Abstract
Receptor subunits in the Cys-loop superfamily assemble to form channels as homopentamers or heteropentamers, expanding functional diversity through modularity. Expression of two or more compatible subunit types can lead to various receptor assemblies or subtypes. However, what may be good for diversity in vivo may be undesirable for the bench scientist, because we often wish to reduce our analyses to a single receptor subtype. By linking two or more subunits, creating tandems or concatamers, we can control stoichiometry and limit expression to exactly one receptor subtype. In this fashion, receptors with mixed subunit subtypes and heterozygous mutations can be separated from a mixture and can be described in detail. However, several recent studies have shown that this may be more easily conceived than accomplished, because several unforeseen problems have arisen. Concatamers can degrade, linkers can sometimes be clipped after or during translation, and one subunit may “loop out” or even become part of a second (now linked) pentamer with different characteristics. Some strategies have been developed to overcome these drawbacks, and the resultant new information that has begun to emerge has revitalized the study of these receptors in heterologous expression systems.
Index Entries: Ligand-gated ion channel, tandem subunits, concatenated proteins, concatamers/concatemers, heterologous expression
Introduction
Ionotropic neurotransmitter receptors are typically composed of symmetrically arranged subunits. In some cases, they can be homomultimeric, whereas in other cases, the receptor is formed from various available subunit types. In the case of ligand-gated ion channels in the Cys-loop superfamily (e.g., nicotinic acetylcholine, glycine, GABAA, and 5-HT3 receptors), the subunits (and subunit subtypes, by definition) are homologous and fairly conserved (Fig. 1). This homology leaves open the possibility—especially in studies using heterologous expression systems—that one subunit might substitute for any another, leading to a vast array of possible pentameric assemblies.
Fig. 1.
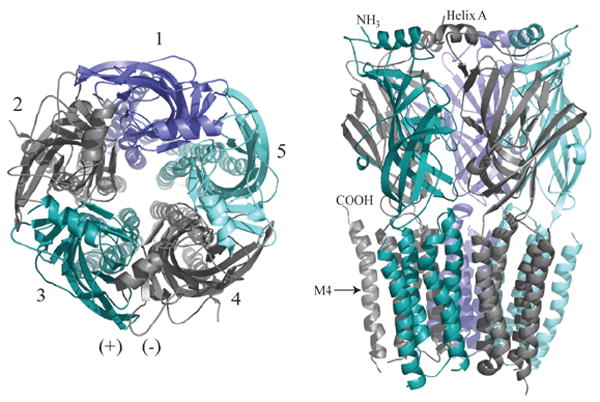
Molecular model of Cys-Loop pentameric receptor. The core GABAAR homology model was constructed as described by Mercado and Czajkowski (51) using Sybyl 7. 1 (Tripos Inc., St. Louis, MO). Left: Top view, as if from the synaptic cleft. Subunits are labeled 1-5 counterclockwise with the principal subunit colored slate (subunits 2 and 4). For the GABAAR, subunits 1-5 correspond to γβαβα, although position 1 can also be occupied by other subunits, such as δ, ε, θ, or π (2); for the muscle nAChR, 1-5 are βαγαδ or βαεαδ; for the neuronal nAChRs and glyRs, 1-5 are (β/α)αβαβ (the first position is still in dispute for glyRs); for 5-HT3Rs 1-5 are BABAB. One (+)/(-) interface is illustrated. Right: A sidelong view shows the extracellular N-termini (mostly β-strands, and one α-helix denoted Helix A) resting atop the transmembrane α-helical spans M1-M4. M4 is labeled to show the position of the C-terminus. The intracellular loop between M3 and M4 is not included, for clarity.
Modularity allows for functionally distinct receptors that might be targeted to different regions of the brain, that may change during development, and that may exhibit different kinetic and pharmacological profiles. For example, in muscle nicotinic acetylcholine receptors (nAChRs), the fetal γ-subunit is later replaced in the pentameric structure by the ε-subunit (1). GABAA receptors (GABAARs) generally have two α- and two β-subunits, with various “fifth” subunits, such as γ, δ, ε, θ or π (2); each imparts different kinetic and pharmacological characteristics to the receptors formed (3,4). If every GABAAR has at least two α- and two β-subunits, the number of possible receptors would be 2268. If a π- or θ-subunit can substitute for one β-subunit (5,6), then the number rises to 4500. Despite the vast potential for assembly diversity, only a few arrangements appear to be expressed functionally at the cell surface, suggesting a stringent underlying selectivity and discriminating mechanisms for receptor assembly.
Because of this diversity of receptor subtypes, functional assays can be confounded by mixed assembly, and more than one receptor subtype is expressed simultaneously. Neuronal nAChRs composed of α4 and β2 subunits have different agonist sensitivities depending on whether the stoichiometry is 3α:2β or 2α:3β (7). Glycine receptors can form α-subunit homomers, but when the β-subunit is present, the preferred form is likely to be heteromeric (8). Whether the stoichiometry is 2α:3β or 3α:2β is debated and is discussed later. GABAAR α- and β-subunits can also form functional receptors without a third type of subunit. Researchers are investigating whether the stoichiometry of this receptor is 3α:2β or 2α:3β, but evidence for both types has been presented (9-12). Some investigators have suggested the possibility of αβ receptors in vivo (13,14), but this has yet to be proven unambiguously. Clearly, however, there are potential problems in interpreting data from expression systems where GABAAR αβ and αβx receptors might be expressed concurrently. GABAARs might also contain multiple subunit subtypes (e.g., one α1, one α6) in a single receptor (15,16). Given the onus of mixed receptor populations in heterologous expression and the desire to separate their effects, it is not surprising that the impetus to yoke Cys-loop receptors into fixed stoichiometries has come, almost by necessity, from the GABAAR subfield.
Concatenation of subunits is used as an attempt to force expression of channels with fixed stoichiometry and arrangement. Tandem design may benefit researchers using heterologous expression systems in two primary ways: (a) predetermining stoichiometry and arrangement eliminates mixed receptor subtypes and their muddled effects, yielding more consistent and appropriate models for structure–function analyses and drug screening; and (b) by localizing mutations in only one of a repeated subunit in a receptor, one may address more subtle questions involving symmetry (or asymmetry), mixed subunit subtype receptors, and perhaps certain heterozygous channelopathies (17-19).
Cys-Loop Receptor Concatamers First Make the Scene
In 1995, Im and coworkers (12) expressed GABAARs using “tandem” constructs, with a two-subunit concatamer linked by decaglutamine (10Q) between the α6- and β2-subunits. They found that this construct gave functional receptors when co-expressed in HEK293 cells with free α6- or γ2-subunits but not with β2-subunits. They concluded that the two likely stoichiometries were 2α:2β:1γ and 3α:2β for these subunits. Similar results were recently obtained using α1-β2 tandems (11), although maximal currents were two- to fivefold smaller than for free subunits (Boileau et al., unpublished observation).
