Abstract
The possible role of CD4- and CD8-bearing T lymphocytes in parasite clearance in vivo was investigated, using Plasmodium chabaudi in C57BL/6 mice as a model. Monoclonal antibodies specific for the CD4 and CD8 molecules were administered in vivo to deplete selectively the appropriate subset of T cells. The efficacy of depletion was ascertained by flow cytometry and functional studies. These mice were then infected with P. chabaudi, and the course of infection was followed. The control groups had maximum parasitemias of approximately 30% 10 days after infection, and the infection was cleared within 27 days. Mice without CD4+ cells had significantly higher parasitemias which they were unable to reduce below 20% for the duration of the experiment. Mice without CD8 cells had slightly higher parasitemias which were cleared after 34 days. Because of the possibility that CD8+ cells alone could not be activated in the absence of growth factors, exogenous interleukin-2 was administered to the mice depleted of CD4 cells. This did not significantly affect parasitemias, and the mice were still unable to clear their infections. The data suggest that CD4+ T cells play a crucial role in the protective immune response to the erythrocytic stages of P. chabaudi.
Full text
PDF

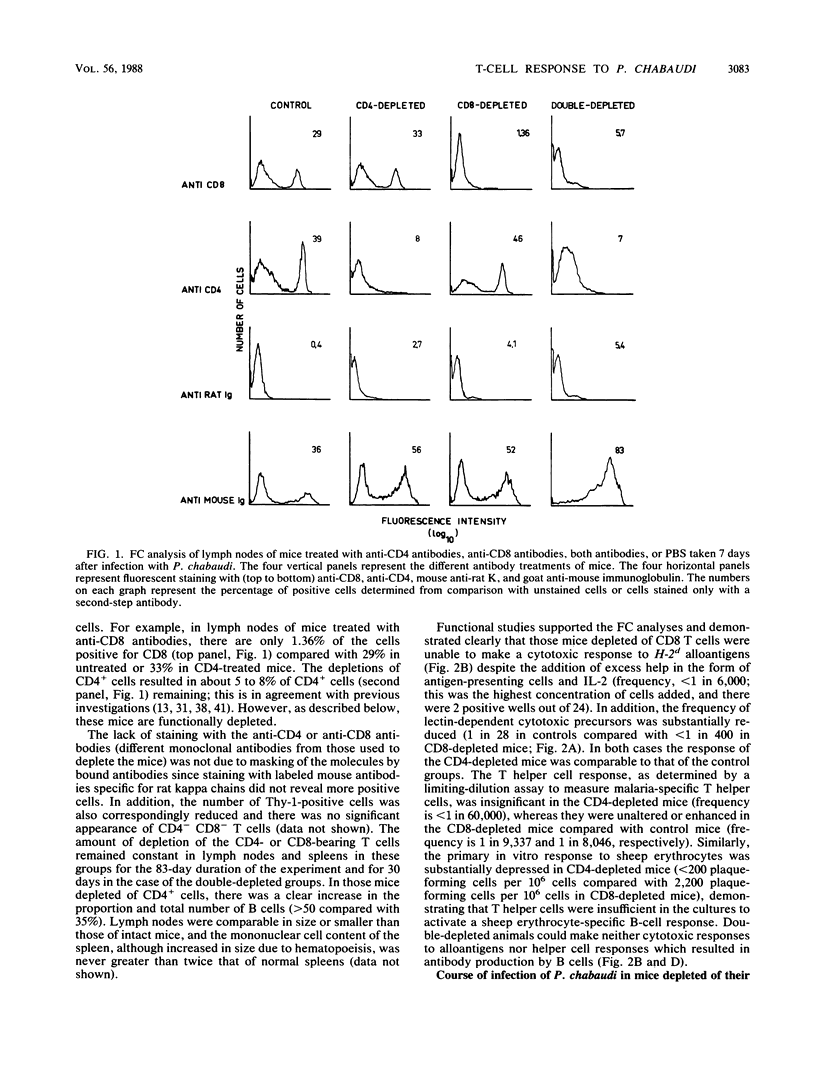
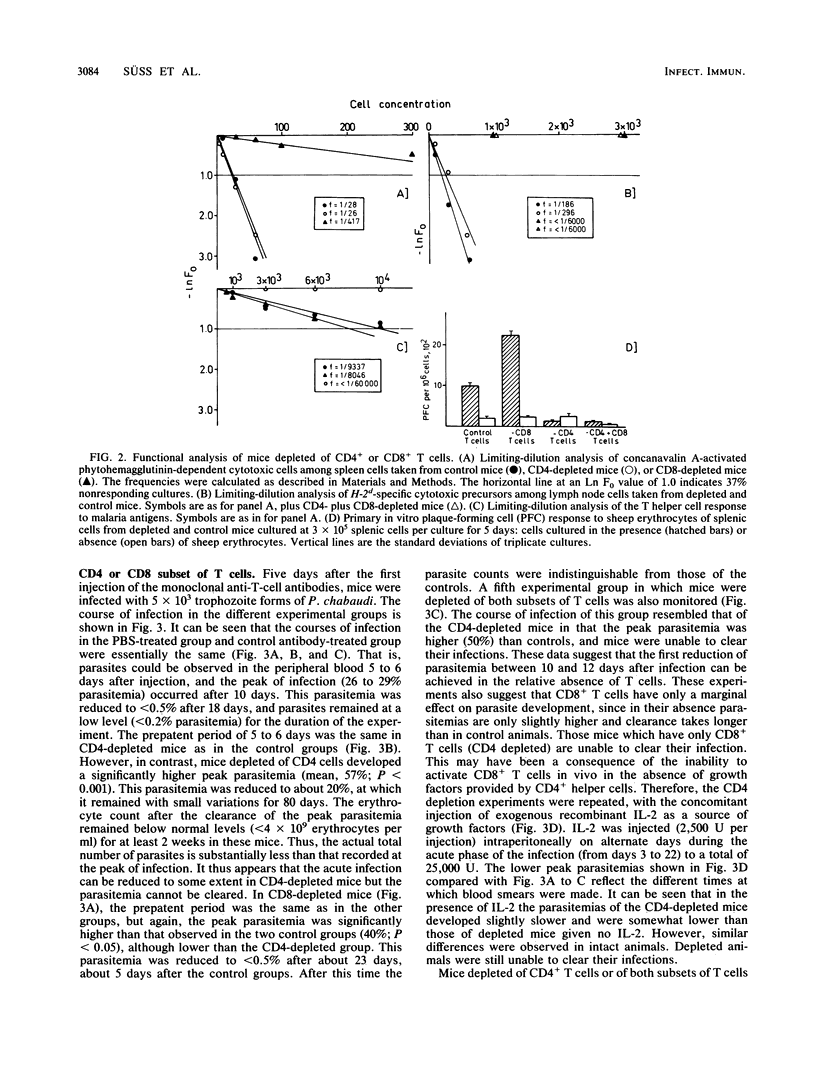
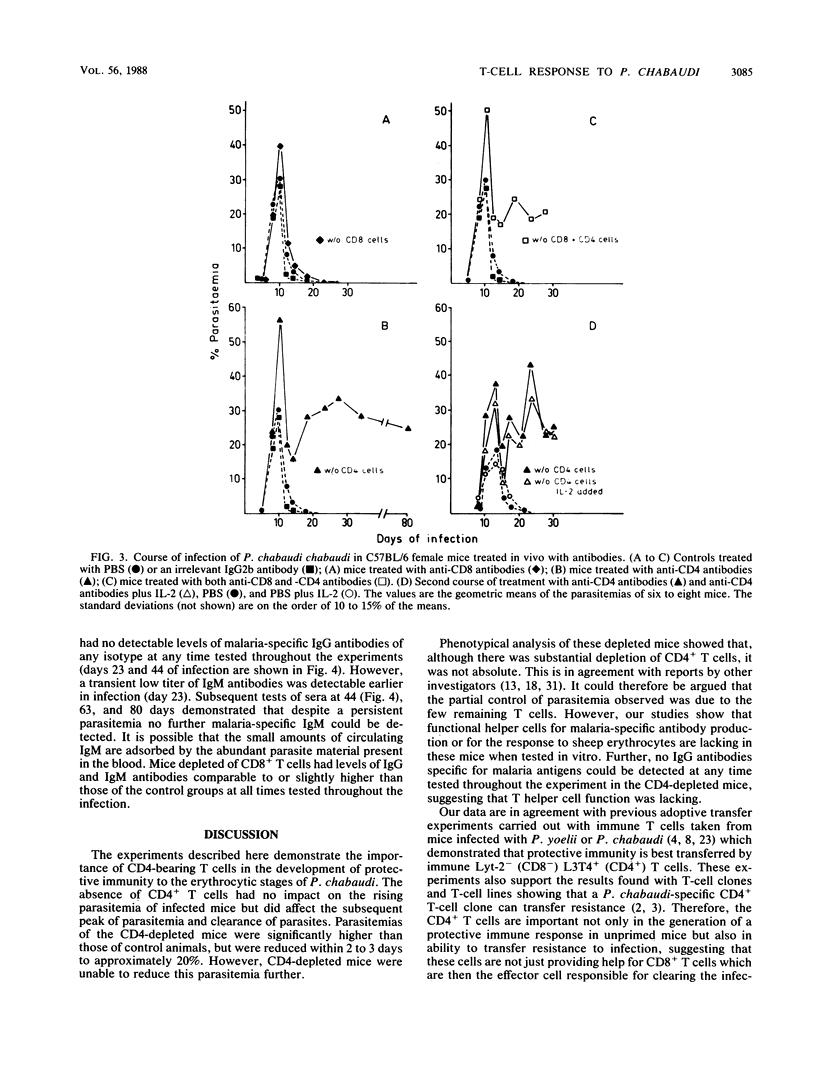
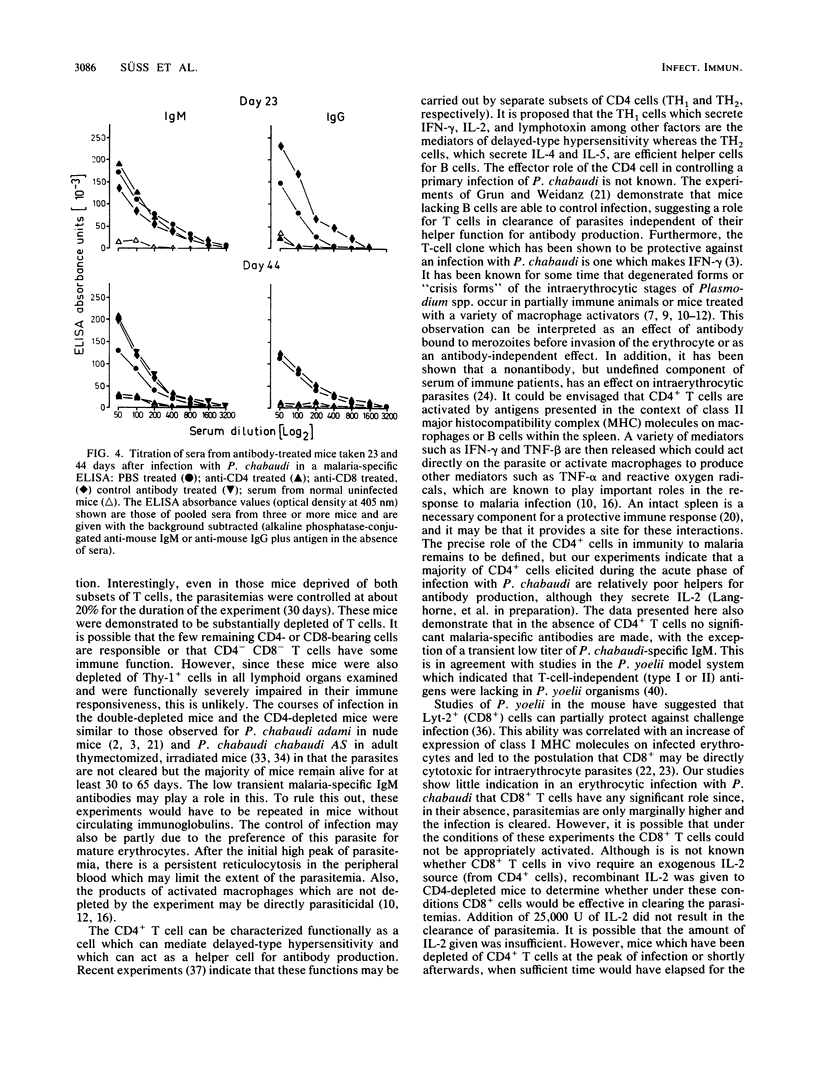


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Brake D. A., Long C. A., Weidanz W. P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned T cell line. J Immunol. 1988 Mar 15;140(6):1989–1993. [PubMed] [Google Scholar]
- Brake D. A., Weidanz W. P., Long C. A. Antigen-specific, interleukin 2-propagated T lymphocytes confer resistance to a murine malarial parasite, Plasmodium chabaudi adami. J Immunol. 1986 Jul 1;137(1):347–352. [PubMed] [Google Scholar]
