Abstract
Levels of dopamine and melatonin exhibit diurnal rhythms in the rat retina. Dopamine is high during daytime adapting the retina to light, whereas melatonin is high during nighttime participating in the adaptation of the retina to low light intensities. Dopamine inhibits the synthesis of melatonin in the photoreceptors via Drd4-receptors located on the cell membrane of these cells. In this study, we show by semiquantitative in situ hybridization a prominent day/night variation in Drd4 expression in the retina of the Sprague Dawley rat with a peak during the nighttime. Drd4 expression is seen in all retinal layers but the nocturnal increase is confined to the photoreceptors. Retinal Drd4 expression is not affected by removal of the sympathetic input to the eye, but triiodothyronine treatment induces Drd4 the expression in the photoreceptors. In a developmental series, we show that the expression of Drd4 is restricted to postnatal stages with a peak at postnatal day 12. The high Drd4 expression in the rat retinal photoreceptors during the night supports physiological and pharmacologic evidence that the Drd4 receptor is involved in the dopaminergic inhibition of melatonin synthesis upon light stimulation. The sharp increase of Drd4 expression at a specific postnatal time suggests that dopamine is involved in retinal development.
Keywords: retina, diurnal rhythm, dopamine, Drd4, development, triiodothyronine
Introduction
The presence of dopamine and dopamine receptors in the vertebrate retina has been known for several decades (Kramer, 1971). Dopamine is synthesized in a subpopulation of retinal amacrine- and interplexiform cells located at the border of the inner nuclear and inner plexiform layer (Savy et al., 1989; Witkovsky, 2004) and the dopaminergic amacrine cells make synaptic contacts with the dendrites of ganglion cells in a narrow horizontal stratum in the outer part of the inner plexiform layer (Dacey, 1990). However, in the retina dopamine is also released extrasynaptically and functions as a paracrine signal acting through dopamine receptors located on cells in outer retinal layers (Witkovsky, 2004).
Retinal dopamine levels exhibit a prominent day/night variation with high levels during the light period and low levels during the dark phase (Melamed et al., 1984; Pozdeyev and Lavrikova, 2000; Zawilska et al., 2003). Within the retina, dopamine synthesis is induced by light due to an increased activity of the rate limiting enzyme in dopamine synthesis, tyrosine hydroxylase (TH) (Boatright et al., 1989; Iuvone et al., 1978). It is thought that dopamine is important for the adaptation of the eye to light (Besharse and Iuvone, 1992; Luft et al., 2004; Megaw et al., 2006).
Melatonin is a hormone, which is synthesized primarily in the pineal gland at night (Klein and Weller, 1970). The synthesis is stimulated by activation of adrenoreceptors on the pinealocyte membrane followed by a cAMP dependent transcriptional and posttranslational activation of arylalkylamine N-acetyltransferase (AANAT, EC 2.3.1.87), a key enzyme in the melatonin synthesis (Klein et al., 1997). Melatonin is also synthesized in the photoreceptors of the vertebrate retina (Vivien-Roels et al., 1981) with a peak in synthesis during the night (Tosini and Fukuhara, 2003). As in the pineal gland, the increased synthesis of melatonin at night is induced by increased levels of cAMP in the photoreceptors (Chaurasia et al., 2006a), which stimulate the synthesis AANAT (Iuvone et al., 2005).
In the retina, melatonin regulates physiological functions involved in the retinal adaptations to low light intensities including dark-adaptive cone elongation in Xenopus (Pierce and Besharse, 1985), activation of rod photoreceptor disc shedding in Xenopus and rat (Besharse and Dunis, 1983; White and Fisher, 1989), enhancement of horizontal cell sensitivity in salamander (Wiechmann et al., 1988), and horizontal cell dark adaptation in fish (Behrens et al., 2000).
Dopamine receptors (Drd) are seven transmembrane G-protein coupled receptors, which are divided into two main classes (Gingrich and Caron, 1993): 1) The excitatory D1-like receptor family, coupled to stimulatory G-proteins, comprises the dopamine receptor D1 (Drd1) and dopamine receptor D5 (Drd5); and 2) receptors of the inhibitory D2-like receptor family are coupled to inhibitory G-proteins and include the dopamine receptor D2 (Drd2), dopamine receptor D3 (Drd3), and dopamine receptor D4 (Drd4).
Among these, Drd4 is highly expressed in the rodent retina (Cohen et al., 1992; Suzuki et al., 1995), and a diurnal rhythm with increased levels of retinal Drd4 mRNA during the dark period has recently been reported (Bai et al., 2008). The inhibitory effect of dopamine on retinal melatonin production is mediated by dopamine Drd2 and/or Drd4 receptors (Nguyen-Legros et al., 1996; Tosini and Dirden, 2000; Zawilska and Iuvone, 1989; Zawilska and Nowak, 1994). Furthermore, D2-like receptor family dopaminergic agonists are capable of mimicking a light stimulus, and inducing a phase shift in melatonin production from perfused vertebrate eyecups (Cahill and Besharse, 1991), and it has recently been shown, that dopamine in the mouse retina regulates the phosphorylation state of the photoreceptor specific protein phosducin via Drd4 receptors (Pozdeyev et al., 2008). Finally, mice lacking the Drd4 show an abnormal light adaptation of the retina (Nir et al., 2002). However, the localization of the Drd4 receptor in retina has not been clarified.
In this study we extend previous studies on expression of Drd4 in the retina by mapping the anatomical distribution of the Drd4 transcripts. Furthermore, we investigate diurnal and ontogenetic patterns as well as regulatory aspects of retinal Drd4 expression.
Materials and methods
Animals
Day-night series
Adult male Sprague-Dawley rats (Charles River, Würtzburg, Germany) were housed for two weeks in LD 12:12 lighting cycles (lights on at 7:00 AM) with food and water ad libitum. Control and ganglionectomized animals were anesthetized and sacrificed by decapitation at both at midday (ZT 6) and midnight (ZT 18). Eyeballs were immediately removed and frozen on crushed solid CO2.
