Abstract
Context
It is essential to understand the effect of parent smoking on offspring tobacco use. In biologically related families, parents who smoke may be transmitting a non-specific genetic risk for offspring disinhibited behavior including tobacco use. Studying adoptive families allows us to control for genetic confounding when examining the environmental effect of exposure to parent smoking.
Objective
1) To examine the genetic and environmental contributions to the risk represented by exposure to parent smoking, and 2) to assess the specificity of that risk.
Design, Setting, and Participants
Adolescents adopted in infancy were systematically ascertained from records of three private Minnesota adoption agencies; non-adopted adolescents were ascertained from Minnesota birth records. Adolescents and their rearing parents participated in an in-person assessment.
Main Outcome Measures
Self- reports of behavioral deviance, substance use, and personality as well as DSM-IV clinical assessments of childhood disruptive disorders.
Results
Data from adoptive families suggest that exposure to parent smoking represents an environmental risk for substance use in adolescent offspring. In biologically related families, the effect of exposure to parent smoking is larger and more diverse, including substance use, disruptive behavior disorders, delinquency, deviant peer affiliations, aggressive attitudes, and preference for risk taking.
Conclusions
This study provides evidence for an environmentally mediated pathway by which parent smoking increases risk specifically for substance use in adolescent offspring. The data are also consistent with a genetically mediated pathway by which non-adoptive parents who smoke may also transmit a non-specific genetic risk to their offspring for disinhibited behavior.
Introduction
Each day in the United States, approximately 4,000 adolescents initiate cigarette smoking1, and data from annual surveys suggest that the decade-long decline in teenage smoking has recently slowed2. Researchers consistently report that lifetime exposure to parent smoking predicts offspring tobacco use3-5. Two issues arise in the evaluation of this literature. The first involves the manner in which smoking behavior is transmitted from parent to offspring. In non-adoptive families, genetic and environmental effects are confounded6. Thus, the observation that the birth children of smoking parents have elevated rates of tobacco use does not tell us whether the basis for this association is environmental (e.g., smoking parents model or otherwise encourage tobacco use) or genetic (e.g., smoking parents transmit genes that increase their offspring's liability for tobacco use). In their review of the genetic epidemiology of smoking, Sullivan and Kendler7 concluded that both genetic and shared familial environmental factors contributed to smoking behavior, suggesting that these factors could be acting jointly to increase the risk for tobacco use in the offspring of smokers.
A second issue concerns the specificity of the risk represented by smoking parents. Previous research suggests that smoking is, in part, an expression of a broad vulnerability to engage in disinhibited behavior, which is distinguished more generally by undersocialized conduct and low levels of dispositional constraint 8, 9. Evidence from twin studies suggests that this broad vulnerability may be genetically transmitted from parent to child10. From this perspective, familial resemblance for disinhibition is general rather than disorder specific. Thus, non-adoptive parents who engage in behavior like smoking may also transmit a non-specific genetic risk that increases the likelihood that their offspring will engage in tobacco use as well as other forms of disinhibited behavior11. Of course this risk would not be present in the adoptive families.
To address these issues we used an adoption design, first elucidated by Scarr and Weinberg12 in 1983, which offers an especially sensitive and precise test for the presence of family-level environmental influences. That is, the correlation between two individuals who are not biologically related, but who have been reared together, provides a direct estimate of the shared environmental effect. Genetic influences are inferred only to the extent that larger effects are demonstrated in biologically related families than adoptive families.
