Abstract
OBJECTIVE
Intrauterine growth restricted newborns (IUGR) have increased risk of obesity-induced “fatty liver” and inflammation. We hypothesized that IUGR-induced inhibition of hepatic peroxisome proliferator activated receptors (PPARs) is associated with an increased inflammatory response.
STUDY DESIGN
Rat Control dams received ad libitum food, whereas study dams were 50% food-restricted from pregnancy day 10 to 21 (IUGR). Pups were nursed by Control dams and weaned to ad libitum feed. Hepatic protein expression of transcription factors, lipid enzymes, triglyceride content and CRP levels were analyzed in 1 day and 9 month old male offspring.
RESULTS
At 1d of age, IUGR pups showed downregulation of PPARγ and PPARα, upregulation of hepatic lipase and CRP. At 9 months of age, IUGR exhibited continued downregulation of PPARγ and PPARα with upregulation of SREBP1 and fatty acid synthase. Furthermore, IUGR adults had increased hepatic triglyceride content and plasma CRP levels.
CONCLUSIONS
The results suggest that developmental hepatic dysregulation may contribute to programmed obesity-induced inflammation in IUGR offspring.
Keywords: Sterol regulatory element-binding proteins, fatty acid synthase, hepatic lipase, fatty liver, C-reactive protein
INTRODUCTION
Intrauterine growth restricted newborns (IUGR) have increased risk of adult metabolic syndrome which is not only associated with obesity and lipid abnormalities, but also with “fatty liver” and inflammation.1,2 The phenomenon of fatty liver, also known as steatosis, occurs due to chronic hepatocyte accumulation of lipids which potentially leads to inflammation.3,4 Though the underlying molecular mechanisms involved remain unclear, obesity and insulin resistance are known to play a major role in the development of fatty liver.4,5 Further, as obesity represents a state of chronic low-level inflammation, lipid transcription factors have recently been implicated in this process.6,7
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors involved in the regulation of lipid metabolism, and lipid-associated inflammatory response.6,7 PPARα in particular is predominantly expressed in the liver and regulates genes involved in fatty acid oxidation,8 whereas PPARγ is mainly detected in the adipose tissue and known to trigger adipocyte differentiation and promote lipid storage.9 Though PPARγ is expressed at very low levels in the liver, PPARγ agonist can ameliorate non-alcohol fatty liver disease in a rat model.10 In addition, PPARα and PPARγ modulate the inflammatory response. PPAR activators have been shown to exert anti-inflammatory activities in various cell types by inhibiting the expression of acute-phase proteins, such as, C-reactive protein (CRP).11,12 CRP is produced by hepatocytes in response to tissue injury, infection, and inflammation and is moderately elevated in obesity, metabolic syndrome, diabetes, and fatty liver.13
A third transcription factor that participates in the development of fatty liver is sterol regulatory element-binding proteins (SREBP) which specifically regulates hepatic lipogenesis14; its activation results in the induction of genes for enzymes that are involved in the biosynthesis of fatty acids and triglycerides.15 Lipogenic fatty acid synthase is the key enzyme of de novo fatty acid synthesis and its expression is stimulated by insulin and glucose,16 whereas hepatic lipase is a lipolytic enzyme that hydrolyzes triglycerides and lipoproteins, thus facilitating plasma lipid metabolism as well as cellular lipid uptake.17
We have previously shown that nutrient restriction in pregnancy results in IUGR pups which develop metabolic syndrome as evidenced by increased body fat, insulin resistance and elevated plasma triglyceride levels.18,19 In view of the role of PPAR transcription factors in regulating lipid metabolism and inflammation, we hypothesized that IUGR-induced obesity inhibits the expression of hepatic PPARα and PPARγ, promotes lipid deposition and increases inflammatory response.
