Abstract
Neovascularization may contribute to functional recovery after neural injury. Combination treatment of stroke with a nitric oxide donor, DETA-NONOate and bone marrow stromal cells promote functional recovery. However, the mechanisms underlying functional improvement have not been elucidated. In this study, we tested the hypothesis that combination treatment upregulates Angiopoietin1 and its receptor Tie2 in the ischemic brain and bone marrow stromal cells, thereby enhances cerebral neovascularization after stroke. Adult wild type male C57BL/6 mice were intravenously administered PBS, bone marrow stromal cells 5×105, DETA-NONOate 0.4 mg/kg or combination DETA-NONOate with bone marrow stromal cells (n=12/group) after middle cerebral artery occlusion. Combination treatment significantly upregulated Angiopoietin-1/Tie2 and tight junction protein (occludin) expression, and increased the number, diameter and perimeter of blood vessels in the ischemic brain compared with vehicle control (mean ± SE, p<0.05). In vitro, DETA-NONOate significantly increased Angiopoietin-1/Tie2 protein (n=6/group) and Tie2 mRNA (n=3/group) expression in bone marrow stromal cells. DETA-NONOate also significantly increased Angiopoietin-1 protein (n=6/group) and mRNA (n=3/group) expression in mouse brain endothelial cells (p<0.05). Angiopoietin-1 mRNA (n=3/group) was significantly increased in mouse brain endothelial cells treated with DETA-NONOate in combination with bone marrow stromal cells conditioned medium compared with cells treated with bone marrow stromal cells conditioned medium or DETA-NONOate alone. Mouse brain endothelial cell capillary tube-like formation assays (n=6/group) showed that Angiopoietin-1 peptide, the supernatant of bone marrow stromal cells and DETA-NONOate significantly increased capillary tube formation compared to vehicle control. Combination treatment significantly increased capillary tube formation compared with DETA-NONOate treatment alone. Inhibition of Angiopoietin-1 significantly attenuated combination treatment-induced tube formation. Our data indicated that combination treatment of stroke with DETA-NONOate and bone marrow stromal cells promotes neovascularization, which is at least partially mediated by upregulation of the Angiopoietin-1/Tie2 axis.
Keywords: Angiopoietin-1/Tie2, bone marrow stromal cell, neovascularization, nitric oxide donor, stroke
The restoration of brain neovascularization after ischemia is a major therapeutic goal in stroke treatment. Reestablishment of functional microvasculature for stroke can be evoked by cellular and pharmacological approaches. As a potential therapeutic strategy, bone marrow stromal cells (BMSCs) when administered intravenously after stroke selectively migrate to the ischemic brain, and increase parenchymal vascular endothelial growth factor (VEGF) expression and promote angiogenesis and vascular stabilization (Li et al., 2001, Chopp and Li, 2002, Al-Khaldi et al., 2003, Chen et al., 2003, Zacharek et al., 2007). Enhancing the targeting of BMSCs to the injured brain significantly increases vascular density in the ischemic cortex and increases functional local cerebral blood flow (Shyu et al., 2008). Treatment of stroke with a nitric oxide (NO) donor, DETA-NONOate [(Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl) amino] diazen-1-ium-1, 2-diolate], as a pharmacological agent, promotes angiogenesis after stroke in rats (Zhang et al., 2001, Chen et al., 2005a, Chen et al., 2006, Zacharek et al., 2006). In earlier studies, both a single dose of 0.4 mg/kg DETA-NONOate and a dose of 5×105 BMSCs did not a significantly improve functional outcome after stroke. However, combination therapy with the sub-therapeutic dose of DETA-NONOate and the sub-therapeutic dose of BMSCs significantly improved functional outcome, indicative of an additive therapeutic effect both in mice (Cui et al., 2007) and in rats (Chen et al., 2004). The mechanism of combination treatment amplified restorative therapy possibly via upregulation of Stromal cell-Derived Factor 1 (SDF1) and its chemokine receptor 4 (CXCR4) and matrix metalloproteinases pathways and promotion the exogenous BMSC migration into the ischemic brain (Cui et al., 2007). In addition, combination treatment also increased the vessel perimeter and endothelial cell proliferation after stroke in young adult rats (Chen et al., 2004). However, whether combination treatment promotion cerebral neovascularization and vascular stabilization and the mechanisms underlying the brain vascular restoration after stroke have not been fully understood.
Angiopoietin-1 (Ang1) is a family of endothelial growth factors that function as ligands for the endothelial-specific receptor tyrosine kinase, Tie2. The Ang1/Tie2 pathway controls pericyte recruitment, endothelial survival, and is implicated in blood vessel formation and vascular stabilization (Iurlaro et al., 2003, Patan, 2004). Ang1-induced angiogenesis required endothelium-derived NO (Babaei et al., 2003). Our previous study showed that the Ang1/Tie2 pathway plays an important role in angiogenesis induced by BMSC transplantation alone (Zacharek et al., 2007) or DETA-NONOate treatment alone (Zacharek et al., 2006) after stroke in rats. In this study, we seek to extend our previous study to further determine whether the expression of the Ang1/Tie2 contributes to the cerebral neovascularization and vascular stabilization induced by a combination of DETA-NONOate and murine BMSCs in the treatment of experimental stroke in vivo and in vitro.
