Abstract
The primarily intravascular MT-independent changes in functional MRI (fMRI) can be separated from magnetization transfer (MT)-dependent changes. This intravascular component is dominated by an arterial blood volume change (ΔCBVa) term whenever venous contributions are minimized. Stimulation-induced ΔCBVa can therefore be measured by a fit of signal changes to MT ratio. MT-varied fMRI data were acquired in 13 isoflurane-anesthetized rats during forepaw stimulation at 9.4 T to simultaneously measure blood-oxygenation-level-dependent (BOLD) and ΔCBVa response in somatosensory cortical regions. Transverse relaxation rate change (ΔR2) without MT was -0.43 ± 0.15 s-1, and increased with MT level. ΔCBVa was 0.46 ± 0.15 ml/ 100 g, which agrees with our previously-presented MT-varied arterial-spin labeled data (0.42 ± 0.18 ml/100 g) in the same animals and also correlates with ΔR2 without MT. Simulations show that ΔCBVa quantification errors due to potential venous contributions are small for our conditions.
Keywords: magnetization transfer, fMRI, BOLD, arterial blood volume, high magnetic field
Introduction
Off-resonance RF pulses can significantly reduce extravascular signals by magnetization transfer (MT) from tissue macromolecule protons to tissue water protons, while intravascular (arterial and venous) signals are much less affected due to low macromolecule concentration in blood (1,2). Signal intensity measurements with vs. without off-resonance irradiation give the MT attenuation factor (1-MTR), where MTR is the MT ratio, which accounts for both MT (spin exchange and cross relaxation) and any potential direct saturation effects of the off-resonance pulse (3). Functional blood-oxygenation-level-dependent (BOLD) studies incorporating off-resonance spin preparation have examined the effects on fMRI contrast (4-6). Initially, human neural activation studies found that MT reduced the BOLD percentage signal change for most activated pixels, and that MTR increased with task activation (5). In contrast, recent hypercapnia studies in rat found that MT increased the BOLD percentage signal change, and that MTR decreased with arterial pCO2 levels (6). Further studies are necessary to understand BOLD signals with MT effects.
Properties of MT-dependent extravascular (tissue) signals and mostly MT-insensitive intravascular (blood) signals can be utilized to enhance arterial and venous contributions to BOLD signal changes. Venous contributions are minimal when capillary water freely exchanges with tissue water, or when the echo time (TE) is long relative to venous blood T2, leaving terms involving the arterial blood volume change (ΔCBVa) as the dominant MT-independent contribution to stimulus-induced changes. A similar concept was applied in the previously-described MT-varied fMRI experiments in our companion article using arterial spin labeling (ASL) (8). For this paper, we utilize these pool-dependent MT properties without ASL to investigate the effects on BOLD fMRI contrast and quantify ΔCBVa from MT-varied BOLD 9.4-T data on rat somatosensory cortex. Individual ΔCBVa values from MT-varied BOLD data are compared with ΔCBVa values from our previously-reported MT-varied ASL data (8), and with transverse relaxation rate changes (ΔR2). Errors in ΔCBVa quantification due to potential venous contributions were simulated.
Theory
It is assumed that an imaging voxel consists of three compartments; intravascular arterial blood, extravascular tissue, and intravascular venous blood. Capillaries are included in the tissue compartment when capillary water freely exchanges with tissue water, while any unexchanged portion is split between arterial and venous compartments. Studies of stationary blood phantoms determined that blood MTR / tissue MTR ≅ 0.4 (9,10). But when flowing blood spins replace those affected by MT (as for certain RF coil geometries), arterial pool MT effects are minimized (8). If the spin-preparation period is long relative to water exchange time = ∼500 ms, (11), capillary water will freely exchange with tissue water, and this upstream exchange could create significant MT effects in venous blood. With complete exchange, venous MTR = tissue MTR; otherwise venous MTR will depend on MT pulse exposure time.
Steady-state spin-echo (SE) signal intensity from all compartments in the presence of MT (SMT) can be expressed as
| [1] |
where subscripts a, t and ν represent intravascular arterial, extravascular tissue, and intravascular venous compartments; ν is the fraction of spins in each blood pool (% units); R2 is the transverse relaxation rate; MTR ≅ tissue MTR, calculated as (1 - SMT/S0), where S0 is baseline signal intensity without MT, and κ = venous blood MTR/ tissue MTR. Gradient-echo implementation would require substitution of effective transverse relaxation rates (R2* for tissue, arterial and venous blood compartments in Eq. [3]).