MATERIAL AND METHODS
Maternal Rat Diets
Studies were approved by the Animal Research Committee of the Los Angeles BioMedical Research Institute at Harbor-UCLA (LABioMed), and were in accordance with the American Association for Accreditation of Laboratory Animal Care (AALC), and National Institutes of Health (NIH) guidelines. The rat model utilized for maternal food restriction during pregnancy and lactation has been previously described.18 Briefly, first time pregnant Sprague Dawley rats (Charles River Laboratories, Hollister, California) were housed in a facility with constant temperature and humidity and controlled 12:12 hour light/dark cycle. At 10 days of gestation, rats were provided either an ad libitum (AdLib; n=12) diet of standard laboratory chow (Lab Diet 5001, Brentwood, MO; protein, 23%; fat, 4.5%; metabolizable energy, 3030kcal/kg), or a 50% food restricted diet (IUGR; n=6) determined by quantification of normal intake in ad libitum fed rats. The respective diets were given from day 10 of gestation to term (21 days).
Offspring
At day 1 after birth, pups were culled to 8 (4 males and 4 females) per litter to normalize rearing. Following birth, both control and IUGR offspring were cross fostered and nursed by ad libitum fed dams. At three weeks of age, all offspring were housed individually, and weaned to ad libitum standard laboratory chow until 9 months of age. Male pups were sacrificed at 1 day (decapitation) and at 9 months of age (pentobarbital 200 mg/kg ip). Blood and liver samples were collected from 1 male from each of 6 litters in both Control and IUGR groups. Liver lobes were removed and frozen at -80°C until processing. Food intake, body weights, body fat and plasma lipids have been previously reported.18,19,20 We elected to study males, as females would have required estrus assessment since estrogen is known to affect adiposity and lipid metabolism.21,22
Plasma CRP
Helica rat C-reactive protein assay (951CRP01R, Helica Biosystems, Inc) was used to determine plasma levels of CRP according to manufacturer’s protocol.
Hepatic Triglyceride Content
Total hepatic triglyceride content was determined using a modification of previously described procedure.23,24 Briefly, liver (100∼200mg) was homogenized in 2.1 ml of chloroform/methanol (2:1) and incubated at 37°C for 40 minutes. Following incubation, 0.5 ml of water was added, and the mixture was stored at 4°C overnight. The lower phase was collected and 0.83 volume of a 47:3:48 mixture of water/chloroform/methanol was added and stored at 4°C overnight. The organic phase was collected and dried under nitrogen gas and, heated at 90°C for 10 minutes. The samples were dissolved in 250μl of 2-propanol and centrifuged at 10,000g for 3 minutes. The resulting supernatant was assayed for total triglyceride (Sigma, Sigma Chemical Company, St. Louis, MO). Data is expressed as mg per grams of liver.
Western Blot
Primary antibodies were obtained, diluted 1:500 and the band density was analyzed as indicated: PPARα (Cayman, 101700; 54 KD), PPARγ (Santa Cruz, SC-8994; 67 KD), SREBP1 (Santa Cruz, SC-8984; Immature, 125KD; mature, 60KD), fatty acid synthase (Santa Cruz, SC-20140; 270KD), hepatic lipase (Santa Cruz, SC-21007; 57 KD), CRP (Santa Cruz, SC-30047) and β-actin (Sigma, A-5441; 40 KD). Secondary horse radish peroxidase conjugated antibodies (1:1000) were anti-rabbit (Bio-Rad 170-6515; used for all primary antibody reactions except β-actin), and anti-mouse (Bio-Rad 170-6515; used for β-actin).
Protein was extracted in RIPA lysis buffer [25 mM Tris·HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS] containing protease inhibitors (HALT inhibitor cocktail, PIERCE, Rockford, IL). Supernatant protein concentration was determined by BCA solution (PIERCE).
Equal amounts of protein (50μg) were mixed with SDS sample buffer, boiled for 3 minutes and separated on a 7.5% or 10% polyacrylamide gel. The separated proteins were transferred electrophoretically to pure nitrocellulose membrane (BIO-RAD Hercules, CA). Non-specific antibody binding was blocked by incubation for 1 hour at room temperature with 5% non-fat dry milk in Tris buffered saline solution (TBST) 0.1% tween 20. The membrane was then incubated with the appropriate primary antibody in 5% milk in TBST overnight at 4°C and washed 3 times for 10 minutes each with TBST at room temperature. Anti-rabbit or anti-mouse IgG secondary antibody labeled with horseradish peroxidase (Bio-Rad Laboratories 1:2000) in 5% milk was added onto the membrane and incubated for 1 hour at room temperature. The membrane was washed 3 times. SuperSignal West Pico Chemiluminescent Substrate (PIERCE) was used to detect the targeted protein. The band density was analyzed by Alpha DigiDoc Gel Documentation & Image Analysis System (Alpho Innotech Corparation San Leandro, CA). Data presented is normalized to β-actin and expressed as fold change.