EXPERIMENTAL PROCEDURES
Middle cerebral artery occlusion (MCAo) and experimental groups
Adult wild type male C57BL/6J mice (22−25 g, Charles River, Wilmington, MA, USA) were employed in all experiments. All experimental procedures have been approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital. Every effort was made to minimize the number of animals used and their suffering. Transient (2.5 hours) monofilament right MCAo was performed by advancing a 6−0 surgical nylon suture (8.5−9.5 mm) with an expanded (heated) tip from the external carotid artery into the lumen of the internal carotid artery to block the origin of the MCA (Chen et al., 2005b). Body temperature is maintained constant using a heating pad. Mice were randomly divided into 4 groups (n=12/group) 24 hours after MCAo, and the following was intravenously administered via the tail vein: a) 0.2 ml PBS for control; b) 5×105 BMSCs (mouse GFP-BMSC, M-126, P9. Cognate Therapeutics, Inc. Baltimore, MD, USA) (Cui et al., 2007); c) DETA-NONOate (0.4 mg/kg in 0.2 ml of PBS; Alexis Biochemical, San Diego, CA, USA); d) Combination BMSCs (5×105) with DETA-NONOate (0.4 mg/kg). In this study, we selected sub-therapeutic doses of 0.4mg/kg DETA-NONOate (single injection) and 5×105 BMSC as the earlier studies.
Histological Assessment
Mice were sacrificed at 14 days after MCAo. The brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin (Chen et al., 2005b). A standard paraffin block was obtained from the center of the lesion (bregma −1 mm to +1 mm). A series of 6 μm thick sections were cut from the block and stained with hematoxylin and eosin (HE) for calculation of the volume of cerebral infarction for each group (Swanson et al. 1990).
Immunohistochemistry
Every 10th coronal section for a total 5 sections was used for immunohistochemical staining. Ang1, Tie2, von Willebrand factor (vWF, a marker of endothelial cells), alpha smooth muscle actin (αSMA, a marker of smooth muscle cells and pericytes) and tight junction protein (occludin) immunostaining were performed. Coronal brain sections were incubated first with antibodies against Ang1 (rabbit polyclonal lgG antibody, 1:2000, Abcam, Cambridge, MA, USA), vWF (goat polyclonal IgG antibody, 1:200, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), and αSMA (mouse monoclonal IgG antibody, 1:200, Santa Cruz), at 4°C overnight and then incubated with avidin-biotin-horseradish peroxidase complex and developed in 3’3’ diaminobenzidine tetrahydrochloride (DAB), respectively. For Tie2 and occludin, the sections were directly incubated with the antibody against Tie2 (rabbit polyclonal IgG antibody, 1:80, Santa Cruz) or occludin (mouse monoclonal IgG antibody, 1:200, Zymed, San Francisco, CA, USA) conjugated with cyanine-3 (Cy3, 1:200, Jackson Immunoresearch Laboratories, West Groove, PA, USA). Control experiments consisted of staining brain coronal tissue sections as outlined above, but the primary antibodies were omitted, as previously described (Li et al., 1998).
Double immunofluorescence staining
To specifically identify Ang1-reactive cells co-localized with endothelial cells, double immunofluorescence staining for Ang1 with vWF was employed. Antibodies against Ang1 (rabbit polyclonal IgG antibody, 1:2000, Abcam) with a monoclonal antibody against vWF (1:400, DAKO, Carpinteria, CA, USA) were used, respectively. FITC (Calbiochem) and CY3 (Jackson Immunoresearch) were used for double-label immunoreactivity. Each coronal section was first treated with the primary anti-Ang1 antibody with Cy3, and was then followed by anti-vWF with FITC staining. Control experiments consisted of staining brain coronal tissue sections as outlined above, but omitted the primary antibodies, as previously described (Li et al., 1998).
Quantitation
For lesion volume evaluation, the seven coronal brain sections were traced using a Global Lab Image analysis system (Data Translation, Marlboro, MA, USA). The indirect lesion area, in which the intact area of the ipsilateral hemisphere was subtracted from the area of the contralateral hemisphere, was calculated (Swanson et al. 1990). Lesion volume is presented as a volume percentage of the lesion compared with the contralateral hemisphere.
For quantitative measurements of Ang1, vascular density and perimeters and diameter, five slides from each brain, with each slide containing 8 fields from the ischemic border zone (between the ischemic core area and non-ischemic area, Fig.1.) (Chen et al., 2003) were digitized under a 20× or 40× objective (BX40; Olympus Optical, Tokyo, Japan) using a 3-CCD color video camera (Sony DXC-970MD, Japan) interfaced with a Micro Computer Imaging Device (MCID) software (Imaging Research, Saint Catharines, ON, Canada). For quantitative detection of Tie2 and occludin, the images were acquired using fluorescent microscopy (Axiophot2, HB0100 W/2, Carl Zeiss, New York, NY, USA) with a digital camera (C4742−95, Hamamatsu, Japan).
Fig.1.