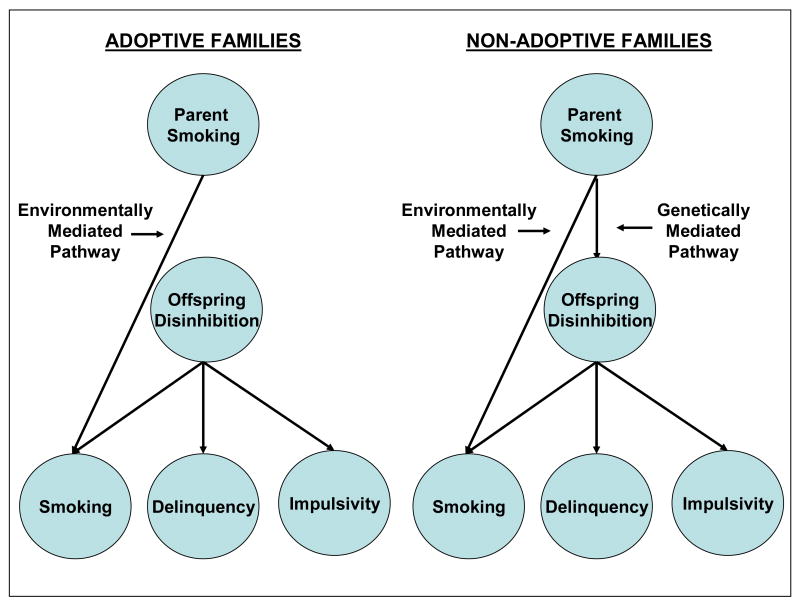
In this study, we compare the effect of being exposed to a non-adoptive parent who smokes, reflecting both genetic and environmental risk, to the effect of being exposed to an adoptive parent who smokes, reflecting environmental risk only. To examine the specificity of the risk, we included a diverse array of adolescent outcomes, both behavioral and dispositional, that tap vulnerability to disinhibition. Figure 1 provides a schematic overview of our adoption design and hypotheses. We hypothesize that exposure to parent smoking represents a specific environmental risk for adolescent tobacco use. Additionally, we hypothesize that non-adoptive parents who smoke may also transmit a non-specific genetic risk for disinhibited behavior not limited to tobacco use. Thus we expect to observe increased tobacco use among both the non-adoptive and adoptive offspring of smoking parents, but increased rates of other disinhibited behavior in the non-adoptive offspring only.
Figure 1.
Adoption design and model predictions. Parent smoking is assumed to affect offspring functioning through both an environmentally mediated specific pathway, which results in an increased risk of adolescent smoking, and a genetically-mediated general pathway, which results in a non-specific increased risk of disinhibited behavior. In non-adoptive families, parent smoking is predicted to be associated with a wide range of offspring outcomes because both pathways are operative. Alternatively, in adoptive families parent smoking is predicted to be associated specifically with offspring smoking because only the environmentally mediated pathway is operative.
Method
Participants
The Sibling Interaction and Behavior Study (SIBS) is a population-based study of 617 families (409 adoptive, 208 non-adoptive) each with an adolescent sibling pair and at least one but typically two rearing parents. Adoptive families were systematically ascertained from infant placements made by three private Minnesota adoption agencies. Reflective of changes in adoption practices over the past 20 years, these agencies minimally screen prospective parents. That is, they must demonstrate a commitment to raising a child, meet a modest minimum income level, and undergo a criminal check, although a criminal record does not preclude placement. Non-adoptive families were ascertained through Minnesota birth records and selected to have a pair of siblings of comparable age and gender to the adoptive sibling pairs. All families were required to live within driving distance of the University of Minnesota
In adoptive families, sibling pairs were biologically unrelated to each other, although one sibling might be the biological offspring of at least one of the adoptive parents. All adoptees were placed permanently in their adoptive home prior to 2 years (average placement age = 4.7 months; SD=3.4). Information is not available on the birth parents of the adoptees. Adoptive parents provided information on the country of birth and ethnicity of participating adolescents. In non-adoptive families, siblings were full, biological siblings. Subsequent to participation, two adolescents were judged ineligible, resulting in a total sample of 1232 adolescents, 613 mothers, and 551 fathers. A complete description of the SIBS recruitment process can be found in McGue et al. (2007)13.
The analyses reported in this paper used a subset of the full sample. Adolescents were included if at least one parent was ever diagnosed with nicotine dependence and used tobacco within their child's lifetime (ever exposed), or if both parents were never diagnosed with nicotine dependence and never used tobacco within their child's lifetime (never exposed). A total of 335 adolescents, with data available on both parents, were excluded based on these criteria. For an additional 112 adolescents, data for one parent was missing, and the other parent did not meet the inclusion criteria. Adolescents excluded because of missing data did not significantly differ from those included on number of disruptive disorder symptoms or measures of lifetime substance use. The final sample included 785 adolescents from 402 families, whose mean age was 14.9 years (SD = 1.9 years). Of these, 414 (53%) had at least one parent who was nicotine dependent and used tobacco during the adolescent's lifetime. Ninety-three adolescents (23%) were exposed by their mother only, 229 (55%) were exposed by their father only, and 92 (22%) were exposed by both parents. Fifty-nine percent of the sample was adopted with 66% of these adoptions originating from South Korea; 94% of the non-adopted adolescents are Caucasian.
Procedure
Families were assessed in our laboratory using a research protocol approved by the University of Minnesota Institutional Review Board. Upon arrival, a complete description of the study was followed by written informed consent from parents and assent from minor offspring. All family members were interviewed simultaneously, each in a separate room by a different interviewer. Interviewers had an M.A. or B.A. in psychology, participated in intensive training, passed written examinations, and satisfied proficiency criteria. Adolescents also completed self-report questionnaires.