Developmental series
Fetal and postnatal Sprague-Dawley rats were obtained from time-pregnant animals (Charles River). The animals were anesthetized and sacrificed during daytime (ZT 6-8). At the earliest stages (E16-E19), the whole fetus was immersion fixed in 4% paraformaldehyde for 2 days. For older fetuses and postnatal animals (E21-P30), the eyeballs were removed and fixed by immersion for 24 hours. Eyeballs were cryoprotected in 25% sucrose in PBS for 24 hours and frozen on crushed solid CO2.
Anesthesia
Animals were anaesthetized by intraperitoneal injection of 1 ml/100 g body weight of a 5% Tribromethanol solution [Tribromethanol (Sigma-Aldrich, Steinheim, Germany,) dissolved in 3-Pentanol (Merck, Darmstadt, Germany), and finally diluted in PBS containing 8% (v/v) ethanol 99%].
Bilateral superior cervical ganglionectomy
Ten adult male Sprague-Dawley rats (Charles River) were anaesthetized and an incision was made in the midline on the anterior surface of the neck. The common carotid arteries were visualized and followed rostrally to the bifurcations. Just medial to the carotid bifurcation, the superior cervical ganglia were isolated and removed with a part of the adjacent caudal sympathetic trunk and the rostral carotid nerve. Both ganglia were removed during the same surgical session. After the ganglionectomy, bilateral ptosis was observed on all animals. The animals survived for ten days before they were sacrificed.
Injection with thyroid hormone
Four male Sprague-Dawley rats were injected intraperitoneally with 0.1 mg/kg body weight triiodothyronine (T3) (Sigma-Aldrich) dissolved in PBS at ZT 5-6. Three animals were injected with the vehicle without T3. The animals were sacrificed four hours after injection (at ZT 9-10). Eyeballs were immediately removed and frozen on crushed solid CO2.
All experiments with animals were performed in accordance with the guidelines of EU Directive 86/609/EEC approved by the Danish Council for Animal Experiments.
Radiochemical in situ hybridization
Preparation of probe
A 32-nucleotide anti-sense probe (5′-GGA TGC GCT CGG AGG CCA CGC TCA CGC AAA CG-3′) directed against rat Drd4 mRNA (NM_12944) was designed using Primer 3 software (Whitehead Institute, Cambridge, MA ). The synthetic probe was end-labeled with S35-dATP by mixing 10 pmol of probe (2 μl) with 10 μl reaction buffer (supplied with the transferase), 24 μl DEPC-treated water, 5 μl CoCl2 (25 mM), 5 μl S35-dATP (Perkin Elmer, Hvidovre, Denmark) and 1 μl recombinant terminal transferase (Roche, Mannheim, Germany ) for 75 min at 37° C. The reaction was stopped by adding 5 μl 0.2M EDTA (pH 8) and the probe was purified by use of an Illustra ProbeQuant™ G-50 microcolumn (GE Healthcare, Hillerød, Denmark ) according to the manufacturer's instructions.
In situ hybridization procedure
14 μm cryostat sections were mounted on SuperFrost® Plus glass slides (Menzel, Braunscweig, Germany). Frozen sections were fixed for 5 min in 4% formaldehyde in PBS, washed twice in PBS and acetylated 10 min in 0.25% acetic anhydride solution (acetic anhydride and 0.1 M triethanolamine in 0.9% NaCl). Sections were then dehydrated in a series of ethanols, delipidated 5 min in chloroform, partially rehydrated and air dried. For hybridization, the probe was diluted in hybridization buffer consisting of 50% (v/v) formamide, 4× sodium chloride and sodium citrate solution (SSC, 150 mM NaCl, 15 mM sodium citrate, pH 7.0), 1X Denhardt's solution (0.02% BSA, 0.02% polyvinylpyrrolidone, 0.02% Ficoll), 10% (w/v) dextran sulfate, 10 mM DTT, 0.5 mg/ml salmon sperm DNA and 0.5 mg/ml yeast tRNA. An aliquot of 150 μl hybridization solution was added onto each slide. Slides were covered with Parafilm® and incubated in a humid chamber overnight at 37° C. Sections were washed in 1X SSC for 4 × 15 min at 55° C and 2 × 30 min at room temperature, rinsed in deionized water and dried under warm air. Sections were exposed to an X-ray film (Agfa, Mortsel, Belgium) for 10 to 14 days (4° C) and developed in a commercial developing machine. Some sections were subsequently dipped in a photographic hypercoat emulsion, (GE Healthcare) diluted 1:1 in distilled water at 43 °C. Slides were allowed to dry in darkness for 2 hours at room temperature and thereafter exposed in a light safe box for 2 weeks at 4°C. The sections were developed 4 ½ min in 0.45% amidol (cat. no. 10641, Merck, Darmstadt), 0.08% potassium bromide (cat. no. 4900, Merck), and 1.8% sodium sulfite (Merck) washed in distilled water, fixed for 10 min in 30% Na2SO2O3 (Merck) in water followed by washing for 1 hour in running tap water. The emulsion was fixed 10 min in 70% ethanol and stained for 1 min in cresyl violet solution (0.1% cresyl violet in 1% acetic acid).
Quantification of hybridization signals
The images on the X-ray film were captured by a high performance black and white CCD-camera (Cohu, San Diego, CA) equipped with a macro zoom lens (Computar, Tokyo, Japan) and quantified densitometrically using Image 1.42 (Wayne Rasband, NIH, Bethesda, MD). Optical densities were converted to dpm/mg tissue by using simultaneously exposed 14C-standards calibrated by comparison with 35S-tissue paste standards. Densitometric quantification was done on four sections from each animal; each experimental consisted normally five animals. For the emulsion-dipped sections, photographic grains were counted in the light microscope equipped with an ocular grid. Three retinal sections from 3 daytime and 3 nighttime animals were quantified. A total of 3,570 μm2 was counted in each retinal layer.