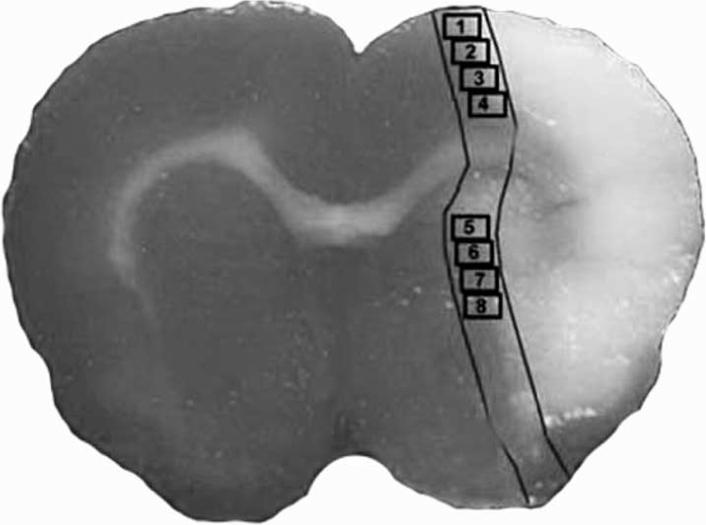
Schematic representation of a coronal brain section shows eight fields selected for quantitative measurements of immunochemical staining in the cortex.
The methods for analysis of morphological and functional features of cerebral vessels include measuring: (a) Profile density (NA), defined as the total number of vascular vessel profiles per unit area of tissue (number of vessels /mm2), NA reflects the neovascularization; (b) Diameter (D), defined by the mean luminal diameter of vessels (μm); (c) Perimeter (P), defined by the mean outer perimeter of the vessels (μm). Vascular diameter and perimeter reflect the vasodilation and vascular structural and functional remodeling (Delp et al., 2000, Manoonkitiwongsa et al., 2001). In this study, the percentage of Ang1, Tie2 and occludin positive areas and the number of vWF-positive or αSMA-positive vessels were quantified throughout each field of view. The total number of vessels was divided by the total tissue-area to determine vascular density. The perimeter of a total of 20 enlarged vWF-positive vessels or the diameter of αSMA-positive artery (mean diameter > 20μm) located in the ischemic border zone of the ischemic lesion were measured in each section using MCID software, respectively (Zhang et al., 2002b, Chen et al., 2003, Chen et al., 2005a).
Cell culture and co-culture
BMSCs and mouse brain endothelial cells (MBECs, CRL-2299, American Type Culture Collection, Manassas, VA) were cultured, as previously described (Cui et al., 2007). Passage 3−5 cells were used. BMSCs were treated with: 1) non-treatment for control; 2) + DETA-NONOate 0.4 μM, respectively. For real time PCR, one set of the experimental groups was terminated at 3 hours after treatment. Two additional sets of experimental groups were terminated at 24 hours after treatment for immunostaining or Western blot, respectively.
For the co-culture test, the conditioned medium from BMSCs was collected after 4 days of culture. MBECs were cultured with: 1) BMSC vehicle medium as the control; 2) conditioned medium from BMSCs; 3) BMSC vehicle medium + DETA-NONOate 0.4 μM; 4) conditioned medium from BMSCs + DETA-NONOate. For real time PCR, one set of the experimental groups was terminated at 3 hours after treatment, and the other experimental groups were terminated at 24 hours after treatment for immunostaining.
Immunocytochemistry
Immunostaining was performed with an antibody against Ang1 (1:2000, Abcam) or Tie2 (1:200, Santa Cruz) conjugated with Cy3 (1:200, Jackson Immunoresearch Laboratories). Nuclei were identified by DAPI (4’, 6-diamidino-2-phenyl-indde, dihydrochloride, 1:10000, Molecular Probes). The percentage of Ang1 or Tie2 positive cells was calculated using MCID software.
Real time polymerase chain reaction
Cells were collected and total RNA were isolated with TRIzol (Invitrogen, Carlsbad, CA, USA) following a standard protocol (Livak and Schmittgen, 2001). Quantitative polymerase chain reaction (PCR) was performed using the SYBR Green real time PCR method on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA) using 3-stage program parameters provided by the manufacturer. Each sample was tested in triplicate, and relative gene expression data was analyzed using the 2−ΔΔCT method. The following primers for real-time PCR were designed using Primer Express software (ABI). Ang1, Fwd: TAT TTT GTG ATT CTG GTG ATT; Rev: GTT TCG CTT TAT TTT TGT AATG. Tie2, Fwd: CGG CCA GGT ACA TAG GAG GAA; Rev: TCA CAT CTC CGA ACA ATC AGC. GAPDH, Fwd: AGA ACA TCA TCC CTG CAT CC; Rev: CAC ATT GGG GGT AGG AAC AC.
Western blot assay
Protein was isolated from the cultured BMSCs with TRIzol (Invitrogen) following standard protocol. Protein concentrations were determined by a DC protein assay kit (Bio-Rad, Hercules, CA, USA). Protein samples were electrophoresed on gradient sodium dodecyl sulfate–polyacrylamide gel (Bio-Rad) and subsequently electrotransferred to nitrocellulose membranes. Membranes were treated with blocking buffer (5% skimmed milk in 25 mM Tris-HCl pH 8.0, 125 mM NaCl, 0.1% Tween 20) for 1 hour at room temperature, followed by incubation with primary antibodies for anti-β-actin (1 : 2000; Sigma, St. Louis, MO, USA), anti-Ang1 antibody (1:500, Abcam) for 16 hours at 4°C. The mem branes were washed with blocking buffer without milk, and then incubated with horseradish peroxidase-conjugated secondary antibody in blocking buffer.