Statistical Analysis
Differences between Control and IUGR groups were compared by unpaired t-test. Values are expressed as means ± SE. Statistical analysis in all cases included n=6 in each group at each age. For clarity purposes, figures for Western blot shows 2 representative bands, depicting the highest and the lowest band intensity in each group.
RESULTS
The phenotypic data for this model has been previously reported.18,19,20 Briefly, at 1 day of age, IUGR newborns had lower body weights with proportionate reduction in liver wet weight. Additionally, the IUGR pups had significantly lower blood glucose and plasma triglyceride levels as compared with Control pups. At 9 months of age, IUGR males were significantly heavier, had increased body fat and demonstrated insulin resistance with higher plasma triglycerides levels.
Hepatic Transcription Factors
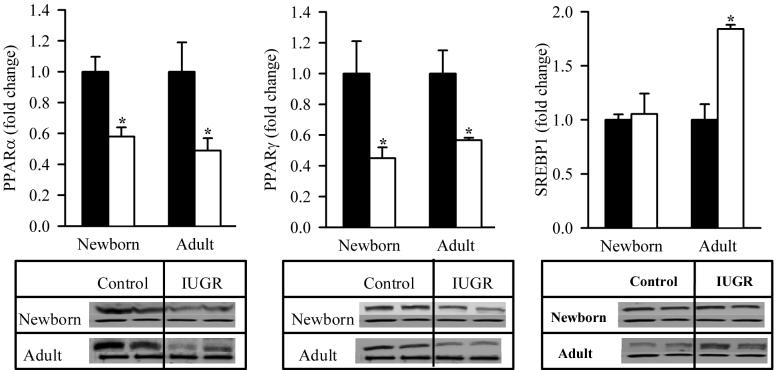
At 1 day of age, IUGR pups showed significantly decreased protein expression of PPARα (0.6-fold) and PPARγ (0.5-fold). At 9 months of age, IUGR males showed continued downregulation of both PPAR transcription factors. Furthermore, at 1 day of age, IUGR pups had comparable SREBP1 expression to Controls though by 9 months of age, it was significantly upregulated (Figure 1).
Figure 1. Protein Expression of Hepatic Transcription Factors.
Hepatic protein expression of PPARα, PPARγ and SREBP1 (upper band) in male offspring from Control (■) and IUGR (□) groups. Data was normalized to β-actin and presented as fold difference. β-actin (lower band) was comparable between IUGR and Control offspring at both ages. The number of animals studied per group per age was 4 males from 4 litters. *P <0.01 vs. Control offspring.
Hepatic Lipids
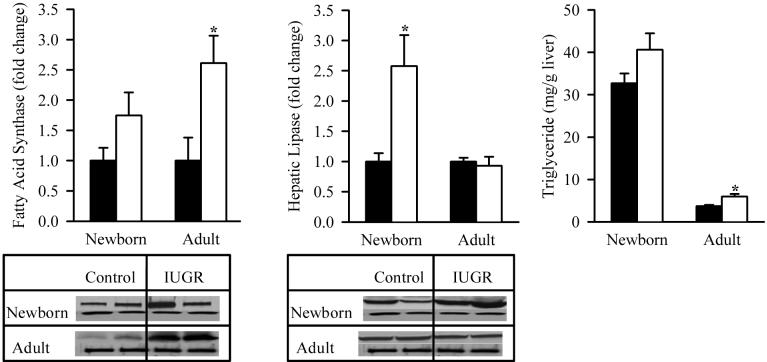
The lipogenic enzyme, fatty acid synthase, demonstrated similar changes to lipogenic transcription factor, SREBP1. At 1 day of age, IUGR pups showed no change in fatty acid synthase expression which was significantly increased by 9 months of age as compared to the Control. In contrast, the hepatic lipase expression in IUGR offspring was significantly upregulated at 1 day though not at 9 months of age. Analogous to the changes in hepatic lipogenic indexes, IUGR males at 1 day of age had comparable hepatic triglyceride content to Controls though it was significantly increased by 9 month of age (Figure 2).