Although the α6-β2 tandem alone did not express functional receptors as measured by Cl-current upon application of agonist, Im et al. (12) observed radiolabeled ligand binding to cell lysates, suggesting that the α6-β2 tandem could assemble into an agonist-binding unit (12). These seminal results using GABAAR tandems suggested that a translated α-subunit followed by a β-subunit can assemble into a proper β(+)/(-)α binding interface (Fig. 1) in the absence of other subunits, but they require other contacts to form a pentameric receptor expressed on the cell surface. Agonists such as GABA and ACh bind at the interface between the principle subunit (β in GABAARs, α in nAChRs) and a neighboring subunit (Fig. 1). Sidedness of the subunit contact sites during assembly, such as β(+)/(-)α vs α(+)/(-)β, is an important question and in discussed in more detail in the following sections.
A series of papers by Erwin Sigel’s group explored numerous possible tandem combinations of GABAAR subunits, all of which were expressed in Xenopus oocytes. Using maximal current as a guideline for appropriate functional expression, Baumann and coworkers (9) explored linker length requirements for α-β and β-α tandem subunits, finding a minimum of 10 amino acid residues required for α1-β2 and 20 to 23 amino acid residues required for β2-α1 to yield currents comparable to free subunits. However, the actual spanning lengths for these α-β vs β-α tandems are nearly equivalent when compensatory differences between mature subunit N- and C-termini lengths are considered. Still, these linkers are shorter than those in which the second subunit retains its signal peptide sequence. For example, the α6-β2 linker used by Im et al. was 10Q plus 24 additional amino acids from the β2 signal sequence (12). Although there was detectable current when either the “linker-optimized” β2-α1 or α1-β2 tandem was co-expressed with free α-subunits, co-expression with a free β-subunit gave much larger currents that were both similar to wild-type maximal currents. And although α-β tandem + γ yielded reasonable currents (approx 25% of wild-type), β-α + γ was superior in expression (9).
Higher Order Concatamers: Symmetry vs Asymmetry
In their second article, Baumann et al. (20) performed an exhaustive study of “triple tandems” and with which dual-tandem (or single) subunits they could combine to express functionally in oocytes. Four “triple + double” combinations were functional, and all gave the same pentameric arrangement of γβαβα (Fig. 1), assuming one triple and one double assembled together without degradation or subunits “looping out” of the pentamer (discussed below). The apparent affinity for GABA was slightly lower in the tandem co-expressions, perhaps because of some structural perturbation or dynamic constraints, but the inhibition by the antagonist bicuculline was unchanged (20). Interestingly, allosteric modulation by the classical benzodiazepine diazepam was enhanced compared to most studies of αβγ receptors, perhaps owing to fixed stoichiometry reducing or eliminating any αβ receptors (11,21,22) that are benzodiazepine-insensitive (23).
With functional concatamers in hand, Sigel’s group went on to answer some fundamental, and previously intractable, questions about the GABAAR. For example, the majority of kinetic models of GABAAR function have assumed equal affinity for the two agonist binding sites. Utilizing previously described point mutations in either the (+) side (β2) or (-) side (α1) of the GABA binding site reduced the apparent agonist affinity by more than 40-fold when expressed in both GABA binding sites of the γβαβα receptor (24). However, a triple + double tandem combination allowed mutation of just one or the other binding site. With this powerful method, a threefold difference in GABA affinity at the two sites became visible. Additionally, an estimate of the open probability for a singly liganded receptor could be obtained. Kinetic models can now be revised to reflect these two important findings. Since then, GABAAR mutations that cause epilepsy have been further characterized using β2-α1 tandems (25) and γ2-β2-α1 + β2-α1 tandems (19).
The next logical step was to determine if either of the two GABA binding sites was influenced more by modulating drugs than the other (26). This is a controversial issue with implications for modeling of both kinetics and structure-function relationships of the receptor. Benzodiazepines shift the GABA concentration-response curve to the left (27), thus making responses to a given subsaturating GABA concentration larger and also delaying deactivation with saturating GABA pulses, similarly to a synapse. Therefore, the in vivo effect is to enhance inhibition by GABA-gated currents. Employing the same mutations that reduce GABA apparent affinity, Baur and Sigel (26) demonstrated that diazepam can affect activity at either GABA-binding site. Their data also showed that retaining high-affinity activation in both GABA-binding sites allows for greater benzodiazepine modulation than if either site is mutated to reduce binding or activation, suggesting that the allosteric influence of the two GABA sites on each other may be further affected by a third modulating molecule at a distinct binding site.
An approach related to separating mutations with tandem subunits is to use different subunit subtypes. Previous studies have shown that two different α-subtypes may exist in the same GABAAR in vivo (28). Minier and Sigel (16) positioned α1 and α6 in the two possible positions of the GABAAR using triple + double tandem combinations. With free subunits, four possible receptors could have been expressed: γβα1βα1, γβα1βα6, γβα6βα1, and γβα6βα6. With the constraints imposed by concatenation, Minier and Sigel were able to produce each of these receptors separately. They found characteristic differences between each receptor using GABA and the partial agonist P4S and discovered that only one α6-subunit was needed to confer sensitivity to the convulsant drug furosemide. A similar approach using β1- and β2-subunits was used to explore loreclezole and etomidate actions (29). Responses for both drugs in mixed tandems tended to be intermediate to β1- or β2-containing receptors or weighted toward the β1-subunit response. However, direct gating by etomidate appeared impaired in the β1-only tandem combination compared to free subunits.
glyRs
Meanwhile, in the related glycine receptor (glyR) field, tandems have been employed to answer questions of stoichiometry as well. Grudzinska et al. (30) reported 2α:3β stoichiometry used a tandem α1-β construct. The linker for this tandem was seven repeats of the tripeptide alanine-glycine-serine, without signal peptide. When expressed alone, the α1-β tandem gave no current in Xenopus oocytes but gave wild-type EC50 for glycine when expressed with β-subunits (which do not form functional homomers). When co-expressed with α1 mutants with lower glycine EC50, the currents behaved as if mutant homomers were dominant—that is, the α1 mutants did not appear to co-assemble with the α1-β tandem. Co-expression with wild-type α1 was not reported, because it would not distinguish between α1 homomers or α1 subunits incorporated with tandems. To corroborate this finding, measurements of radiolabeled methionine into purified α1- and β-subunits, either from whole oocyte or from surface receptors, yielded approximately 2α:3β stoichiometry (30).
