 | 240,000 | 97% domestic, 3% industrial |
| 5 | West | P + TF + AS | June 2004 | 64,000 | 202,000 | 60% domestic, 10% industrial, 30% business |
| 6 | West South Central | P + AS | June 2004 | 26,000 | 65,000 | 99% domestic, 1% light industry |
| 7 | West North Central | P + AS + MMF | June 2004 | 42,000 | 110,000 | 50% papermill effluent, 50% domestic/commercial |
| 8 | West | P + AS + MMF | June 2004 | 98,000 | 220,000 | 99% domestic, 1% industry |
| 9 | West North | P + TF + AS | June 2004 | 238,000 | 415,000 | 85% domestic, 10% light industry, 5% heavy industry |
| 10 | Northeast | P + AS | June 2004 | 11,000 | 17,000 | 80% domestic, 10% leachate, 10% industrial |
P = primary gravitational settling, TF = trickling filter, AS = activated sludge, MMF = mixed media filters (medium grain anthracite coal, silica sand, garnet, fine gravel, and medium-sized gravel)
based on 2000 population census
Prior to analysis, all samples were prepared by centrifugation at 13,200 rpm for 10 min followed by transferring a portion (1.8 mL) of the supernatant into a glass autosampler vial. Vials were then spiked with 0.045 ng each of the primary internal standard, PFEES, and the secondary internal standard, [1,2-13C2]PFOA. Raw influent and final effluent WWTP samples were analyzed with two replicate injections.
Quality Assurance and Quality Control
Accuracy and precision determinations were made for analytes that were detected in preliminary scans of wastewater influent and effluent from WWTPs 1–3. To determine the accuracy of the analytical method and for quality assurance and quality control, four additional HDPE bottles were sent to each of the ten WWTPs: one travel blank, one travel spike (spiked Milli-Q water), and two field matrix spikes (one influent field matrix spike and one effluent field matrix spike). See Supporting Information for details on the preparation of field blanks, travel spikes, and field spikes. All quality assurance and quality control samples were handled and stored in a manner that was identical to that of the wastewater samples received from each WWTP, with the exception of the travel blanks and travel spikes, which were transferred directly to autosampler vials without centrifugation. Each field matrix spike was analyzed and the endogenous concentrations in the sample matrix were subtracted, if the analyte was detected. A single matrix spike was analyzed for each of the ten WWTP samples such that the accuracy of recovery, as indicated by the standard error, was determined from two-factor ANOVA calculations using the software functions included in a conventional spreadsheet program (Excel, Microsoft Corporation, Redmond, WA). To determine the precision of the analytical method, additional spike and recovery experiments were performed by spiking standards into eight replicate portions of both raw influent and final effluent samples collected from WWTP 1. Precision was determined as the percent relative standard deviation for the eight replicate analyses. See Supporting Information for details the spike and recovery experiments to determine method precision. The endogenous concentration in the raw influent and final effluent, if any, was subtracted in order to determine the recoveries of the analytes spiked into the replicate samples of raw influent and final effluent.
Liquid Chromatography/Mass Spectrometry
All separations were performed on a Waters 2690 HPLC system (Milford, MA). All accessible polytetrafluoroethylene lines in the instrument were replaced with polyetheretherketone (PEEK) tubing (Upchurch Scientific, Oak Harbor, WA). A 500 μL injection loop made of PEEK tubing was used to load samples onto a 4 mm × 3 mm C18 guard cartridge (Phenomenex, Torrance, CA) that was connected to a 150 mm × 2 mm Betasil C-18 column (Thermo Hypersil-Keystone, Bellefonte, PA) heated to 35ºC. Samples were injected onto the LC column and eluted with the following methanol (Optima Grade; Fisher Scientific, Pittsburgh, PA)/2 mM aqueous ammonium acetate (98%; Aldrich Chemical, Milwaukee, WI) gradient cycle: an initial hold time of 5 min at 50% methanol to account for the large 3 minute void volume associated with the 500 μL sample loop, followed by an increase to 90% methanol over 5 min. The 90% methanol condition was held for 5 min and then decreased back to 50% methanol over 5 min. The column flow rate was 200 μL/min.
Mass spectrometry was performed on a Waters Quattro Micro system (Beverly, MA). See Supporting Information for details on the ESI operating parameters and the fragments monitored for quantitation of a given analyte. Analyte quantitation was performed using conventional internal standard calibration with PFEES serving as the internal standard. See Table S1 in Supporting Information for details on the construction of calibration curves, calibration data treatment, and data quality parameters. Background contamination developed periodically within the instrument as detected in solvent blanks. When the background contamination reduced the signal-to-noise (S/N) of the lowest calibration standard to a S/N<10:1, a solvent mixture consisting of 10% (v/v) formic acid (97%) (Sigma-Aldrich, St. Louis, MO) in optima grade isopropanol (Fisher Scientific, Pittsburgh, PA) was run overnight through the system. The background contamination was usually attributed to the analysis of highly concentrated samples; however, it may have also resulted from accumulation from high throughput analyses over multiple days.
