Abstract
Ngn3 is a bHLH transcription factor critical for the specification of endocrine cells in the pancreatic Islets of Langerhans. Previous studies in mouse embryos have reported transient expression of Ngn3 in scattered cells within the developing pancreatic epithelium during midgestation (Schwitzgebel et al., 2000). Specifically, these Ngn3-expressing cells have been shown to be progenitor cells fated to give rise to islet endocrine cells (Gradwohl et al., 2000). Here, we characterize the expression of Ngn3 transcripts and protein throughout pancreatic development. Interestingly, we identify and define a dramatic and previously unnoticed gap in developmental Ngn3 expression. We show that both Ngn3 transcript and protein expression occur in two distinct temporal waves, the first occurring early from approximately E8.75 to E11.0, and the second initiating at approximately E12.0. Strikingly, this observed biphasic expression correlates with the ‘first’ and ‘second’ transitions, which encompass two distinct waves of embryonic endocrine differentiation. In addition, our studies demonstrate that Ngn3 transcripts are markedly more widespread in the pancreatic epithelium than NGN3 protein, indicating that post-transcriptional regulation is likely to play a critical role during endocrine differentiation.
Keywords: Ngn3, endocrine, pancreas, first transition, second transition, Pdx1
Introduction
The study of pancreatic development has gained significant momentum in the last few decades, as a direct consequence of the recognition that proper pancreatic growth and homeostasis is of critical medical importance. Diabetes is a chronic disease that results from either the autoimmune destruction of insulin-producing beta cells within pancreatic islets (Type I), or alternatively from ineffective use of insulin (Type II). This disease affects over 180 million people worldwide (www.who.int/en). Understanding the ontogeny and differentiation of beta cells is likely to pave the way for regenerative therapies. Classical studies (Golosow and Grobstein, 1962; Pictet et al., 1972) and more recent work (Jorgensen et al., 2007; Zhou et al., 2007) have elucidated the landmarks of pancreatic development and identified key regulators of endocrine cell fate. However, while a few groundbreaking studies have significantly advanced our ability to drive stem cells towards the beta cell fate (D’Amour et al., 2005; D’Amour et al., 2006; Kroon et al., 2008), much remains to be understood about the molecular pathways involved in differentiation of pancreatic cell lineages.
In mouse, the pancreas originates around embryonic day 9.5 (E9.5) from dorsal and ventral evaginations of the gut tube endoderm. These pancreatic ‘buds’ invade and grow into the visceral mesoderm. Shortly after E10.5, the gut tube rotates, resulting in the fusion of the dorsal and ventral pancreatic buds and the formation of a single organ. The pancreatic epithelium then begins to branch, initially extending short fingerlike lobules into the mesoderm. From E12.5 to birth, branching expands, creating a highly branched, three-dimensional organ that grows dramatically throughout gestation. During this time, the pancreatic epithelium undergoes a number of dynamic cellular changes, giving rise to a tree-like, tubular epithelial network. Along the trunk of the developing pancreatic tree, endocrine progenitors delaminate as individual cells from the endodermal epithelium. These progenitors migrate and coalesce into small islet-like clusters, which progressively join and proliferate into larger endocrine aggregates. Postnatally, these take on recognizable islet anatomy, consisting of a core of insulin-expressing β-cells, surrounded by a mantle of mostly α-cells (glucagon), but also δ-(somatostatin), ε-(ghrelin) and PP cells (pancreatic polypeptide) (Murtaugh and Melton, 2003; Cleaver and Melton, 2004; Jensen, 2004).
During pancreas development, two temporal waves of endocrine differentiation have been identified and their initiation have been termed the ‘first’ and ‘secondary transitions’ (Pictet et al., 1972). The first wave encompasses the generation of ‘early’ endocrine cells, between E9.5 and approximately E12.5. During this time, individual cells within the early pancreatic endoderm initiate expression of glucagon, with a smaller subset transiently co-expressing insulin, with glucagon, pancreatic polypeptide or peptide YY (Herrera et al., 1991; Upchurch et al., 1994). There is interesting controversy and uncertainty regarding the precise fate and potential function of these early endocrine cells. It has been reported that early glucagon- and insulin-expressing (and co-expressing) cells do not contribute to the mature endocrine pancreas (Herrera et al., 1998; Herrera, 2000). However lineage labeling, using indelible tracing of early endocrine cells with the Cre-Lox system, has demonstrated that at least some early endocrine cells (or their progenitors) can contribute to adult islets (Gu et al., 2002). It therefore remains unclear how these early endocrine cells might contribute to either embryonic pancreatic function or later pancreas homeostasis. The second wave of endocrine differentiation, on the other hand, initiates around E12.5 (Pictet et al., 1972), and results in the generation of single hormone-expressing cell types (β-cells, α-cells, δ-cells, ε-cells and PP cells). The second transition also encompasses a dramatic pancreatic transformation, characterized by rapid expansion and differentiation of exocrine and endocrine tissues. The branching epithelium reshapes itself into finer tubular ducts, with the tips of the branches differentiating into exocrine cells and endocrine progenitors delaminating along the central axis. Although initiating at E12.5, the cellular and morphological changes associated with this ‘second transition’ become most evident between E13.5–E14.5.
Recent work in the field of pancreas development suggests that different cascades of molecular regulation regulate these two temporally separate waves of islet endocrine cell generation. Pdx1 has been thought to regulate endocrine cells of the second transition, since only first transition endocrine cells can be found in Pdx1 null embryos (Jonsson et al., 1994; Ahlgren et al., 1996; Offield et al., 1996). However, recent data demonstrates that although the early endocrine lineage does not require Pdx1 for specification, Pdx1 plays a role in its proper expansion and maintenance (Burlison et al., 2008). In addition, some molecules are expressed differently during the two waves of pancreatic development. For example, the transcription factor HLXB9 is transiently expressed early in the pancreatic epithelium, but diminishes following the first transition. Later, however, it reinitiates expression in differentiated β-cells, coinciding with the onset of secondary transition (Li and Edlund, 2001). During the second transition, the expression of many critical pancreatic transcription factors becomes differentially restricted to either endocrine or exocrine cell types. For instance, the transcription factors Pdx1, Nkx2.2, Nkx6.1 and Sox9, which are initially expressed widely in the pancreas, gradually become restricted to the endocrine lineage during this time, while Ptf1a becomes restricted to exocrine cells (Kim and MacDonald, 2002). Even more strikingly, some factors only initiate expression after the secondary transition. For instance, it has been shown that the expression of the transcription factor MafA is specifically restricted to the endocrine cells of the second wave (Artner et al., 2007), thus supporting the hypothesis that there exist distinct molecular cascades underlying the first and second wave of pancreatic endocrine differentiation.