Statistical analysis
Data are presented as mean ± SEM. Two-tailed Student's t-test was used for comparison of experimental groups (Excel 2003, Microsoft). A p-value of < 0.05 was considered to represent statistical significance.
Results
Expression of Drd4 in the retina exhibits a day/night variation
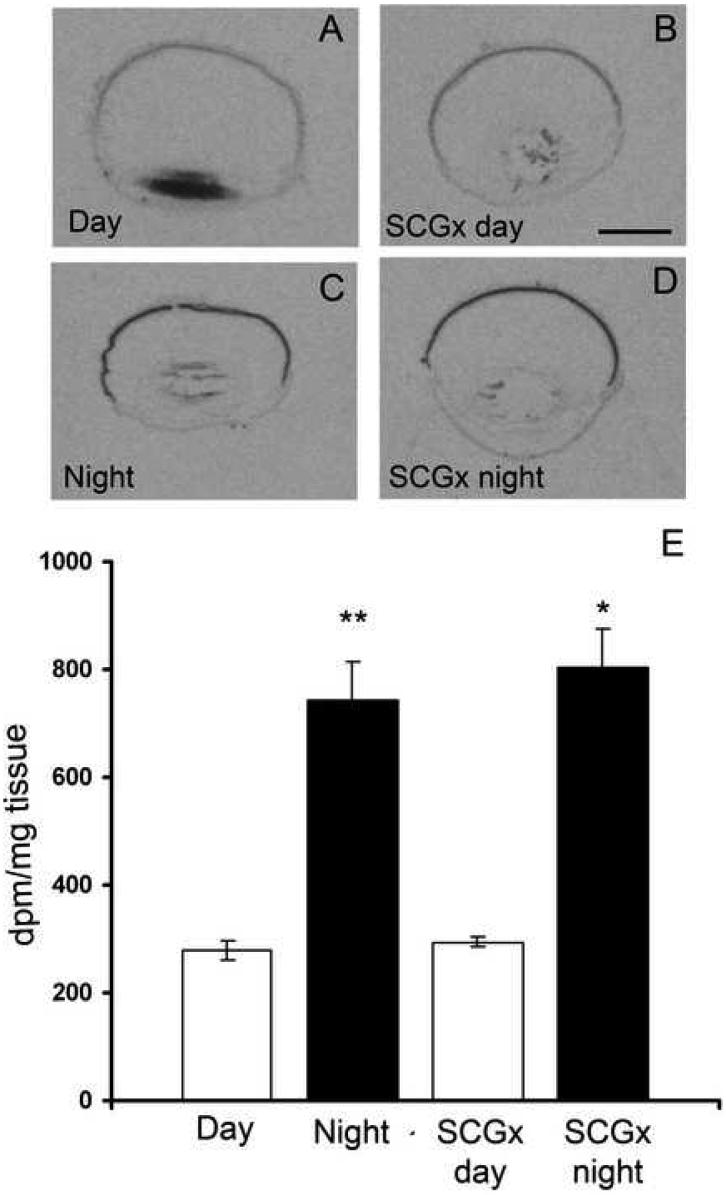
To examine day/night variation in retinal expression of Drd4 mRNA, animals were sacrificed at midday (ZT 6) and midnight (ZT 18) and radioactive in situ hybridization was performed on sagittal sections of the eye. Images on the exposed X-ray film revealed the presence of Drd4 mRNA in the retina of the rat during both day and night (Fig. 1A and C). Densitometric quantification Drd4 mRNA expression showed a significant day/night difference with a 2.70 ± 0.30 fold increase during the dark period as compared to daytime levels (Fig. 1E). This expression was not influenced by removal of the sympathetic input to the pineal and eye (Fig. 1B and D). Drd4 expression was not observed in non-retinal parts of the eye including the ciliary body, lens, cornea, as well as choroid- and scleral layers.
Fig. 1. Day/night variation in the retinal Drd4 expression in the Sprague-Dawley rat.
X-ray images of sagittal sections, hybridized for the Drd4-receptor, of rat eyeballs from a control rat (A and C) and from a rat (B and D), in which the superior cervical ganglia have been removed bilaterally (SCGx) ten days before sacrifice. Note the high nighttime expression, which is unaffected by ganglionectomy. E: densitometric quantification of the signals showing mean ±SEM of 5 animals. dpm = disintegrations per minute. * = p<0.05, ** = p<0.01. Scale bar = 2 mm.
The nighttime up-regulation of retinal Drd4 mRNA is confined to the photoreceptors
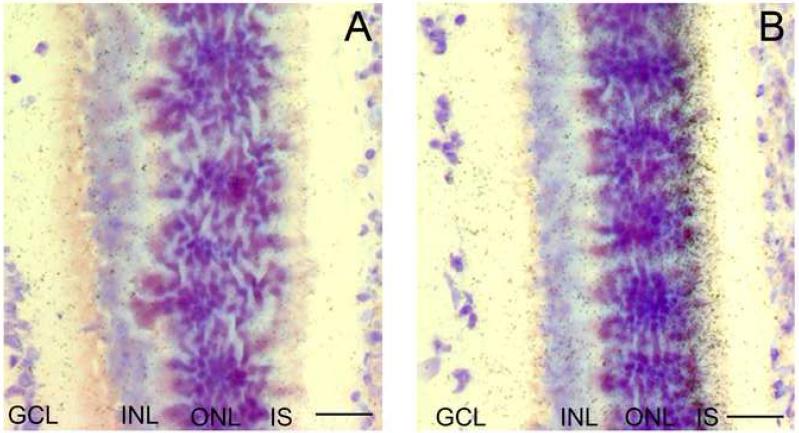
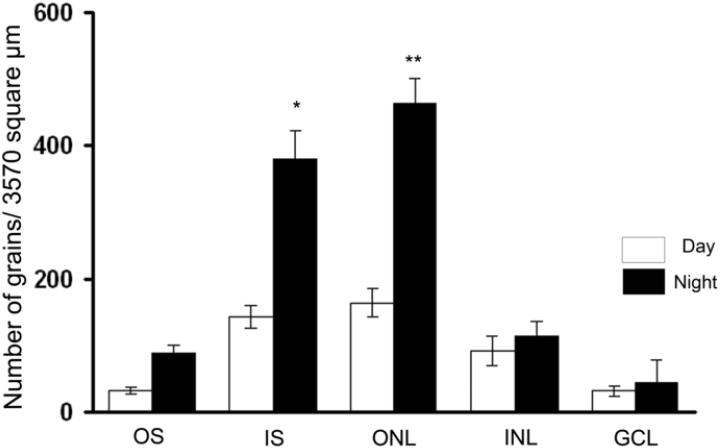
To examine the cellular localization of Drd4 mRNA in the rat eye, hybridized sections were dipped in a photographic emulsion. During daytime, Drd4 mRNA was detected in all retinal layers (Figs.2 and 3). However, during nighttime the level of Drd4 mRNA was increased only in the photoreceptors. Thus, in the inner segments the ratio between the night/day expression was 2.65 ± 0.44 and in the outer nuclear layer the ratio was 2.83 ± 0.42 (Figs. 2 and 3).