MBEC capillary-like tube formation assay
The methods employed for the MBEC capillary-like tube formation assay are as previous by described (Haralabopoulos et al., 1994). Briefly, 0.1 ml Matrigel (Becton Dickinson) was added per well to a 96 well plate, and then MBECs (2×104 cells) were added and incubated in: 1) BMSC vehicle medium as the control; 2) BMSC vehicle medium + Ang1 (200 ng/ml); 3) BMSC conditioned medium; 4) BMSC vehicle medium + DETA-NONOate (0.4 μM); 5) DETA-NONOate + BMSC conditioned medium; 6) DETA-NONOate + BMSC conditioned medium + anti-Ang1 antibody (1 μg/ml, Rabbit anti-Ang1 affinity purified polycolonal antibody, Chemicon) for 24 hours. All assays were performed in n=6/group. For quantitative measurements of capillary-like tube, Matrigel wells were digitized under a 10× objective (Olympus BX40) for measurement of total tube length of capillary-like tube using a video camera (Sony DXC-970MD) interfaced with the MCID image analysis system (Imaging Research, St. Catharines, Canada) at 6 hours after culture. Tracks of endothelial cells organized into networks of cellular cords (tubes) were counted and averaged in randomly selected 3 microscopic fields (Rikitake et al., 2002).
Statistical analysis
Independent Samples T-Test was used for testing the expression of Ang1 and Tie2 gene or protein expression between two groups. One-way ANOVA and Least Significant Difference (LSD) analysis after Post Hoc Test were used for testing the data in vivo and in vitro. The data are presented as mean ± SE. P < 0.05 is considered significant.
RESULTS
Treatments do not decrease cerebral ischemic lesion after stroke in mice
The infarction volume was not significantly decreased in the BMSC (15.01 ± 4.61%), DETA-NONOate (17.27% ± 5.12%) and combination treatment group mice (13.38% ± 3.61%) compared with the MCAo alone group (20.87% ± 6.44%).
Combination treatment increases ischemic brain neovascularization after stroke in mice
In this study, to test whether combination treatment of stroke increases ischemic neovascularization and vascular integrity, the number of vWF-positive blood vessels and αSMA-positive arteries, and the perimeter of vWF-positive vessels or the diameter of αSMA-positive arteries were counted and measured using MCID software. Fig. 2A-D show the vWF-immunoreactive positive vessels present in the ischemic border zone (A: MCAo alone; B: + BMSCs; C: + DETA-NONOate; D: Combination treatment). Fig. 2E and 2F show that the density (E) and the perimeter (F) of vWF-immunoreactive positive vessels significantly increased in the combination treatment group compared with the MCAo alone group after 14 days of stroke (P < 0.05). Fig. 2G-J show the αSMA-immunoreactive positive vessels present in the ischemic border zone (G: MCAo alone; H: + BMSCs; I: + DETA-NONOate; J: Combination treatment). Fig. 2K and 2L show the density (K) and the diameter (L) of αSMA-immunoreactive positive arteries significantly increased in the combination treatment group compared with MCAo alone group after 14 days of stroke (P < 0.05). In addition, the densities both vWF-positive vessels and αSMA-positive arteries are significantly increased in the combination treatment group compared with the DETA-NONOate monotherapy group, and the density of vWF-positive vessels in the combination group significantly increased compared with the BMSC monotherapy group (P < 0.05, Fig. 2E and 2K). These data indicate that combination treatment significantly enhances angiogenesis and arteriogenesis compared with the MCAo control group in the ischemic brain after stroke.
Fig.2.
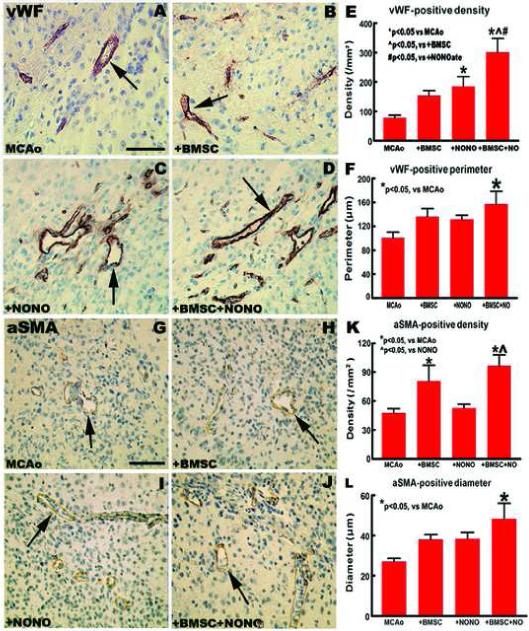
Combination DETA-NONOate with BMSCs treatment of stroke increases vascular density and diameter/perimeter in the ischemic brain in mice. (A-F) vWF-immunoreactive positive vessels present in the ischemic border zone at 14 days after MCAo (A: MCAo alone; B: MCAo + BMSCs; C: MCAo + DETA-NONOate; D: Combination treatment; E: Quantitative density data; F: Quantitative perimeter data). (G-J) αSMA-immunoreactive positive vessels present in the ischemic border zone at 14 days after MCAo (G: MCAo alone; H: MCAo + BMSCs; I: MCAo + DETA-NONOate; J: Combination treatment; K: Quantitative density data; L: Quantitative diameter data). Scale bar = 50 μm. n = 12 / group.