- Brinkmann V., Kaufmann S. H., Simon M. M. T-cell-mediated immune response in murine malaria: differential effects of antigen-specific Lyt T-cell subsets in recovery from Plasmodium yoelii infection in normal and T-cell-deficient mice. Infect Immun. 1985 Mar;47(3):737–743. doi: 10.1128/iai.47.3.737-743.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. N., Jarra W., Hills L. A. T cells and protective immunity to Plasmodium berghei in rats. Infect Immun. 1976 Oct;14(4):858–871. doi: 10.1128/iai.14.4.858-871.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Cavacini L. A., Long C. A., Weidanz W. P. T-cell immunity in murine malaria: adoptive transfer of resistance to Plasmodium chabaudi adami in nude mice with splenic T cells. Infect Immun. 1986 Jun;52(3):637–643. doi: 10.1128/iai.52.3.637-643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A. Cell-mediated immunity in protection and pathology of malaria. Parasitol Today. 1987 Oct;3(10):300–305. doi: 10.1016/0169-4758(87)90187-6. [DOI] [PubMed] [Google Scholar]
- Clark I. A. Does endotoxin cause both the disease and parasite death in acute malaria and babesiosis? Lancet. 1978 Jul 8;2(8080):75–77. doi: 10.1016/s0140-6736(78)91386-7. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H., Butcher G. A., Cowden W. B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987 Nov 15;139(10):3493–3496. [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Diggs C. L., Osler A. G. Humoral immunity in rodent malaria. II. Inhibition of parasitemia by serum antibody. J Immunol. 1969 Feb;102(2):298–305. [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of blood-stage murine malaria parasites by hydrogen peroxide. Infect Immun. 1983 Jan;39(1):456–459. doi: 10.1128/iai.39.1.456-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A., Schofield L., Enea V., Schellekens H., van der Meide P., Collins W. E., Nussenzweig R. S., Nussenzweig V. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986 May 16;232(4752):881–884. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- Goronzy J., Weyand C. M., Fathman C. G. Long-term humoral unresponsiveness in vivo, induced by treatment with monoclonal antibody against L3T4. J Exp Med. 1986 Sep 1;164(3):911–925. doi: 10.1084/jem.164.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravely S. M., Kreier J. P. Adoptive transfer of immunity to Plasmodium berghei with immune T and B lymphocytes. Infect Immun. 1976 Jul;14(1):184–190. doi: 10.1128/iai.14.1.184-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Long C. A., Weidanz W. P. Effects of splenectomy on antibody-independent immunity to Plasmodium chabaudi adami malaria. Infect Immun. 1985 Jun;48(3):853–858. doi: 10.1128/iai.48.3.853-858.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Mogil R., Murphy D. B., Burger D., Gershon R. K. Enhanced expression of H-2K and H-2D antigens on reticulocytes infected with Plasmodium yoelii. Nature. 1983 Apr 14;302(5909):623–626. doi: 10.1038/302623a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Murphy D. B., Janeway C. A., Gershon R. K. T cell-mediated immunity in malaria. I. The Ly phenotype of T cells mediating resistance to Plasmodium yoelii. J Immunol. 1982 Jul;129(1):377–381. [PubMed] [Google Scholar]
- Jensen J. B., Boland M. T., Akood M. Induction of crisis forms in cultured Plasmodium falciparum with human immune serum from Sudan. Science. 1982 Jun 11;216(4551):1230–1233. doi: 10.1126/science.7043736. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Evans C. B., Asofsky R., Taylor D. W. Immunoglobulin isotype distribution of malaria-specific antibodies produced during infection with Plasmodium chabaudi adami and Plasmodium yoelii. Cell Immunol. 1984 Sep;87(2):452–461. doi: 10.1016/0008-8749(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Lindahl K. F. Role of non-H-2 antigens in the cytotoxic T cell response to allogeneic H-2. Eur J Immunol. 