Results and Discussion
Method Optimization
Initial attempts to analyze municipal wastewaters by direct injection (25 μL) LC/MS/MS proved unsuccessful because the concentrations of fluorinated alkyl substances were at or below detection limits (12). Solid-phase extraction (SPE) was avoided as a sample concentration step because initial SPE experiments with C18 yielded low and variable analyte recovery (50–90%) for PFBS, PFHxS, PFOS, PFHxA, PFHpA, and PFOA. Alternatively, large-volume injection requires less time and resources with a lower potential for analyte loss than preconcentration onto SPE phases. For this reason, a 500 μL PEEK sample loop was constructed and a 5 min hold was added to the beginning of the gradient. As analyte peak area increased in proportion to injection volume, a 500 μL volume was chosen to allow for maximum signal. PEEK material was chosen to minimize significant background contamination that may arise from large volume injections. The addition of the large volume sample loop allowed an increase in sensitivity that lead directly to lower detection limits for fluorinated alkyl substances at environmentally relevant concentrations.
Once large-volume injection was selected and optimized, filtration was explored as a means for removing particles from wastewater samples. However, the four types of filters tested, including glass fiber, nylon, cellulose acetate, and polyethersulfone filters, either removed selected analytes from spiked deionized water or increased the concentration of analytes in the deionized water that had passed through the filter (see Table S2 in Supporting Information). Yamashita et al. (25) also found PFOS and PFOA in three types of filters. Since all filters either retained analytes or resulted in an increase due to the presence of fluorinated alkyl substances in the filters themselves, filtration was not considered a viable sample preparation step. As a result, centrifugation was selected as the only sample clean-up step.
Method Accuracy and Precision
The travel blanks were analyzed upon return to the laboratory, and in all ten cases, no fluorinated alkyl substances were detected above the LOQs. To assess the analytical method accuracy and precision for the list of target analytes, single samples of spiked municipal wastewater influents and effluents from each of the ten WWTPs sampled and ten Milli-Q water travel spikes were analyzed. Recoveries from travel spikes (spiked Milli-Q) water ranged from 87% to 98% (Table 2). The recovery of analytes for the ten samples of raw influent field matrix spikes ranged from 82 to 100%. The recovery of analytes from the ten final effluent field matrix spikes ranged from 86 to 100% (Table 2). The standard error of the average recovery for the field matrix spikes was 2% as determined by two-factor ANOVA. For the list of reported analytes, the precision of the analytical method, indicated by the relative standard deviation (RSD) from 8 replicates of a single influent and effluent ranged from 2 to 18% for the raw influent and from 4 to 22% for the final effluent (see Table S3 in Supporting Information).
Table 2.
Method accuracy as indicated by the average recovery for analytes spiked into Milli-Q water (n = 10) and into single samples of raw influent (raw influent field matrix spikes) and final effluent (final effluent field matrix spikes) collected from ten different WWTPs.a
| Analyte | Milli-Q waterb (%) | Raw Influent (%) | Final Effluent (%) |
|---|---|---|---|
| PFBS | 98 | 100 | 100 |
| PFHxS | 88 | 98 | 95 |
| PFOS | 91 | 95 | 88 |
| PFDS | 92 | 94 | 92 |
| 6:2 FtS | 97 | 97 | 95 |
| PFHxA | 91 | 88 | 93 |
| PFHpA | 94 | 92 | 89 |
| PFOA | 97 | 92 | 95 |
| [1,2-13C2]PFOA | 93 | 88 | 89 |
| PFNA | 88 | 92 | 89 |
| PFDA | 91 | 90 | 86 |
| FOSA | 87 | 82 | 92 |
Because single samples of ten different influents and effluents were analyzed, the variability of the average recovery (2%) was determined by two-factor analysis of variance (ANOVA).
Endogenous concentrations in raw influent and effluent were subtracted to determine spike recovery and are available in Table 3.
Milli-Q water samples (n=10) were spiked with analytes in the laboratory and sealed during transport and not opened until analysis.
Limit of Quantitation
The limit of quantitation (LOQ) was defined as the higher of either the lowest point on the calibration curve (0.5 ng/L) or the analyte concentration required to produce a S/N of 10:1 in the wastewater matrix. The lower LOQs for the fluorinated alkyl substances range from 0.5 ng/L to 3.0 ng/L (see Table S1 in Supporting Information).