Measures
The adolescent outcome battery incorporated diverse indicators of vulnerability to disinhibition including measures of delinquent behavior, antisocial attitudes, aggressive orientation, harm avoidance, substance use, and disruptive behavior disorders. We also assessed nicotine dependence in the parents.
Self Reports of Behavioral Deviance and Substance Use
Adolescents completed the Delinquent Behavior Inventory (DBI)14, a 36-item checklist of minor (e.g., “Skipping school”) and more serious (e.g., “Using a weapon in a fight”) delinquent behaviors (α = .89). Adolescents also completed two 8-item scales assessing the cognitive factors that underlie delinquent behavior: Aggressive Orientation (e.g., “If I didn't like someone, I might try to hurt him or her just for the heck of it,” α = .87) and Antisocial Attitudes (e.g., I see nothing wrong in trying a little beer with my friends,” α = .87). Finally, adolescent lifetime use (at least one time without parents' permission) of tobacco, alcohol, and marijuana was assessed via a private, computer-administered questionnaire. In our relatively young sample, lifetime use is best viewed as experimental use. Among those using substances in the last year, the modal response was less than once a month for each substance. The experimental use phenotype is especially appropriate for this age group in that research has consistently shown that substance use early in adolescence is a potent predictor of later substance misuse15-17.
Personality
The personality trait of Harm Avoidance (HA) was assessed using the Multidimensional Personality Questionnaire18, a self-report instrument assessing personality characteristics in normal populations. Those scoring highly on HA tend to avoid danger and prefer safer activities; they are low in risk taking (α = .84).
Clinical Assessments
Participants were interviewed with the revised version of the Diagnostic Interview for Children and Adolescents (DICA-R)19, 20, modified to ensure lifetime coverage of DSM-IV childhood disorders. All questions asked of the child were asked of the mother as they pertained to the child. Disruptive behavior disorders included conduct disorder (CD), oppositional defiant disorder (ODD, assessed without regard for the presence of CD), and attention-deficit/hyperactivity disorder (ADHD).
Nicotine dependence in parents was assessed with the expanded substance abuse module (SAM), developed by Robins et al.21 as a supplement to the World Health Organization's Composite International Diagnostic Interview22, modified for DSM-IV. Nicotine dependence was assessed over the lifetime of the parent. Parents also provided information on the tobacco products used most heavily, the largest amount consumed, and the age at which they last used tobacco.
Adolescent and parent clinical interviews were reviewed by at least two individuals with advanced clinical training, who were blind to the diagnoses of other family members. This information was used to code, by consensus, every relevant DSM-IV symptom and diagnostic criterion. For the behavior disorders assessed in adolescents, the mother and child reports were combined using the best-estimate method in which a symptom was considered present if endorsed by either reporter23. Diagnoses were considered positive if all (definite) or all but one (probable) of the DSM-IV criteria were met. The disorder symptoms were also summed to provide a quantitative index of clinically significant disruptive behavior. In the parents, nicotine dependence diagnoses were considered positive if present at the definite or probable level. Because the DSM was developed to assess acute rather than lifetime psychopathology, probable diagnoses were included to minimize the likelihood of false negatives due to imperfect recall. Kappa coefficients for our assessments of childhood disruptive disorders are: .73 (ODD), .77 (ADHD), and .80 (CD). Kappa coefficients for substance use disorders, including nicotine dependence, exceed .91.
Family Socioeconomic Status
Parents' education level was coded on a 5-point scale (1 = less than high school, to 5 = professional degree). For parents employed on a full-time basis, occupational status was coded on a 6-point reflected Hollingshead scale (1 = manual laborer, to 6 = professional/managerial). Following procedures outlined in McGue et al. (2007)13, we standardized the educational and occupational status scores for each parent using the mean and standard deviation from the distribution of scores for the non-adoptive families. We then summed these standardized scores for the parents in each family to form a composite socioeconomic (SES) status indicator.
Statistical Analyses
The relationship between adoption status (adopted/non-adopted), exposure to parent smoking (ever/never), and adolescent adjustment was investigated using analysis of variance (AVOVA) for quantitative outcomes and logistic regression for categorical outcomes. The basic model included adoption status, exposure, and their interaction as independent variables. The significance of each variable was measured net the other variables in the model, a regression approach to the analysis of genetically informative data24. Adolescent age and ethnicity (Caucasian/non-Caucasian), and family SES were included as covariates. Dependent variables included a diverse array of adolescent substance use and other disinhibitory outcomes. The correlated nature of the family data was taken into account with hierarchical linear methods as incorporated into PROC MIXED (for quantitative outcomes) and generalized estimating equations (GEE) as incorporated into PROC GENMOD (for categorical outcomes) from the Statistical Analysis System (SAS)22. Analyses were structured to estimate the simple main effect of exposure to parent smoking in the adopted and non-adopted adolescents separately. For quantitative outcomes, effect sizes (ESs) were estimated by dividing the difference in the covariate-adjusted means by the residual standard deviation. For categorical outcomes, covariate-adjusted odds ratios (ORs) and confidence intervals are reported.