One regulatory factor that is critical to endocrine differentiation is the bHLH transcription factor Ngn3 (Neurogenin3 or Neurog3). Ngn3 is transiently expressed in endocrine progenitor cells and it is never co-expressed with endocrine hormones in pancreatic islets (Schwitzgebel et al., 2000). It therefore marks transitional endocrine progenitors. Genetic ablation of Ngn3 in mouse results in the complete failure of all pancreatic endocrine cell differentiation and neonatal lethality (Gradwohl et al., 2000). Conversely, gain-of-function studies demonstrate that Ngn3 can induce differentiation of the four endocrine lineages (Apelqvist et al., 1999; Schwitzgebel et al., 2000; Grapin-Botton et al., 2001). The complete failure of both early and late endocrine differentiation in Ngn3 null embryos suggests that NGN3 is required for endocrine cell specification, during both the first and secondary transition.
Recent work on Ngn3 has highlighted differences between early versus late embryonic endocrine cells. Using a transgenic temporally-controlled ‘addback’ system to express Ngn3 in an Ngn3 null background, Grappin-Botton and colleagues demonstrated that Ngn3 expression drives endocrine precursors to differentiate, however endodermal progenitors pass through different stages of ‘competence’, resulting in the differentiation of precursors down various lineages at different embryonic timepoints (Johansson et al., 2007). Early embryonic endoderm displays the capacity to generate mostly glucagon-expessing cells, while later endoderm is able to generate the full range of endocrine cell types, including β cells. This suggests that there is likely to be temporal regulation of Ngn3 expression during different stages of pancreatic development.
Previous reports describe Ngn3 expression at various stages (Apelqvist et al., 1999; Gradwohl et al., 2000; Schwitzgebel et al., 2000; van Eyll et al., 2006; Murtaugh, 2007; Burlison et al., 2008), however, no detailed characterization currently exists that examines either Ngn3 expression initiation or maintenance throughout pancreatic development. Here, we present a detailed developmental profile of Ngn3 mRNA and protein expression throughout all stages of pancreatic budding and branching. Clarifying the temporal expression of Ngn3 expression, both during the first and secondary transition, will advance our understanding of both early and late islet endocrine cells.
Results and Discussion
Ngn3 expression initiation
To assess early embryonic Ngn3, we used whole mount in situ hybridization and compared its expression to that of the pancreatic duodenal homeobox gene, Pdx1 (Fig. 1), utilizing a Pdx1-lacZ reporter line (Offield et al., 1996). We first examined mouse embryonic endoderm and budding pancreas, from E8.0 (5 somites, or 5s) to E9.5 (24s). We find that Ngn3 expression initiates around E8.25 (Fig. 1A–C and Supplement Fig. 1), slightly earlier than previously reported (Apelqvist et al., 1999). Specifically, it initiates as early as the 9-somite stage, in a few cells within the dorsal pre-pancreatic endoderm. Soon after, at 12 somites, Ngn3 expression has quickly become quite robust, in a rather punctate pattern, with cells expressing either higher or lower levels of transcript (Fig. 1C). Given that early Ngn3 expression is clearly punctate, and thus not in all cells of the pancreatic bud, it is likely that these early cells already represent progenitors that give rise to endocrine cell lineages.
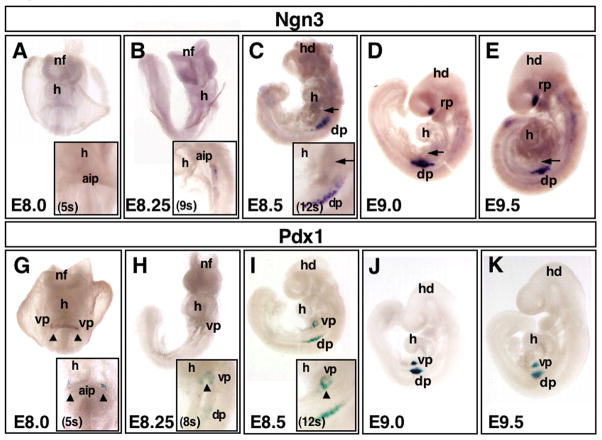
Figure 1. Initiation of Ngn3 and Pdx1 expression during embryogenesis.
Developmental profile comparison of early expression of Ngn3 and Pdx1, using whole mount in situ hybridization of Ngn3 (A–E) and whole mount β-galactosidase staining of Pdx1-lacZ embryos (G–K) from E8.0 to E9.5. Embryos in A,B,G,H face forward. Embryos in C–E and I–K face left. Ngn3 and Pdx1 initiate in the dorsal pancreas at relatively similar timepoints shortly after turning, between E8.25 and E8.5 (A–C Ngn3, G–I Pdx1). Ngn3 initiates in the dorsal pancreas at 9s (inset in B). Pdx1 is expressed in two ventral pancreas domains, as early as E8.0 (5s) (arrowheads in G). These ventral domains fuse as the embryo turns (arrowheads in H,I). While Pdx1 marks the ventral pancreas from early stages (G–K), Ngn3 is not expressed in the ventral pancreas (arrows in C–E) at any time during early development (prior to E10.0). See insets in (A–C) for close-ups on early dorsal and ventral domains for onset of Ngn3 expression, and insets in (G–I) for close-ups of Pdx1 initiation. Note also that Ngn3 marks anterior neurectoderm in the forebrain (head staining in D,E), ventral to Rathke’s pouch. dp, dorsal pancreatic bud; h, heart; hd, head; nf, neural folds; rp, Rathke’s pouch; vp, ventral pancreatic bud.
