Abstract
Background
Superior mesenteric artery (SMA) flow increases after a feeding to meet the intestines’ increased metabolic demands. Although a PDA can affect SMA perfusion in non-feeding infants, there is no information about its effects on the hyperemic response that follows a feeding.
Objective
To study the effects of a PDA on SMA perfusion in preterm baboons.
Design
Preterm baboons were delivered at 67% gestation and ventilated for 14 days. Enteral feedings were begun and advanced per protocol. Feeding studies were performed between days 10 and 14. Thirty-one studies were performed in animals with a closed ductus; 21 studies in those with a moderate PDA shunt (Qp/Qs≥2:1). 2-D Echo and Doppler exams were performed before, 10 and 30 min after a feeding. The groups were similar in birth weights, feeding volumes, and age at time of study.
Results
During the preprandial period, baboons with a moderate PDA had significantly lower blood pressures and systemic blood flows than animals with a closed ductus. Preprandial SMA-blood flow velocities did not differ between the open and closed ductus groups. Animals with a closed ductus increased their SMA-velocities (diastolic and mean) and decreased their SMA relative-vascular-resistance (mean BP/mean SMA-velocity) by 10 min after the feeding. By 30 min after the feeding, the values were returning to their preprandial values. In contrast, in the PDA group, there were no significant changes in SMA-velocity or resistance following the feeding, and SMA-velocities were significantly lower than the closed ductus group.
Conclusions
A moderate PDA shunt limits the ability of the preterm newborn baboon to increase its postprandial mesenteric blood flow velocity. We speculate that this may interfere with its ability to meet increased intestinal metabolic demands and may contribute to feeding difficulties.
Keywords: patent ductus arteriosus, mesenteric blood flow, necrotizing enterocolitis, feeding problems, premature newborn, blood flow velocity, pulsatility index
Introduction
In full term infants, enteral feeding is accompanied by an increase in mesenteric blood flow to meet the intestines’ new, increased, metabolic demands (1, 2). The postprandial increase in mesenteric blood flow is usually accomplished by decreasing mesenteric vascular resistance (2). In contrast with older infants, preterm infants have less mesenteric blood flow reserve and develop near maximal intestinal blood flow and oxygen extraction during feedings (3, 4). Their diminished reserve may increase their risk for developing intestinal ischemia when alterations in intestinal blood flow occur.
Numerous studies have shown an association between a persistent PDA and feeding intolerance in preterm infants (5, 6). However, the exact role of the PDA, if any, has yet to be defined (7). Although a PDA can affect mesenteric perfusion and resistance in non-feeding infants (8-10), there is no information about its effect on the hyperemic response that normally accompanies a feeding. This question is quite relevant since the regulation of intestinal blood flow appears to depend more on its metabolic demands and less on alterations in systemic blood pressure (1, 9, 11).
We designed the following study to examine the effects of a PDA on intestinal perfusion, both before and after an enteral feed, in preterm baboons. We used the SMA end-diastolic velocity as a surrogate measure of perfusion since a) it correlates with mesenteric blood flow (12) and b) alterations in SMA velocity are predictive of subsequent feeding intolerence (13) and NEC (14, 15). We hypothesized that the presence of a PDA would inhibit the immature baboon's ability to increase its SMA enddiastolic velocity when challenged with an enteral feeding.
Methods
General Animal care
Studies were performed at the Southwest National Primate Research Center, Southwest Foundation for Biomedical Research in San Antonio, TX and were approved by the Institutional Animal Care and Use Committee. Details of animal care have been published elsewhere (16-18). Briefly, baboon (Papio papio) dams, with timed pregnancies, were treated with 6 mg of intramuscular betamethasone 48 and 24 h before elective C-section delivery at 125 ± 2 days gestation (full term = 185 days). At birth, the infants were weighed, sedated, intubated, given surfactant (Survanta, courtesy of Ross Laboratories, Columbus, OH) prior to initiation of ventilator support (InfantStar, Infrasonics, San Diego, CA), and ventilated for 14 days.