Application to Wastewater Samples
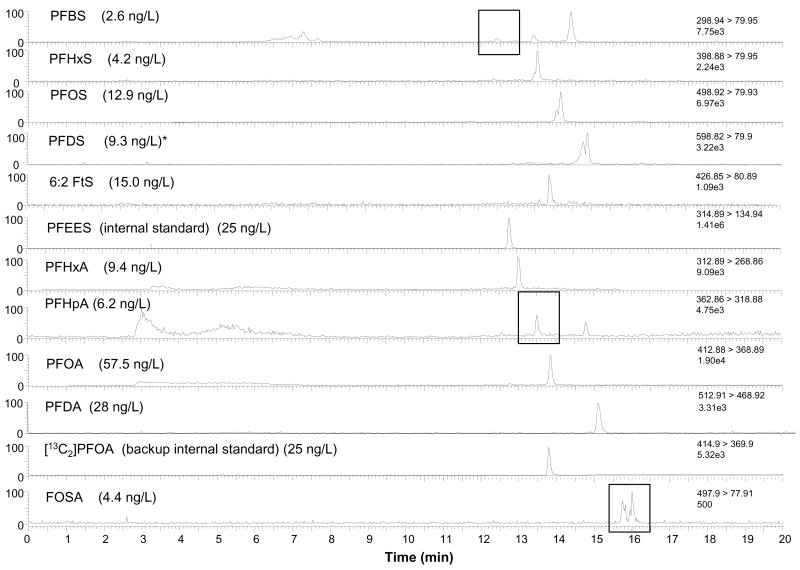
The large-volume-injection LC/MS/MS method was applied to municipal wastewater raw influents and final effluents collected from ten WWTPs nationwide. The four different classes of fluorinated alkyl substances screened for in this study are chromatographically presented in Figure 1 for the final effluent collected at WWTP 6. The observed split chromatographic peaks of PFHxS, PFOS, and FOSA, indicate the branched (early) and linear (largest) isomers common to the products of electrochemical fluorination (1). Quantitation of these analytes was based on the branched plus linear isomer peaks. Calibration standards of PFHxS and PFOS contained branched isomers in a similar ratio to what was observed in the wastewater. A chromatogram of these standards is shown in Schultz et al. (12). The FOSA standard also contained a branched isomer; however, it contained less of the branched isomer than what was present in the wastewater (chromatogram of FOSA standard not shown).
Figure 1.
LC/MS/MS chromatograms for target analytes observed in effluent collected from WWTP 6 except for PFDS, which is from WWTP 3. The concentrations of each analyte are presented in parentheses. If multiple peaks are present for a specific transition, the peak at the correct retention time is depicted in the box.
Perfluoroalkyl Sulfonates
Perfluoroalkyl sulfonates were measured in wastewaters collected from all WWTPs included in this study (Table 3). LC/MS/MS chromatograms indicated that peaks were detectable for each perfluoroalkyl sulfonate and were consistent with chemicals produced by electrofluorination that have branched (first peak) and linear (second peak) isomers (Figure 1) (1). Of those perfluoroalkyl sulfonates quantitatively determined in this study (PFBS, PFHxS, PFOS, and PFDS), PFOS was the most frequently detected and often the highest concentration perfluoroalkyl sulfonate except for WWTP 10, which had higher a concentration of PFHxS. The highest concentration of PFOS (400 ng/L) was that observed for the raw influent of WWTP 2, which was also the highest concentration of any individual analyte evaluated in this study (Table 3). The detection of PFOS in wastewater despite the phase out in 2002 (26) indicates that products containing the C8-based chemicals are still actively being used and released to U.S. municipal wastewater facilities.
Table 3.
