Abstract
The assessment of patients presenting to the emergency department (ED) with suspected cardiac chest pain (CP) and a non-diagnostic electrocardiogram (EKG) is lengthy and costly. We hypothesized that myocardial contrast echocardiography (MCE) can be cost-efficient in such patients by detecting those whose CP is non-cardiac in nature. Accordingly, cost-efficiency was evaluated in 957 patients presenting to the ED with suspected cardiac CP, but no ST segment elevation on the EKG, who underwent MCE. Economic outcome calculations were based on costs estimated from national average Medicare charges adjusted by a cost-charge ratio. Based on routine clinical criteria, 641 (67%) patients were admitted to the hospital while 316 (33%) were discharged directly from the ED. The average cost per patient using routine evaluation was $5000. Patients with normal MCE (n=523) had a very low primary event rate (death, AMI) of 0.6 % within 24 hours after presentation making it relatively safe to have discharged patients directly from the ED with a normal MCE. Hence, if MCE had been used for decision-making 523 (55%) patients would have been discharged directly from the ED and 434 (45%) would have been admitted to the hospital. Preventing unnecessary admissions and tests would have saved an average of $900 per patient, in addition to reducing their ED stay. In conclusion, by excluding cardiac causes in patients presenting to the ED with CP and a non-diagnostic ECG, MCE can prevent unnecessary admissions and downstream resource utilization, making it a cost-efficient tool in the evaluation of these patients.
Keywords: myocardial contrast echocardiography; chest pain, emergency department; cost-efficiency
Introduction
We have previously shown that regional function (RF) and myocardial perfusion (MP) on myocardial contrast echocardiography (MCE) have excellent diagnostic and prognostic utility in patients who present to the emergency department (ED) with suspected cardiac chest pain (CP) but a non-diagnostic initial electrocardiogram (EKG)1,2. For the present analysis, we hypothesized that the excellent negative predictive value of MCE can identify low risk patients who could be safely discharged from the ED, and that it could also prevent downstream resource utilization in these patients, making it a cost-efficient method to risk stratify CP patients who do not have a diagnostic EKG.
Methods
This patient population has been described previously1. Consecutive patients, >30 years of age, who presented to the ED with complaints suspicious for cardiac CP, and who did not ST-elevation on their initial EKG were included. At the time of presentation, a complete history, physical exam, and 12-lead EKG were performed. Blood was drawn for troponin-I (cTNI), which was repeated twice at 6 h intervals. MCE was performed within 12 h from the patient’s last episode of CP. The median time from the last episode of CP to MCE was 3 hours3. RF or MP information was not shared with ED or consulting physicians. The decision to admit or discharge the patient as well as the need for any follow-up investigations was determined by the ED or consulting physicians.
The evaluations of RF and MP on MCE have been previously described1,2. RF and MP were scored separately by experienced observers blinded to all other clinical data as normal, abnormal, or not interpretable using a 14-segment model4. If RF was not normal in a segment then it was interpreted as abnormal. Delayed or absent perfusion in a segment denoted abnormal perfusion. The segments were then grouped into anteroapical, lateral, or inferior-posterior territories. Studies were called abnormal if either RF or MP was abnormal in 1 or more territory. If a territory could not be assessed from any view, the study was classified as not interpretable and the patient was excluded from analysis.
Events occurring with 24 hours of presentation were analyzed for this study. Primary events included non-fatal AMI (cTNI level >0.6 ng·mL-1) and all-cause mortality. Secondary events included unstable angina pectoris (CP of >30 min duration associated with dynamic EKG changes and/or a cTNI of between 0.08 and 0.6 ng·mL-1, and requiring hospitalization and/or revascularization), congestive heart failure requiring hospitalization, and percutaneous or surgical revascularization. In patients with both primary and secondary events, only the primary event was included in the analysis. If a patient had multiple secondary events, only the first event was included in the analysis.
The disposition of the patient from the ED (admission versus discharge), and any follow-up diagnostic tests performed (including stress echocardiography or single photon emission computed tomography (SPECT) and cardiac catheterization) were obtained from the hospital records. Only tests ordered before the patient’s discharge and only costs incurred as a direct result of a patient’s presentation to the ED for CP evaluation were considered for analysis.