Ventilator adjustments were made based on chest radiograph, clinical examination, arterial blood gas measurements, and tidal volume measurements (17). Target goals for PaO2 were 55 to 70 mm Hg, for PaCO2 were 45 to 55 mm Hg, and for tidal volume were 4 to 6 ml/kg. Chest radiographs were obtained daily. None of the animals developed septicemia or pneumonia during the study period. Nutritional, fluid, transfusion, antibiotic and blood pressure management have been previously described (17). None of the animals received postnatal steroids.
Enteral feedings (by orogastric tube over a period of 5 minutes) were initiated on day 4 with a premature infant formula (Primilac (20 cal/oz) or Similac Special Care (20 cal/oz)). Thirty percent of the baboons received Similac Special Care because of a shortage of Primilac. There were no differences between the study groups (see below) in the type of formula (Primilac or Similac Special Care) that they were fed. Feedings were administered every 3 hours. The initial daily feeding volume was 5 ml/kg/feed and the feeding volume was increased by 8−10 ml/kg/day as tolerated (17).
Blood pressures (BP), heart rate (HR), arterial blood gases, and FiO2 were recorded every 4 hours throughout the study. Oxygenation Index (OI= mean airway pressure (cm H2O) × FiO2 × 100/ PaO2) and Ventilation Index (VI= peak inspiratory pressure × ventilator rate × PaCO2/ 1000) were calculated at the same times and averaged over 12 hour intervals.
A complete echocardiographic exam, including assessment of ductal patency, was performed daily using an 8-mHz transducer interfaced with a Biosound AU3 (Genoa, Italy) echocardiographic system (19, 20). In the presence of a ductus left-to-right shunt, we used the measurement of flow, returning from the body, across the pulmonary valve, to estimate the effective systemic output (Qs), and the flow, returning from the lungs, across the aortic valve, to estimate the pulmonary blood flow (Qp). These measurements did not take into account any left-to-right shunt at the level of the foramen ovale. By disregarding the foramen ovale shunt we may have underestimated the magnitude of the left-to-right PDA shunt. At the time of the feeding studies, most animals appeared to have either no or small amounts of foramen ovale flow.
Study design
SMA velocity responses to enteral feedings were measured in animals that were at least 10 days old and that had been receiving enteral feedings for at least 4 days prior to the study. Ventilator settings, pH, blood gases, hematocrit, weight and 2-D echocardiographic and Doppler measurements of the ductus were recorded at the beginning of each study. Blood pressure, heart rate, and SMA velocity measurements were recorded before, and at 10 and 30 minutes after a feeding. The baboons were studied in a supine, quiet state. The temperature was maintained by servo control and none were receiving phototherapy. We used end-diastolic velocity as a surrogate for blood flow since this has been shown to be well correlated with mesenteric blood flow (12). The hemodynamic measurements were made by a single investigator (DM). A 7.5 mHz transducer was used for tissue imaging and a 5.0 mHz transducer was used for Doppler recordings. Color flow mapping was used to identify the arteries and pulsed-wave was applied to measure SMA velocities. The Doppler sample volume was placed in the proximal portion of the SMA near its origin from the aorta and correction was made for the angle of insonation. Peak-systolic, end-diastolic, and time-averaged mean SMA velocity measurements were obtained from the peak velocity envelope of three consecutive cardiac cycles. We calculated two separate pulsatility indices [PI (mean) and PI (peak)] for each set of velocity measurements, since each of these measurements has been used as a surrogate for SMA impedance {PI (mean) = (peak systolic velocity – end diastolic velocity)/ time-averaged mean velocity (14); PI (peak) = (peak systolic velocity – end diastolic velocity)/ peak systolic velocity (21)}. We also calculated the SMA relative vascular resistance as a surrogate measure of resistance (relative vascular resistance = mean arterial blood pressure divided by mean SMA velocity) (22, 23).