Delay of Ngn3 expression in the ventral pancreas
Early endodermal Ngn3 expression, in the dorsal pancreatic region, is rather remarkable at E8.5 (12s) in that it precedes pancreatic morphogenesis and it initiates long before ventral pancreas Ngn3 expression (Fig. 1C, and Supplement Fig. 1). The dorsal bud in mouse evaginates from the pre-pancreatic endoderm at embryonic day E9.0–E9.5 (Jorgensen et al., 2007) and we observe Ngn3 expression prior to this stage. This indicates that cells turn on Ngn3 expression before the epithelium thickens, stratifies and begins the cellular morphogenesis that initiates pancreatic differentiation. In addition, although endocrine hormone transcription is reported to be detectable as early as E9.25 (Gittes and Rutter, 1992), endocrine precursor cells cannot yet be identified by hormone protein immunohistochemistry (Jorgensen et al., 2007).
The expression of Pdx1, on the other hand, initiates earlier than Ngn3 and is also first observed only in the ventral pancreatic domain. Specifically, we observe two clusters of Pdx1 expressing cells in the ventral endoderm of the anterior intestinal portal (AIP), as early as the 5-somite stage (E8.0), slightly earlier than previously described (Fig. 1G)(Li et al., 1999; Jorgensen et al., 2007). Faint expression of Pdx1 in the dorsal pancreatic region is detected later, around the 8-somite stage as the embryo turns (Fig. 1H). By 12 somites, we can detect strong Pdx1 expression in both the ventral and dorsal pancreas (Fig. 1I) (Guz et al., 1995; Apelqvist et al., 1999). In contrast to the close correlation of Pdx1 and Ngn3 expression initiation in the dorsal pancreas, we find a dramatic delay in Ngn3 expression initiation in the ventral pancreas (compare Fig. 1C–E to Fig. 1I–K), where Ngn3 is not detectable until about two days later (about E10.0). Interestingly, it has been shown that endocrine differentiation occurs later in the ventral pancreas, compared to dorsal (Spooner et al., 1970; Pictet et al., 1972; Apelqvist et al., 1999), and the mature ventral pancreas is known to contain fewer α– and β– cells in many species (Beaupain and Dieterlen-Lievre, 1974; Stefan et al., 1982; Cleaver, 2004). It is likely that this notable delay in Ngn3 expression is correlated with this differential β cell composition of the dorsal and ventral pancreas.
Neural expression of Ngn3
Separately, during our analysis of early Ngn3 expression, we noted prominent expression in a region of the ventral telencephalon/anterior neuroepithelium (Fig. 1D,E). In this region, we observe early and strong Ngn3 expression in two small patches flanking the midline (Supplement Fig. 2). Although this domain is close to the pre-pituitary oral ectoderm and Rathke’s pouch, it is slightly more ventral and is likely to represent pre-hypothalamus ectoderm. Indeed, expression of Ngn3 in the later hypothalamus has been previously noted in different species, including mouse (Huang et al., 2000; Wang et al., 2001) (www.genepaint.org).
Biphasic expression of Ngn3
In order to elucidate whether Ngn3 is regulated throughout pancreatic growth, we examined Ngn3 expression during dorsal pancreatic bud growth and branching (Fig. 2). Surprisingly, we observed that Ngn3 is expressed in a biphasic manner and that each wave of expression precedes the first and secondary transitions of pancreas development. In the dorsal pancreatic epithelium, we detected high Ngn3 expression from E10.0 to E10.5 (28s–35s) (Fig. 2A–C). Shortly after E10.5, we identified a dramatic and precipitous decrease in Ngn3 transcript in the dorsal pancreas. Careful examination revealed that the downregulation of Ngn3 expression starts to occur about E10.75 (36s–38s) (Fig. 2D) and continues to decrease until about E11.25 (41–42s), when we found distinct downregulation, as assayed by in situ hybridization (Fig. 2F). Afterwards, levels of Ngn3 mRNA start to recuperate and before the onset of the secondary transition, around E12.5, higher Ngn3 transcript levels can be observed (Fig. 2G–K). This high level of expression is maintained as the dorsal bud begins to lobulate and extend along the flank of the stomach. After approximately E13.5, expression of Ngn3 becomes restricted to axial, endodermally-derived epithelial cells that undergo extensive branching (Fig. 2M–O). This region is known to generate endocrine and duct cells, while the more peripheral regions consisting of the tips of lobules/branches are known to generate acinar cells (Zhou et al., 2007).
Figure 2. Biphasic transcription of Ngn3 in the developing pancreas.