Figure 2. Protein Expression of Hepatic Lipid Enzymes and Hepatic Triglyceride Content.
Hepatic protein expression of fatty acid synthase and hepatic lipase (upper band), and hepatic triglyceride content in male offspring from Control (■) and IUGR (□) groups. Data was normalized to β-actin and presented as fold difference. β-actin (lower as band) fold was comparable between IUGR and Control offspring at both ages. The number of animals studied per group per age was 4 males from 4 litters. *P <0.01 vs. Control offspring.
CRP
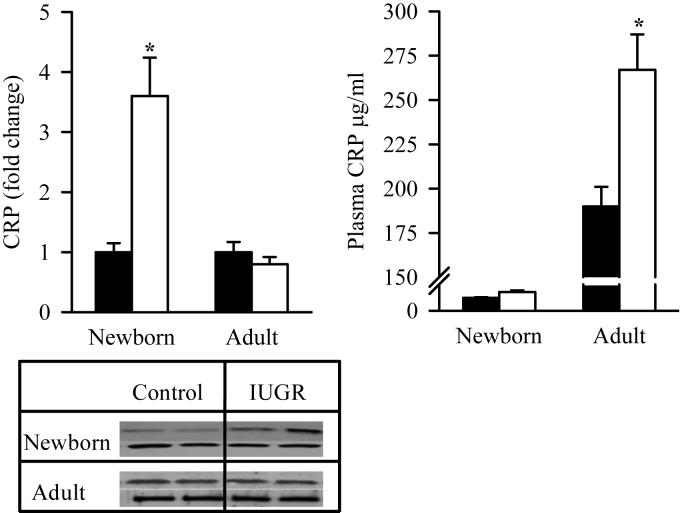
At 1 day of age, IUGR offspring exhibited significantly increased protein expression of hepatic CRP as compared to the Control. However at 9 months of age, hepatic CRP expression was similar in IUGR and Control males. Conversely, the changes in plasma CRP levels paralleled the changes seen in hepatic triglyceride content in IUGR offspring. At 1 day of age, no differences were noted in plasma CRP levels, however by 9 months of age IUGR males had significantly increased levels as compared to Control (Figure 3).
Figure 3. Protein Expression of Hepatic CRP and Plasma CRP Levels.
Hepatic protein expression of CRP (upper band) and plasma CRP levels in male offspring from Control (■) and IUGR (□) groups. Data was normalized to β-actin and presented as fold difference. β-actin (lower band) was comparable between IUGR and Control offspring at both ages. The number of animals studied per group per age was 4 males from 4 litters. *P <0.001 vs. Control offspring.
COMMENT
The findings of this study demonstrate a potential role of transcription factor (PPAR) in the pathogenesis of hepatic lipid dysregulation and inflammation in IUGR offspring. Notably, the hepatic transcription factors that modulate inflammation are downregulated in IUGR newborns and this trend persists in the adult IUGR offspring. Further, the relative upregulation of hepatic SREBP1 and fatty acid synthase favors the expression of lipogenic pathways, which leads to increased lipid synthesis and deposition. Thus, developmental hepatic dysregulation leads to programmed obesity-induced inflammation in IUGR offspring. It should be noted that only male newborns were examined due to the confounding effects of estrogen on adiposity and lipid metabolism.21,22
In the present study maternal food-restriction during pregnancy results in IUGR newborns with decreased protein expression of hepatic PPARα and PPARγ, increased expression of hepatic lipase and CRP, and no change seen in SREBP1 and fatty acid synthase. These results indicate that factors modulating inflammation are altered prior to the development of adiposity and hepatic steatosis. The reduced expression of hepatic PPARα and PPARγ is consistent with increased expression of hepatic CRP and normal hepatic triglyceride content. Studies have confirmed the anti-inflammatory responses of PPARα and PPARγ.4,12 This has been shown to occur via inhibition of inflammatory gene expression or via reduced secretion of cytokines and chemokines.7 Also, PPAR agonists are known to reduce circulating levels of inflammatory markers.4,7 In addition, PPARα prevents hepatic lipid storage by upregulating the expression of genes involved in fatty acid oxidation.5 Studies in mice fed a high-fat diet show that PPARα reduces hepatic steatosis by inducing mitochondrial, peroxisomal, and microsomal fatty acid oxidation.25 In contrast, PPARγ is known to facilitate lipogenesis by induction of SREBP1 which in turn activates fatty acid synthase.9 The increased expression of hepatic lipase seen in our study suggests increased fatty acid uptake by the liver though this would be dependent upon substrate availability. We have previously shown that IUGR newborns have decreased plasma triglycerides at 1 day of age.19 Thus, the results of the present study, in conjunction with the known effects of PPARα on fatty acid oxidation25 suggest that reduced fatty acid oxidation in concert with reduced lipogenesis prevents hepatic steatosis while enhancing inflammatory responses in IUGR newborns. Further, it is unclear if hepatic PPARα and PPARγ are regulated at a transcriptional or post-transcriptional level. It is believed that changes at the protein level would be most relevant to any potential impact on programmed inflammation. Transcriptional changes are not always evident in protein changes and post-transcriptional modification or degradation may also play a role.