Average concentrations ± 95% confidence interval of fluorinated alkyl substances in wastewater treatment plant influent and effluent.a
| WWTP | PFBS ng/L | PFHxS ng/L | PFOS ng/L | PFDS ng/L | 6:2 FtS ng/L | PFHxA ng/L | PFHpA ng/L | PFOA ng/L | PFNA ng/L | PFDA ng/L | FOSA ng/L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1- Influent | 4.8±0.8 | 8.6±1.9 | 21±2.1 | ND | 11±0 | 31±1 | 6.6±1.1 | 13±1 | 1.0±0.5 | ND | ND |
| Effluent | < LOQ | 4.5±1.6 | 11±4 | ND | 3.9±1.2 | ND | ND | 2.5±0.8 | 0.7±0.7 | ND | 1.0±0.8 |
| 2- Influent | 27±5 | 9.3±1.1 | 400±29 | 6.1±0.2 | 38±2 | ND | 6.5±2.1 | 16±3 | 5.3±0.2 | 1.0±0.7 | ND |
| Effluent | 20±3 | 5.0±1.1 | 130±11 | ND | 370±19 | 3.4±1.1 | 7.5±0.8 | 28±2 | 2.3±2.2 | 3.3±2.2 | ND |
| 3- Influent | 5.2±1.70 | 2.3±2.1 | 20±3 | 9.3±2.2 | 2.1±0.7 | 11±0 | ND | 7.4±2.2 | 7.3±1.0 | ND | ND |
| Effluent | 5.5±2.2 | 2.4±0.4 | 6.2±3.1 | < LOQ | 4.4±1.4 | 16±1 | 1.8±2.28 | 6.6±1.45 | 5.8±1.7 | ND | 4.4±0.3 |
| 4- Influent | 7.0±1.0 | 11±1 | 26±3 | ND | 57±1 | 9.0±0.2 | 15±3 | 89±3 | 5.1±0.9 | ND | ND |
| Effluent | 10±0 | 17±1 | 24±3 | ND | 15±1 | 17±1 | 15±3 | 97±6 | 6.1±1.0 | 2.1±1.1 | 1.6±0.2 |
| 5- Influent | 3.3±1.6 | 11±2 | 12±3 | ND | 12±3 | 8.3±0.2 | 0.7±0.3 | 4.9±1.0 | ND | ND | 5.5±1.6 |
| Effluent | 3.1±0.5 | 5.3±0.5 | 5.3±0.6 | ND | 6.4±1.4 | 7.2±0.3 | 3.7±0.4 | 15±1 | ND | ND | 10±1 |
| 6- Influent | < LOQ | 6.0±2.6 | 12±1 | ND | < LOQ | 11±1 | 7.2±2.2 | 29±2 | ND | 1.7±1.7 | ND |
| Effluent | 2.6±0.4 | 4.2±1.0 | 13±1 | ND | 15±5 | 9.4±3.1 | 6.2±1.3 | 58±10 | ND | 28±7 | 4.9±0.5 |
| 7- Influent | ND | 4.2±2.1 | 14±3 | 10±3 | 9.0±0.3 | 23±01 | 0.8±0.8 | 1.7±0.8 | ND | ND | ND |
| Effluent | ND | ND | 11±2 | < LOQ | 6.5±0.3 | 8.3±0.5 | 2.4±0.7 | 7.7±1.1 | ND | ND | ND |
| 8- Influent | ND | 7.7±0.6 | 13±1 | 8.8±2.0 | 9.4±1.2 | 13±0 | ND | 8.5±0.3 | ND | ND | ND |
| Effluent | ND | 7.1±0.5 | 7.1±1.1 | ND | 11±2 | 17±2 | 1.0±0.7 | 12±2 | ND | ND | ND |
| 9- Influent | < LOQ | 5.6±0.7 | 27±6 | ND | 16±2 | 17±1 | 1.6±0.2 | 13±2 | ND | < LOQ | ND |
| Effluent | 1.8±0.5 | 4.9±0.9 | 25±6 | ND | 24±4 | 20±0 | ND | 11±2 | ND | < LOQ | 2.4±0.4 |
| 10- Influent | 5.5±0.5 | 12±23 | 1.4±0.4 | ND | 5.8±0.8 | 20±1 | 25±3 | 49±2 | 7.2±1.5 | < LOQ | ND |
| Effluent | < LOQ | 5.7±1.8 | 1.1±0.7 | ND | ND | 18±2 | 23±4 | 65±0 | 0.7±1.0 | < LOQ | ND |
LOQ = detected but not above the LOQ (see Table 2) ND = not detected
Average concentration from four observations (i.e. two replicate injections of two replicate samples). Shaded cells indicate that the difference in concentration between influent and effluent concentrations was determined to be significant at the 95% confidence level.
The decrease or increase of perfluoroalkyl sulfonates (and other analytes) was determined as significant when the computed average concentration values at the 95% confidence level did not overlap. The confidence level was computed from the pooled standard deviation of the influent and effluent samples with 2 degrees of freedom. PFBS concentrations decreased (26–100%) in three WWTPs but no statistically-significant decrease at the 95% confidence level was detected between effluent and influent concentrations for two WWTPs (Table 3). Significant increases in PFBS concentrations were observed for three WWTPs. The increase in PFBS concentrations during wastewater treatment may be due to the degradation of precursors such as N-methyl perfluorobutane sulfonamide, which was identified in sludge from WWTP 1 (27). PFHxS concentrations decreased (46–100%) in five of the ten WWTPs studied; however, PFHxS was not detected in sludges from these WWTPs (23). No statistically significant removal was found for four WWTPs and a single WWTP showed a 55% increase in effluent concentrations compared to the influent (Table 3).