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Comparisons of continuous variables were performed using Student's t-test and categorical data using Fisher's exact test. Two-way ANOVA with repeated measures and post hoc analysis (Tukey's test) was used to compare differences within and between groups. Statistical significance was determined at 2-sided values of p<0.05.
Results
The hemodynamic responses to feeding were grouped according to the degree of left-to-right shunt through the ductus arteriosus: a) closed ductus (Qp/Qs ≤ 1.2), b) small shunt (Qp/Qs ≥1.4 and <2), or c) moderate shunt (Qp/Qs ≥ 2) (Table 1).
Table 1.
Pre-prandial measurements (at the time of the feeding study) in baboons with a closed, small and moderate PDA
| Closed (Qp/Qs ≤1.2) |
Small Shunt (Qp/Qs ≥1.4 and <2) |
Moderate Shunt (Qp/Qs >2) |
|
|---|---|---|---|
| Number of animals | 13 | 13 | 11 |
| Number of studies | 31 | 20 | 21 |
| Postnatal age (d) | 11.5 ± 1.1 | 11.6 ± 1.0 | 11.6 ± 1.3 |
| Weight (gm) | 365 ± 47 | 374 ± 40 | 382 ± 49 |
| Qp/Qs | 1.00 ± 0.07 | 1.65 ± 0.18* | 2.26 ± 0.40* |
| LVEDD (mm) | 0.9 ± 0.1 | 1.0 ± 0.1* | 1.1 ± 0.1* |
| Effective systemic output (ml/kg/min) | 228 ± 45 | 254 ± 57 | 192 ± 43* |
| BP systolic (mm Hg) | 65 ± 8 | 56 ± 7* | 56 ± 8* |
| BP diastolic (mm Hg) | 34 ± 7 | 30 ± 7 | 31 ± 7 |
| BP mean (mm Hg) | 46 ± 5 | 41 ± 5* | 42 ± 6* |
| HR (beats/min) | 151 ± 15 | 158 ± 12* | 162 ± 13* |
| Feeding (ml/kg/feed) | 4.2 ± 1.0 | 3.8 ± 0.8 | 3.9 ± 0.9 |
| PaO2 (mm Hg) | 71.9 ± 4.7 | 69.9 ± 0.6 | 71.4 ± 3.5 |
| PaCO2 (mm Hg) | 51.4 ± 0.9 | 50.3 ± 0.2 | 52.1 ± 1.5 |
| pH | 7.34 ± 0.1 | 7.35 ± 0.1 | 7.33 ± 0.1 |
| Hematocrit (%) | 37±3 | 38±2 | 36±3 |
| VI | 36.7 ± 3.3 | 38.9 ± 0.6 | 40.0 ± 4.9 |
| OI | 5.3 ± 0.6 | 5.3 ± 0.1 | 4.5 ± 0.5 |
Values are means ± SD.
p<0.05 versus closed ductus group
The three groups did not differ in the degree of initial respiratory distress during the early neonatal course {OI (at 24 hours after birth): Closed=5.3±2.2, Small=5.1±2.0, Moderate=4.4±2.8); VI (at 24 hours after birth): Closed=33.8±6.8, Small=34.1±9.9, Moderate=32.7±17}. Similarly, at the time of the feeding studies, there were no differences between the groups in postnatal age, weight, or in the volume or type of formula that was being fed. Nor were there difference in the VI, OI, or arterial blood gases prior to the feeding (Table 1).
During the baseline period prior to the feeding, animals with a closed ductus had significantly lower LVEDD and HR, and significantly higher mean blood pressure than the animals in the open ductus groups (Table 1). The effective systemic blood flow was significantly reduced by the presence of a PDA shunt, but only when the shunt was moderate in size (Qp/Qs>2) (Table 1). Since effective systemic blood flow was reduced only by a moderate size PDA shunt, we focused our comparisons on the closed ductus and moderate PDA shunt groups.
Enteral feeding had no effect on BP, HR or effective systemic output in either the closed or moderate shunt groups (Figure 1). The significant differences in BP, HR and systemic output, that existed prior to the feeding study, between the closed and moderate shunt groups, persisted throughout the postprandial period.