Combination treatment increases Ang1/Tie2 and occludin expression and promotes vascular stabilization after stroke in mice
The Ang1/Tie2 system promotes angiogenesis and vascular stability. Occludin is a tight junction protein, and an index of microvascular integrity (Mark and Davis, 2002). To identify the mechanism by which combination treatment increases vascular stabilization, Ang1/Tie2 and occludin expression were measured in the ischemic brain after stroke in mice. Fig. 3A-O show that DETA-NONOate combination treatment with BMSCs significantly increases Ang1 (A: MCAo alone; B: + BMSCs; C: + DETA-NONOate; D: Combination treatment; E: quantitative data of Ang1-immunoreactive positive cells), Tie2 (F: MCAo alone; G: + BMSCs; H: + DETA-NONOate; I: Combination treatment; J: quantitative data of Tie2-immunoreactive positive cells) and occludin (K: MCAo alone; L: + BMSCs; M: + DETA-NONOate; N: Combination treatment; O: quantitative data of occludin-immunoreactive positive cells) expression in the ischemic border area compared with MCAo control animals (P < 0.05), respectively.
Fig.3.
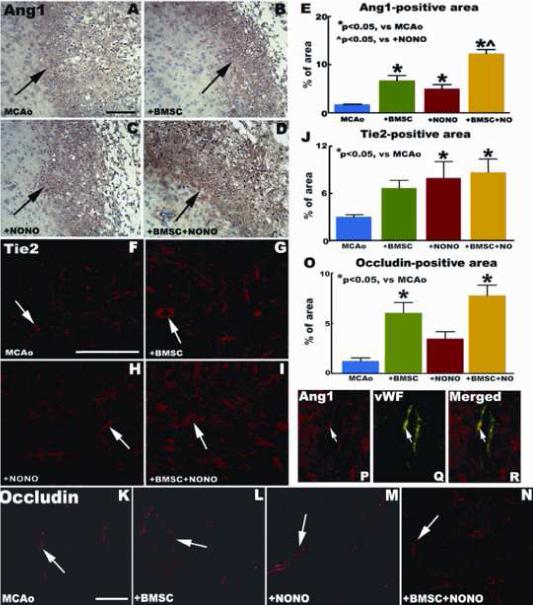
Combination DETA-NONOate with BMSCs treatment of stroke upregulates endogenous ischemic brain Ang1/Tie2 and occludin in mice. (A-E) show Ang1 immunohistochemical expression in MCAo alone (A), MCAo + BMSCs (B), MCAo + DETA-NONOate (C) and combination treatment (D) group in the ischemic border zone. (F-J) Tie2 immunofluorescent expression in MCAo alone (F), MCAo + BMSCs (G), MCAo + DETA-NONOate (H) and combination treatment (I) group in the ischemic border zone. (K-M) show occludin immunofluorescent expression in MCAo alone (K), MCAo + BMSCs (L), MCAo + DETA-NONOate (M) and combination treatment (N) group in the ischemic border zone. (P-R) Images of double immunofluorescent staining of (P) Ang1 (red) with (Q) vWF (green), and (R) Ang1 with vWF merged (yellow). Scale bar = 50 μm. n = 12 / group.
Our previous study showed that Ang1 positive cells primarily co-localize with glial fibrillary acidic protein (GFAP, an astrocyte marker) and αSMA (smooth muscle cells and pericytes) reactive cells. Tie2 positive cells primarily co-localize with vWF (endothelial cells) and αSMA reactive cells (Zacharek et al., 2007). In this study, we showed that Ang1 positive cells also co-localize with vWF reactive cells (Fig. 3P-R).
DETA-NONOate alone or combination with the conditioned medium from BMSCs Increase Ang1 gene and protein expression in BMSCs and MBECs
To test whether DETA-NONOate alone or combination with the conditioned medium from BMSCs up-regulates BMSC and MBEC Ang1/Tie2 expression, BMSCs and MBECs were cultured with DETA-NONOate (0.4 μM) alone or co-cultured with DETA-NONOate (0.4 μM) and the conditioned medium from BMSCs (the supernatant of 4 day incubation). Fig. 4A-D show DETA-NONOate significantly increased BMSC Ang1 protein expression (A: BMSC control; B: + DETA-NONOate; C: quantitative data of Ang1-immunoreactive positive cells; D: Ang1 protein tested by Western blot) compared to non-treatment control after 24 hours of culture (P < 0.05). Fig. 4E-H show DETA-NONOate significantly increased BMSC Tie2 protein and mRNA expression (E: BMSC control; F: + DETA-NONOate; G: quantitative data of Tie2-immunoreactive positive cells; H: Tie2 mRNA level tested by real time PCR) compared to non-treatment (p<0.05).
Fig.4.