PFOS concentrations decreased significantly (21–70%) in the aqueous waste stream in six of ten WWTPs and no removal occurred in the remaining four WWTPs. Boulanger et al. also reported the reduction of PFOS concentrations for a single WWTP (22). Removal of PFOS and PFDS can be attributed to sorption onto sludge because, to date, there is no known biodegradation pathway for members of this class of fluorochemicals. Furthermore, PFOS and PFDS were detected in the sludge samples collected from seven of these WWTPs (WWTP 1, 2, 5–8, and 10)(23). Potential precursors of PFOS, including perfluorooctanesulfonamidoacetate (FOSAA) and its N-ethyl and N-methyl derivatives (23), are present in anaerobically-digested sludge but were not detected during initial scans of WWTP 1–3 (this study). However, Boulanger et al. (22) reported N-EthylFOSAA in the influent and effluent from a single WWTP and in river water. Other potential precursors of PFOS including N-methyl perfluorooctane sulfonamidoethanol (N-MeFOSE), N-ethyl perfluorooctane sulfonamidoethanol (N-EtFOSE), and N-methyl perfluorooctane sulfonamide ethyl acrylate were detected in anaerobically-digested sludge from WWTP 1 by gas chromatography MS/MS (27).
PFDS concentrations decreased significantly (93–100%) in all four WWTPs in which it was detected (Table 3). With the exception of a report that documents the occurrence of PFDS in ospreys (13), little is known about the occurrence of PFDS and its potential sources or precursors. Because PFOS can be formed during the biodegradation of sulfonamide alcohols (28), sludges were scanned for the potential presence of C10 sulfonamide precursors of PFDS; however, none were detected (Chris Higgins, personal communication). More work and authentic standards are needed to determine if PFDS precursors are present in wastewater and to verify if PFDS occurs as a biodegradation product of precursors.
Fluorotelomer Sulfonates
The 6:2 FtS was the only fluorotelomer sulfonate detected in the initial scans of wastewater from WWTPs 1–3. For this reason, it was the only fluorotelomer sulfonate monitored in subsequent wastewater influents and effluents. The 6:2 FtS was detected in wastewater from all ten WWTPs and occurred as a single peak (Figure 1), which is consistent with fluorochemicals produced by the telomerization process (1). The highest concentration of 6:2 FtS (370 ng/L, Table 3) was observed in the final effluent of WWTP 2, which was a ten-fold increase over the influent concentration (38 ng/L).
No statistically significant decrease or increase (at the 95% confidence level) in the 6:2 FtS occurred in WWTP 8 while significant decreases (29–100%), as indicated by student’s t-test, was found for five out of ten WWTPs (Table 3). It is likely that 6:2 FtS is being removed either by sorption onto sludge and/or by biodegradation. The latter is possible, as others have found that the 6:2 FtS is susceptible to biodegradation under sulfur-limiting and aerobic conditions (29). In contrast, the concentration of the 6:2 FtS significantly increased (50–874%) in four of the ten WWTPs (WWTPs 2, 3, 6 and 9) and the increase was an order of magnitude in two of the four plants. The observed increase is likely due to the biodegradation of precursors that form 6:2 FtS. If any removal by sorption onto sludge or biodegradation is occurring in these WWTPs, it is effectively being masked by processes leading to the formation of 6:2 FtS. Given that 6:2 FtS concentrations can increase as a result of wastewater treatment, more research is needed to better understand potential precursors of fluorotelomer sulfonates.
Perfluoroalkylcarboxylates
Perfluorocarboxylates including PFHxA, PFHpA, PFOA, PFNA, and PFDA were quantified in wastewaters for this study. PFHxA, PFHpA, and PFOA were detected in each of the ten WWTPs while PFNA and PFDA were detected in five out of ten and three out of ten WWTPs, respectively (Table 3). Although chromatograms for the perfluorocarboxylates appear as single symmetrical peaks (Figure 1), additional experiments need to be performed to determine if the apparent single peak actually consists of branched and linear isomers.
PFHxA concentrations decreased significantly (13–100%) in three of ten WWTPs, while no significant decrease or increase at the 95% confidence level was observed for two WWTPs and concentrations of PFHxA increased significantly (17–89%) in the remaining five WWTPs (Table 3). PFHpA concentrations decreased 100% in two WWTPs while four WWTPs showed no significant removal and four showed a 200–430% increase (Table 3). No sludge data are available to support the observed removal of PFHxA and PFHpA because the method of Higgins et al. was not validated for these analytes (23).