Results
All of the mothers and 95% of the fathers in the exposure group reported that their most heavily used tobacco product was cigarettes. During their period of heaviest use, these fathers averaged about 30 cigarettes and these mothers almost 25 cigarettes per day. Although there was a higher proportion of non-adopted relative to adopted adolescents who were exposed to parent smoking (59% vs. 48%, X2=5.81, p < .05), severity of risk indices within the exposure group were not associated with adoption status. That is, within the exposure group, non-adoptive mothers or fathers were no more likely to smoke currently than were adoptive mothers or fathers. Further, adopted and non-adopted adolescents were equally likely to have been exposed to more than one smoking parent.
Table 1 presents demographic data on offspring ethnicity, sex, age, and family SES. It also includes the lifetime prevalence of tobacco, alcohol, and marijuana use and any DSM-IV disruptive behavior disorder (ODD, ADHD, or CD) in exposed and non-exposed adolescents by adoption status. Finally, Table 1 provides the means and standard deviations (SD) for quantitative variables including self reports of delinquency, antisocial attitudes, aggressive orientation, and harm avoidance as well as best-estimate symptom counts for the disruptive behavior disorders.
Table 1.
Demographic and outcome characteristics in adopted and non-adopted adolescents as a function of exposure to parent smoking.
| Non-Adopted Adolescents | Adopted Adolescents | |||
|---|---|---|---|---|
| Never Exposed
(N=130) |
Ever Exposed
(N=192) |
Never Exposed
(N=241) |
Ever Exposed
(N=222) |
|
| %Male | 47.7 | 45.8 | 45.2 | 42.3 |
| %Caucasian | 89.2 | 97.9 | 19.1 | 23.9 |
| Assessment Age (Years) | 14.5 (1.8) | 15.1 (1.9) | 14.7 (2.0) | 15.2 (2.0) |
| Family SES | .01 (1.0) | -0.2 (1.0) | 0.7 (0.8) | 0.3 (0.9) |
| Categorical Outcomes – Lifetime Prevalence (%) | ||||
| Ever Use Tobacco | 10.9 | 35.6 | 18.9 | 31.7 |
| Ever Use Alcohol | 10.9 | 38.7 | 22.7 | 32.1 |
| Ever Use Marijuana | 5.5 | 19.9 | 9.7 | 18.6 |
| Any Disruptive Disorder | 13.8 | 29.3 | 38.5 | 33.9 |
| Quantitative Outcomes -- Mean (SD) | ||||
| Delinquency | 3.2 (4.8) | 7.6 (9.5) | 5.0 (6.1) | 6.1 (7.3) |
| Antisocial Attitudes | 12.2 (4.1) | 15.2 (5.7) | 13.2 (4.9) | 14.1 (5.3) |
| Aggressive Attitudes | 13.1 (4.5) | 15.3 (5.4) | 14.1 (4.7) | 14.1 (5.2) |
| Harm Avoidance | 50.9 (10.8) | 44.2 (11.1) | 49.1 (11.0) | 48.6 (10.5) |
| Symptoms of Disruptive Disorders | 2.4 (4.1) | 4.1 (5.3) | 5.5 (6.2) | 4.3 (5.0) |
Note: Exposed group had at least one parent with a lifetime diagnosis of nicotine dependence who had smoked during the offspring's lifetime; in non-exposed group both parents had never been nicotine dependent or smoked during the offspring's lifetime. Disruptive disorders include oppositional defiant disorder (ODD), attention-deficit/hyperactivity disorder (ADHD), and conduct disorder (CD).
Focusing on the demographic variables, offspring sex was unrelated to exposure risk or adoption status. Ethnicity was associated with adoption status only; Caucasian participants constituted 94% of the non-adopted sample and but 21% of the adopted sample (X2=134.90, p <.001). Assessment age was related only to exposure risk with older adolescents more likely to be exposed to parent smoking (15.1 yrs. vs. 14.6 yrs., F=13.43, p<.001). Family SES was also related to exposure risk (F=28.41, p < .001) as well as to adoption status (F=60.55, p < .001), but not their interaction. The mean family SES for both exposed and non-adopted adolescents was significantly lower when compared to unexposed and adopted adolescents, respectively. Thus, all adolescent outcome variables included in our analyses were statistically adjusted for age, ethnicity, and family SES.