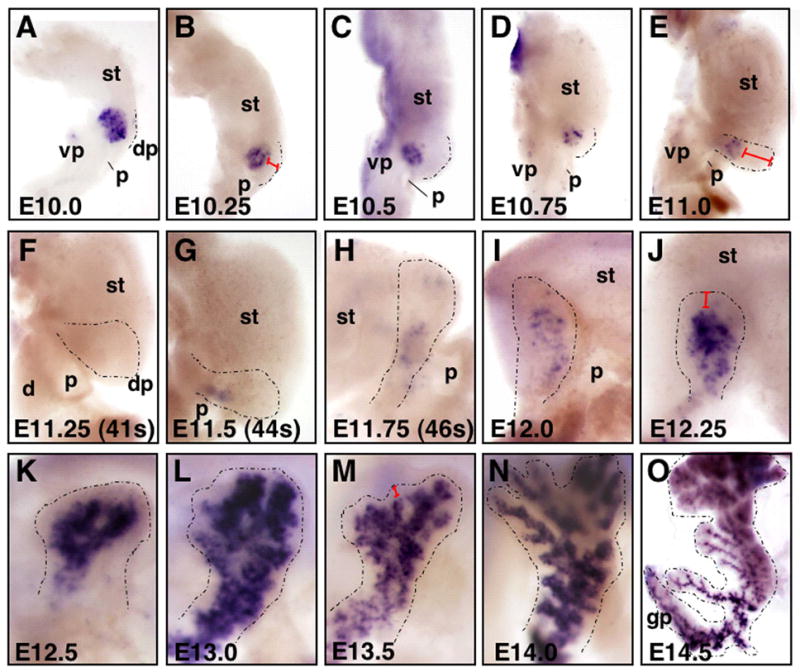
Whole mount in situ hybridization of Ngn3 in wildtype embryos from E10.0 to E14.5. A–G) Dissected gut tubes, anterior is up, dorsal is to the right; H) gut tube in process of turning, anterior is up, but pancreas is shifting relative to stomach, and focus is on pancreas near portal vein; I–O) Dorsal pancreatic bud, with stomach seen in background, except in O where pancreas is isolated. Dorsal pancreas in all panels is outlined with a black dotted line. A–C) Note robust expression of Ngn3 in dorsal pancreatic bud at E10.0 to E10.5. D,E) Decline of Ngn3 expression begins at E10.75, and F) is undetectable at E11.25. G–K) Expression then begins to increase from E11.5–12.5, after which expression is strong in cells of the branching epithelium (L–O). A–E) Note that Ngn3 expression in ventral pancreas is low at all stages shown. Ventral pancreas is not shown in (F–O). After E11.0, the ventral pancreas grows posteriorly, along the mesentery of the duodenum, and is not visible in images as presented. Note also that distal pancreatic mesenchyme (or ‘cap’) is thickest around E11.0, but becomes progressively thinner as development proceeds (compare red brackets in B,E,J,M). Precise staging of embryos was accomplished by counting somites (see Table 1). dp, dorsal pancreatic bud; d, duodenum; gp, gastric pancreas; p, portal vein; st, stomach; vp, ventral pancreatic bud.
Of interest, we note the temporal coincidence of Ngn3 downregulation with two anatomical phenomena. First, we observe that Ngn3 transcription decreases precipitously at precisely the time when the midgut undergoes dramatic remodeling. Before this, from E9.5–E10.5, the pancreatic buds are bilaterally symmetric along the midline. However, between E10.75 to E12.0, the stomach and duodenum fold relative to each other and rotate. During this turning, the pancreas swings left and comes to lie against the left flank of the stomach (Fig. 2D–I). It is precisely during this rotation that Ngn3 expression ceases. We observe that Ngn3 cessation also correlates with a change in the thickness of the distal pancreatic mesoderm or ‘cap’. Initially, this ‘cap’ is of moderate thickness, but during gut turning it expands dramatically, more than doubling in thickness (compare red brackets in Fig. 2B and 2E). Subsequently, as the pancreas branches, this mesoderm progressively thins again, until it is a rather minor component enveloping the pancreas (compare brackets in Fig. 2E with Fig. 2J,M). Given that mesenchyme is known to repress or delay Ngn3 induction (Duvillie et al., 2006), it is possible that when the mesenchyme is at maximal thickness, Ngn3 is repressed by mesenchymal factors. Alternatively, the mesenchyme may change molecularly during this time and affect the underlying endoderm differently. Although tight correlation of Ngn3 transcription cessation with both gut turning and changes in mesenchymal thickness is intriguing, it remains unclear what is causative, what might regulate this transcriptional activity and what purpose it may serve.
The observed biphasic expression of Ngn3 was highly reproducible, and restricted to a narrow developmental window. To ensure that this was not an experimental artifact, we assayed large numbers of systematically staged embryos (n=142 pancreases assayed between E9.5–E14.5; n=23 at E11.0–11.5) (Supplemental Fig. 3A,B and Table 1). At E11.25, we consistently detect a significant decrease of Ngn3 transcripts by in situ hybridization and expression was low shortly before and after this timepoint (Fig. 3G,H). Close examination of sections reveal that this downregulation in developmental Ngn3 expression is due to both a decrease in the number of cells expressing high levels of Ngn3 and to an overall decrease of transcript levels in individual cells (Fig. 3, and data not shown). In addition, we confirmed these observations using semi-quantitative RT-PCR (Supplemental Fig. 3C). While we detect Ngn3 transcript by PCR at E10.5 and E13.5, we detect little Ngn3 expression at E11.5.
Table 1. Staging of embryos.
To establish a developmental profile of Ngn3 expression, precise staging of embryos is accomplished by counting somite number. This table is based on staging by M.H.Kaufman (Kaufman, 1992), but provides increased resolution within each stage. Staging shown in figures is based on somite counts as presented here.
| Embryonic Stage | No. of Somites |
|---|---|
| E7.5 | 1–2 |
| E7.75 | 3–4 |
| E8.0 | 5–7 |
| E8.25 | 8–10 |
| E8.5 | 11–13 |
| E8.75 | 14–16 |
| E9.0 | 17–19 |
| E9.25 | 20–22 |
| E9.5 | 23–25 |
| E9.75 | 26–27 |
| E10.0 | 28–30 |
| E10.25 | 31–32 |
| E10.5 | 33–35 |
| E10.75 | 36–38 |
| E11.0 | 39–40 |
| E11.25 | 41–42 |
| E11.5 | 43–44 |
| E11.75 | 45–46 |
| E12.0 | 47–48 |
| E12.25 | 49–50 |
| E12.5 | 51–52 |
| E12.75 | 53–55 |
| E13.0 | 56–60 |
| E13.5 | ~61–63 |
Figure 3. Ngn3 expression in pancreatic epithelium.