During neural stimulation, it is reasonable to assume that R2,a does not change. Signal intensity during stimulation (Sstim,MT) accounting for potential changes in R2,t and R2,ν (ΔR2,t and ΔR2,ν), changes in arterial and venous blood volumes (Δνa and Δνν) and a decrease in tissue volume is then
| [2] |
Under assumptions that arterial and venous blood contributions to baseline are very small (i.e., S0 ≅ M0 · exp(-R2,t · TE)), and that both ΔR2,t · TE and ΔR2,ν · TE are very small, the stimulation-induced signal change in the presence of MT (ΔSstim,MT = Sstim,MT - SMT) normalized to S0 for the general case becomes
| [3] |
Eq. [3] can be simplified for either of the following conditions. i) If capillary and tissue water freely exchange (κ = 1), venous MTR = tissue MTR, and both tissue (second) and venous (last) terms can then be combined into a single term with a factor (A) multiplied by (1 - MTR). ii) Even for κ ≠ 0, the venous (third) term is minimized whenever TE is appropriately long relative to venous blood T2 - a condition easily satisfied at 9.4 T where R2,ν = ∼ 150 to 200 s-1 (7,12). Under either of those conditions,
| [4] |
At 9.4 T, the arterial term is approximately Δνa, since R2 values of arterial blood and tissue are similar (R2,a ≅ R2,t ≅ 25 s-1 (7)), leaving
| [5] |
so that for a linear fit of ΔSstim,MT / S0 vs. (1 - MTR), the intercept yields Δνa, which is converted to ΔCBVa (units of ml blood/g tissue) by multiplying by the tissue-to-blood partition coefficient of 0.9 ml/g (13).
Conventional fMRI responses have volume and relaxation components from arteries, tissue and veins. Pure BOLD responses (i.e. with origins in tissue and veins) can be obtained by removal of terms directly related to Δνa under conditions where Δνν is minimal (8); this is accomplished by subtracting Δνa · (exp[-(R2,a - R2,t) · TE] - 1) from fMRI signal change without MT (see Eq. [3] with MTR = 0). At 9.4 T, this correction is unnecessary since R2,a = R2,t.
Methods
Animal Preparation
Animal protocol was approved by the University of Pittsburgh Animal Care and Use Committee. Thirteen male Sprague-Dawley rats weighing 350-450 g (Charles River Laboratories, Wilmington, MA) were studied under 1.3 - 1.5% isoflurane anesthesia. Details of animal preparation were described previously (8). Animals were maintained within normal physiological ranges; PaCO2 = 38.1 ± 4.6 mmHg, PaO2 = 110.2 ± 11.6 mmHg, and mean arterial blood pressure = 93.1 ± 8.01 mmHg (n = 13). Forepaw stimulation parameters were pulse duration = 3 ms, pulse current = 1.5 - 1.8 mA, pulse frequency = 6 Hz (14), stimulus duration = 15 s, and interstimulus period > 1 min. No significant blood pressure changes were observed during somatosensory stimulation.
MRI Methods
Images were acquired at 9.4 T (magnet bore size = 31-cm diameter), with a Unity INOVA console (Varian, Palo Alto, CA). A 2.3-cm diameter surface coil on the head provided for variable MT effects and image acquisition capability; arterial MT effects were negligible due to the flow of arterial blood into the limited region of imaging coil coverage (8). A butterfly-shaped surface coil over the neck provided the arterial-spin labeling required only for our previously-presented data (8). Both RF coils were actively-detunable. There is no possibility for interference between spin labeling and MT effects with this coil configuration. Magnetic field homogeneity was manually optimized on a slab double the imaging slice thickness. Data acquisition used an adiabatic version of the single-shot SE echo planar imaging (EPI) technique with coronal slice thickness = 2 mm, matrix size = 64 (readout) × 32 (phase-encode) and field of view = 3.0 × 1.5 cm2, repetition time = 2.5 s, and TE = 40 ms (n = 7), or 30 ms (n = 6).