Logistic regression results along with associated ORs and confidence intervals for the lifetime prevalence of tobacco, alcohol, and marijuana use are given in Table 2. Across all adolescents, adoption status was unrelated to ever using any of these substances. Exposure to parent smoking was, however, associated with ever having used each of these substances. Within the adoptee subsample, those exposed to parent smoking were slightly but significantly more likely to use tobacco (OR=1.68) and marijuana (OR=1.95). Within the non-adopted subsample, those exposed to parent smoking were significantly more likely to use not only tobacco (OR=4.17) and marijuana (OR=3.43), but also alcohol (OR=5.08). Thus, exposure to parent smoking placed adolescents at risk for tobacco and marijuana use, whether the rearing parent was biologically related or adoptive. When the parent was biologically related, the adolescent was at risk for tobacco, marijuana, and alcohol use. The interaction between adoption status and exposure was significant for tobacco and alcohol use, indicating a larger exposure effect in biologically related families.
Table 2.
Odds ratios (OR) and p-values from Adoption Status (ADOP) by Exposure to Parent Smoking (EXP) Logistic Regression of Categorical Outcomes
| ADOP | EXP | ADOP × EXP | EXP in Adopted Adolescents | EXP in Non-Adopted Adolescents | ||||
|---|---|---|---|---|---|---|---|---|
| X2 | p | X2 | p | X2 | p |
OR (95% CI) |
OR (95% CI) |
|
| Lifetime Prevalence | ||||||||
| Tobacco Use | 0.71 | .40 | 20.46 | <.001 | 4.79 | <.05 |
1.68
(1.0-2.8) |
4.17
(2.2-8.1) |
| Alcohol Use | 0.40 | .53 | 20.91 | <.001 | 11.53 | <.001 | 1.31
(0.8-2.2) |
5.08
(2.7-9.4) |
| Marijuana Use | 0.00 | .97 | 13.18 | <.001 | 1.15 | .28 |
1.95
(1.0-3.7) |
3.43
(1.5-7.7) |
| Any Disruptive Disorder | 13.58 | <.001 | 2.47 | .12 | 8.92 | <.01 | 0.77
(0.5-1.2) |
2.34
(1.3-4.3) |
Note: Covariates include sex, age, and ethnicity. Significant effects are highlighted in bold. Odds ratios (OR) reflect the increase in the odds of substance use in the offspring of ever nicotine dependent parents relative to the offspring of never nicotine dependent parents. Disruptive disorders include oppositional defiant disorder (ODD), attention-deficit/hyperactivity disorder (ADHD), and conduct disorder (CD).
Table 2 also provides ORs and confidence intervals for the lifetime prevalence of any assessed disruptive behavior disorder. In contrast to the results for substance use, but consistent with other published reports6, 25, adoption status was associated with an increased prevalence of any disruptive behavior disorder. Importantly, this increased risk for adoptees was not associated with exposure to parent smoking. However, non-adopted adolescents exposed to parent smoking were significantly more likely than those not exposed to be diagnosed with any disruptive behavior disorder (OR=2.34). The interaction between adoption status and exposure was significant, indicating, as before, a larger exposure effect in biologically related families.
ANOVA results along with effect-size estimates for the quantitative indicators of disinhibited behavior are given in Table 3. Across all adolescents, adoption status was unrelated to any quantitative indicator of disinhibited behavior save disruptive disorder symptom counts. Exposure to parent smoking was associated with three quantitative indicators of disinhibition: delinquency, antisocial attitudes, and harm avoidance. Adoptees exposed to parent smoking were not at increased risk for any of the quantitative indicators of disinhibition when compared with adoptees who were not exposed. Conversely, non-adopted adolescents exposed to parent smoking reported significantly higher levels of delinquent behavior, more tolerance of antisocial behavior, greater acceptance of aggressive behavior, and lower levels of harm avoidance than non-adopted adolescents who were not exposed. In addition, the best-estimate symptom counts for disruptive behavior disorders were significantly higher in non-adopted adolescents exposed to parent smoking when compared with those who were unexposed. Significant effect sizes (ES) were moderate, ranging from .36 to .59. Finally, the interaction between adoption status and exposure was significant for all indicators of disinhibition, indicating a larger exposure effect in biologically related families.
Table 3.