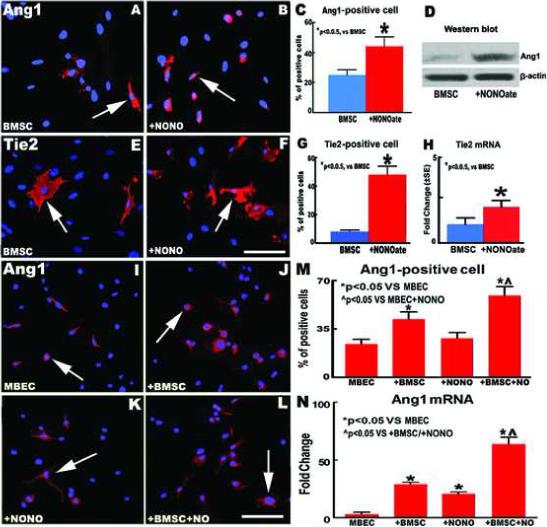
DETA-NONOate alone or combination with the conditioned medium from BMSCs Increase Ang1 gene and protein expression in BMSCs and MBECs. (A-C) Ang1 expression in the non-treatment control (A) and DETA-NONOate (0.4 μM) treatment (B) groups in BMSCs at 24 hours after treatment. (D) Ang1 protein measured by western blot at 24 hours after treatment. (E-G) Tie2 expression in non-treatment control (E) and DETA-NONOate treatment (F) groups in BMSCs at 24 hours after treatment. (H) Tie2 mRNA measured by real time polymerase chain reaction (PCR) at 3 hours after treatment. (I-M) Ang1 expression in non-treatment control (I), BMSC conditioned medium alone (J), DETA-NONOate alone (K), and combination DETA-NONOate with BMSC conditioned medium (L) in mouse brain endothelial cells (MBECs) at 24 hours after treatment. (N) Ang1 mRNA in MBECs measured by real time PCR at 3 hours after treatment. Scale bar = 25 μm. n = 6 / group in (A-C), (E-G), (I-M) and n = 3 / group in (D), (H), (N).
Ang1 expression in MBECs was also performed. Fig. 4I-M show DETA-NONOate combination with the conditioned medium from BMSCs significantly increased Ang1 protein expression in MBECs (I: normal medium of BMSCs; J: conditioned medium from BMSCs; K: normal medium of BMSCs + DETA-NONOate (0.4 μM); L: DETA-NONOate (0.4 μM) + conditioned medium from BMSCs; M: quantitative data of Ang1-immunoreactive positive MBECs) compared with non-treatment control and DETA-NONOate alone groups after 24 hours of culture, respectively (P < 0.05). In addition, Fig. 4N shows the Ang1 mRNA in MBECs (tested by real time PCR after 3 hours of culture) also significantly increased in the DETA-NONOate combination treatment with the conditioned medium from the BMSCs group compared with DETA-NONOate or BMSC conditioned medium alone groups, respectively (P < 0.05).
DETA-NONOate co-cultured with the conditioned medium from BMSCs increases angiogenesis and capillary tube formation
To test whether DETA-NONOate in combination with BMSCs upregulates angiogenesis via the Ang1/Tie2 system, MBEC capillary tube-like formation assays were performed in vitro. Ang1, the conditioned medium from BMSC alone, DETA-NONOate alone, and DETA-NONOate co-culture with BMSC conditioned medium were used in the tube formation assay. Fig. 5 A-G show the data of MBEC capillary tube formation after 6 hours of culture. Ang1 (B: 200 ng/ml) significantly increased capillary tube formation (P < 0.05). Three treatments (C: conditioned medium from BMSCs alone; D: DETA-NONOate 0.4 μM; E: conditioned medium from BMSCs + DETA-NONOate 0.4 μM) significantly increase capillary tube formation compared to the non-treatment control (A) at 6 hours after culture (P < 0.05). In addition, DETA-NONOate combination with the conditioned medium from BMSCs significantly increased capillary tube formation compared with the DETA-NONOate alone group (+26.7%), and inhibition of Ang1 with anti-Ang1 antibody (F: conditioned medium from BMSCs + DETA-NONOate 0.4 μM + anti-Ang1 antibody 1μg/ml) significantly attenuated combination treatment-induced tube formation (−32.9%) after 6 hours of culture (G: quantitative data of capillary-like tube), respectively (P < 0.05). These data indicate that DETA-NONOate in combination with BMSCs significantly promotes angiogenesis (capillary tube-like formation) in vitro, which is consistent with the enhancement of neovascularization formed in the ischemic brain, and these data also support the hypothesis that the angiogenesis induced by combination treatment is regulated by the Ang1/Tie2 system.
Fig.5.
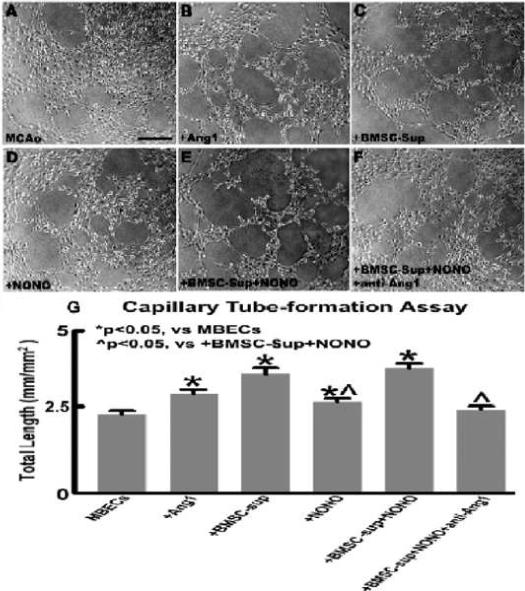
DETA-NONOate co-cultured with the conditioned medium from BMSCs enhances angiogenesis and capillary tube formation which are mediated by increases of the Ang1/Tie2 axis. (A-F) Data of MBEC capillary tube formation in non-treatment control (A), Ang1 200 ng/ml (B), conditioned medium from BMSCs (C), DETA-NONOate 0.4 μM (D), conditioned medium from BMSCs + DETA-NONOate 0.4 μM (E), conditioned medium from BMSCs + DETA-NONOate 0.4 μM + anti-Ang1 antibody 1 μg/ml (F) after 6 hours of culture. (G) Quantitative data of capillary tube formation. Scale bar=100 μm, n=6/group.