Figure 1.
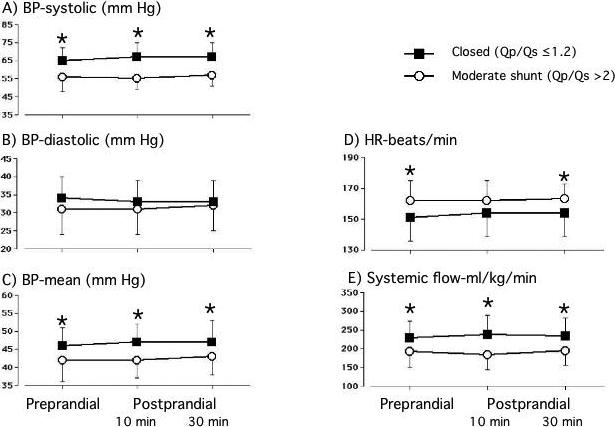
Blood pressure, heart rate and effective systemic output are significantly different between the closed ductus (n=31) and moderate shunt (n=21) groups. Enteral feeding had no effect on any of these variables. Values are mean±sd. * p<0.05, moderate shunt versus closed ductus; # p<0.05, postprandial value versus preprandial value.
Although the animals in both groups had forward flow in the SMA throughout the cardiac cycle, the response of the SMA velocity profiles to feeding differed significantly between the two groups. In the closed ductus group, there was a significant increase in diastolic and mean SMA velocities, and a significant decrease in the relative vascular resistance, by 10 minutes after the feeding (compared with determinations made prior to the feeding) (Figure 2). By 30 minutes after the feeding, the velocities and relative vascular resistance were returning towards their baseline pre-prandial values (Figure 2).
Figure 2.
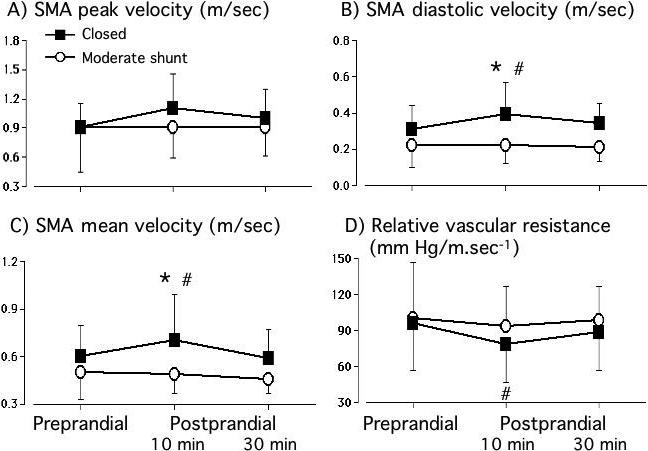
Superior Mesenteric Artery flow velocities and relative vascular resistances before and after a feeding. There were significant increases in diastolic and mean SMA velocities and a significant decrease in relative vascular resistance at 10 minutes after a feeding in the closed ductus (n=31) group but not in the group with a moderate shunt (n=21). * p<0.05, moderate shunt versus closed ductus; # p<0.05, postprandial value versus preprandial value.
In contrast, animals in the moderate shunt group had no change in SMA flow velocity (peak, mean or diastolic) or in relative vascular resistance at either 10 or 30 minutes after the feeding (Figure 2).
The PI, our surrogate measure of SMA impedance, was significantly elevated in the pre-prandial period in the moderate shunt group, and continued to be significantly greater than the PI in the closed ductus group throughout the postprandial period (Figure 3).
Figure 3.