Standardized effect size estimates (ES) and p-values from Adoption Status (ADOP) by Exposure to Parent Smoking (EXP) ANOVA of Quantitative Outcomes
| ADOP | EXP | ADOP × EXP | EXP in Adopted Adolescents | EXP in Non-Adopted Adolescents | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ES | ES | |
| Quantitative Indicators | ||||||||
| Delinquency1 | 3.12 | .08 | 11.16 | <.001 | 8.22 | <.01 | .07 | .58*** |
| Antisocial Attitudes | 0.00 | .99 | 9.85 | <.01 | 5.91 | <.05 | .08 | .51*** |
| Aggressive Attitudes | 1.35 | .25 | 3.64 | .06 | 7.18 | <.01 | -.06 | .42** |
| Harm Avoidance | 0.59 | .44 | 11.92 | <.001 | 12.38 | <.001 | -.01 | -.59*** |
| Disruptive Disorder Symptoms1 | 28.20 | <.001 | 0.59 | .44 | 11.38 | <.001 | -.22 | .36** |
Variables were log-transformed prior to analysis to reduce skew.
Note: Covariates include sex, age, and ethnicity. Disruptive disorder symptoms include symptoms of oppositional defiant disorder (ODD), attention-deficit/hyperactivity disorder (ADHD), and conduct disorder (CD). Significant effects are highlighted in bold. Standardized effect size, ES, are computed as the mean of the offspring of ever nicotine dependent parents minus the mean of the offspring of never nicotine dependent parents divided by the residual standard deviation. Significance of ES:
p < .05,
p < .01,
p < .001.
Discussion
Data from adoptive families suggest that exposure to parent smoking represents an environmental risk factor for the lifetime use of tobacco and marijuana in adolescent offspring. When contrasted with data from biologically related families, however, the findings indicate that the effect of exposure to parent smoking is both larger and more diverse when adolescents and their parents share a common genetic endowment. Thus, smoking parents may provide an environment that increases their offspring's risk for tobacco and marijuana use, and non-adoptive parents may also transmit genes that increase their offspring's liability for disinhibited behavior, including substance use, disruptive behavior disorders, delinquency, antisocial attitudes, aggressive orientation, and preference for risk taking.
The environmental effect of exposure to parent smoking, demonstrated in adoptive families, is consistent with studies showing a reduced risk for tobacco use in offspring of parents who have stopped smoking when compared to adolescents whose parents are current smokers26. Interestingly, adopted adolescents exposed to parent smoking were also at increased risk for marijuana use as well as alcohol use, although the latter did not reach conventional levels of statistical significance. This suggests that the environmental mechanism at play may not be behavioral modeling or access per se, but rather increased parent tolerance for offspring substance use or reduced authority in regulating offspring substance use when the parent is a regular smoker. Alternatively, it may reflect the role of smoking as a gateway to the use of other substances27.
The larger and more diverse effects demonstrated in biologically related families are consistent with other family studies in implicating the existence of a common inherited liability across multiple indicators of disinhibition. For example, relatives of alcoholics have elevated rates of smoking and illicit drug dependence28, and the inherited factors that underlie conduct disorder overlap extensively with those for alcoholism29, 30 and drug dependence31, 32. More generally, there appears to be a common familial liability to disinhibitory disorders10, due in large part to a general genetic vulnerability33. Research in adolescent samples also confirms the existence of a common genetic liability underlying both behavioral and dispositional markers of risk34, 35.
Critics of adoption studies maintain that because adoptive parents are better educated and have greater economic resources than the general population, environmental effects measured in adoptive families are greatly attenuated36. Indeed, the socioeconomic status of adoptive families in our sample was significantly higher than that of the non-adoptive families. However, statistically adjusting for family SES did not eliminate the larger and more diverse effects of parent smoking in non-adoptive families. Of course, the larger effects in non-adoptive families may be due to factors independent of SES that are differentially present in the environments of adoptive and non-adoptive families. In addition, the increase in disruptive behavior disorder symptoms in the adoptees could suggest that they represent a sample at high genetic risk where the effect of exposure to parental smoking might be diminished. Although we cannot rule this out, we believe it is an unlikely explanation for our findings. In fact, we and others25, 37 have shown that the majority of adoptees are psychologically well-adjusted with only a modest, albeit significant, increase in risk for symptoms of disruptive disorders.
To our knowledge, only one other adoption study has examined the effect of parent smoking in adoptees38. These authors reported that both adoptive and birth parent smoking were unrelated to ever smoking in adopted adults. However, this Danish, registry-based sample included adoptions from 1924-1947, a period during which smoking was highly prevalent. Although restriction in range likely attenuated the association between parents and offspring in that study, the factors that influenced risk for smoking behavior during that period may be different from those that are operating today39.