When IUGR newborns are provided normal nursing and post-weaning diet, the adult offspring show continued downregulation of PPARα and PPARγ though now with new upregulation of lipogenic factor (SREBP1) and enzyme (fatty acid synthase). This is paralleled by increased hepatic triglyceride content in conjunction with increased plasma levels of CRP. The persistent reduced expression of hepatic PPARα and PPARγ is again consistent with increased plasma CRP levels though not with hepatic CRP expression. The apparent rationale for the dichotomy between liver and plasma CRP levels is unclear at this stage. However, since adipose tissue is also known to produce CRP, it is likely that in IUGR adult offspring, both liver and adipose tissue contribute to the circulating plasma CRP levels. In view of the fact that IUGR adult offspring are obese, the increased body fat may be a major source of elevated plasma CRP levels. Human studies have shown that in severely obese patients, plasma CRP levels are not a good diagnostic predictor of non-alcoholic steatohepatitis.26 The relative upregulation of both hepatic SREBP1 and fatty acid synthase in IUGR adult offspring favor the expression of lipogenic pathways, and thus increased hepatic lipid synthesis and deposition. Indeed, there is a concomitant increase in hepatic triglyceride content. However, despite decreased expression of PPARγ, SREBP1 gene expression is increased, which may be due to the combined effects of transcriptional signaling at the SREBP1c promoter as well as its post-transcriptional regulation. In addition to PPARγ, both insulin and liver X receptor alpha are known to induce SREBP1c transcription.16 Notably, these changes are seen in the adult IUGR offspring that are obese and exhibit elevated plasma triglyceride and insulin levels.18,19 Thus in IUGR adult offspring, increased hepatic lipogenesis with likely reduced fatty acid oxidation (though this needs further confirmation) contribute to hepatic steatosis. To demonstrate more pronounced manifestations of programmed hepatic steatosis future studies on IUGR offspring receiving a high fat diet may be required.
Studies in humans and animals have highlighted the influencial role of early nutrition on lipid metabolism.27-31 Barker et al32 have suggested that nutritionally impaired growth, particularly of the liver, during late gestation could result in permanent changes in lipid metabolism that persist until adult life. Recent studies on small-for gestational age infants report increased prevalence of abnormal lipid metabolism.33 Similarly, animal studies report enhanced hepatic lipogenesis due to increased expression of SREBP134,35 and fatty acid synthase36 in adult offspring of maternally protein/nutrient restricted dams. Numerous studies have further shown the association of increased SREBP1 expression with fatty liver.37 These changes may be due to both hepatic based gene expression as well as signaling from other programmed tissues such as skeletal muscle and adipose.
In conclusion, these results demonstrate that IUGR offspring exhibit reduced expression of hepatic PPARγ and PPARα which may be associated with the elevated hepatic CRP levels and triglyceride content. These findings suggest that developmental hepatic dysregulation may contribute to programmed obesity-induced inflammation in IUGR offspring.