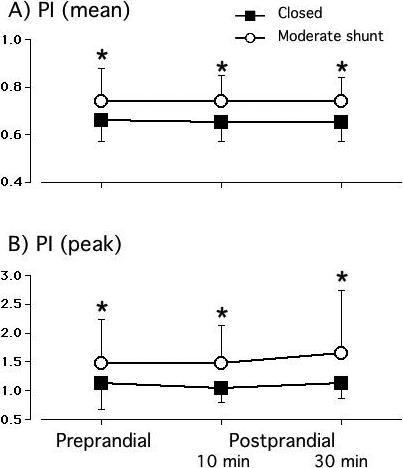
Both mean and peak pulsatility indices are significantly increased in the moderate shunt (n=21) group compared with the closed ductus (n=31) group. * p<0.05, moderate shunt versus closed ductus; # p<0.05, postprandial value versus preprandial value.
Animals in the small PDA shunt group (Qp/Qs ≥1.4 and <2) had SMA flow velocities (peak, mean and diastolic), relative vascular resistances and PIs (both prior to and following the feeding) that fell midway between the values in the closed ductus and moderate shunt groups, respectively (data not shown). Our study was not powered to be able to detect significant differences in values between the small PDA shunt group and the groups with either a closed or moderate PDA shunt.
Discussion
The mesenteric circulation is a “pressure-passive” system. Its blood flow and oxygen extraction are primarily regulated by the intestine's metabolic demands. Autoregulation plays only a minor role in its control in both the fed and fasted state (1, 9). The introduction of feedings into the intestinal tract increases its metabolic requirements and increases its oxygen uptake (1, 2). In the adult, the intestine's increased postprandial oxygen uptake is initially achieved by an increase in blood flow. This is followed by an increase in oxygen extraction as the postprandial hyperemia wanes (2).
Although a left-to-right aortopulmonary shunt has a profound impact on mesenteric perfusion (24), full term infants, with systemic-to-pulmonary arterial shunts, are able to maintain their mesenteric blood flow, both at rest and during feedings, by vasodilating their splanchnic circulation (24). The immature infant, on the other hand, has a limited ability to increase its mesenteric blood flow and oxygen extraction at rest (3, 4). Prior studies have shown that, during the fasting state, a left-to-right PDA shunt causes a decrease in arterial perfusion pressure, an increase in localized mesenteric vascular resistance, and a decrease in mesenteric blood flow (8-10).
The presence of a PDA produces similar changes in other “pressure-passive” vascular beds; however, the presence of a PDA does not necessarily impede their ability to increase local perfusion when metabolic demand increases. For example, the presence of a PDA decreases blood flow to resting skeletal muscle by nearly 50% (25). However, once the muscle starts to contract, its vascular resistance decreases precipitously and blood flow increases markedly. As a result, blood flow, oxygen delivery and muscle performance are the same whether the ductus is open or closed (25).
Our findings in the mesenteric circulation, contrast markedly with what has been observed in skeletal muscle. We found that preterm baboons, with a moderate PDA shunt, have lower systemic BP, lower mean and diastolic SMA velocities, and increased PI during the baseline, preprandial period (Figures 1, 2 and 3). Preterm animals, with a closed ductus, are able to increase their SMA velocities and decrease their relative resistance index following a feeding (Figure 2); in contrast, animals with an open ductus are not able to make the same compensatory changes (Figure 2).
The inability to increase postprandial flow, in the presence of a PDA, may increase the risk for intestinal ischemia, feeding intolerance, and NEC (5, 6, 14, 15, 26). Although it is unlikely that a PDA is sufficient to produce the ischemic injury itself, the inability to increase flow following a feeding might render the intestine more vulnerable to other cardiovascular stresses or ischemic insults. For example, small increases in intra-abdominal pressure (produced by increased diaphragmatic breathing) have no effect on intestinal blood flow in newborn animals with a closed ductus; in contrast, in the presence of a PDA, the same changes in intra-abdominal pressure lead to decreased intestinal blood flow (25). This hypothesis is consistent with the study by Palder et al (27), which showed that the severity of NEC was increased in the presence of a PDA.