Fig. 2. Localization of Drd4 mRNA in the retina of the Sprague-Dawley rat.
Microautoradiographs of retinal sections hybridized for detection of Drd4 mRNA. A: section from a rat sacrificed during daytime (ZT6). B: section from a rat sacrificed during nighttime (ZT18). Note the high number of grains above the inner segments of the photoreceptors. INL=inner nuclear layer, IS=inner segments of photoreceptors, ONL=outer nuclear layer. Scale bar = 50 μm.
Fig. 3. Quantification of the Drd4 expression in retinal layers during day- and nighttime.
Bar graph showing the increase in nighttime Drd4 expression above the layer containing the inner segments of the photoreceptors and the inner nuclear layer with the cell bodies of the photoreceptors. GCL=ganglion cell layer, INL=inner nuclear layer, IS=inner segments of photoreceptors, ONL=outer nuclear layer, OS= outer segments of photoreceptors. Values are expressed as mean ± SEM in each group. * = p<0.05, ** = p<0.01.
Drd4 expression is upregulated by thyroid hormone
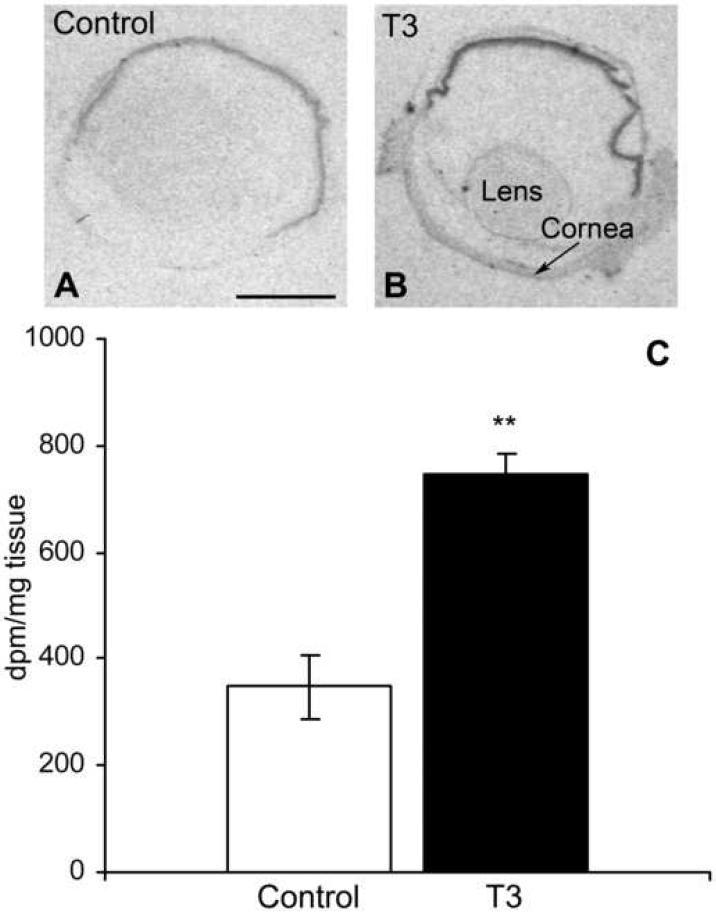
We have recently shown an upregulation of Drd4 mRNA in the pineal gland in response to treatment with thyroid hormone (Kim et al., unpublished). To investigate the possible influence of thyroid hormone on retinal Drd4 expression, animals were injected during daytime with triiodothyronine (T3) and control animals were injected with vehicle. In situ hybridization followed by densitometric quantification revealed a 2.10 ± 0.38 fold increase in daytime Drd4 mRNA upon T3 stimulation (Fig. 4).
Fig. 4. Retinal Drd4 expression is increased by injection of triiodothyronine (T3).
A: X-ray images of rat eye section from daytime animal hybridized with S35-labeled antisense probes against Drd4-mRNA. B: section from an animal, which has been injected with 0.1 mg T3/kg body weight for 4 hours before sacrifice. C. bar graph of the densitometric quantification of the signals in the hybridized sections showing mean ± SEM of 3 animals in each group. dpm = disintegration per minute. ** = p<0.01. Scale bar = 2 mm.