Acknowledgments
Financial support:
This work was supported by the National Institutes of Health K01 DK 063994, LABioMed/Seed Research Grant 405001and NIH/RIMI 5P20MD000545
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONDENSATION
Nutrient-restriction during pregnancy results in IUGR newborns with reduced expression of hepatic PPARα and PPARγ which may be associated with elevated hepatic CRP levels and triglyceride content.
This study was presented at the 28th Annual Clinical Meeting of the Society for Maternal-Fetal Medicine, Dallas, TX, January 31- February 2, 2008.
REFERENCES
- 1.Desai M, Babu J, Ross MG. Programmed metabolic syndrome: prenatal undernutrition and postweaning overnutrition. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2306–14. doi: 10.1152/ajpregu.00783.2006. [DOI] [PubMed] [Google Scholar]
- 2.Roberts A, Nava S, Bocconi L, Salmona S, Nicolini U. Liver function tests and glucose and lipid metabolism in growth-restricted fetuses. Obstet Gynecol. 1999;94:290–4. doi: 10.1016/s0029-7844(99)00235-5. [DOI] [PubMed] [Google Scholar]
- 3.Leclercq IA. Pathogenesis of steatohepatitis: insights from the study of animal models. Acta Gastroenterol Belg. 2007;70:25–31. [PubMed] [Google Scholar]
- 4.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradbury MW. Lipid Metabolism and Liver Inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G194–8. doi: 10.1152/ajpgi.00413.2005. [DOI] [PubMed] [Google Scholar]
- 6.Anghel SI, Wahli W. Fat poetry: a kingdom for PPARγ. Cell Research. 2007;17:486–511. doi: 10.1038/cr.2007.48. [DOI] [PubMed] [Google Scholar]
- 7.Moller DE, Berger JP. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation. Intern J Obesity. 2003;27:S17–21. doi: 10.1038/sj.ijo.0802494. [DOI] [PubMed] [Google Scholar]
- 8.Berger J, Moller DE. The mechanism of action of PPARs. Ann Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 9.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–14. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 10.Seo YS, Kim JH, Jo NY, Choi KM, Baik SH, Park JJ, Kim JS, Byun KS, Bak YT, Lee CH, Kim A, Yeon JE. PPAR agonists treatment is effective in a nonalcoholic fatty liver disease animal model by modulating fatty-acid metabolic enzymes. J Gastroenterol Hepatol. 2008;23:102–9. doi: 10.1111/j.1440-1746.2006.04819.x. [DOI] [PubMed] [Google Scholar]
- 11.Watkins SM, Reifsnyder PR, Pan H, German BJ, Leiter EH. Lipid metabolome-wide effects of the PPARγ agonist rosiglitazone. J Lipid Res. 2002;43:1809–17. doi: 10.1194/jlr.m200169-jlr200. [DOI] [PubMed] [Google Scholar]
- 12.Kleemann R, Verschuren L, Rooij BJ, Lindeman J, Maat MM, Szalai AJ, Princen HM, Kooistra T. Evidence for anti-inflammatory activity of statins and PPAR-activators in human C-reactive protein transgenic mice in vivo and in cultured human hepatocytes in vitro. Blood. 2004;103:4188–94. doi: 10.1182/blood-2003-11-3791. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Koike T, Ichikawa T, Hatakeyama K, et al. C-reactive protein in atherosclerotic lesions: its origin and pathophysiological significance. Am J Pathol. 2005;167:1139–48. doi: 10.1016/S0002-9440(10)61202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horton JD, Shimomura I, Ikemoto S, Bashmakov Y, Hammer RE. Overexpression of sterol regulatory element-binding protein-1a in mouse adipose tissue produces adipocyte hypertrophy, increased fatty acid secretion, and fatty liver. J Biol Chem. 2003;278:36652–60. doi: 10.1074/jbc.M306540200. [DOI] [PubMed] [Google Scholar]
- 15.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–332. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 16.Kim JB, Sarraf P, Wright M, et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest. 1998;101:1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santamarina-Fojo S, Navarro HG, Freeman L, Wagner E, Nong Z. Hepatic Lipase, Lipoprotein Metabolism, and Atherogenesis. Arterioscler Thromb Vasc Biol. 2004;24:1750–54. doi: 10.1161/01.ATV.0000140818.00570.2d. [DOI] [PubMed] [Google Scholar]