PFOA concentrations increased from 9–352% in seven of ten WWTPs (Table 3). An 81% decrease in PFOA was observed only for WWTP 1, while the two remaining WWTPs showed no significant change in PFOA concentrations (Table 3). PFOA was quantified in primary sludge for WWTP 5 and in aerobically-digested sludge from WWTP 6 (23). PFOA was detected but not quantified in anaerobically-digested sludge from WWTPs 1, 2, 7, and 8 and none was detected in sludge from WWTP 10 (23). In contrast to PFOS, removal onto sludge appears to be a minor process affecting PFOA during wastewater treatment. Biodegradation of precursors is likely responsible for the observed increases. For example, perfluorocarboxylates are known to form as biodegradation products of fluorotelomer alcohols in microcosms containing activated sludge (30) and in enrichment cultures obtained from a contaminated aquifer (31). Of these known precursors, only the fluorotelomer alcohols have been identified in anaerobically-digested sludge from WWTP 1 (27). Further research is needed to determine if additional classes of fluorochemicals are present in wastewater that could degrade to form PFOA and other perfluorocarboxylates.
Of the five WWTPs in which PFNA was detected, two were characterized by statistically-significant decreases in PFNA concentration (Table 3). PFDA was detected in only three WWTPs and the concentrations in the final effluent were 230–1,547% higher than those of the raw influents (Table 3). Of the sludges analyzed by Higgins et al., PFNA was measured in only two sludges (WWTP 1 and 6) whereas PFDA occurred in all seven sludges (23). Increases for perfluorocarboxylates may be due to precursors such as fluorotelomer alcohols undergoing aerobic biodegradation to form carboxylates (30,31). Aerobic treatment of sludge from WWTP 6 may explain why this sludge has the highest concentrations of perfluorocarboxylates (23).
Fluoroalkyl Sulfonamides
FOSA was the only fluoroalkyl sulfonamide detected in the wastewater samples and monitored for this study. In all six WWTPs in which FOSA was detected, the concentration of FOSA significantly increased 82% in WWTP 5 and from below detection (0.5 ng/L) in raw influent up to 1–10 ng/L in the other five WWTPs (Table 3). The appearance of FOSA in effluents suggests that FOSA occurs as a biodegradation product of precursors entering WWTPs. The FOSA detected in effluent is comprised of branched and linear isomers (Figure 1), which indicates that it is derived from branched precursors such as N-EtFOSE and N-ethyl perfluorooctanesulfonamide, which are known to degrade to FOSA (22,32).
Fluorochemical Occurrence in Wastewater
Each of the WWTPs sampled exhibited a unique fingerprint of fluorinated alkyl substances. The fluorochemicals evaluated in this study did not exhibit consistent removal and/or enhancement trends across all WWTPs. Specific treatment processes present at a WWTP did not appear to influence the fate of fluorinated alkyl substances. For example, WWTPs 1, 3, 5, and 9 all incorporate primary gravitational settling, trickling filters, and activated sludge in their treatment processes (Table 1). Because no trends in the removal (or enhancement) were observed, it suggests that the observed distributions are not solely influenced by the treatment processes. With a limited data set (i.e. single sampling of influents and effluents at ten WWTPs on a single day), observed trends merit postulations of sources; however, definitive conclusions cannot be drawn with the data currently available. An improved understanding of the occurrence and behavior of the individual fluorochemicals requires a better understanding of the precursors that are present in municipal wastewater. Higher time resolution through repetitive sampling, hourly grab samples over a defined period of time, sampling after each treatment stage, and seasonal sampling are only a few of the research studies needed to address whether the observed trends described here are representative of the fate of fluorinated alkyl substances throughout the wastewater treatment process.
Supplementary Material
Supplementary text and tables that provide additional information in regards to quality assurance and quality controls, method precision, and mass spectrometric details are located in Supporting Information.
Acknowledgments
The authors thankfully recognize financial support in part by a grant (R-82902501-0) from the National Center for Environmental Research (NCER) STAR Program, U.S. Environmental Protection Agency, and in part by a grant (P30 ES00210) from the National Institute of Environmental Health Sciences. The authors thank Carin Huset of Oregon State University and Chris Higgins of Stanford University for fruitful insights in regards to data interpretation. The authors are also indebted to the 3M Company for authentic standards. We would especially like to thank Bodgan Szostek for his suggestion to investigate large-volume injection.