Previous studies contended that glycine receptors were composed of 3α:2β-subunits (31-33). Early biochemical purifications (33) may have been contaminated with native α homomers. It may also be possible that other glyR α subunit subtypes (α2-4) assemble differently, as preparations from spinal cord (33) contain moderate levels of α3-subunits (34). Two other studies support the conclusion of 3α:2β stoichiometry. Co-expression of wild-type α1 with a chimeric β/α1-subunit containing a mutation to shift agonist EC50 to the right, and the converse experiment with a shifted α1 mutant plus an α1/β-chimera suggesting a 3:2 α:β stoichiometry, based on theoretical curve fits (32). Using a more powerful technique, Burzomato and coworkers (31) made mutations in the so-called “central leucine” of the M2 transmembrane region, believed to line the pore of the ion channel. This residue is conserved throughout the Cys-loop superfamily, and mutations cause leftward shifts in EC50. Because HEK293 cells were transfected in a 1:40 α1:β ratio, homomeric α1-receptor currents were unlikely to confuse the results. Again, curve fits suggested a 3α:2β stoichiometry to be much more likely than 2α:3β. However, the extreme cases could not be measured (i.e., mutant α1 homomers or mutant β homomers), so the multiplicative shifts in EC50 for multiple mutations could not be determined (31). It has been suggested that the glyR stoichiometry debate might be settled by the use of a pentamer concatamer, or pentaconcatamer (35).
nAChRs
The predominant subtype of neuronal nicotinic ACh receptors is formed from α4- and β2-subunits. When Zhou et al. (36) linked these two subunits in a tandem construct and expressed them in Xenopus oocytes, several interesting (and vexing) things happened. In this case, either α-β or β-α tandems with linkers of 6 or 12 repeats of the alanine-glycine-serine tripeptide, (also without signal peptide) formed functional receptors; the β-α tandem with no linker did not. A combination of sucrose density gradients, radioactive immuno-assays, and electrophysiological recordings revealed that two pentamers could share one tandem, forming a “dipentamer.” Most monopentamers were retained in the endoplasmic reticulum (ER), but some with a “dangling” or “looping out” subunit did express on the surface, as evidenced by the ability of two subunit tandems to form a pentamer, thereby leaving one subunit unincorporated (36). By combining β-α tandems with free α4 or β2, they were able to form either 2α3β or 3α2β receptors and to confirm agonist EC50 differences between the two subtypes observed previously using limiting subunit cRNA injections (7). Oddly, 3α2β receptors from tandems expressed more strongly and yielded larger currents per receptor than even free subunit expression (36). Perhaps this is an example of gain of function caused by restricted stoichiometry.
Groot-Kormelink et al. (37) confirmed the “loop-out” hypothesis in neuronal nicotinic ACh receptors, in a nice example of a strong article built on a negative result. These workers also found tandem-alone expression for several constructs of α2-4 or α6 concatenated with β2 or β4. Luckily, one construct assembled well into functional receptors only when co-expressed with a monomer (β4-α3 + β4) in Xenopus oocytes. Tests for protein degradation in oocytes or HEK293 cells were inconclusive, but because the tandem did not form functional receptors on its own or with monomers other than β4, broken subunits forming receptors was unlikely (37). However, when either the tandem or the monomer was mutated in the M2 “central leucine” (causing a subunit dose-dependent leftward shift in EC50), the deed was undone. Results clearly showed that the monomer subunit was taking multiple positions in the receptor. In the case of tandems with mutant-free β4-subunits co-expressed with free wild-type β4 monomer, the EC50 for ACh was near wild-type, suggesting that perhaps both of the β4-subunits contributed by tandems were looping out. In the reverse case, with “wild-type” tandems plus free mutant β4, the concentration-response curve showed at least two components, suggesting incorporation of the mutant β4 into one, two, or all three of the available positions if the β4 from the β4-α3 was looping out, resulting in mixed receptor type expression. Therefore, one or more of the linked tandem subunits was looping out of the way and allowing insertion of a monomer.
All-in-One Concatamers: No Accessories Needed?
Groot-Kormelink and coworkers (35) required higher order concatamers to obtain functional receptors that did not form dipentamers or have dangling particles and took the ambitious approach of constructing pentamer tandems rather than triple + double tandem combinations. Although this group showed full-length messenger RNA (mRNA) transcripts rather than sucrose density gradients or Western blots to test whether smaller protein products were made, they took a mutational approach to the question of concatamer breakdown, focusing on function in Xenopus oocytes. Again taking advantage of central leucine mutations to shift EC50 for ACh, they showed that monomers of mutated α3 or β4 were not incorporated, even in excess, with the pentaconcatamer (β4-β4-α3-β4-α3). Therefore, the most likely subunits to loop out (i.e., the first and last) did not, nor did the middle ones for that matter. They also tested whether β4-β4-α3 triplets might have been made as byproducts of the pentaconcatamer by co-expressing the remainder, β4-α3 tandems, with the central leucine mutation in the α3 segment. Their results suggested no functional β4-β4-α3 tandems as products of proteolytic cleavage or mRNA clipping and suggested that the pentamer remained intact (35).
Another possibility is that β4-β4 tandems could be produced from β4-β4-α3-β4-α3 expression, which could be tested by co-expression with mutated α3-β4-α3 triple tandems. Although Groot-Kormelink and coworkers did not address this possibility, we submit that it might not matter. Even if β4-β4 + α3-β4-α3 demonstrated functional assembly, the utility of the pentamer tandem would be the same, and mutations could still be targeted to particular subunits. In another set of experiments, they showed that mutation in the pentamer tandem of one or two subunits at particular positions, and in α- vs. β-subunits, yielded predictable shifts in ACh sensitivity. This is the power of being able to separate one identical subunit from another and can be achieved with pentaconcatamers, triple + double combinations, tetraconcatamers + free single subunits, or other combinations of nonidentical tandems for nAChRs, which use four subunits to form pentamers.
Very recently, a pentaconcatamer of GABAAR subunits was engineered and expressed (38). This α1-β2-α1-γ2-β2 construct gave near wild-type responses to agonist, antagonist, and a benzodiazepine modulator. The authors mentioned a possible difference in the number of desensitization time constants, but because of the vagaries of kinetic measurements in whole Xenopus oocytes and because the weighted time constant was the same in both cases, this is probably not significant. The pentaconcatamer did express at a lower level than free subunits in oocytes (approx 40%)—still a better ratio than for the nAChR pentaconcatamer (35)—but even lower in HEK293 cells (approx 2%) for unknown reasons. One possibility may involve the lower temperature at which Xenopus oocytes are generally stored (approx 16–18°C), which may be more permissive for assembly of certain constructs, or may affect other processes such as degradation and turnover. Temperature effects are part of a general caution when comparing expression data from amphibian oocytes to mammalian expression systems.