Target MTR values were 0, 0.3, and 0.5 for gray matter (corresponding B1 field strengths were 0, 0.11 and 0.16 gauss). The head surface coil transmitted MT pulses at +8500 Hz offset relative to water to guarantee irradiation of macromolecular protons, without direct saturation of any water protons (6). The 2.4-s spin-preparation period contained twelve 100-ms MT pulses separated by 100-ms intervals, during which spin-tagging pulses were applied both for labeled and unlabeled data, but only unlabeled data were used for MT-varied BOLD studies. Sixteen pairs of arterial-spin labeled and unlabeled images (in alternating order) were acquired for each MT level with five pre-stimulation control pairs, three stimulation pairs and eight post-stimulus pairs. Although the MT-induced signal may not reach steady state during a single 2.4-s spin-preparation period, the 0.1-s acquisition time allowed virtually-continuous spin preparation during fMRI studies, ensuring steady-state MT conditions. Other experimental details appear in our previous report on MT-varied ASL measurement of ΔCBVa during somatosensory stimulation (8), where ΔCBF and ΔCBVa were obtained from both arterial-spin labeled and unlabeled images. In this work, BOLD and ΔCBVa responses were determined solely from unlabeled images with varied MT. ΔCBVa values computed by both approaches were compared.
Data Processing
For each animal, all runs from identical conditions were averaged, where the baseline condition included all pre-stimulation data, and the stimulation condition included data acquired between 5 and 15 s after stimulation onset. Data were subsequently processed as previously described for ΔCBVa quantification with MT-varied ASL (8) except that only unlabeled images were used. Since a rat brain atlas (15) shows a forelimb somatosensory cortical area of ∼1.5 × 1.5 mm2 in coronal plates 0.2 and 0.3 mm anterior to bregma, a 9-pixel region of interest (ROI) (1.4 × 1.4 mm2) centered over this anatomically-defined area on the side contralateral to stimulation was defined for all quantifications in each animal. The presence of MT-independent contributions to BOLD fMRI was initially assessed by plotting -ΔR2 values [= (ΔSstim,MT/SMT)/TE] vs. MTR calculated from baseline data. Normalized functional signal changes (ΔSstim,MT/S0) were linearly fitted against normalized signals from baseline conditions (SMT/S0 = 1 - MTR), and ΔCBVa was calculated from the intercept in each study. These ΔCBVa values for individual animals were compared with companion report values (8) for the same ROI obtained by MT-varied ASL. Individual results were averaged and group data are reported as mean ± SD. Statistical analyses were performed using paired t-tests (Origin 7.0, Northampton, MA). Maps of ΔSstim,MT/SMT were generated for active pixels with P-values < 0.05 for illustrative purpose only.
Simulations of ΔCBVa quantification errors
If assumptions for our conditions about negligible venous-term contributions in Eq. [3] are incorrect, then Eq. [5] may be oversimplified. Errors in our ΔCBVa values were therefore assessed using Eq. [3] for the following conditions: at baseline νa = 0.9%, νa + νν = 3.5%; venous oxygenation level (Y) = 0.6 or 0.7 (8); R2,a = R2,t = 25 s-1 and R2,ν = 478 - (458·Y) s-1 (7); ΔR2,t ≅ ΔR2 = -0.43 s-1, ΔR2,ν = -458·ΔY s-1 (derived from (7)); and TE = 35 ms. Since Δνν is minimal for our conditions (8), we assess errors in ΔCBVa quantification with Δνν = 0%, as a function of κ values and stimulus-induced changes in Y (ΔY) = 0.04, 0.06, 0.08 and 0.1.