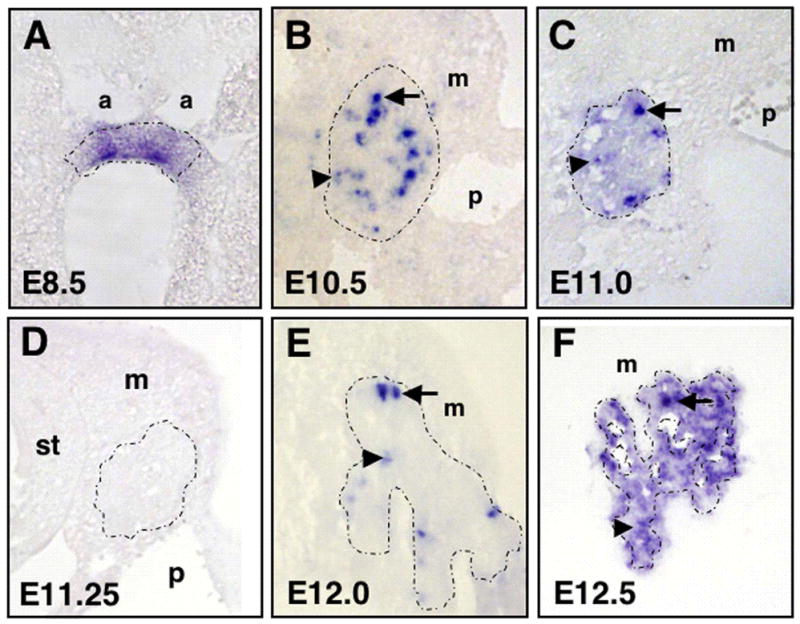
Sections showing in situ hybridization of Ngn3 in wildtype embryos from E8.5 to E12.5. A) Note more homogeneous expression in early endoderm (E8.5). At this stage, the endoderm has not yet thickened or stratified and is still a cuboidal epithelium. B) At E10.5, expression becomes heterogeneous with ‘high’ (arrow) and ‘low’ (arrowhead) expressing cells. C) By E11.0, fewer scattered cells still express higher levels of Ngn3 (arrow), while expression levels decline in the rest of the epithelium (arrowhead). D) Ngn3 expression is undetectable at E11.25. E) Cells expressing Ngn3 can be easily detected at E12.0. F) Expression then increases, both at higher levels in scattered cells and at lower levels throughout the epithelium (E12.5 shown). Pancreatic epithelium is outlined with a dotted line. a, aortae; m, mesenchyme; p, portal vein; st, stomach.
Biphasic expression of NGN3 protein
In order to determine whether the observed biphasic transcriptional profile of Ngn3 translates into differences in protein levels at different stages, we assayed NGN3 protein using immunofluorescence (Fig. 4). Previous reports had identified small numbers of NGN3 protein expressing cells at approximately E11.5 (Schwitzgebel et al., 2000), so we examined NGN3 protein expression profile during the timeframe encompassing the observed Ngn3 transcriptional cessation (n=24 pancreases between E10.5–E14.5; n=7 at E11.25–E11.5). Again, using carefully staged embryos from E10.5 to E14.5, we found that protein levels did indeed experience a similar downregulation (E14.5 data not shown). At E10.5 we can clearly detect appreciable NGN3 protein in many cells within the stratified pancreatic epithelium (Fig. 4A). These levels start decreasing by E10.75–E11.0 (data not shown), are very low at E11.25–11.5 (Fig. 4B,C), and start to increase by E12.0 (Fig. 4D), in accordance with observed mRNA levels. By E13.5 and thereafter, we observed a dramatic increase in NGN3 protein that correlates with increasing Ngn3 transcript levels (Fig. 4F). In contrast to in situ hybridization analysis, we did not observe a total absence of NGN3 protein during the E11.25–11.5 window, although protein levels were significantly lower at these stages (Fig. 4G).
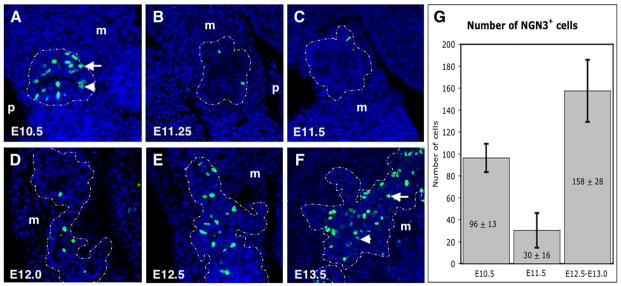
Figure 4. Biphasic NGN3 expression in pancreatic epithelium.
NGN3 protein detected by immunofluorescence on sectioned pancreas, from E10.5 to E13.5. A) NGN3 protein is readily detected in the stratified epithelium of the E10.5 bud. B,C) From E11.25 to E11.5, few if any cells show NGN3 staining in single sections. D–F) At E12.0, we start to detect a steady increase in the number of cells expressing NGN3 that continues to E13.5. Pancreatic epithelium is outlined with a white dotted line. High (arrows) and low (arrowheads) levels of NGN3 protein can be observed in individual cells. m, mesenchyme; p, portal vein. G) Quantification of the number of cells showing NGN3 protein expression at E10.5, E11.5 and E12.5–E13.0 (n=3 for each stage). The average number of cells showing protein expression at E11.5 is approximately 30, while at E10.5 it is 96 and between E12.5–E13.5 it is 158. Note the statistiscally significant reduction of NGN3+ cells at E11.5 (p≤0.02).
Expression of Ngn3 transcripts is more widespread than NGN3 protein
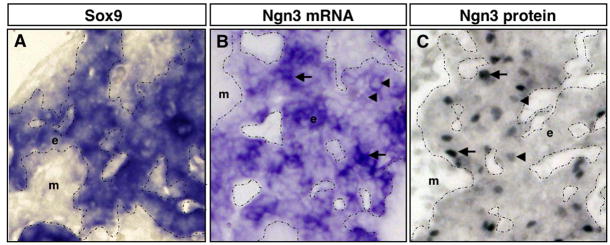
We noticed that expression patterns of Ngn3 transcripts and NGN3 protein are distinctly different. Although we could observe rather extensive expression of Ngn3 throughout the branching epithelium by in situ hybridization (Fig. 2L–O), NGN3 protein was clearly restricted to individual scattered cells (Fig. 4). To assess the extent of epithelial expression, we compared Ngn3 to Sox9, which marks the entire early pancreatic epithelium (Fig. 5A,B). We found that although not all cells of the epithelium expressed Ngn3, many more than expected did, when compared to published reports (Apelqvist et al., 1999; Schwitzgebel et al., 2000) and to our own protein analyses (Fig. 5C). Thus, we find that Ngn3 transcripts are unexpectedly more widespread in the pancreatic epithelium than NGN3 protein. While it is possible that in situ hybridization is simply more sensitive than immunostaining at detecting low levels of Ngn3, these observations may alternatively suggest that post-transcriptional regulation is likely to be important during endocrine differentiation.