Problems and Promise
Breakdown: Fraying at the Hem
Some tandem combinations appear to be more prone to breakdown than others (39). Whether the degradation happens before or after assembly is unknown, and it could occur as a result of subunit recycling machinery. Figure 2A shows one example and compares the signal from HEK293 cells transfected with free subunits, an α1-β2 GABAAR tandem, and triple tandems α1-β2-α1 and α1-β2-γ2. Although reasonable currents were obtained from the cells transfected with α-β-γ + α-β tandems (data not shown), the α-β-γ tandem displayed significant degradation when examined in Western blots (Fig. 2A shows the worst case we observed). In each sample shown, α-subunits were tagged in the N-terminus with the FLAG epitope (between residues 6 and 7 of the mature peptide sequence), excluding the possibility of differences in antibody affinity or positional effects of the epitope. Antibodies against the N-terminus of the β2/β3-GABAA subunit lose affinity when the β2-subunit is not the first subunit in a tandem (19,24). We also have seen that M2 antibodies against the FLAG epitope have a similar, but unexpected N-terminal requirement in our constructs (Fig. 2B). Perhaps the epitope becomes occluded, twisted, or stretched such that antibody recognition is impaired. To fully assess breakdown, other antibodies must be used, such as one directed against an internal segment (e.g., the large cytoplasmic loop in each subunit of the superfamily). However, having broken subunit remnants that are “immuno-invisible” is not always a concern if other functional controls are in place.
Fig. 2.
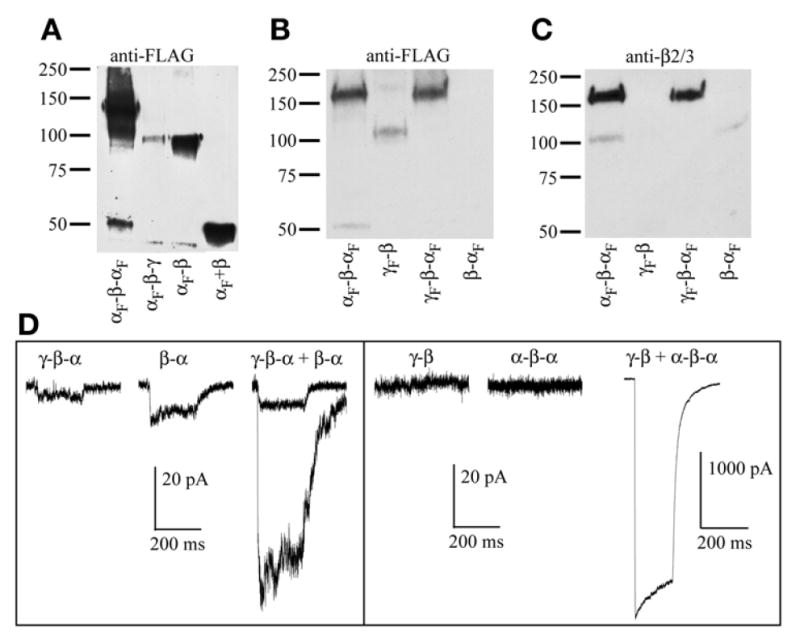
Western blots of several GABAAR concatamers. All constructs shown include signal peptide in the linker region. (A) Blot probed with anti-FLAG M2 monoclonal antibodies (Sigma). Subunits and tandems were immunoprecipitated from HEK293 cells transfected with α FLAG-tagged α1-β2-α1 (Lane 1), α1-β2-γ2 (Lane 2), α1-β2 (Lane 3), or free α1 plus β2 (Lane 4). The triple concatamer is expected at approx 165 kD, the tandems at approx 110 kD, and the monomers near 50–55 kD. The first lane was overloaded to show breakdown products, presumably free FLAG-tagged α1-subunit. Lane 2 shows no signal at 165 kD for αF-β-γF but, rather, a fragment that runs at the size of a double subunit tandem. Conversely, Lane 3 shows a robust signal for αF-β. (B) Blot probed with anti-FLAG antibody again showing breakdown of αF-β-αF (Lane 1), but full-length γF-β (Lane 2) and γF-β-αF (Lane 3) with no apparent breakdown, and no signal for β-αF (Lane 4). Note that both anti-FLAG and anti-β2 antibodies are less efficient out of first position. (C) Same blot as in (B) reprobed with polyclonal antibodies that recognize β2 or β3. Lane 1 shows a product of 110 kD, presumably β-αF as a second breakdown product of αF-β-αF. Lane 4 shows low expression of β-αF. The same result was obtained multiple times with multiple β-αF constructs, whereas γF-β constructs expressed better in other experiments, with no appreciable breakdown (data not shown). (D) Representative traces from whole-cell recordings of HEK293 cells transfected with tandem constructs, alone or in combinations designed to yield γβαβα pentamers. All constructs include 9Q (for the α-β linker) or 10Q (all others) plus signal peptide. Each trace shows current from exposure to 10 mM of GABA for 20 ms, and current scale bars for all recordings are 20 pA, with the exception of “γ-β + α-β-α,” which is 1000 pA. Left: γ-β-α and β-α tandems exhibit currents when expressed alone. Two traces for “γ-β-α + β-α” are overlaid to show range, and the rather small amplitude of the currents. Right: In at least eight cells each, neither γ-β nor α-β-α showed any current when expressed on their own, whereas robust currents were seen when these two constructs were co-expressed at a 1:1 ratio. (Portions of these data are reprinted with permission (11); Copyright 2005 by the Society for Neuroscience.)
Signal Peptide Clipping: When a One-Piece Becomes a Two-Piece
The first Cys-loop tandem was engineered to retain the signal peptide of the C-terminal linked subunit (12). Normally, this segment is cleaved from the mature peptide in the ER. We have gathered some unexpected evidence that cleavage can also occur deep in the protein, albeit with lower efficiency (Fig. 2B). In α1-β2-α1 tandems with signal peptides retained at the two junctions, Western blots show that both β-α and free α-subunits are being “clipped” from the full-length protein (Fig. 2B). Again, as in the case of degradation, this is only a concern if these minority clipped subunits are capable of assembling with each other or with any other subunit or tandem co-expressed. Most often, a series of controls, including expression of tandems alone or tandems with free subunits that should not form a pentamer coupled with Western blot data that clearly demonstrate whether monomers have formed, should be included to reduce the chance of misinterpretation of stoichiometry.