Several caveats are needed when interpreting our results. The two animal groups were not randomly chosen. They were comprised of those that spontaneously closed their ductus before day 10 and those that failed to close their ductus by that date. Therefore, our experimental design does not allow us to determine whether the differences between the groups are due to the PDA shunt, itself, or to factors that might coexist in animals that fail to close their ductus spontaneously. Although the distribution of several factors known to affect the postprandial, hyperemic response (e.g., initial degree of illness and postnatal age (28, 29), prior exposure to feedings (11, 28), feeding volume (22, 29), exposure to phototherapy (30), and degree of mechanical ventilation at the time of study (21)) were similar between the two groups, other unknown conditions may have contributed to our findings. Our experimental model, also, does not allow us to comment on the role of a PDA with larger feeding volumes, or of the immature intestine's ability to increase its oxygen extraction. It should also be noted that the investigator who performed the hemodynamic assessments was not blinded to the status of the ductus shunt. This may have introduced an unconscious bias when performing the SMA-specific hemodynamic measurements.
There are also several differences between our results in baboons and those that have been reported in humans. In general, the postprandial hyperemic response in the baboon was of shorter duration than that observed in the human (10, 28, 29). The PDA shunts in our animals were only moderate in size; and, none of the animals developed NEC. In addition, none of the animals had reversal of flow in the descending aorta or mesenteric bed. In contrast, reversal of flow has been observed in the human studies (10, 31).
Acknowledgements
The authors thank all the personnel that support the BPD Resource Center: the animal husbandry group led by Drs. D. Carey and M. Leland, the NICU staff (H. Martin, D. Correll, L. Kalisky, L Nicley, R. Degan, S. Salazar, S. Ali), Deborah Catland, NNP, the Wilford Hall Medical Center neonatal fellows who assist in the care of the animals, and the UTHSCSA pathology staff (L Buchanan, K Symank, Y Valdes and K Mendoza) who perform necropsies. Francoise Mauray performed the artwork. We especially thank Vickie Winter, who has skillfully managed to categorize and keep track of all the animal data over the years, and Dr. Jackie Coalson, who provided invaluable leadership, guidance, and scientific oversight for the BPD Resource Center and with whom we have had many thought provoking discussions.
This research is supported in part by NIH Grants HL 63399, HL56061, HL46691, HL52636 BPD Resource Center, P51RR13986 Primate Center facility support and a gift from the Jamie and Bobby Gates Foundation.
Abbreviations
- SMA
superior mesenteric artery
- PDA
patent ductus arteriosus
- Qp
pulmonary blood flow
- Qs
effective systemic blood flow
- Qp/Qs
pulmonary-to-systemic blood flow ratio
- HR
heart rate
- BP
blood pressure
- LVEDD
left ventricular end diastolic diameter
- NEC
necrotizing enterocolitis
- VI
ventilation index
- OI
oxygenation index
- PI
pulsatility index
Footnotes
Disclosures: We have no financial disclosures.
Conflict of interests: We have no conflict of interests.