Results
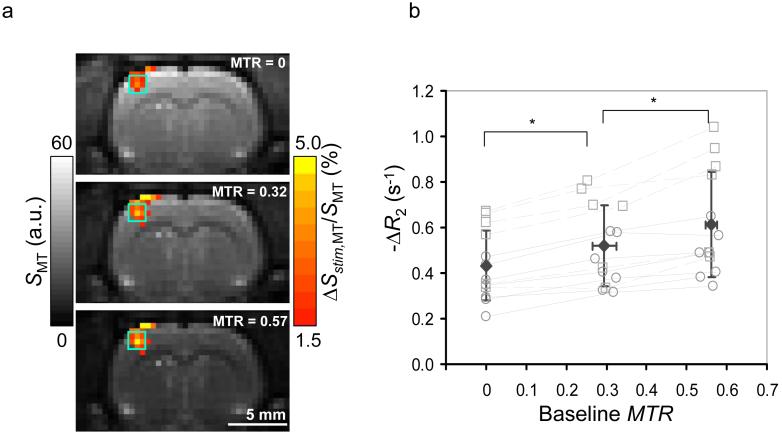
Baseline EPI signals (SMT) decreased with MTR as expected, as demonstrated in the grayscale image from one animal (Fig. 1a). Substantial functional changes (ΔSstim,MT/SMT) observed for all MTR values increased with MTR in the somatosensory ROI (color maps, Fig. 1a). Individual subject’s -ΔR2 values within somatosensory ROIs plotted against MTR values calculated from pre-stimulus data show that mean BOLD signal changes also increased with MTR (Fig. 1b), with ΔR2 = -0.43 ± 0.15, -0.52 ± 0.17 and -0.61 ± 0.23 s-1 at baseline MTR = 0, 0.294 ± 0.030 and 0.561 ± 0.013, respectively (n = 13). Corresponding stimulation-condition MTR values = 0, 0.292 ± 0.029 and 0.558 ± 0.0130 (n = 13); although these stimulation vs. baseline MTR decreases are small relative to SD, the decreases were seen for each animal and are statistically significant (P < 0.01).
Fig. 1.
MT-varied BOLD fMRI responses to somatosensory stimulation. (a) Grayscale baseline EPI images from one animal are overlaid with color maps of stimulation-induced percentage change (no spatial smoothing was performed). The hemisphere contralateral to stimulation appears on the left in images. As MTR increases, baseline signal intensity (a.u. = arbitrary units) decreases, while percentage signal change (ΔSstim,MT/SMT) increases in the contralateral somatosensory area. Green square overlays show 9-pixel ROI in the somatosensory area. (b) Inter-animal comparisons within somatosensory ROIs show stimulus-induced changes to R2 [-ΔR2 = (ΔSstim,MT/SMT)/TE] vs. MTR calculated from the baseline data. The statistically-significant change in ΔR2 between target MTR values (*P < 0.01, n = 13) reflects the increasing intravascular contribution to ΔR2 with MTR. Open symbols show individual animal’s data at each MTR value for TE = 30 ms (squares connected with dashed lines) or TE = 40 ms (circles connected with solid lines), while filled diamonds show mean data, where error bars are SD.
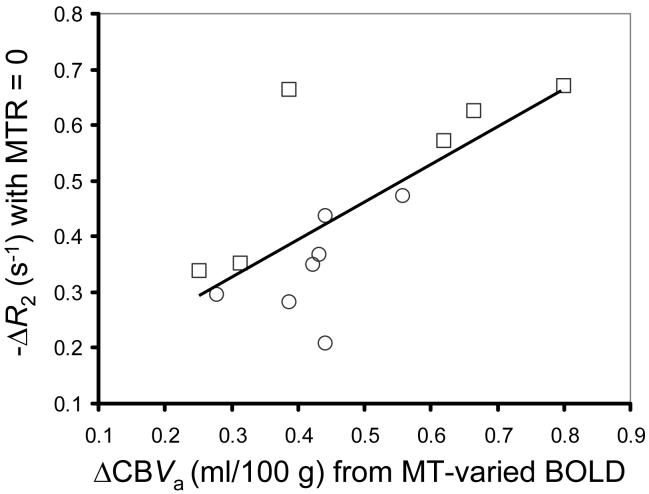
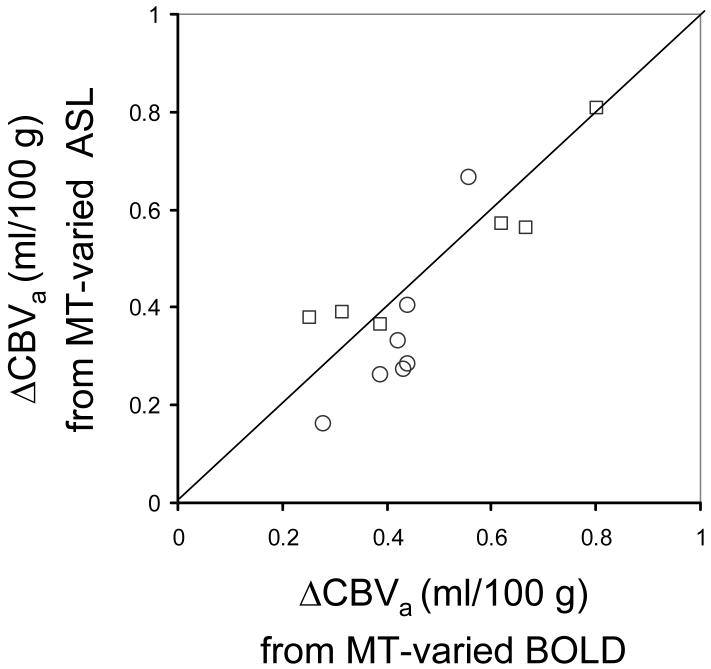
Neural activity-induced ΔCBVa was determined from a linear fit of ΔSstim,MT/S0 vs. SMT/S0 (= 1 - baseline MTR) (Fig. 2). The intercept yields νa = 0.51 ± 0.17 % (n = 13), which converts to ΔCBVa = 0.46 ± 0.15 ml/100 g. Individual animal’s BOLD signal changes (-ΔR2 values without MT) vs. ΔCBVa values are well correlated (Fig. 3, R2 = 0.49). Comparison of ΔCBVa determined for individual animals with the MT-varied BOLD approach vs. the MT-varied ASL approach (Fig. 4) shows a good correlation with no statistical differences (R2 = 0.72, P = 0.15).