Figure 5. Ngn3 transcripts are more widespread in pancreatic epithelium than NGN3 protein.
Sections through E13.0 dorsal pancreas. A,B) In situ hybridzation. C) Immunohistochemistry for NGN3. A–C) High magnification of the pancreatic epithelium (20X). A) Sox9 is expressed throughout the pancreatic epithelium. B) Ngn3 is expressed in many cells within the pancreatic epithelium, displaying both high (arrows) and low (arrowheads) expressing cells. C) NGN3 protein is concentrated in scattered cells of the pancreatic epithelium. In addition, cells are observed that express either high (arrows) or low (arrowheads) levels of NGN3 protein. Note that there are significantly more cells that express the Ngn3 transcript (B), than cells that express NGN3 protein (C). Pancreatic epithelium is outlined with a dotted line. e, epithelium; m, mesenchyme.
We also observed that Ngn3-expressing cells did not all express equal levels of either transcript or protein. Our sections revealed clear ‘high’ and ‘low’ Ngn3-expressing cells, present within the dorsal pancreatic endoderm, both by RNA (Fig. 3B,E,F) and protein (Fig. 4A,F). Moreover, the appearance of ‘high expressors’ at E10.5 (Fig. 3B) seems to correlate with the first differentiated endocrine cells, which are detected as early as E10.0 (Jorgensen et al., 2007). We speculate that certain threshold levels of total Ngn3 mRNA are required for NGN3 protein accumulation. In addition, it is possible that only Ngn3high cells ultimately express enough NGN3 protein to give rise to endocrine cells. This may be similar to reported ‘high’ and ‘low’ Pdx1 expressing cells, where the Pdx1high cells are thought to give rise to β cells (Fujitani et al., 2006; Nishimura et al., 2006). Finally, it is possible that Ngn3 is initially expressed in a broader domain of the pancreatic epithelium than the future endocrine compartment. This is supported by lineage analysis using Cre-mediated recombination, which demonstrates that at least some proportion of both ductal and acinar progenitors express Ngn3 at some point during their development (Schonhoff et al., 2004).
Summary
We thus report the novel observation that Ngn3 expression is biphasic during early pancreatic development. To our knowledge, this is one of few demonstrations of a molecular correlation with the first and secondary transitions of pancreatic endocrine differentiation. We find that the first period of Ngn3 expression is temporally correlated with the primary transition endocrine lineage, while the reactivation of Ngn3 expression initiates just prior to the secondary transition. Given that very little is known regarding the regulation of these two separate waves of endocrine differentiation, it is a step towards identifying similarities and differences between ‘early’ versus ‘late’ embryonic endocrine cells.
Recent data from Grapin-Botton and colleagues demonstrate that these two populations of endocrine cells have different developmental potentials (Johansson et al., 2007), with early Ngn3-expressing precursors giving rise mostly to α-cells and later precursors giving rise to a full range of endocrine cell types, including insulin-expressing β cells. It is likely that these different pools of Ngn3-expressing endocrine precursors experience different molecular contexts, which change over time and result in different differentiation outcomes. It is therefore important for pancreatic studies to identify and recognize whether similar fluctuations occur in the expression of other essential endocrine differentiation factors and to better understand the profile of factors expressed in endocrine cells, ‘early’ versus ‘late’. Studies support the hypothesis that pancreatic progenitors are inherently different during the first and secondary waves. Melton and colleagues, for instance, have recently identified pancreatic multipotent progenitor cells (MPCs) that give rise to three pancreatic lineages, including endocrine, exocrine and ductal, however these are only maintained until E12.5 (Zhou et al., 2007). After E12.5, their potential becomes restricted and they give rise solely to acinar cells. How and why these precursors undergo an abrupt restriction in cell fate during the secondary transition remains unclear, but the answer is likely to lie in the complex interplay of molecular cascades that change as the pancreas develops. Clarifying the role of first and secondary transition endocrine cells and elucidating whether they emerge as a result of distinct genetic programs will undoubtedly forward the field of pancreatic islet studies, ES cell differentiation to β cells and regenerative therapies for diabetes.
Experimental procedures
Mouse embryos handling
CD1 embryos were collected from pregnant females (E8.0 through E14.5) by dissection in ice-cold 1xPBS buffer (phosphate buffered saline) and fixed in 4% paraformaldehyde (PFA) in PBS solution overnight at 4°C with gentle rocking. The amnion and body wall were removed during dissection for better probe penetration to the pancreatic anlage, or alternatively, the entire gut tube of older embryos (E10.5–E14.5) were isolated prior to analysis. Embryos or isolated gut tubes were washed twice in PBS for 10 min at 4°C, and dehydrated using an ethanol series, and stored in 75% ethanol at −20°C.
Whole mount in situ hybridization and RNA probes
Whole mount in situ hybridization was carried out using a protocol adapted from D. Wilkinson’s Method (Wilkinson, 1999). Briefly, embryos stored in 75% EtOH at −20°C, were rehydrated in stepwise fashion to PBST. Then, the embryos were treated with proteinase K, fixed in a 0.25% gluteraldehyde/4% PFA solution, and pre-hybridized at 60°C for 1 hour. The samples were transferred into hybridization mix, containing either Ngn3 coding region (539 bp) or Sox9 full-length transcript (2305bp, Clone Image ID 5320371 Open Biosystems) Digoxigenin-labeled probe. The in situ hybridization steps were carried out using a Biolane HTI automated incubation liquid handler (Holle & Huttner). Development of color reaction was done using BM Purple (Roche). Images were taken using a Lumar dissecting microscope (Zeiss) and a DP-70 camera (Olympus).