Drd4 is strongly expressed postnatally during rat eye development
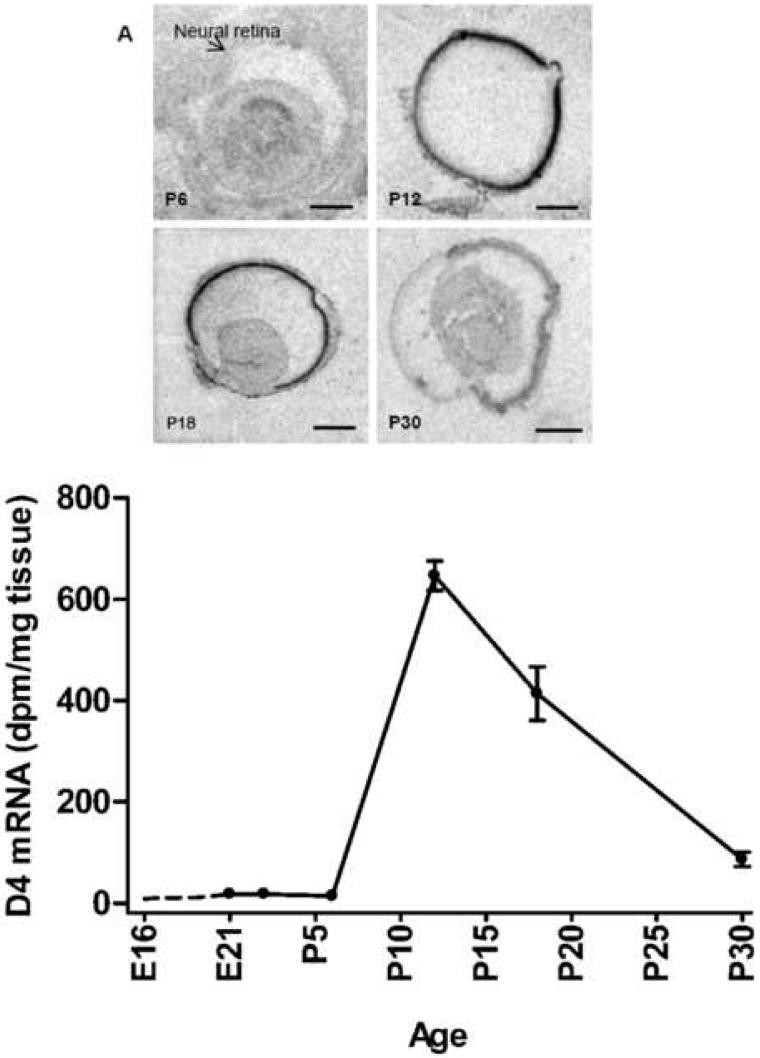
To investigate the expression pattern of Drd4 mRNA in rat retina during eye development, in situ hybridization was performed on eye sections from animals in a developmental series ranging from embryonic day 16 (E16) to postnatal day 30 (P30). During prenatal stages, a signal above background was not observed. During the first postnatal week, a very weak signal was detected; however at P12 a very strong expression was observed (Fig. 5). In the following developmental stages, the level of Drd4 mRNA decreased to adult daytime level at postnatal day 30 (Fig. 5).
Fig. 5. Ontogenetic Drd4 expression in the developing rat eye.
Upper panel: X-ray images of sections hybridized with a S35-labeled antisense probe against Drd4-mRNA in a developmental series (postnatal day 6, 12, 18, and 30) of the rat eye. Lower panel: line graph showing Drd4 mRNA expression during development from embryonic day 16 to postnatal day 30. Drd4 mRNA is barely detectable before postembryonic day 12, where a dramatic increase in expression is observed. Scale bars: P6 = 0.5 mm, P12 = 2 mm, P18 = 2 mm, P30 = 2 mm.
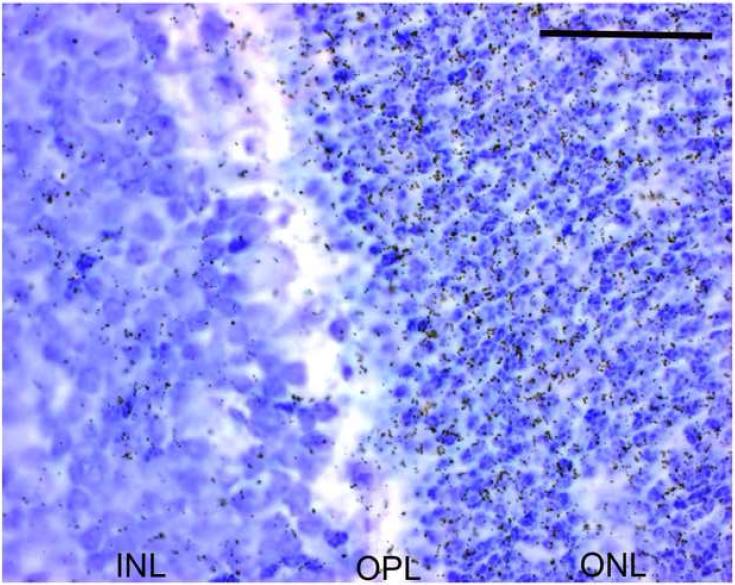
Hybridized sections from P12 were dipped in a photographic emulsion. This revealed a high number of grains, and thus a high level of Drd4 mRNA in the outer nuclear layer as compared to the other layers of the retina (Fig.6).
Fig. 6. The high Drd4 expression in the rat retina at postnatal day 12 occurs in the photoreceptors.
Microautoradiograph of emulsion-dipped section hybridized for Drd4-mRNA from a rat at postnatal day 12. The Drd4 expression is seen primarily in the outer nuclear layer. Scale bar = 50 μm.
Discussion
The in situ hybridizations performed in this study on sections through the rat eye showed a high specific expression of Drd4 in the rat retina. The hybridizations further showed a prominent diurnal rhythm with an increased Drd4 expression during the dark phase as compared to the light period. This diurnal Drd4 mRNA expression is in accord with other studies using qRT-PCR for detection of day/night variation in Drd4 expression in both pineal gland and retina (Fukuhara and Tosini, 2008; Bai et al., 2008; Kim et al., unpublished). However, in the present study the spatial resolution has been increased to show that Drd4 is expressed in all retinal layers, whereas the increase in Drd4 mRNA expression during the dark phase is confined to the photoreceptors and especially to the parts of the cells containing the endoplasmic reticulum.