Linker Length/Structure: Choosing the Right Fastenings
The choice of linker length and structure varies considerably among tandem engineers. An appropriate linker sequence must satisfy two basic conditions. Obviously, the linker must constrain oligomerization to a desired arrangement and, therefore, be short enough to limit oligomerization possibilities. On the other hand, the linker must not produce functional artifacts and, therefore, should extend sufficiently to “loosely” span subunits, allowing them to fold properly and to behave as their analogous, discrete monomers, once assembled. To demonstrate this point, in tests of various linker lengths (9), sequences below a certain threshold length did not express. Furthermore, consider the significant variation in subunit termini length (linker anchor points), as observed from multiple sequence alignments. The core Cys-loop receptor subunit ends with a conserved transmembrane helix (M4), followed by variable lengths of nonconserved C-terminal sequence that extend extracellularly. Similar variability is observed in the N-terminal sequence prior to the first conserved secondary structure element, α-helix A (Fig. 1). The lengths of these native “extensions” also should contribute to the spanning potential of the linker (9).
Other physicochemical properties of the engineered linker polypeptide sequence are also considered. Early tandem studies used polyglutamine linkers because of their hydrophilicity and lack of secondary structure that may hinder flexibility and extensibility (40). More recent GABAAR linker designs have taken advantage of these characteristics but have also employed various patterns of proline, polyalanine, and polyglutamine to avoid excessive redundancy and thus not deplete a particular tRNA pool during translation and to (hopefully) avoid secondary structures (9). Similarly, others working with glyR and nAChRs link with repeating trimers of alanine, glycine, and serine (30,36). In some cases, the second subunit’s signal peptide is included in the linker to ensure sufficient length between subunits. However, possible problems with clipping, trafficking, or undesirable secondary structure (41) may justify the extra time spent designing a novel linker sequence. It is unknown whether the same problems might also occur with some linker designs that do not include the signal peptide.
To gain some perspective on the influence of different linker structures, we constructed and optimized various published linker structures and arrangements into a GABAAR homology model (Fig. 3) based on acetylcholine binding protein and nAChR structures (42,43). We then looked at the capacity of reported linker designs to span subunit types and positions within the canonical GABAAR pentamer γβαβα (counterclockwise as viewed from the synaptic cleft). Molecular mechanical models provide a means to calculate the theoretical strain upon a given linker as it bridges various subunits in the pentamer, comparing final product energies (not transitions). We found that there was essentially no significant difference in energetic strain between counterclockwise or clockwise tandem linkages for any published GABAAR tandem (9,11,12). Although strain may increase or decrease upon solvation, we could not predict an N-to-C counterclockwise dimer arrangement dictated merely by linker-spanning length limits. By visual inspection, the β-10Q-α linker, which does not express in Xenopus oocytes (9), does appear to be near its unstrained limit (Fig. 3E), although this is not reflected in the molecular mechanics calculation. A small change in flexibility constraints for the adjoining residues could begin to stretch the linker and increase strain beyond relaxed capacity.
Fig. 3.
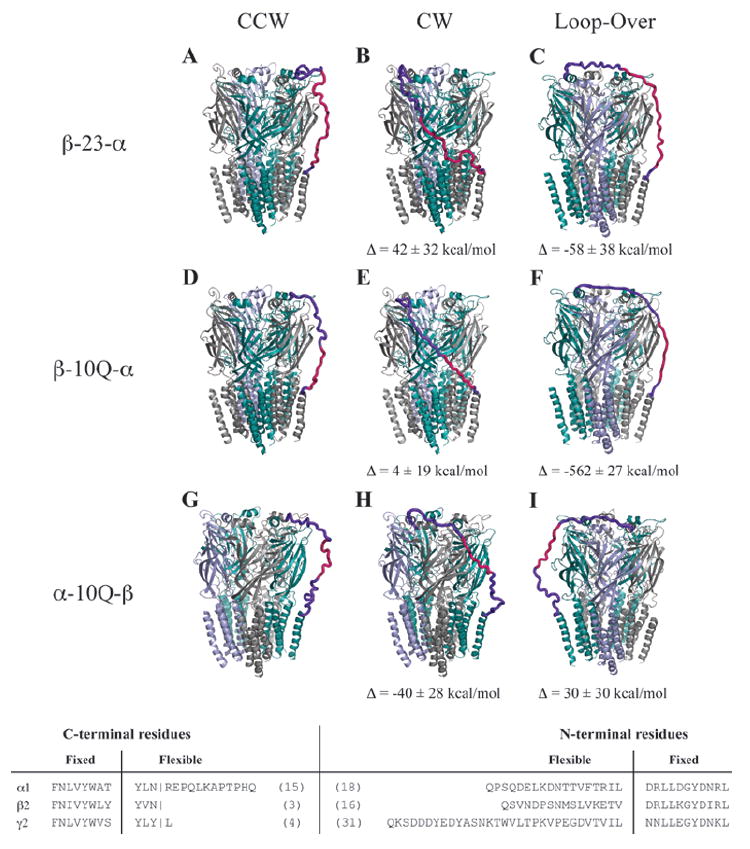
Hypothetical tandem linker conformations. Several linker architectures reported in the literature for GABAARs were evaluated for linker strain using molecular mechanics models. Here, we present the optimized tandem-containing receptors in each possible tandem configuration: counterclockwise (left), clockwise (center), and loop-over (right). The color code for subunits is α1 (teal), β2 (gray), and γ2 (blue). Linkers are hot pink, and regions of the native subunit allowed flexibility are shown in purple. Evaluated linkers were β-23-α (A–C) with linker composed of Q3(Q2A3PA)2AQ5, β-10Q-α (D–F), α-10Q-β (G–I), all tested in oocytes by Baumann and coworkers (9). Also tested but not shown were α-(Q9+signal peptide)-β (11,12), β-(10Q+signal peptide)-α from Fig. 2, and γ-26-β (20). The latter three showed no energetic differences in linker strain for counterclockwise, clockwise, or loop-over conformations. To see differences in relative strain among the tandem arrangements for each linker, ΔE was calculated by subtracting the converged potential energy of linker-imposed strain from the energy for the counterclockwise conformation. Data are mean ± SD from three or more converged cycles. For these calculations, all parts of the pentamer were held fixed except for linker sequence and adjoining terminal regions (3-31 residues, bottom). Linker and signal peptides were added to our core model and assigned Gasteiger-Marsili charges and Sybyl atom types from the Tripos forcefield by default. Each structure was minimized 4000 steps by default methods and was then subjected to 8 to 15 2-picosecond cycles of simulated annealing in vacuo with a linear temperature gradient from 700 to 200 K, followed by a quenching minimization after each cycle. Cycles were repeated until apparent convergence of system potential energy.