This research is not without limitations. The SIBS sample is predominantly in mid-adolescence, so all participants have not yet experienced the full risk period for substance misuse. Consequently, our analysis of substance use necessarily focused on ever using rather than patterns of regular use or abuse. Further, behavioral genetic research suggests that the impact of family environmental influences on substance use and mental health outcomes diminishes with age as genetic influences gain in importance40-42. It will be important to follow the SIBS sample through late adolescence and early adulthood as more of the participants initiate substance use and as others transition to regular or abusive use patterns to determine whether the effects we observe will endure.
In summary, this study presents evidence for an environmentally mediated pathway by which parent smoking increases risk specifically for substance use in adolescent offspring. The data are also consistent with a genetically mediated pathway by which non-adoptive parents who smoke may also transmit a non-specific genetic risk to their offspring for disinhibited behavior.
Acknowledgments
Supported by grants from the National Institute on Alcohol Abuse and Alcoholism (AA11886) and the National Institute on Mental Health (MH066140). These funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. All authors had full access to all the data, and none has any conflicts of interest or financial disclosures relevant to this manuscript. Dr. Keyes takes responsibility for the integrity of the data and accuracy of data analyses.
References
- 1.Administration SAaMHS. Results from the 2005 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2006. [Google Scholar]
- 2.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Decline in daily smoking by younger teens has ended. Ann Arbor, MI: Dec 21, 2006. [Google Scholar]
- 3.Otten R, Engels R, van de Ven MOM, Bricker JB. Parental smoking and adolescent smoking stages: The role of parents' current and former smoking, and family structure. Journal of Behavioral Medicine. 2007 Apr;30(2):143–154. doi: 10.1007/s10865-006-9090-3. [DOI] [PubMed] [Google Scholar]
- 4.Blokland E, Engels R, Hale WW, Meeus W, Willemsen MC. Lifetime parental smoking history and cessation and early adolescent smoking behavior. Preventive Medicine. 2004 Mar;38(3):359–368. doi: 10.1016/j.ypmed.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Bailey SL, Ennett ST, Ringwalt CL. Potential mediators, moderators, or independent effects in the relationship between parents former and current cigarette use and their childrens cigarette use. Addictive Behaviors. 1993 Nov-Dec;18(6):601–621. doi: 10.1016/0306-4603(93)90015-2. [DOI] [PubMed] [Google Scholar]
- 6.Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007 May;12(5):432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine & Tobacco Research. 1999;1:S51–S57. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- 8.Hicks BM, Bernat E, Malone SM, et al. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007 Jan;44(1):98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGue M, Iacono WG, Krueger R. The association of early adolescent problem behavior and adult psychopathology: A multivariate behavioral genetic perspective. Behavior Genetics. 2006 doi: 10.1007/s10519-006-9061-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: A twin-family study. Archives of General Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- 11.Button TMM, Maughan B, McGuffin P. The relationship of maternal smoking to psychological problems in the offspring. Early Human Development. 2007 Nov;83(11):727–732. doi: 10.1016/j.earlhumdev.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarr S, Weinberg RA. The Minnesota Adoption Studies - Genetic-Differences and Malleability. Child Development. 1983;54(2):260–267. [PubMed] [Google Scholar]
- 13.McGue M, Keyes M, Sharma A, et al. The environments of adopted and non-adopted youth: Evidence on range restriction from the Sibling Interaction and Behavior Study (SIBS) Behavior Genetics. 2007;37:449–462. doi: 10.1007/s10519-007-9142-7. [DOI] [PubMed] [Google Scholar]
- 14.Gibson HB. Self-report delinquency among school boys and their attitudes to police. British Journal of Social and Clinical Psychology. 1967;20:303–315. doi: 10.1111/j.2044-8260.1967.tb00517.x. [DOI] [PubMed] [Google Scholar]
- 15.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 16.Everett SA, Warren CW, Sharp D, Kann L, Husten CG, Crossett LS. Initiation of Cigarette Smoking and Subsequent Smoking Behavior among U.S. High School Students. Preventive Medicine. 1999 Nov;29(5):327–333. doi: 10.1006/pmed.1999.0560. [DOI] [PubMed] [Google Scholar]
- 17.McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25(8):1156–1165. [PubMed] [Google Scholar]
- 18.Tellegen A. Manual for the Multidimensional Personality Questionnaire. Minneapolis: University of Minnesota Press; in press. [Google Scholar]