The high Drd4 expression in the photoreceptors supports physiological, biochemical, and pharmacological studies on the relationship between light, dopamine release, and melatonin production in the vertebrate retina. Thus, light inhibits retinal melatonin production (Tosini and Fukura, 2003; Zawilska et al., 2007) but stimulates the dopamine release from the amacrine- and interplexiform cells. The dopamine release during the light period results in an inhibition of melatonin synthesis in the retina. Pharmacological studies reveal that dopamine inhibition of the photoreceptor production of melatonin occurs via Drd2 and Drd4 receptors located on the cell membrane of the photoreceptors (Nguyen-Legros et al., 1996; Tosini and Dirden, 2000). Further, in the mouse, dopamine Drd4 receptors, negatively coupled to adenylyl cyclase, are present in the photoreceptor cells (Cohen et al., 1992). Our data support the concept that dopamine inhibits melatonin production via the Drd4 receptor. Light might also decrease melatonin synthesis during daytime by disrupting the binding of the AANAT protein to the 14-3-3 protein, which protects AANAT from being dephosphorylated and proteolytically degraded by the proteasome system (Pozdeyev et al., 2006).
Melatonin, on the other hand, inhibits the dopamine release from amacrine cells in the vertebrate retina (Dubocovich et al., 1997) via melatonin receptors present in the cell membrane of the amacrine cells (Fujieda et al., 2000; Scher et al., 2002; Scher et al., 2003). However, studies on the chick retina suggest that this inhibition of dopamine release might be an indirect mechanism via an enhancement of GABAergic inhibition of light-evoked dopamine release in the retina (Boatright et al., 1994). Melatonin might also participate in the dark adaption by counteracting the cAMP accumulation in retinal cells caused by dopamine receptor D1 activation (Iuvone and Gan, 1995).
In constant darkness, melatonin production in the photoreceptors and dopamine synthesis by amacrine cells are under endogenous circadian control (Besharse and Iuvone, 1983; Tosini and Menaker, 1996) possibly by a clock located in the retina itself as indicated by the expression of clock genes in both photoreceptors and in dopaminergic amacrine cells of the retina (Chaurasia et al., 2006b; Dorenbos et al., 2007; Tosini et al., 2007).
The Drd4 receptors might also have a function in the inner retina where low amounts of Drd4 transcripts were observed. Melanopsin-containing neurons are located in the ganglion cell layer (Beaulé et al., 2003); these cells are directly responsive to light and transmit the non-visual photic inputs to the neurons of the suprachiasmatic nucleus (Foster and Hankins, 2007). Dopamine is capable of upregulating the expression of melanopsin in these cells possibly via a receptor belonging to the dopamine D2-family (Sakamoto et al., 2005); whether Drd4 is the responsible receptor has not been evaluated.
Drd4 receptors are also present in the pineal gland and exhibits a prominent day/night rhythm (Humphries et al., 2002; Fukuhara and Tosini, 2008; Kim et al., unpublished). Perikarya located in the superior cervical ganglia project sympathetic axons to the mammalian pineal gland (Møller and Baeres, 2002; Møller et al. 2006). Removal of both superior cervical ganglia in the rat abolishes the diurnal Drd4 receptor rhythm of the rat pineal verifying the well-known important sympathetic influence on rodent pineal metabolism. The sympathetic nerve fibers originating in the superior cervical ganglia do not innervate the retina but only the iris and choroidea (Steinle et al., 2000). However, an indirect sympathetic influence on the retina via the vascular system would have been possible. But in this study the retinal Drd4 expression and day/night variation was not influenced by removal of the superior cervical ganglia.
We also in this paper show that triiodothyronine stimulates the expression of Drd4 in the rat retina. Such an effect of triiodothyronine has also been described in the rat pineal gland (Kim et al., unpublished), but never in the vertebrate retina. In the rat pineal it was shown that cAMP and T3 are able to stimulate Drd4 expression and that the two factors might function as an “AND” gate being important for biological mechanisms in several tissues. A possible explanation for the stimulatory function of T3 on the Drd4 expression might be that T3 could target the promoter region of the Drd4 gene.
We show in this paper a fairly late expression of Drd4 in the rat retina during ontogenesis. From P6 to P12 the retinal expression level of Drd4 increases dramatically followed by a decrease to the adult level at P30. In a study by Fujieda and colleagues using quantitative RT-PCR, a small increase in Drd4 expression was seen already at P1 increasing through P7 to a maximum at P14, which showed only a minor decline at P54. (Fujieda et al., 2003). Our data are well correlated with this study with regard to the onset of Drd4 expression, but the decline in expression after P14 shown in our study was several folds larger than the one demonstrated by Fujieda et al. Currently, we do at the moment not have an explanation for this discrepancy. The expression levels and cellular localization of Drd4 mRNA in the photoreceptors correlate ontogenetically with the postnatal photoreceptor maturation (Weidman and Kuwabara, 1969; Fujieda et al., 2003), but the Drd4 receptors are not directly involved in photoreceptor maturation, since morphology and survival of photoreceptors have been shown to be equal in both Drd4 knockout mice and wild type mice (Nir et al., 2002).
In summary, this study shows the presence of Drd4 expression in the rat retina with the highest expression confined to the photoreceptors. The nighttime upregulation of the retinal Drd4 receptors occurs in the photoreceptors; furthermore, retinal Drd4 expression is upregulated by triiodothyronine. These data are in accordance with a main function of retinal Drd4 in the dopaminergic light adaptation of the retinal photoreceptors.