Is it possible to make a linker too long? One can imagine that a long enough linker might allow a subunit to take a position far from its partner, with interleafed subunits (looping over or reaching around). Stoichiometry and arrangement in this case would be a virtual black box. Modeled tandem structures adopted a surprisingly feasible “loop-over” topology, without sharp increases in system strain relative to that observed for comparable adjacent tandems (Fig. 3). However, with the short Q10 linker as in β-10Q-α, such a loop-over conformation is clearly disfavored (Fig. 3F). When the model was forced into this arrangement, it encountered prohibitive strain. However, the loop-over topology may be quite unlikely given other dynamic constraints on assembly (discussed below). It is possible that excessive linker length may sometimes impede expression by greater tendency for loop-overs or loop-outs or by other confounding effects that interfere with the assembly mechanism.
Loop-Outs: A Tandem Faux Pas
Even if a tandem subunit retains integrity, it is possible that one of the subunits may assemble into a functional receptor, and the other remains unincorporated (37). Having a subunit dangling in or above the membrane might be energetically unfavorable, and thus dipentamers may form more readily, with each subunit of the tandem contributing to a different (linked) pentamer (36). If the two pentamers in the dipentamer have different characteristics, as upon expression of α4-β2 nAChR tandems, then a mixed functional read-out will result. One solution to this problem might be to co-express a tandem composed of identical subunits (e.g., β-β) with a nonidentical tandem (e.g., α-β) and form dipentamers with identical pentamers (ββαβα). This possible configuration may be of little concern, unless one pentamer displays allosteric actions on the other, as has been suggested (36).
Loop-outs can still pose a problem in the case of a tandem that does not form dipentamers on its own (37). When tested with a diagnostic mutation, β4-α3 nAChR tandems clearly show that receptors can have unincorporated subunits, even without forming dipentamers. Unfortunately, dipentamers, semi-incorporated tandems, and degraded subunits can appear to express “functional” receptors quite well, suggesting that maximal expression approaching that of nonconcatenated subunits is not a sufficient test for concatamer integrity.
On the opposite end of the spectrum, poor expression still plagues the tandem designer. Many interesting tandem constructs show low expression for reasons that remain unclear. For example, the nAChR pentamer tandem required orders of magnitude more cRNA to express currents similar to free subunits (35), and the GABAA pentaconcatamer also showed reduced expression (38). Even simpler α-β tandems have shown reduced functional expression (9,11). On Western blots, β2-α1 GABAAR tandems express poorly compared to others (one of three constructs we attempted is shown in Fig. 2). This tandem gave small currents when expressed alone (as did its partner γ2-β2-α1; Fig. 2D), perhaps by combining with the native human β3 that has been observed in HEK293 cells (44,45).
Many concatamers that express well in Xenopus oocytes do not fare well in mammalian expression systems. For example, the GABAAR γ2-β2-α1+β2-α1 pair expresses well in oocytes (24) but not particularly well in HEK293 cells (Fig. 2D; ref. 19). The GABAAR pentaconcatamer expressed reasonably well in oocytes (38), but in HEK293 cells, expression was badly impaired, similarly to the nAChR pentaconcatamer (35). Clearly, work will continue to optimize the length and composition of these linkers to enhance expression while maintaining specificity of stoichiometry and arrangement.
With free subunits, assembly order appears to rely upon hierarchical oligomerization steps guided by selective recognition among subunits. Evidence points toward a pentamer assembly mechanism that is not concerted but proceeds rapidly in the ER through specific dimer and trimer intermediates prior to channel formation, trafficking from the ER to cell surface (2,46-48). Selectivity of subunit interactions in the oligomerization process is generally conferred by specific regions in the N-terminal domain.
The assembly of tandem subunits appears to be heavily influenced by the translation order. Results with GABAA tandems suggest that tandems prefer counterclockwise assembly order as they are translated in the ER (N-to-C). For example, β followed by α in the β-α tandem construct is well-tolerated because the β(+)/(-)α dimer is preferred. A second β-α and free γ may then join to make a γ/βα/βα pentamer. In the case of the α-β tandem, the α(+)/(-) β dimer may be formed first. If joined with another α-β tandem dimer and a free γ, then γ/αβ/αβ pentamers might form, but these should not behave properly and would have only one GABA binding site. Therefore, the α-β tandems must rearrange to the β(+)/(-)α dimer, possibly accounting for their lower expression levels (9). Theoretical strain calculations suggest that such a rearrangement is not unfavorable if the linker length is sufficient; yet even with longer linkers, α-β tandems assemble somewhat less efficiently with free γ-subunits (as determined by functional assembly at the cell surface) than free α1-, β2-, and γ2-GABAAR subunits assemble.
The reason that GABAAR α-β tandems only form αβ-receptors with free α if the tandem includes the β signal peptide (11,12) or with free β if it does not (9) may be an issue of assembly order, loop-outs, or linker strain and requires further investigation. In a counterclockwise arrangement, α-β/α-β/α is highly favored over α-β/α-β/β, suggesting that the β(+)/(-)β interaction may not be allowed, except as the final contact in pentamer formation, as in β-α/β-α/β (β-α tandem with free β; ref. 9). It also suggests either that tandems lead the assembly process or that adding a whole tandem last is also not favored, because another possible outcome of α-β tandems plus β is β/α-β/α-β. Similarly, the α(+)/(-)α interaction that would be required for β-α tandems plus free α to form receptors is also not favored (9). Perhaps these sorts of association and “sidedness” requirements dictate glyR assembly as well and might help explain why 2α:3β is observed using concatamers (30). It will be interesting to see whether pentaconcatamers of glyRs and GABAARs engineered with either β-α-β-α-β or α-β-α-β-α stoichiometry can faithfully assemble and express. Similarly, it may be interesting to observe the assembly of A-B or B-A tandems in the 5-HT3A/B receptor, which has recently been determined to have B-B-A-B-A stoichiometry using atomic force microscopy (49).
The idea that translation order may dictate tandem assembly processes is further evidenced by the high level of expression of α-β-α triple tandems in oocytes (20) and mammalian cells (Fig. 2), with or without signal peptide linkers. In this case, the α(+)-(-)β interface is formed first, followed by the β(+)-(-)α interface. The α-β-α triple is then free to assemble with γ-β (20) or even free β to form αβα/γβ or αβα/β/β , respectively.