RNA isolation, cDNA synthesis and RT-PCR
Individually dissected pancreata from different developmental stages (n ≥4 for each stage) were collected either in RNAlater (Ambion) or flash-frozen in liquid nitrogen for RNA extraction. RNA extraction was performed using an RNeasy Micro Kit (QIAGEN). RNA was converted into cDNA utilizing M-MLV RT (Promega), oligos (IDT DNA) and by following instructions from Promega. cDNA concentration was quantified/normalized by gel band intensity and RT-PCR was carried out on a 2720 Thermal Cycler (Applied Biosystems). The annealing temperature used was 57 °C. Normalization of cDNA amounts was carried out by first analyzing actin and Pdx1 mRNA levels. The following primers were used to identify amount of Ngn3 transcript present at each stage: Ngn3: 5′-AGTCGGGAGAACTAGGATGG-3′, 5′-TGGAACTGAGCACTTCGTGG-3′ yields 113bp product size (32 cycles), Pdx1: 5′ GAAATCCACCAAAGCTCACG-3′, 5′ CCCGCTACTACGTTTCTTATCTTCC-3′yields a 271bp product size (35 cycles), and Actin: 5′-GACGGCCAGGTCATCACTAT-3′, 5′-AGGGAGACCAAAGCCTTCAT-3′ yields a 995bp product size (28 cycles).
Pdx-lacZ embryo generation and β-Galactosidase reaction
Pdx-lacZ embryos were generated by mating Pdx-lacZ heterozygous males (generously provided by Chris Wright, Vanderbilt) and CD1 females. Embryos were dissected manually and fixed in 4% paraformaldehyde/PBS for 15 min at room temperature on a nutator. After fixation, the embryos were rinsed 3 times in PBS for 5 min each. After rinsing embryos, a lacZ staining solution was made using the following: 20 mM K4Fe(CN)6. 3H2O, 20 mM K3Fe(CN)6, 2 mM MgCl2, 0.02% NP-40, and 1xPBS to a final volume of 500rl. Addition of 4μl of 100 mg/ml X-Gal stock (in dimethyl formamide) was added after the staining solution was warmed to 37°C to avoid X-Gal precipitation. Embryos remained in staining solution overnight, shielded from light, to allow the color reaction to develop. Then, the embryos were washed with PBS 3 times for 5 min each, and transferred to 80% glycerol for viewing using a NeoLumar stereomicroscope (Zeiss) and photographed using a DP70 camera (Olympus).
Sectioning and histology
For paraplast sectioning of embryos following in situ hybridization, the embryos were post-fixed in 4%PFA and dehydrated to 75% ethanol. Embryos were then rinsed twice in 80%, 95% and 100% ethanol for 5 min, twice in xylene at room temperature for 5 min, then 1:1 paraplast:xylene at 60°C for 5 min, then a series of rinses in 100% Paraplast Plus tissue embedding medium (McCormick) at 60°C. The embryos were then embedded and sectioned with a 2030 Reichert-Jung microtome. For examination, the sections were mounted on SuperfrostPlus glass slides (Fisher), deparaffinized in Xylene twice for 5 min each and mounted with glass coverslips using Permount (Fisher).
Immunofluorescence
Dorsal pancreata were dissected from E10.5–E14.5 and were fixed overnight in 4% PFA in 1xPBS at 4°C. The next day they were dehydrated and paraffin-embedded in Paraplast Plus tissue embedding medium (McCormick). 10μm sections of complete pancreata were mounted on SuperfrostPlus slides (Fisher). For immunofluorescence, sections were de-waxed in xylene; rehydrated via an ethanol series; washed several times in 1xPBS; treated with Antigen Unmasking Solution (Vector Labs); quenched for endogenous peroxidases with 3% H2O2; blocked for 1hr at room temperature (RT) with 1% Blocking Reagent (TSA kit); and incubated with 1:4000 mouse anti-NGN3-AB2013 (Beta Cell Biology Consortium, kindly provided by Dr. Raymond MacDonald) diluted in blocking solution overnight at RT. Signal was detected the following day by incubating the slides with mouse secondary antibody (TSA kit) and DAPI (1:1000). Slides were mounted with ProLong Gold Antifade Reagent (Invitrogen). Images were acquired on a LSM510META (Zeiss) confocal microscope.
NGN3 protein quantification
The entire pancreas from several embryos (n ≥3 for each developmental stage) were sectioned (10μm) and processed for NGN3 immunofluorescence. NGN3 positive cells were counted on every section using an Axiovert 200M inverted fluorescence microscope (Zeiss). At each stage, the overall cell number per pancreas were added and averaged. Means and standard deviations were calculated and graphed using Microsoft Excel. Mean differences were tested for statistical significance by using the Student-T test.
Immunohistochemistry
E13.5 dorsal pancreata were fixed overnight in 4% PFA, dehydrated and paraffin-embedded in Paraplast Plus tissue embedding medium (McCormick). 10μm sections of complete pancreata were mounted on SuperfrostPlus glass slides (Fisher). Sections were de-waxed with xylene; rehydrated using decreasing concentrations of ethanol; washed several times in PBS; treated with Antigen Unmasking Solution (Vector Labs); quenched for endogenous peroxidases with 3% H2O2 for 45 min; blocked for 1hr at RT with 10% NDS (Sigma); and incubated with 1:3000 mouse anti-NGN3-AB2013 (Beta cell biology Consortium kindly provided by Raymond MacDonald) diluted in 5% NDS blocking solution overnight at room temperature. Slides were then washed with 1X PBS and incubated with 1:200 Biotin anti-mouse antibody for 1hr and then treated with ABC solution (vectastain system from vector labs) for 15 min at room temperature. Signal was detected utilizing DAB substrate (Vector labs). Images were acquired on an Axiovert 200M inverted fluorescence microscope (Zeiss).