Bibliography
- 1.Granger HJ, Norris CP. Intrinsic regulation of intestinal oxygenation in the anaesthetized dog. Am J Physiol. 1980;239:H156–H162. doi: 10.1152/ajpheart.1980.238.6.H836. [DOI] [PubMed] [Google Scholar]
- 2.Sit SP, Chou CC. Time course of jejunal blood flow, O2 uptake, and O2 extraction during nutrient absorption. Am J Physiol. 1984;247(3 Pt 2):H395–402. doi: 10.1152/ajpheart.1984.247.3.H395. [DOI] [PubMed] [Google Scholar]
- 3.Nowicki PT, Miller CE. Autoregulation in the developing postnatal intestinal circulation. Am J Physiol. 1988;254:G189–G193. doi: 10.1152/ajpgi.1988.254.2.G189. [DOI] [PubMed] [Google Scholar]
- 4.Crissinger KD, Granger DN. Intestinal blood flow and oxygen consumption: responses to hemorrhage in the developing piglet. Pediatr Res. 1989;26(2):102–5. doi: 10.1203/00006450-198908000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Patole SK, Kumaran V, Travadi JN, Brooks JM, Doherty DA. Does patent ductus arteriosus affect feed tolerance in preterm neonates? Arch Dis Child Fetal Neonatal Ed. 2007;92(1):F53–5. doi: 10.1136/adc.2006.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr. 2005;40(2):184–8. doi: 10.1097/00005176-200502000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Kanto WP, Jr., Wilson R, Breart GL, Zierler S, Purohit DM, Peckham GJ, et al. Perinatal events and necrotizing enterocolitis in premature infants. Am J Dis Child. 1987;141(2):167–9. doi: 10.1001/archpedi.1987.04460020057026. [DOI] [PubMed] [Google Scholar]
- 8.Clyman RI, Mauray F, Heymann MA, Roman C. Cardiovascular effects of a patent ductus arteriosus in preterm lambs with respiratory distress. J. Pediatr. 1987;111:579–587. doi: 10.1016/s0022-3476(87)80126-9. [DOI] [PubMed] [Google Scholar]
- 9.Meyers R, Alpan G, Clyman RI. Effect of patent ductus arteriosus and indomethacin on intestinal blood flow in the newborn lamb. Pediatr Res. 1990:216A. doi: 10.1203/00006450-199106010-00010. [DOI] [PubMed] [Google Scholar]
- 10.Coombs RC, Morgan MEI, Durin GM, Booth IW, McNeish AS. Gut blood flow velocities in the newborn: effects of patent ductus arteriosus and parenteral indomethacin. Arch. Dis. Child. 1990;65:1067–1071. doi: 10.1136/adc.65.10_spec_no.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiel JW, Bishop VS. Effect of fasting and refeeding on mesenteric autoregulation in conscious rabbits. Am J Physiol. 1992;262(5 Pt 2):H1407–14. doi: 10.1152/ajpheart.1992.262.5.H1407. [DOI] [PubMed] [Google Scholar]
- 12.Martinussen M, Odden JP, Brubakk AM, Vik T, Bratlid D, Yao AC. Validity of Doppler measurements of superior mesenteric artery blood flow velocity: comparison with blood flow measured by microsphere technique. Eur J Ultrasound. 1996;4:55–62. [Google Scholar]
- 13.Fang S, Kempley ST, Gamsu HR. Prediction of early tolerance to enteral feeding in preterm infants by measurement of superior mesenteric artery blood flow velocity. Arch Dis Child Fetal Neonatal Ed. 2001;85(1):F42–5. doi: 10.1136/fn.85.1.F42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murdoch EM, Sinha AK, Shanmugalingam ST, Smith GC, Kempley ST. Doppler flow velocimetry in the superior mesenteric artery on the first day of life in preterm infants and the risk of neonatal necrotizing enterocolitis. Pediatrics. 2006;118(5):1999–2003. doi: 10.1542/peds.2006-0272. [DOI] [PubMed] [Google Scholar]
- 15.Carlo WF, Kimball TR, Michelfelder EC, Border WL. Persistent diastolic flow reversal in abdominal aortic Doppler-flow profiles is associated with an increased risk of necrotizing enterocolitis in term infants with congenital heart disease. Pediatrics. 2007;119(2):330–5. doi: 10.1542/peds.2006-2640. [DOI] [PubMed] [Google Scholar]
- 16.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999;160(4):1333–46. doi: 10.1164/ajrccm.160.4.9810071. [DOI] [PubMed] [Google Scholar]