DISCUSSION
One of the fundamental principles underlying tissue-engineering strategies using cell transplantation is that a newly formed tissue must acquire and maintain sufficient vascularization in order to support its growth. Vascular endothelial permeability is maintained by the regulated apposition of adherent and tight junctional proteins (Aurrand-Lions et al., 2002, Bazzoni and Dejana, 2004). The tight junctions of the endothelial and epithelial regions of the BBB are regulated by interactions with the neighboring tissue. In the present study, we showed that combination treatment of stroke with DETA-NONOate and BMSCs significantly increases the expression of occludin, a tight junction protein, and the number and the perimeter/diameter both of venous and arterial blood vessels in the ischemic border zone compared with MCAo control animals and DETA-NONOate or BMSC monotherapy groups, respectively. Thus, combination treatment promotes neovascularization and vascular stabilization after stroke.
Ang1 signaling mediates angiogenesis, vascular maturation and remodeling of blood vessels through Tie2 receptor, which is expressed on blood vessel endothelial cells and pericytes (Patan, 2004, Zacharek et al., 2007). Ang1 reduces endothelial permeability and enhances vascular stabilization and maturation (Suri et al., 1996, Pfaff et al., 2006). Transgenic over-expression of Ang1 increases vascular stabilization (Suri et al., 1998), prevents plasma leakage in the ischemic brain and consequently decreases ischemic lesion volume (Thurston et al., 2000, Zhang et al., 2002a). Ang1/Tie2 cooperates with vascular endothelial growth factor (VEGF) to control angiogenesis to form large and small vessels in the mature vascular system and to establish dynamic blood vessel structures (Marti and Risau, 1999, Satchell et al., 2004). Thus, upregulation of Ang1/Tie2 in the endogenous ischemic brain may increase angiogenesis and vascular integrity. The present study showed that DETA-NONOate combination treatment with BMSCs significantly upregulates Ang1/Tie2 and occludin in the ischemic border zone.
In vitro, BMSCs promoted proliferation and migration of endothelial cells and smooth muscle cells in a dose-dependent manner and enhanced collateral vascular flow recovery and remodeling through paracrine signaling in tissue ischemia (Kinnaird et al., 2004). BMSCs could be recruited at the sites of active tumor neovascularization through paracrine regulation of their angiogenic properties (Annabi et al., 2004). BMSCs contribute to revascularization via vessel remodeling after cerebral ischemia (Kokovay et al., 2006). Moreover, BMSC induces angiogenesis and vascular stability via up-regulation of the Ang1/Tie2 pathway (Zacharek et al. 2007). Thus, up-regulation of Ang1/Tie2 in the transplanted BMSCs may increase angiogenesis and arteriogenesis and augment BMSC neovascularization treatment of stroke. NO plays an important role in angiogenesis and vascular integrity in ischemic brain after stroke (Zhang et al., 2001, Chen et al., 2004, Zacharek et al., 2006). Endogenous eNOS-derived NO directly increases angiogenesis and mural cell recruitment to immature angiogenic sprouts (Yu et al., 2005). Ang1 expression co-localizes with pericyte reactive cells (αSMA) in the ischemic brain, and pericyte-derived Ang1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie2 activation in vitro (Hori et al., 2004). In this study, DETA-NONOate not only significantly increased endogenous brain Ang1/Tie2, but also increased Ang1/Tie2 expression in BMSCs. In addition, DETA-NONOate also increases Ang1 protein and mRNA expression in MBECs. Ang1 mRNA expression was significantly increased in MBECs in DETA-NONOate combination treatment with BMSC conditioned medium compared to DETA-NONOate treatment alone. The capillary-like tube formation test shows that DETA-NONOate co-cultured with the conditioned medium from BMSCs significantly increased capillary tube formation compared to DETA-NONOate alone (+26.7%). Inhibition of Ang1 significantly attenuated DETA-NONOate combination with BMSC conditioned medium induced tube formation; therefore, combination treatment induced angiogenesis and vascular stabilization is, at least partially, mediated by upregulation of the Ang1/Tie2 system in MBECs and BMSCs in the ischemic brain.
CONCLUSION
In summary, combination treatment of stroke with DETA-NONoate and BMSCs upregulates the Ang1/Tie2 axis in the endogenous ischemic brain as well as in the transplanted BMSCs, and increases tight junction protein expression, neovascularization and vascular stabilization in the ischemic brain.
Acknowledgements
The authors wish to thank Qinge Lu, Yuping Yang and Supata Santra for technical assistance. This work was supported by National Institute of Neurological Disorders and Stroke grants P01 NS042345 and R01 NS047682 and American Heart Association grant 0750048Z.