Expression suggested that clockwise association of two subunits in a tandem is not efficient, although very little theoretical strain was calculated among the alternative arrangements in these systems. Our calculations estimate only the relative energies of arrangement products and do not account for the influence of the path activation barriers to the various dimer arrangements. Tethering by linkers may promote an unnaturally high “effective concentration” of one concatenated subunit in the vicinity of its partner. If assembly order is sensitive to kinetic accessibility of particular arrangements, then tethering may drive oligomerization toward unproductive arrangements that may, in turn, not assemble effectively as pentamers. There is still much to learn about the relative timescales of subunit folding, oligomerization, and assembly mechanisms. Ultimately, the constraints imposed by linkers may be defined and exploited through clever engineering to tune and elucidate aspects of the quality control of ligand-gated ion channel assembly.
Function Over Fashion
Beware of Low Expression
Although low expression might cause some consternation, the goal of tandem use is generally to assess characteristics of a certain stoichiometry of receptor subunits, or mutations therein. Therefore, if function is assessed as “normal” and demonstrations can be made that other “broken” parts are not likely forming the receptor, then we are generally satisfied that our tandem studies will provide useful information about the native receptor. Low expression may just be a good indication that we have not replicated the in vivo assembly order. When kinetic and pharamacological controls for normal function have been executed exhaustively, we can then begin to swap subunit subtypes and make mutations that address functional questions.
Use Appropriate Antibodies
What about unseen degradation? In the case of GABAAR concatamers, antibodies directed against the N-terminus of the α- or β-subunit, or an N-terminally FLAG-tagged subunit, will not recognize subunits that have undergone limited degradation in the epitope region. One might have concerns that an α-β tandem could then yield proteins with partial α linked to β, or even something approximating β alone. However, if those α-β tandems do not form receptors when co-expressed with β in a given expression system (11,12), the concern is ameliorated, and a secondary probe for partially degraded α would not be necessary. Moreover, because α-β tandems do not form functional receptors when expressed alone, the fear of breakdown into free α- and β-subunits, which can form receptors, is also allayed.
Incorporate Discriminating Mutations
In fact, complete Western blot studies are frequently wanting in many tandem studies, often for lack of an antibody that recognizes internal segments of each of the subunits. Functional tests for agonist and drug binding do not always exclude the possibility of unincorporated subunits or incorporated products of degradation or signal peptide clipping. Adding mutations that exclude these possibilities is an elegant solution (37), such that a Western blot might not even be required in some instances (35), although it might help to quell any doubts, especially if antibodies to internal epitopes are used. Indeed, using mutations that shift function can even be used to separate mixtures of free subunits (50), but hopefully the effort required will be substantially reduced with tandems. Luckily, we have the experiences (and sweat) of our colleagues to draw upon; lessons learned about linker design, loop-outs, dipentamers, mutational testing, and concatamer order will help us with future experimental designs that can be best addressed with tandems.
Acknowledgments
We thank Dr. Ken Satyshur for initial modeling, Dr. Cynthia Czajkowski, Jeremy Bushman, Lee Wheeler, and Abdalla Saad for tandem construction and testing and Diana Eng for inspiration. This work was funded by, and NIH R01 grant MH66406-03 to C.C./A.B.
References
- 1.Lindstrom JM. Nicotinic acetylcholine receptors of muscles and nerves: comparison of their structures, functional roles, and vulnerability to pathology. Ann N Y Acad Sci. 2003;998:41–52. doi: 10.1196/annals.1254.007. [DOI] [PubMed] [Google Scholar]
- 2.Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol Neurobiol. 2002;26:251–268. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- 3.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 4.Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- 5.Bonnert TP, McKernan RM, Farrar S, et al. theta, a novel gamma-aminobutyric acid type A receptor subunit. Proc Natl Acad Sci U S A. 1999;96:9891–9896. doi: 10.1073/pnas.96.17.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neelands TR, Macdonald RL. Incorporation of the pi subunit into functional gamma-aminobutyric Acid(A) receptors. Mol Pharmacol. 1999;56:598–610. doi: 10.1124/mol.56.3.598. [DOI] [PubMed] [Google Scholar]
- 7.Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- 8.Rajendra S, Lynch JW, Schofield PR. The glycine receptor. Pharmacol Ther. 1997;73:121–146. doi: 10.1016/s0163-7258(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 9.Baumann SW, Baur R, Sigel E. Subunit arrangement of gamma-aminobutyric acid type A receptors. J Biol Chem. 2001;276:36,275–36,280. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- 10.Horenstein J, Wagner DA, Czajkowski C, Akabas MH. Protein mobility and GABA-induced conformational changes in GABA(A) receptor pore-lining M2 segment. Nat Neurosci. 2001;4:477–485. doi: 10.1038/87425. [DOI] [PubMed] [Google Scholar]
- 11.Boileau AJ, Pearce RA, Czajkowski C. Tandem subunits effectively constrain GABAA receptor stoichiometry and recapitulate receptor kinetics but are insensitive to GABAA receptor-associated protein. J Neurosci. 2005;25:11,219–11,230. doi: 10.1523/JNEUROSCI.3751-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Im WB, Pregenzer JF, Binder JA, Dillon GH, Alberts GL. Chloride channel expression with the tandem construct of alpha 6-beta 2 GABAA receptor subunit requires a monomeric subunit of alpha 6 or gamma 2. J Biol Chem. 1995;270:26,063–26,066. doi: 10.1074/jbc.270.44.26063. [DOI] [PubMed] [Google Scholar]
- 13.Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sieghart W, Sperk G. Subunit Composition, Distribution and Function of GABA(A) Receptor Subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 15.Jechlinger M, Pelz R, Tretter V, Klausberger T, Sieghart W. Subunit composition and quantitative importance of hetero-oligomeric receptors: GABAA receptors containing alpha6 subunits. J Neurosci. 1998;18:2449–2457. doi: 10.1523/JNEUROSCI.18-07-02449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minier F, Sigel E. Positioning of the alpha-subunit isoforms confers a functional signature to gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:7769–7774. doi: 10.1073/pnas.0400220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engel AG, Ohno K, Milone M, et al. New mutations in acetylcholine receptor subunit genes reveal heterogeneity in the slow-channel congenital myasthenic syndrome. Hum Mol Genet. 1996;5:1217–1227. doi: 10.1093/hmg/5.9.1217. [DOI] [PubMed] [Google Scholar]