Fig. 2.
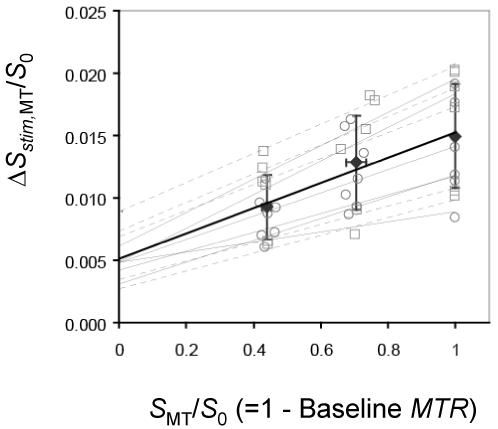
Normalized stimulus-induced BOLD fMRI changes (ΔSstim,MT/S0) vs. baseline MT attenuation factor within somatosensory ROIs (n = 13). A linear fit to all data (thick solid line) yields ΔSstim,MT/S0 = 0.0101 · (1 - MTR) + 0.0051, R2 = 0.9708. The 0.51% intercept represents the MT-independent change for the fraction of spins in the arterial blood pool due to neural activation, and corresponds to a ΔCBVa value of 0.46 ml/100 g. Open symbols show individual animal’s data at each MTR value for TE = 30 ms (squares connected with gray dashed lines) or TE = 40 ms (circles connected with gray solid lines), while filled diamonds show mean data, where error bars are SD.
Fig. 3.
Conventional BOLD signal changes (-ΔR2 without MT) vs. ΔCBVa calculated from MT-varied BOLD within somatosensory ROIs (n = 13). Measured ΔCBVa is well correlated with BOLD; line shows fit to -ΔR2 = 0.68 · ΔCBVa + 0.12 (R2 = 0.49). Individual animal’s data for TE = 30 ms (squares) or TE = 40 ms (circles).
Fig. 4.
Inter-animal comparison of stimulus-induced ΔCBVa calculated from MT-varied ASL data vs. MT-varied BOLD data within somatosensory ROIs (n = 13). A line of unity shows that ΔCBVa values determined for individual animals by both methods are statistically similar (P = 0.15, R2 = 0.72, n = 13). Open symbols show individual animal’s data for TE = 30 ms (squares) and TE = 40 ms (circles).
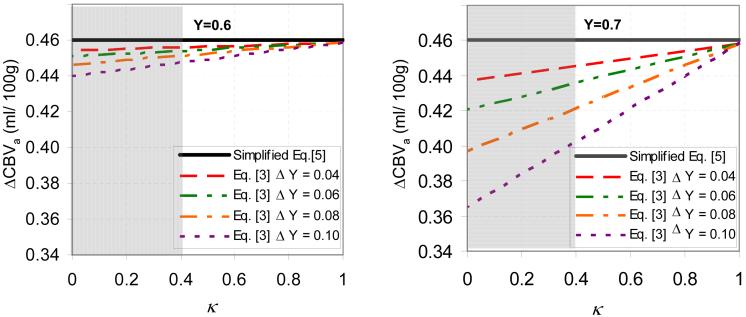
In the absence of free exchange, quantification of our MT-varied BOLD data accounting for non-negligible venous-term contributions (the approximation of Eq. [5]) yields ΔCBVa values that are reduced as Y increases, ΔY increases, or venous/tissue MT effects (κ) are reduced (Fig. 5). For reasonable κ values (> 0.4), the maximum overestimation of ΔCBVa is 0.06 ml/ 100g for 0.46 ml/ 100g.