Acknowledgments
This study was supported by the Lundbeck Foundation, the Danish Medical Research Council (grant number 271-06-0754), the Novo Nordisk Foundation, the Carlsberg Foundation, the Simon Fougner Hartmann's Family Foundation, the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. We wish to thank Mrs. Ursula Rentzmann for expert histological assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bai L, Zimmer S, Rickes O, Rohleder N, Holthues H, Engel L, Leube R, Spessert R. Daily oscillation of gene expression in the retina is phase-advanced with respect to the pineal gland. Brain Res. 2008;1203:89–96. doi: 10.1016/j.brainres.2008.01.073. [DOI] [PubMed] [Google Scholar]
- Beaulé C, Robinson B, Lamont EW, Amir S. Melanopsin in the circadian timing system. J. Mol. Neurosci. 2003;21:73–89. doi: 10.1385/JMN:21:1:73. [DOI] [PubMed] [Google Scholar]
- Behrens UD, Douglas RH, Sugden D, Davies DJ, Wagner HJ. Effects of melatonin agonists and antagonists on horizontal cell spinule formation and dopamine release in a fish retina. Cell Tissue Res. 2000;299:299–306. doi: 10.1007/s004419900161. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Dunis DA. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science. 1983;219:1341–1343. doi: 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Iuvone PM. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature. 1983;305:133–135. doi: 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Iuvone PM. Is dopamine a light-adaptive or a dark-adaptive modulator in retina? Neurochem. Int. 1992;20:193–199. doi: 10.1016/0197-0186(92)90167-p. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Rubimm NM, Iuvonem P,M. Regulation of endogenous dopamine release in amphibian retina by melatonin: the role of GABA. Vis. Neurosci. 1994;11:1013–1018. doi: 10.1017/s0952523800003941. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Hoel MJ, Iuvone PM. Stimulation of endogenous dopamine release and metabolism in amphibian retina by light- and K+-evoked depolarization. Brain Res. 1989;482:164–168. doi: 10.1016/0006-8993(89)90555-6. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J. Neurosci. 1991;11:2959–2971. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Rhythmic regulation of retinal melatonin: metabolic pathways, neurochemical mechanisms, and the ocular circadian clock. Cell Mol. Neurobiol. 1991;11:529–560. doi: 10.1007/BF00734814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Haque R, Pozdeyev N, Jackson CR, Iuvone PM. Temporal coupling of cyclic AMP and Ca/calmodulin-stimulated adenylyl cyclase to the circadian clock in chick retinal photoreceptor cells. J. Neurochem. 2006a;99:1142–1150. doi: 10.1111/j.1471-4159.2006.04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Pozdeyev N, Haque R, Visser A, Ivanova TN, Iuvone PM. Circadian clockwork machinery in neural retina: evidence for the presence of functional clock components in photoreceptor-enriched chick retinal cell cultures. Mol. Vis. 2006b;12:215–223. [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S, O'Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 1992;89:12093–12097. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. The dopaminergic amacrine cell. J. Comp. Neurol. 1990;301:461–489. doi: 10.1002/cne.903010310. [DOI] [PubMed] [Google Scholar]
- Dorenbos R, Contini M, Hirasawa H, Gustincich S, Raviola E. Expression of circadian clock genes in retinal dopaminergic cells. Vis. Neurosci. 2007;24:573–580. doi: 10.1017/S0952523807070538. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Masana MI, Iacob S, Sauri DM. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn Schmiedebergs Arch. Pharmacol. 1997;355:365–375. doi: 10.1007/pl00004956. [DOI] [PubMed] [Google Scholar]
- Foster RG, Hankins MW. Circadian vision. Curr. Biol. 2007;17:R746–751. doi: 10.1016/j.cub.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Fujieda H, Scher J, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM. Dopaminergic and GABAergic amacrine cells are direct targets of melatonin: immunocytochemical study of mt1 melatonin receptor in guinea pig retina. Vis. Neurosci. 2000;17:63–70. doi: 10.1017/s0952523800171068. [DOI] [PubMed] [Google Scholar]
- Fujieda H, Scher J, Lukita-Atmadja W, Brown GM. Gene regulation of melatonin and dopamine receptors during eye development. Neuroscience. 2003;120:301–307. doi: 10.1016/s0306-4522(03)00298-7. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Tosini G. Analysis of daily and circadian gene expression in the rat pineal gland. Neurosci. Res. 2008;60:192–198. doi: 10.1016/j.neures.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich JA, Caron MG. Recent advances in the molecular biology of dopamine receptors. Annu. Rev. Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- Humphries A, Klein D, Baler R, Carter DA. cDNA array analysis of pineal gene expression reveals circadian rhythmicity of the dominant negative helix-loop-helix protein-encoding gene. J. Neuroendocrinol. 2002;14:101–108. doi: 10.1046/j.0007-1331.2001.00738.x. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Galli CL, Neff NH. Retinal tyrosine hydroxylase: comparison of short-term and long-term stimulation by light. Mol. Pharmacol. 1978;14:1212–1219. [PubMed] [Google Scholar]
- Iuvone PM, Gan J. Functional interaction of melatonin receptors and D1 dopamine receptors in cultured chick retinal neurons. J.Neurosci. 1995;15:2179–2185. doi: 10.1523/JNEUROSCI.15-03-02179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog. Retin. Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kim JS, Bailey MJ, Weller JL, Sugden D, Rath MF, Møller M, Klein DC. Pineal Drd4 Expression: Daily rhythm is controlled by an adrenergic mechanism involving a thyroid hormone/cAMP operated “AND” gate. unpublished. submitted. [Google Scholar]
- Klein DC, Weller JL. Indole metabolism in the pineal gland: a circadian rhythm in N-acetyltransferase. Science. 1970;169:1093–1095. doi: 10.1126/science.169.3950.1093. [DOI] [PubMed] [Google Scholar]
- Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriguez IR, Bégay V, Falcón J, Cahill GM, Cassone VM, Baler R. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog. Horm. Res. 1997;52:307–357. [PubMed] [Google Scholar]