- 19.Reich W. Diagnostic interview for children and adolescents (DICA) Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Welner Z, Reich W, Herjanic B, Jung K, Amado H. Reliability, validity, and parent-child agreement studies of the Diagnostic Interview for Children and Adolescents (DICA) Journal of the Academy of Child and Adolescent Psychiatry. 1987;26:649–653. doi: 10.1097/00004583-198709000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Robins LN, Babor TF, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. St. Louis: Authors; 1987. [Google Scholar]
- 22.Robins LN, Wing J, Wittchen HU, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1988;45(12):1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 23.Leckman JK, Scholomskas D, Thompson W, Belanger A, Weisman M. Best-estimate of lifetime psychiatric diagnosis: A methodological study. Archives of General Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 24.Defries JC, Fulker DW. Multiple-regression analysis of twin data. Behavior Genetics. 1985;15(5):467–473. doi: 10.1007/BF01066239. [DOI] [PubMed] [Google Scholar]
- 25.Keyes M, Sharma A, Elkins I, Iacono WG, McGue M. The mental health of US adolescents adopted in infancy. Archives of Pediatrics & Adolescent Medicine. 2008 doi: 10.1001/archpedi.162.5.419. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farkas AJ, Distefan JM, Choi WS, Gilpin EA, Pierce JP. Does parental smoking cessation discourage adolescent smoking? Preventive Medicine. 1999 Mar;28(3):213–218. doi: 10.1006/pmed.1998.0451. [DOI] [PubMed] [Google Scholar]
- 27.Kandel DB, Yamaguchi K. Developmental stages of involvement in substance use. In: Ott PJ, Tarter RE, Ammerman RT, editors. Sourcebook on substance abuse: Etiology, epidemiology, assessment, and treatment. Allyn & Bacon; Needham Heights: 1999. pp. 50–74. [Google Scholar]
- 28.Bierut LJ, Dinwiddie SH, Begleiter H, et al. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry. 1998;55(11):982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- 29.Haber JR, Jacob T, Heath AC. Paternal alcoholism and offspring conduct disorder: Evidence for the ‘common genes’ hypothesis. win Research and Human Genetics. 2005;8:120–131. doi: 10.1375/1832427053738782. [DOI] [PubMed] [Google Scholar]
- 30.Slutske WS, Heath AC, Dinwiddie SH, et al. Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychol. 1998;107(3):363–374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- 31.Button TMM, Hewitt JK, Rhee SH, Young SE, Corley RP, Stallings MC. Examination of the causes of covariation between conduct disorder symptoms and vulnerability to drug dependence. Twin Research and Human Genetics. 2006;9(1):38–45. doi: 10.1375/183242706776402993. [DOI] [PubMed] [Google Scholar]
- 32.Button TMM, Rhee SH, Hewitt JK, Young SE, Corley RP, Stallings MC. The role of conduct disorder in explaining the comorbidity between alcohol and illicit drug dependence in adolescence. Drug and Alcohol Dependence. 2007;87(1):46–53. doi: 10.1016/j.drugalcdep.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003 Sep;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 34.Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111(3):411–424. [PubMed] [Google Scholar]
- 35.Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics. 2000;96(5):684–695. [PubMed] [Google Scholar]
- 36.Stoolmiller M. Implications of restricted range of family environments for estimates of heritability and nonshared environment in behavior-genetic adoption studies. Psychological Bulletin. 1999;125:392–409. doi: 10.1037/0033-2909.125.4.392. [DOI] [PubMed] [Google Scholar]
- 37.Juffer F, van Ijzendoorn MH. Behavior problems and mental health referrals of international adoptees: A meta-analysis. Journal of the American Medical Association. 2005;293:2501–2515. doi: 10.1001/jama.293.20.2501. [DOI] [PubMed] [Google Scholar]
- 38.Osler M, Holst C, Prescott E, Sorensen TIA. Influence of genes and family environment on adult smoking behavior assessed in an adoption study. Genetic Epidemiology. 2001 Nov;21(3):193–200. doi: 10.1002/gepi.1028. [DOI] [PubMed] [Google Scholar]
- 39.Kendler KS, Thornton LM, Pedersen NL. Tobacco consumption in Swedish twins reared apart and reared together. Archives of General Psychiatry. 2000 Sep;57(9):886–892. doi: 10.1001/archpsyc.57.9.886. [DOI] [PubMed] [Google Scholar]
- 40.Lyons MJ, True WR, Eisen SA, et al. Differential heritability of adult and juvenile antisocial traits. Archives of General Psychiatry. 1995 Nov;52:906–915. doi: 10.1001/archpsyc.1995.03950230020005. [DOI] [PubMed] [Google Scholar]
- 41.Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol Bull. 2002 May;128(3):490–529. [PubMed] [Google Scholar]
- 42.Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: Regional residency moderates longitudinal influences on alcohol use. Alcoholism: Clinical and Experimental Research. 2001;25:637–643. [PubMed] [Google Scholar]