Literature Cited
- 1.Kissa E. Fluorinated surfactants: Synthesis, Properties, and Applications. Vol. 50 Marcel Dekker; New York: 1994. [Google Scholar]
- 2.Martin JW, Muir DCG, Moody CA, Ellis DA, Kwan WC, Solomon KR, Mabury SA. Collection of airborne fluorinated organics and analysis by gas chromatography/chemical ionization mass spectrometry. Anal Chem. 2002;74:584–590. doi: 10.1021/ac015630d. [DOI] [PubMed] [Google Scholar]
- 3.Stock NL, Lau FK, Ellis DA, Martin JW, Muir DCG, Mabury SA. Polyfluorinated telomer alcohols and sulfonamides in the North American troposphere. Environ Sci Technol. 2004;38:991–996. doi: 10.1021/es034644t. [DOI] [PubMed] [Google Scholar]
- 4.Reagen W, Lindstrom K, Thompson K, Flaherty J. Analytical techniques and method validation for the measurement of selected semi-volatile and non-volatile organofluorochemicals in air. J Occup Environ Hygiene. 2004;1:559–569. doi: 10.1080/15459620490485891. [DOI] [PubMed] [Google Scholar]
- 5.Moody CA, Kwan WC, Martin JW, Muir DCG, Mabury SA. Determination of perfluorinated surfactants in surface water samples by two independent analytical techniques: liquid chromatography/tandem mass spectrometry19F NMR. Anal Chem. 2001;73:2200–2206. doi: 10.1021/ac0100648. [DOI] [PubMed] [Google Scholar]
- 6.Hansen KJ, Johnson H, Eldridge J, Butenhoff J, Dick L. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ Sci Technol. 2002;36:1681–1685. doi: 10.1021/es010780r. [DOI] [PubMed] [Google Scholar]
- 7.Taniyasu S, Kannan K, Horii Y, Hanari N, Yamashita N. A survey of perfluorooctane sulfonate and related perfluorinated organic compounds in water, fish, birds, and humans from Japan. Environ Sci Technol. 2003;37:2634–2639. doi: 10.1021/es0303440. [DOI] [PubMed] [Google Scholar]
- 8.Saito N, Harada K, Inoue K, Sasaki K, Yoshinaga T, Koizumi A. Perfluorooctanoate and perfluorooctane sulfonate concentrations in surface water in Japan. J Occup Health. 2004;46:49–59. doi: 10.1539/joh.46.49. [DOI] [PubMed] [Google Scholar]
- 9.So MK, Taniyasu S, Yamashita N, Giesy JP, Zheng J, Fang Z, Im SH, Lam PKS. Perfluorinated compounds in coastal waters of Hong Kong, South China, and Korea. Environ Sci Technol. 2004;38:4056–4063. doi: 10.1021/es049441z. [DOI] [PubMed] [Google Scholar]
- 10.Boulanger B, Vargo J, Schnoor J, Hornbuckle K. Detection of perfluorooctane surfactants in Great Lakes water. Environ Sci Technol. 2004;38:4064–4070. doi: 10.1021/es0496975. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita N, Kannan K, Taniyasu S, Horii Y, Okazawa T, Petrick G, Gamo T. Analysis of perfluorinated acids at parts-per-quadrillion levels in seawater using liquid chromatography-tandem mass spectrometry. Environ Sci Technol. 2004;38:5522–5528. doi: 10.1021/es0492541. [DOI] [PubMed] [Google Scholar]
- 12.Schultz MM, Barofksy DF, Field JA. Quantitative determination of fluorotelomer sulfonates in groundwater by LC MS/MS. Environ Sci Technol. 2004;38:1828–1835. doi: 10.1021/es035031j. [DOI] [PubMed] [Google Scholar]
- 13.Toschik P, Rattner B, McGowan P, Christman M, Carter D, Hale R, Matson C, Ottinger M. Effects of contaminant exposure on reproductive success of ospreys (Pandion Haliaetus) nesting in Delaware River and Bay, USA. Environ Tox Chem. 2005;24 doi: 10.1897/04-141r.1. [DOI] [PubMed] [Google Scholar]
- 14.Kannan K, Koistinen J, Beckman K, Evans T, Gorzelany JF, Hansen KJ, Jones PD, Helle E, Nyman M, Giesy JP. Accumulation of perfluorooctane sulfonate in marine mammals. Environ Sci Technol. 2001;35:1593–1598. doi: 10.1021/es001873w. [DOI] [PubMed] [Google Scholar]
- 15.Martin JW, Smithwick MM, Braune BM, Hoekstra PF, Muir DCG, Mabury SA. Identification of long-chain perfluorinated acids in biota from the Canadian Arctic. Environ Sci Technol. 2004;38:373–380. doi: 10.1021/es034727+. [DOI] [PubMed] [Google Scholar]
- 16.Martin JW, Whittle DM, Muir DCG, Mabury SA. Perfluoroalkyl contaminants in a food web from lake Ontario. Environ Sci Technol. 2004;38:5379–5385. doi: 10.1021/es049331s. [DOI] [PubMed] [Google Scholar]