Supplementary Material
Acknowledgments
We are very grateful to Chris Wright (Vanderbilt) for the Pdx1-lacZ mice. We also thank Ray MacDonald for invaluable discussions, Ella and Cormac Carroll for support, and Anne Grapin-Botton, Yuval Dor, Ray MacDonald and Jenny Hsieh for helpful comments on the manuscript. This work was supported by grants JDRF Award 99-2007-472, NIH R01 grant DK079862-01 and the Basil O’Connor March of Dimes Award to OC.
References
- Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409–1416. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R. MafB is required for islet beta cell maturation. Proc Natl Acad Sci U S A. 2007;104:3853–3858. doi: 10.1073/pnas.0700013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaupain D, Dieterlen-Lievre F. An immunocytological study of differentiation of the endocrine pancreas of the chick embryo. II. Glucagon. Gen Comp Endocrinol. 1974;23:421–431. doi: 10.1016/0016-6480(74)90040-9. [DOI] [PubMed] [Google Scholar]
- Burlison JS, Long Q, Fujitani Y, Wright CV, Magnuson MA. Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev Biol. 2008;316:74–86. doi: 10.1016/j.ydbio.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O, Melton DA. Development of the endocrine pancreas. In: Kahn CR, Smith RJ, Jacobson AM, Weir GC, King GL, editors. Joslin’s Diabetes Mellitus. 14. Lippincott Williams & Wilkins; 2004. pp. 21–40. [Google Scholar]
- Cleaver O, Melton DA. Development of the Endocrine Pancreas 2004 [Google Scholar]
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Duvillie B, Attali M, Bounacer A, Ravassard P, Basmaciogullari A, Scharfmann R. The mesenchyme controls the timing of pancreatic beta-cell differentiation. Diabetes. 2006;55:582–589. doi: 10.2337/diabetes.55.03.06.db05-0839. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Fujitani S, Boyer DF, Gannon M, Kawaguchi Y, Ray M, Shiota M, Stein RW, Magnuson MA, Wright CV. Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation. Genes Dev. 2006;20:253–266. doi: 10.1101/gad.1360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes GK, Rutter WJ. Onset of cell-specific gene expression in the developing mouse pancreas. Proc Natl Acad Sci U S A. 1992;89:1128–1132. doi: 10.1073/pnas.89.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golosow N, Grobstein C. Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev Biol. 1962;4:242–255. doi: 10.1016/0012-1606(62)90042-8. [DOI] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapin-Botton A, Majithia AR, Melton DA. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 2001;15:444–454. doi: 10.1101/gad.846001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Herrera PL, Huarte J, Sanvito F, Meda P, Orci L, Vassalli JD. Embryogenesis of the murine endocrine pancreas; early expression of pancreatic polypeptide gene. Development. 1991;113:1257–1265. doi: 10.1242/dev.113.4.1257. [DOI] [PubMed] [Google Scholar]
- Herrera PL, Orci L, Vassalli JD. Two transgenic approaches to define the cell lineages in endocrine pancreas development. Mol Cell Endocrinol. 1998;140:45–50. doi: 10.1016/s0303-7207(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol Cell Biol. 2000;20:3292–3307. doi: 10.1128/mcb.20.9.3292-3307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. Gene regulatory factors in pancreatic development. Dev Dyn. 2004;229:176–200. doi: 10.1002/dvdy.10460. [DOI] [PubMed] [Google Scholar]
- Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell. 2007;12:457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Jorgensen MC, Ahnfelt-Ronne J, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. Atlas of Mouse Development. San Diego, CA USA: Elsevier Academic Press; 1992. [Google Scholar]
- Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Curr Opin Genet Dev. 2002;12:540–547. doi: 10.1016/s0959-437x(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Li H, Arber S, Jessell TM, Edlund H. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nat Genet. 1999;23:67–70. doi: 10.1038/12669. [DOI] [PubMed] [Google Scholar]
- Li H, Edlund H. Persistent expression of Hlxb9 in the pancreatic epithelium impairs pancreatic development. Dev Biol. 2001;240:247–253. doi: 10.1006/dbio.2001.0440. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC. Pancreas and beta-cell development: from the actual to the possible. Development. 2007;134:427–438. doi: 10.1242/dev.02770. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Melton DA. Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol. 2003;19:71–89. doi: 10.1146/annurev.cellbio.19.111301.144752. [DOI] [PubMed] [Google Scholar]
- Nishimura W, Kondo T, Salameh T, El Khattabi I, Dodge R, Bonner-Weir S, Sharma A. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol. 2006;293:526–539. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Pictet RL, Clark WR, Williams RH, Rutter WJ. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972;29:436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Spooner BS, Walther BT, Rutter WJ. The development of the dorsal and ventral mammalian pancreas in vivo and in vitro. J Cell Biol. 1970;47:235–246. doi: 10.1083/jcb.47.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan Y, Orci L, Malaisse-Lagae F, Perrelet A, Patel Y, Unger RH. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes. 1982;31:694–700. doi: 10.2337/diab.31.8.694. [DOI] [PubMed] [Google Scholar]
- Upchurch BH, Aponte GW, Leiter AB. Expression of peptide YY in all four islet cell types in the developing mouse pancreas suggests a common peptide YY-producing progenitor. Development. 1994;120:245–252. doi: 10.1242/dev.120.2.245. [DOI] [PubMed] [Google Scholar]
- van Eyll JM, Passante L, Pierreux CE, Lemaigre FP, Vanderhaeghen P, Rousseau GG. Eph receptors and their ephrin ligands are expressed in developing mouse pancreas. Gene Expr Patterns. 2006;6:353–359. doi: 10.1016/j.modgep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Wang X, Chu LT, He J, Emelyanov A, Korzh V, Gong Z. A novel zebrafish bHLH gene, neurogenin3, is expressed in the hypothalamus. Gene. 2001;275:47–55. doi: 10.1016/s0378-1119(01)00648-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. In situ hybidization: A practical approach. Oxford, New York, USA: Oxford University Press; 1999. [Google Scholar]
- Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.