1982 Feb;12(2):101–106. doi: 10.1002/eji.1830120202. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Titus J. A. Expression of Fc gamma receptors on splenic T cells of mice infected with Plasmodium chabaudi adami. Eur J Immunol. 1988 Jan;18(1):1–6. doi: 10.1002/eji.1830180102. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Lindahl K. F., Langhorne J., Coutinho A. Quantitative studies on concanavalin A-induced, TCGF-reactive T cells. I. Correlation between proliferation and lectin-dependent cytolytic activity. J Immunol. 1981 Sep;127(3):1081–1085. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Cobbold S. P., Waldmann H., Aguet M., Zinkernagel R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987 Apr 1;138(7):2278–2281. [PubMed] [Google Scholar]
- Maier B., Bühring H. J., Simon M., Eichmann K., Melchers I. Limiting dilution analysis of proliferating and helper T cells in the in vivo immune response to KLH: derepression of helper T cells at moderately increased frequencies. J Mol Cell Immunol. 1986;2(5):293–305. [PubMed] [Google Scholar]
- McDonald V., Phillips R. S. Plasmodium chabaudi: adoptive transfer of immunity with different spleen cell populations and development of protective activity in the serum of lethally irradiated recipient mice. Exp Parasitol. 1980 Feb;49(1):26–33. doi: 10.1016/0014-4894(80)90052-1. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil R. J., Patton C. L., Green D. R. Cellular subsets involved in cell-mediated immunity to murine Plasmodium yoelii 17X malaria. J Immunol. 1987 Mar 15;138(6):1933–1939. [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Taylor D. W., Bever C. T., Rollwagen F. M., Evans C. B., Asofsky R. The rodent malaria parasite Plasmodium yoelii lacks both types 1 and 2 T-independent antigens. J Immunol. 1982 Apr;128(4):1854–1859. [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]