Fig. 5.
Simulations show that potential ΔCBVa quantification errors from our MT-varied BOLD data are small using simplified Eq. [5] vs. Eq. [3] with Δνν = 0%. Eq. [3] accounts for non-negligible venous blood contributions in the absence of free exchange between capillary and tissue water; simulations are for baseline venous blood oxygenation levels (Y) of 0.6 (a) and 0.7 (b), as a function of stimulus-induced venous oxygenation changes (ΔY) and ratio of venous MTR to tissue MTR (κ). See text for parameters. Horizontal lines in (a) and (b) represent the ΔCBVa value of 0.46 ml/100 g obtained with Eq. [5], which ignores the venous (third) term of Eq. [3]. A reasonable lower limit for κ is 0.4, based on measurements of a stationary blood phantom (9,10), and the fact of that significant exchange between blood and tissue during the MT spin-preparation period should increase κ; regions that are not gray therefore indicate likely in vivo κ values. It should be noted that Eq. [5] is valid under the conditions of free exchange (κ = 1), even with a non-negligible venous blood term in Eq. [3].
Discussion
Our MT-varied BOLD rat forepaw stimulation studies at 9.4 T show enhanced signal changes due to MT-insensitive intravascular contributions. Under the conditions of the present 9.4-T MT-varied BOLD study, venous-term contributions to the MT-independent signal should be minimal, leaving primarily ΔCBVa contributions; if any venous blood contributions do exist, ΔCBVa is only slightly overestimated. At low magnetic field, the potential of a significant venous term in Eq. [3] means that successful ΔCBVa quantification depends on κ; when κ ≠ 1, our approach is not applicable, whenever κ = 1 (independent of field), the intercept of a linear fit of ΔSstim, MT/S0 vs. (1 - MTR) from Eq. [4] can be used to quantify ΔCBVa if R2,a and R2,t are known.
Quantification of ΔCBVa from the same group of animals (n = 13) with MT-varied BOLD vs. MT-varied ASL (8) are well matched (0.46 ± 0.15 ml/100 g vs. 0.42 ± 0.18 ml/100 g, respectively) (Fig. 4). Our Δνa values of 0.51 ± 0.17 % are considerably smaller than those of 1.2 ± 0.7 % for human finger movement at 3 T recently measured with Look-Locker EPI acquisition of ASL data with multiple inversion times following one inversion pulse (16). The discrepancy may be due to differences in species, anesthesia and/or technique-dependent measurement errors.
Our findings of BOLD signal increases with MT due to increased intravascular contributions agree with other recent studies (6), but our theory also incorporates any potential MT sensitivity within the vasculature. These findings are not consistent with one study using short TR and low MTR, where there may have been more progressive saturation in blood than in tissue (5). At 9.4 T, T1 of tissue and blood (2.0 s vs. 2.3 s) are similar (17), minimizing any progressive saturation differences. A spectroscopic finding of decreased MTR with neuronal depolarization was attributed to relaxation time changes, as opposed to any change in the true MT effect (18). However, under our normal physiological conditions, any relaxation time changes due to neural activation are likely to be very small.
Our method to measure ΔCBVa from MT-varied BOLD fMRI is a simple technique implementable with a single coil of limited RF coverage that could also be used in human studies. This approach only determines ΔCBVa, but could provide higher temporal resolution than MT-varied ASL (17), although the latter approach provides baseline CBVa, functional ΔCBVa and functional perfusion changes.
Acknowledgments
This study was supported by NIH R01 grants (EB003375, EB003324, NS44589). The 9.4 T system was funded in part by an NIH grant (RR17239).