- 17.Yoder BA, Siler-Khodr T, Winter VT, Coalson JJ. High-frequency oscillatory ventilation: effects on lung function, mechanics, and airway cytokines in the immature baboon model for neonatal chronic lung disease. Am J Respir Crit Care Med. 2000;162(5):1867–76. doi: 10.1164/ajrccm.162.5.9912145. [DOI] [PubMed] [Google Scholar]
- 18.McCurnin DC, Yoder BA, Coalson J, Grubb P, Kerecman J, Kupferschmid J, et al. Effect of ductus ligation on cardiopulmonary function in premature baboons. Am J Respir Crit Care Med. 2005;172(12):1569–74. doi: 10.1164/rccm.200502-230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seidner SR, Chen Y-Q, Oprysko PR, Mauray F, Tse MM, Lin E, et al. Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res. 2001;50(3):365–373. doi: 10.1203/00006450-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Yoder B, Martin H, McCurnin DC, Coalson JJ. Impaired urinary cortisol excretion and early cardiopulmonary dysfunction in immature baboons. Pediatr Res. 2002;51(4):426–32. doi: 10.1203/00006450-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Havranek T, Madramootoo C, Carver JD. Nasal continuous positive airway pressure affects pre- and postprandial intestinal blood flow velocity in preterm infants. J Perinatol. 2007;27(11):704–8. doi: 10.1038/sj.jp.7211808. [DOI] [PubMed] [Google Scholar]
- 22.Martinussen M, Brubakk AM, Linker DT, Vik T, Yao AC. Mesenteric blood flow velocity and its relation to circulatory adaptation during the first week of life in healthy term infants. Pediatr Res. 1994;36(3):334–9. doi: 10.1203/00006450-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Van Bel F, Van Zoeren D, Schipper J, Guit GL, Baan J. Effect of indomethacin on superior mesenteric artery blood flow velocity in preterm infants. J Pediatr. 1990;116(6):965–70. doi: 10.1016/s0022-3476(05)80662-6. [DOI] [PubMed] [Google Scholar]
- 24.Cheung YF, Ho MH, Cheng VY. Mesenteric blood flow response to feeding after systemic-to-pulmonary arterial shunt palliation. Ann Thorac Surg. 2003;75(3):947–51. doi: 10.1016/s0003-4975(02)04627-1. [DOI] [PubMed] [Google Scholar]
- 25.Alpan G, Mauray F, Clyman RI. The effects of the patent ductus arteriosus on diaphragmatic blood flow and function. Pediatr. Res. 1990;28:437–445. doi: 10.1203/00006450-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Cassady G, Crouse DT, Kirklin JW, Strange MJ, Jonier CH, Godoy G, et al. A randomized, controlled trial of very early prophylactic ligation of the ductus arteriosus in babies who weighed 1000 g or less at birth. N Engl J Med. 1989;320:1511–1516. doi: 10.1056/NEJM198906083202302. [DOI] [PubMed] [Google Scholar]
- 27.Palder SB, Schwartz MZ, Tyson KRT, Marr CC. Association of closure of patent ductus arteriosus and development of necrotizing enterocolitis. J. Pediatr. Surg. 1988;23:422–423. doi: 10.1016/s0022-3468(88)80439-1. [DOI] [PubMed] [Google Scholar]
- 28.Gladman G, Sims DG, Chiswick ML. Gastrointestinal blood flow velocity after the first feed. Arch Dis Child. 1991;66(1 Spec No):17–20. doi: 10.1136/adc.66.1_spec_no.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanowitz TD, Yao AC, Pettigrew KD, Werner JC, Oh W, Stonestreet BS. Postnatal hemodynamic changes in very-low-birthweight infants. J Appl Physiol. 1999;87(1):370–80. doi: 10.1152/jappl.1999.87.1.370. [DOI] [PubMed] [Google Scholar]
- 30.Yao AC, Martinussen M, Johansen OJ, Brubakk AM. Phototherapy-associated changes in mesenteric blood flow response to feeding in term neonates. J Pediatr. 1994;124(2):309–12. doi: 10.1016/s0022-3476(94)70325-6. [DOI] [PubMed] [Google Scholar]
- 31.Groves AM, Kuschel CA, Knight DB, Skinner JR. Does retrograde diastolic flow in the descending aorta signify impaired systemic perfusion in preterm infants? Pediatr Res. 2008;63(1):89–94. doi: 10.1203/PDR.0b013e31815b4830. [DOI] [PubMed] [Google Scholar]