- 18.Desai M, Gayle DA, Jooby B, Ross MG. Programmed obesity in intrauterine growth restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–6. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 19.Desai M, Gayle D, Babu J, Ross MG. The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am J Obstet Gynecol. 2007;196:555.e1–e7. doi: 10.1016/j.ajog.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai M, Gayle D, Han G, Ross MG. Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine Growth Restricted Offspring. Reproductive Sciences. 2007;14:329–337. doi: 10.1177/1933719107303983. [DOI] [PubMed] [Google Scholar]
- 21.Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med. 2004;229:127–135. doi: 10.1177/153537020422901107. [DOI] [PubMed] [Google Scholar]
- 22.Homma H, Kurachi H, Nishio Y, Takeda T, Yamamoto T, Adachi K, Morishige K, Ohmichi M, Matsuzawa Y, Murata Y. Estrogen suppresses transcription of lipoprotein lipase gene. J Biol Chem. 2000;275:11404–11411. doi: 10.1074/jbc.275.15.11404. [DOI] [PubMed] [Google Scholar]
- 23.Inoue Y, Inoue J, Lambert G, Yim SH, Gonzalez FJ. Disruption of hepatic C/EBPalpha results in impaired glucose tolerance and agedependent hepatosteatosis. J Biol Chem. 2004;279:44740–8. doi: 10.1074/jbc.M405177200. [DOI] [PubMed] [Google Scholar]
- 24.Basso F, Freeman L, Knapper CL. Role of hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J Lipid Res. 2003;44:296–302. doi: 10.1194/jlr.M200414-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Matsusue K, Haluzik M, Lambert G, Yim S, et al. Liver-specific leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anty R, Bekri S, Luciani N, Saint-Paul MC, et al. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, Type 2 diabetes, and NASH. Am J Gastroenterol. 2006;10:1824–33. doi: 10.1111/j.1572-0241.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 27.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–7. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 28.Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15:183–7. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2000;72:1101–06. doi: 10.1093/ajcn/72.5.1101. [DOI] [PubMed] [Google Scholar]
- 30.Lucas A, Baker BA, Desai M, Hales CN. Nutrition in pregnant or lactating rats programs lipid metabolism in the offspring. Br J Nutr. 1996;76:605–12. doi: 10.1079/bjn19960066. [DOI] [PubMed] [Google Scholar]
- 31.Desai M, Hales CN. Role of fetal and infant growth in programming metabolism in later life. Biol Rev. 1997;72:329–48. doi: 10.1017/s0006323196005026. [DOI] [PubMed] [Google Scholar]
- 32.Barker DJP, Martyn CN, Osmond C, Hales CN, Fall CHD. Growth in utero and serum cholesterol concentrations in adult life. BMJ. 1993;307:1524–7. doi: 10.1136/bmj.307.6918.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Cui Y, Tong X, Ye H, Li S. Glucose and Lipid Metabolism in Small-for-Gestational-Age Infants at 72 Hours of Age. J Mat Fetal Invest. 1997;7:172–4. doi: 10.1210/jc.2006-1281. [DOI] [PubMed] [Google Scholar]
- 34.Erhuma A, Salter AM, Sculley DV, Langley-Evans SC, Bennett AJ. Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat. Am J Physiol Endocrinol Metab. 2007;292:E1702–14. doi: 10.1152/ajpendo.00605.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi GY, Tosh D, Garg A, Mansano R, Ross MG, Desai M. Gender specific programmed hepatic lipid dysregulation in intrauterine growth restricted offspring. Am J Obstet Gynecol. 2007;196:477.e1–e7. doi: 10.1016/j.ajog.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Thompson NM, Norman AM, Donkin SS, Shankar RR, Vickers MH, Miles JL, Breier BH. Prenatal and Postnatal Pathways to Obesity: Different Underlying Mechanisms, Different Metabolic Outcomes. Endocrinology. 2007;148:2345–54. doi: 10.1210/en.2006-1641. [DOI] [PubMed] [Google Scholar]
- 37.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]