- 18.Vergouwe MN, Tijssen MA, Peters AC, Wielaard R, Frants RR. Hyperekplexia phenotype due to compound heterozygosity for GLRA1 gene mutations. Ann Neurol. 1999;46:634–638. doi: 10.1002/1531-8249(199910)46:4<634::aid-ana12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher MJ, Song L, Arain F, Macdonald RL. The juvenile myoclonic epilepsy GABA(A) receptor alpha1 subunit mutation A322D produces asymmetrical, subunit position-dependent reduction of heterozygous receptor currents and alpha1 subunit protein expression. J Neurosci. 2004;24:5570–5578. doi: 10.1523/JNEUROSCI.1301-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46,020–46,025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- 21.Boileau AJ, Baur R, Sharkey LM, Sigel E, Czajkowski C. The relative amount of cRNA coding for gamma2 subunits affects stimulation by benzodiazepines in GABA(A) receptors expressed in Xenopus oocytes. Neuropharmacology. 2002;43:695–700. doi: 10.1016/s0028-3908(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 22.Boileau AJ, Li T, Benkwitz C, Czajkowski C, Pearce RA. Effects of gamma2S subunit incorporation on GABAA receptor macroscopic kinetics. Neuropharmacology. 2003;44:1003–1012. doi: 10.1016/s0028-3908(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 23.Pritchett DB, Sontheimer H, Shivers BD, et al. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- 24.Baumann SW, Baur R, Sigel E. Individual properties of the two functional agonist sites in GABA(A) receptors. J Neurosci. 2003;23:11,158–11,166. doi: 10.1523/JNEUROSCI.23-35-11158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang JQ, Macdonald RL. The GABAA receptor gamma2 subunit R43Q mutation linked to childhood absence epilepsy and febrile seizures causes retention of alpha1beta2 gamma2S receptors in the endoplasmic reticulum. J Neurosci. 2004;24:8672–8677. doi: 10.1523/JNEUROSCI.2717-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baur R, Sigel E. Benzodiazepines affect channel opening of GABAA receptors induced by either agonist binding site. Mol Pharmacol. 2005;67:1005–1008. doi: 10.1124/mol.104.008151. [DOI] [PubMed] [Google Scholar]
- 27.Campo-Soria C, Chang Y, Weiss DS. Mechanism of action of benzodiazepines on GABA(A) receptors. Br J Pharmacol. 2006;148:984–990. doi: 10.1038/sj.bjp.0706796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieghart W, Fuchs K, Tretter V, et al. Structure and subunit composition of GABA(A) receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 29.Boulineau N, Baur R, Minier F, Sigel E. Consequence of the presence of two different beta subunit isoforms in a GABA(A) receptor. J Neurochem. 2005;95:1724–1731. doi: 10.1111/j.1471-4159.2005.03495.x. [DOI] [PubMed] [Google Scholar]
- 30.Grudzinska J, Schemm R, Haeger S, et al. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Burzomato V, Groot-Kormelink PJ, Sivilotti LG, Beato M. Stoichiometry of recombinant heteromeric glycine receptors revealed by a pore-lining region point mutation. Recept Chan. 2003;9:353–361. doi: 10.3109/714041016. [DOI] [PubMed] [Google Scholar]
- 32.Kuhse J, Laube B, Magalei D, Betz H. Assembly of the inhibitory glycine receptor: identification of amino acid sequence motifs governing subunit stoichiometry. Neuron. 1993;11:1049–1056. doi: 10.1016/0896-6273(93)90218-g. [DOI] [PubMed] [Google Scholar]
- 33.Langosch D, Thomas L, Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988;85:7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. Embo J. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groot-Kormelink PJ, Broadbent S, Beato M, Sivilotti LG. Constraining the expression of nicotinic acetylcholine receptors by using pentameric constructs. Mol Pharmacol. 2006;69:558–563. doi: 10.1124/mol.105.019356. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. Human alpha4beta2 acetylcholine receptors formed from linked subunits. J Neurosci. 2003;23:9004–9015. doi: 10.1523/JNEUROSCI.23-27-09004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groot-Kormelink PJ, Broadbent SD, Boorman JP, Sivilotti LG. Incomplete incorporation of tandem subunits in recombinant neuronal nicotinic receptors. J Gen Physiol. 2004;123:697–708. doi: 10.1085/jgp.200409042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baur R, Minier F, Sigel E. A GABA(A) receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS Lett. 2006;580:1616–1620. doi: 10.1016/j.febslet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Stoop R, Thomas S, Rassendren F, et al. Contribution of individual subunits to the multimeric P2X(2) receptor: estimates based on methanethiosulfonate block at T336C. Mol Pharmacol. 1999;56:973–981. doi: 10.1124/mol.56.5.973. [DOI] [PubMed] [Google Scholar]
- 40.Altschuler EL, Hud NV, Mazrimas JA, Rupp B. Random coil conformation for extended polyglutamine stretches in aqueous soluble monomeric peptides. J Pept Res. 1997;50:73–75. doi: 10.1111/j.1399-3011.1997.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 41.Minier F, Sigel E. Techniques: Use of concatenated subunits for the study of ligand-gated ion channels. Trends Pharmacol Sci. 2004;25:499–503. doi: 10.1016/j.tips.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 43.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 44.Downing SS, Lee YT, Farb DH, Gibbs TT. Benzodiazepine modulation of partial agonist efficacy and spontaneously active GABA(A) receptors supports an allosteric model of modulation. Br J Pharmacol. 2005;145:894–906. doi: 10.1038/sj.bjp.0706251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueno S, Zorumski C, Bracamontes J, Steinbach JH. Endogenous subunits can cause ambiguities in the pharmacology of exogenous gamma-aminobutyric acidA receptors expressed in human embryonic kidney 293 cells. Mol Pharmacol. 1996;50:931–938. [PubMed] [Google Scholar]
- 46.Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Millar NS. Assembly and subunit diversity of nicotinic acetylcholine receptors. Biochem Soc Trans. 2003;31:869–874. doi: 10.1042/bst0310869. [DOI] [PubMed] [Google Scholar]
- 48.Betz H, Kuhse J, Schmieden V, Laube B, Kirsch J, Harvey RJ. Structure and functions of inhibitory and excitatory glycine receptors. Ann N Y Acad Sci. 1999;868:667–676. doi: 10.1111/j.1749-6632.1999.tb11343.x. [DOI] [PubMed] [Google Scholar]
- 49.Barrera NP, Herbert P, Henderson RM, Martin IL, Edwardson JM. Atomic force microscopy reveals the stoichiometry and subunit arrangement of 5-HT3 receptors. Proc Natl Acad Sci U S A. 2005;102:12,595–12,600. doi: 10.1073/pnas.0503253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akk G, Sine S, Auerbach A. Binding sites contribute unequally to the gating of mouse nicotinic alpha D200N acetylcholine receptors. J Physiol. 1996;496:185–196. doi: 10.1113/jphysiol.1996.sp021676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mercado J, Czajkowski C. Charged residues in the alpha1 and beta2 pre-M1 regions involved in GABAA receptor activation. J Neurosci. 2006;26:2031–2040. doi: 10.1523/JNEUROSCI.4555-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