- Kramer SG. Dopamine: A retinal neurotransmitter. I. Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo. Invest. Ophthalmol. 1971;10:438–452. [PubMed] [Google Scholar]
- Luft WA, Iuvone PM, Stell WK. Spatial, temporal, and intensive determinants of dopamine release in the chick retina. Vis. Neurosci. 2004;21:627–635. doi: 10.1017/S0952523804214110. [DOI] [PubMed] [Google Scholar]
- Megaw PL, Boelen MG, Morgan IG, Boelen MK. Diurnal patterns of dopamine release in chicken retina. Neurochem. Int. 2006;48:17–23. doi: 10.1016/j.neuint.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Melamed E, Frucht Y, Lemor M, Uzzan A, Rosenthal Y. Dopamine turnover in rat retina: a 24-hour light-dependent rhythm. Brain Res. 1984;305:148–151. doi: 10.1016/0006-8993(84)91130-2. [DOI] [PubMed] [Google Scholar]
- Møller M, Baeres FMM. The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res. 2002;309:139–150. doi: 10.1007/s00441-002-0580-5. [DOI] [PubMed] [Google Scholar]
- Møller M, Osgaard O, Grønbech-Jensen M. Influence of sympathectomy in humans on the rhythmicity of 6-sulphatoxymelatonin urinary excretion. Mol. Cell. Endocrinol. 2006;252:40–45. doi: 10.1016/j.mce.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Chanut E, Versaux-Botteri C, Simon A, Trouvin JH. Dopamine inhibits melatonin synthesis in photoreceptor cells through a D2-like receptor subtype in the rat retina: biochemical and histochemical evidence. J. Neurochem. 1996;67:2514–2520. doi: 10.1046/j.1471-4159.1996.67062514.x. [DOI] [PubMed] [Google Scholar]
- Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M, Iuvone PM. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J. Neurosci. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce ME, Besharse JC. Circadian regulation of retinomotor movements I: interaction of melatonin and dopamine in control of cone length. J. Gen. Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozdeyev NV, Lavrikova EV. Diurnal changes of tyrosine, dopamine, and dopamine metabolites content in the retina of rats maintained at different lighting conditions. J. Mol. Neursci. 2000;15:1–9. doi: 10.1385/JMN:15:1:1. [DOI] [PubMed] [Google Scholar]
- Pozdeyev N, Taylor C, Haque R, Chaurasia SS, Visser A, Thazyeen A, Du Y, Fu H, Weller J, Klein DC, Iuvone PM. Photic regulation of arylalkylamine N-acetyltransferase binding to 14-3-3 proteins in retinal photoreceptor cells. J. Neurosci. 2006;26:9153–9161. doi: 10.1523/JNEUROSCI.1384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozdeyev N, Tosini G, Li L, Ali F, Rozov S, Lee RH, Iuvone PM. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur. J. Neurosci. 2008;27:2691–2700. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, Tosini G. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur. J. Neurosci. 2005;22:3129–3136. doi: 10.1111/j.1460-9568.2005.04512.x. [DOI] [PubMed] [Google Scholar]
- Scher J, Wankiewicz E, Brown GM, Fujieda H. MT (1) melatonin receptor in the human retina: expression and localization. Invest. Ophthalmol Vis. Sci. 2002;43:889–897. [PubMed] [Google Scholar]
- Scher J, Wankiewicz E, Brown GM, Fujieda H. AII amacrine cells express the MT1 melatonin receptor in human and macaque retina. Exp. Eye. Res. 2003;77:375–382. doi: 10.1016/s0014-4835(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Steinle JJ, Krizsan-Agbas D, Smith PG. Regional regulation of choroidal blood flow by autonomic innervation in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R202–R209. doi: 10.1152/ajpregu.2000.279.1.R202. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kobayashi K, Nagatsu T. Genomic structure and tissue distribution of the mouse dopamine D4 receptor. Neurosci. Lett. 1995;199:69–72. doi: 10.1016/0304-3940(95)12021-u. [DOI] [PubMed] [Google Scholar]
- Tosini G, Dirden JC. Dopamine inhibits melatonin release in the mammalian retina: in vitro evidence. Neurosci. Lett. 2000;286:119–122. doi: 10.1016/s0304-3940(00)01117-4. [DOI] [PubMed] [Google Scholar]
- Tosini G, Fukuhara C. Photic and circadian regulation of retinal melatonin in mammals. J. Neuroendocrinol. 2003;15:364–369. doi: 10.1046/j.1365-2826.2003.00973.x. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21:3866–3871. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivien-Roels B, Pevet P, Dubois MP, Arendt J, Brown GM. Immunohistochemical evidence for the presence of melatonin in the pineal gland, the retina and the Harderian gland. Cell Tissue Res. 1981;217:105–115. doi: 10.1007/BF00233830. [DOI] [PubMed] [Google Scholar]
- Weidman TA, Kuwabara TOIC. Development of the rat retina. Invest. Ophthalmol. Vis. Sci. 1969;8:60–69. [PubMed] [Google Scholar]
- White MP, Fisher LJ. Effects of exogenous melatonin on circadian disc shedding in the albino rat retina. Vision Res. 1989;29:167–179. doi: 10.1016/0042-6989(89)90122-3. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Ziong-Li Y, Wu SM, Hollyfield JG. Melatonin enhances horizontal cell sensitivity in salamander retina. Brain Res. 1988;453:377–380. doi: 10.1016/0006-8993(88)90182-5. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc. Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Zawilska J, Iuvone PM. Catecholamine receptors regulating serotonin N-acetyltransferase activity and melatonin content of chicken retina and pineal gland: D2-dopamine receptors in retina and alpha-2 adrenergic receptors in pineal gland. J. Pharmacol. Exp. Ther. 1989;250:86–92. [PubMed] [Google Scholar]
- Zawilska JB, Nowak JZ. Dopamine receptor regulating serotonin N-acetyltransferase activity in chick retina represents a D4-like subtype: pharmacological characterization. Neurochem. Int. 1994;24:275–280. doi: 10.1016/0197-0186(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Bednarek A, Berezinska M, Nowak JZ. Rhythmic changes in metabolism of dopamine in the chick retina: the importance of light versus biological clock. J. Neurochem. 2003;84:717–724. doi: 10.1046/j.1471-4159.2003.01559.x. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Lorenc A, BerezĺDnska M, Vivien-Roels B, Pévet P, Skene DJ. Photoperiod-dependent changes in melatonin synthesis in the turkey pineal gland and retina. Poult. Sci. 2007;86:1397–1405. doi: 10.1093/ps/86.7.1397. [DOI] [PubMed] [Google Scholar]