Abbreviations
- Ang1
Angiopoietin-1
- αSMA
alpha smooth muscle actin
- BMSCs
bone marrow stromal cells
- MBECs
mouse brain endothelial cells
- MCAo
middle cerebral artery occlusion
- NO
nitric oxide
- vWF
von Willebrand factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Al-Khaldi A, Al-Sabti H, Galipeau J, Lachapelle K. Therapeutic angiogenesis using autologous bone marrow stromal cells: improved blood flow in a chronic limb ischemia model. Ann Thorac Surg. 2003;75:204–209. doi: 10.1016/s0003-4975(02)04291-1. [DOI] [PubMed] [Google Scholar]
- Annabi B, Naud E, Lee YT, Eliopoulos N, Galipeau J. Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors. J Cell Biochem. 2004;91:1146–1158. doi: 10.1002/jcb.10763. [DOI] [PubMed] [Google Scholar]
- Aurrand-Lions M, Johnson-Leger C, Lamagna C, Ozaki H, Kita T, Imhof BA. Junctional adhesion molecules and interendothelial junctions. Cells Tissues Organs. 2002;172:152–160. doi: 10.1159/000066967. [DOI] [PubMed] [Google Scholar]
- Babaei S, Teichert-Kuliszewska K, Zhang Q, Jones N, Dumont DJ, Stewart DJ. Angiogenic actions of angiopoietin-1 require endothelium-derived nitric oxide. Am J Pathol. 2003;162:1927–1936. doi: 10.1016/S0002-9440(10)64326-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y, Lu M, Zhang Z, Chopp M. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Research. 2004;1005:21–28. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Li Y, Li A, Wang L, Katakowski M, Roberts C, Lu M, Chopp M. N-cadherin mediates nitric oxide-induced neurogenesis in young and retired breeder neurospheres. Neuroscience. 2006;140:377–388. doi: 10.1016/j.neuroscience.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005a;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005b;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circulation Research. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurology. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Cui X, Chen J, Zacharek A, Li Y, Roberts C, Kapke A, Savant-Bhonsale S, Chopp M. Stem Cells (Dayton, Ohio) 2007;25:2777–2785. doi: 10.1634/stemcells.2007-0169. (Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine. CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Colleran PN, Wilkerson MK, McCurdy MR, Muller-Delp J. Structural and functional remodeling of skeletal muscle microvasculature is induced by simulated microgravity. American Journal of Physiology. 2000;278:H1866–1873. doi: 10.1152/ajpheart.2000.278.6.H1866. [DOI] [PubMed] [Google Scholar]
- Haralabopoulos GC, Grant DS, Kleinman HK, Lelkes PI, Papaioannou SP, Maragoudakis ME. Inhibitors of basement membrane collagen synthesis prevent endothelial cell alignment in matrigel in vitro and angiogenesis in vivo. Lab Invest. 1994;71:575–582. [PubMed] [Google Scholar]
- Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89:503–513. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- Iurlaro M, Scatena M, Zhu WH, Fogel E, Wieting SL, Nicosia RF. Rat aorta-derived mural precursor cells express the Tie2 receptor and respond directly to stimulation by angiopoietins. J Cell Sci. 2003;116:3635–3643. doi: 10.1242/jcs.00629. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circulation Research. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Li L, Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab. 2006;26:545–555. doi: 10.1038/sj.jcbfm.9600214. [DOI] [PubMed] [Google Scholar]
- Li H, Brodsky S, Basco M, Romanov V, De Angelis DA, Goligorsky MS. Nitric oxide attenuates signal transduction: possible role in dissociating caveolin-1 scaffold. Circulation Research. 2001;88:229–236. doi: 10.1161/01.res.88.2.229. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–1980. doi: 10.1161/01.str.29.9.1972. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Manoonkitiwongsa PS, McMillan PJ, Schultz RL, Jackson-Friedman C, Lyden PD. A simple stereologic method for analysis of cerebral cortical microvessels using image analysis. Brain Res Brain Res Protoc. 2001;8:45–57. doi: 10.1016/s1385-299x(01)00087-3. [DOI] [PubMed] [Google Scholar]
- Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. American Journal of Physiology. 2002;282:H1485–1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti HH, Risau W. Angiogenesis in ischemic disease. Thrombosis and haemostasis. 1999;82(Suppl 1):44–52. [PubMed] [Google Scholar]
- Patan S. Vasculogenesis and angiogenesis. Cancer Treatment and Research. 2004;117:3–32. doi: 10.1007/978-1-4419-8871-3_1. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Fiedler U, Augustin HG. Emerging roles of the Angiopoietin-Tie and the ephrin-Eph systems as regulators of cell trafficking. J Leukoc Biol. 2006;80:719–726. doi: 10.1189/jlb.1105652. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Hirata K, Kawashima S, Ozaki M, Takahashi T, Ogawa W, Inoue N, Yokoyama M. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:108–114. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- Satchell SC, Anderson KL, Mathieson PW. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol. 2004;15:566–574. doi: 10.1097/01.asn.0000115397.22519.03. [DOI] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Yen PS, Su CY, Chen DC, Wang HJ, Li H. Stromal cell-derived factor-1alpha promotes neuroprotection, angiogenesis, and mobilization/homing of bone marrow-derived cells in stroke rats. J Pharmacol Exp Ther. 2008;324:834–849. doi: 10.1124/jpet.107.127746. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10(2):290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nature Medicine. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci USA. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Zhang C, Cui X, Roberts C, Jiang H, Teng H, Chopp M. Nitric oxide regulates Angiopoietin1/Tie2 expression after stroke. Neuroscience Letters. 2006;404:28–32. doi: 10.1016/j.neulet.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001;50:602–611. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002a;113:683–687. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circulation Research. 2002b;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]