References
- 1.Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10(1):135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- 2.Balaban RS, Chesnick S, Hedges K, Samaha F, Heineman FW. Magnetization transfer contrast in MR imaging of the heart. Radiology. 1991;180(3):671–675. doi: 10.1148/radiology.180.3.1871277. [DOI] [PubMed] [Google Scholar]
- 3.Henkelman RM, Huang X, Xiang QS, Stanisz GJ, Swanson SD, Bronskill MJ. Quantitative interpretation of magnetization transfer. Magn Reson Med. 1993;29(6):759–766. doi: 10.1002/mrm.1910290607. [DOI] [PubMed] [Google Scholar]
- 4.Song AW, Wolff SD, Balaban RS, Jezzard P. The effect of off-resonance radiofrequency pulse saturation on fMRI contrast. NMR Biomed. 1997;10(45):208–215. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<208::aid-nbm467>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Zhang R, Cox RW, Hyde JS. The effect of magnetization transfer on functional MRI signals. Magn Reson Med. 1997;38(2):187–192. doi: 10.1002/mrm.1910380205. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Payen JF, van Zijl PC. The interaction between magnetization transfer and blood-oxygen-level-dependent effects. Magn Reson Med. 2005;53(2):356–366. doi: 10.1002/mrm.20348. [DOI] [PubMed] [Google Scholar]
- 7.Lee SP, Silva AC, Ugurbil K, Kim SG. Diffusion-weighted spin-echo fMRI at 9.4 T: microvascular/tissue contribution to BOLD signal changes. Magn Reson Med. 1999;42(5):919–928. doi: 10.1002/(sici)1522-2594(199911)42:5<919::aid-mrm12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Kim T, Hendrich KS, Masamoto K, Kim SG. Arterial versus total blood volume changes during neural activity-induced cerebral blood flow change: implication for BOLD fMRI. J Cereb Blood Flow Metab. 2007;27(6):1235–1247. doi: 10.1038/sj.jcbfm.9600429. [DOI] [PubMed] [Google Scholar]
- 9.Niemi PT, Komu ME, Koskinen SK. Tissue specificity of low-field-strength magnetization transfer contrast imaging. J Magn Reson Imaging. 1992;2(2):197–201. doi: 10.1002/jmri.1880020213. [DOI] [PubMed] [Google Scholar]
- 10.Pike GB, Hu BS, Glover GH, Enzmann DR. Magnetization transfer time-of-flight magnetic resonance angiography. Magn Reson Med. 1992;25(2):372–379. doi: 10.1002/mrm.1910250217. [DOI] [PubMed] [Google Scholar]
- 11.Sanders JAOW. Functional Brain Imaging. Mosby; St Louis: 1995. Functional magnetic resonance imaging; pp. 239–326. [Google Scholar]
- 12.Jin T, Wang P, Tasker M, Zhao F, Kim SG. Source of nonlinearity in echo-time-dependent BOLD fMRI. Magn Reson Med. 2006;55(6):1281–1290. doi: 10.1002/mrm.20918. [DOI] [PubMed] [Google Scholar]
- 13.Herscovitch P, Raichle ME. What is the correct value for the brain--blood partition coefficient for water? J Cereb Blood Flow Metab. 1985;5(1):65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- 14.Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17(4):942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Watson C. The rat brain in sterotaxic coordinates. n. Academic Press; San Diego: 1986. editor. [Google Scholar]
- 16.Brookes MJ, Morris PG, Gowland PA, Francis ST. Noninvasive measurement of arterial cerebral blood volume using Look-Locker EPI and arterial spin labeling. Magn Reson Med. 2007;58(1):41–54. doi: 10.1002/mrm.21199. [DOI] [PubMed] [Google Scholar]
- 17.Kim T, Kim SG. Quantification of Cerebral Arterial Blood Volume and Cerebral Blood Flow using MRI with Modulation of Tissue and Vessel (MOTIVE) Signals. Magn Reson Med. 2005;54(2):333–342. doi: 10.1002/mrm.20550. [DOI] [PubMed] [Google Scholar]
- 18.Stanisz GJ, Yoon RS, Joy ML, Henkelman RM. Why does MTR change with neuronal depolarization? Magn Reson Med. 2002;47(3):472–475. doi: 10.1002/mrm.10071. [DOI] [PubMed] [Google Scholar]
- 19.Kim T, Kim SG. Quantification of cerebral arterial blood volume using arterial spin labeling with intravoxel incoherent motion-sensitive gradients. Magn Reson Med. 2006;55(5):1047–1057. doi: 10.1002/mrm.20867. [DOI] [PubMed] [Google Scholar]