- 17.Hansen KJ, Clemen LA, Ellefson ME, Johnson HO. Compound-specific quantitative characterization of organic fluorochemicals in biological matrices. Environ Sci Technol. 2001;35:766–770. doi: 10.1021/es001489z. [DOI] [PubMed] [Google Scholar]
- 18.Kuklenyik Z, Reich JA, Tully JS, Needham LL, Calafat AM. Automated solid-phase-extraction and measurement of perfluorinated organic acids and amides in human serum and milk. Environ Sci Technol. 2004;38 doi: 10.1021/es040332u. [DOI] [PubMed] [Google Scholar]
- 19.Flaherty J, Connolly P, Decker E, Kennedy S, Szostek N, Ellefson M, Reagen W. Quantitative determination of perfluorooctanoic acid in serum and plasma by liquid chromatography tandem mass spectrometry. J Chrom B. 2005;819:329–338. doi: 10.1016/j.jchromb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.3M “Executive Summary: Environmental Monitoring - Multi-City Study. Water, Sludge, Sediment, POTW Effluent and Landfill Leachate Samples. U.S. Environmental Protection Agency Docket AR226–1030a,”; 2001. [Google Scholar]
- 21.Alzaga R, Bayona JM. Determination of perfluorocarboxylic acids in aqueous matrices by ion-pair solid-phase microextraction-in-port derivatization-gas chromatography-negative ion chemical ionization mass spectrometry. J Chromatogr. 2004;1042:155–162. doi: 10.1016/j.chroma.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Boulanger B, Vargo J, Schnoor J, Hornbuckle K. Evaluation of perfluorooctane surfactants in a wastewater treatment system and in a commercial surface protection product. Environ Sci Technol. 2005;39:5524–5530. doi: 10.1021/es050213u. [DOI] [PubMed] [Google Scholar]
- 23.Higgins C, Field J, Criddle C, Luthy R. Quantitative determination of perfluorochemicals in sediments and domestic sludge. Environ Sci Technol. 2005;39:3946–3956. doi: 10.1021/es048245p. [DOI] [PubMed] [Google Scholar]
- 24.Moody CA, Martin JW, Kwan WC, Muir DCG, Mabury SA. Monitoring perfluorinated surfactants in biota and surface waters following an accidental release of fire-fighting foam into Etobicoke creek. Environ Sci Technol. 2002;36:545–551. doi: 10.1021/es011001+. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita N, Kannan K, Taniyasu S, Horii Y, Petrick G, Gamo T. A global survey of perfluorinated acids in oceans. Marine Pollution Bulletin. doi: 10.1016/j.marpolbul.2005.04.026. In Press. [DOI] [PubMed] [Google Scholar]
- 26.Weppner WA. 3M Company. 2000. Phase-out plan for POSF-based products, U.S. Environmental Protection Agency Docket AR226-0600. [Google Scholar]
- 27.Huset C, Barofsky D, Field J. Determination of semi-volatile fluoroalkyl substances in municipal wastewater treatment plant biosolids. 53rd ASMS Conference on Mass Spectrometry and Allied Topics; June 5–9; San Antonio, Texas. 2005. [Google Scholar]
- 28.3M “The 18 day aerobic biodegradation study of perfluorooctanesulfonyl-based chemistries. 3M Report No. E01-0415; U.S. Environmental Protection Agency Docket AR 226-10301,” 2001.
- 29.Key BD, Howell RD, Criddle CS. Defluorination of organofluorine sulfur compounds by Pseudomonas Sp. Strain D2. Environ Sci Technol. 1998;32:2283–2287. [Google Scholar]
- 30.Wang N, Szostek B, Folsom PW, Sulecki LM, Capka V, Buck RC, Berti WR, Gannon JT. Aerobic biotransformation of C-14-labeled 8-2 telomer B alcohol by activated sludge from a domestic sewage treatment plant. Environ Sci Technol. 2005;39:531–538. doi: 10.1021/es049466y. [DOI] [PubMed] [Google Scholar]
- 31.Dinglasan MJA, Ye Y, Edwards EA, Mabury SA. Fluorotelomer alcohol biodegradation yields poly- and perfluorinated acids. Environ Sci Technol. 2004;38:2857–2864. doi: 10.1021/es0350177. [DOI] [PubMed] [Google Scholar]
- 32.3M “The aerobic biodegradation of N-EtFOSE alcohol by the microbial activity present in municipal wastewater treatment sludge. 3M Report No. E00-2252; U.S. Environmental Protection Agency Docket AR 226–10301,” 2000.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary text and tables that provide additional information in regards to quality assurance and quality controls, method precision, and mass spectrometric details are located in Supporting Information.