The national average of Medicare-allowable charges for ED visits and all diagnostic tests as well as Medicare reimbursement rates for the final diagnosis-related group (DRG) billed (based upon the discharge diagnosis of each patient) were determined for 2000-2003. Costs were obtained by applying the cost-to-charge ratios provided in the Medicare Cost Report for each year, and all costs were inflation-corrected to 2003 dollars using estimates from the medical care sector of the Bureau of Labor Statistics.
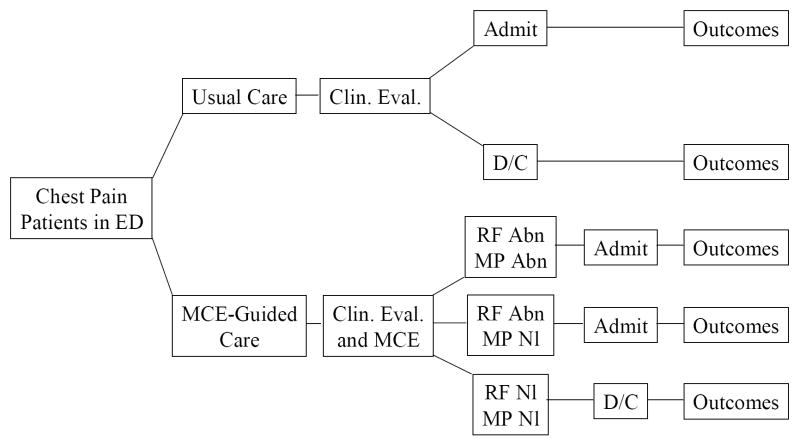
Cost-efficiency was calculated using a decision-analysis software (TreeAge Pro Healthcare)5. A decision tree was constructed (Figure 1) to analyze the costs of “Usual Care” (what actually occurred) versus that of a MCE-guided approach. The overall cost per patient was calculated by adding the costs of the ED evaluation, the final inpatient DRG (if applicable), and outpatient diagnostic tests performed based on actual patient data. A sensitivity analysis was performed to determine the highest MCE cost that would still result in cost savings using the MCE-guided compared to Usual Care approach. To this end the cost of MCE was varied and the total cost per patient for each value was re-calculated using the decision-analysis software.
Figure 1.
Decision tree for cost-efficiency analysis. See text for details.
Results
A total of 1194 consecutive patients were enrolled between October 2000 and January 2003. Of these, 957 had complete RF, MP, and follow-up data, and form the basis of this report. Approximately half were male (n=498, 52%), with a median age of 60 years (range 32 to 92).
Based on clinical criteria, 316 (33%) patients were discharged directly from the ED. The majority was (n=263 or 83%) discharged after a complete “rule out” that included at least 2 cTNI determinations and >8 h stay in the ED. Of these patients, there was only 1 early event (unstable angina pectoris). The average cost for usual-care patient evaluation prior to ED discharge was $805.
A total of 641 patients were admitted to the hospital. Their final diagnoses, events, and costs are shown in Table 1. Most patients (n=503, 78%) were discharged from the hospital within 24 hours without any events. Thus, including the patients discharged after a full ‘rule-out’ in the ED, a total of 819 of patients in the usual care arm (86%) required a prolonged stay for the management of their CP.
Table 1.
Final Diagnosis and Costs for Patients Admitted to Hospital (n=641)
| DRG | Number of Patients | 2003 Medicare Reimbursement ($) | Total Cost ($) |
|---|---|---|---|
| Chest Pain (without a primary or secondary event) | 513 | 4,343 | 2,227,959 |
| Acute Myocardial Infarction | 63 | 7,277 | 458,451 |
| Death | 2 | 12,079 | 24,158 |
| Unstable Angina Pectoris | 48 | 3,904 | 187,392 |
| Congestive Heart Failure | 12 | 7,896 | 94,752 |
| Coronary Artery Bypass Surgery | 3 | 41,665 | 124,995 |
The most common test performed after early discharge from the ED was SPECT: 72 patients (24%) directly discharged directly from the ED underwent this test. The average total cost per patient in the Usual Care branch (including downstream diagnostic tests) was $5,000.
RF was found to be normal in 523/957 (55%) of patients with suspected cardiac CP. Because the early (within 48 hours) event rate is very low in patients with normal MCE1,2, these patients could have theoretically been discharged directly from the ED as shown in the algorithm in Figure 1. The events in these patients are shown in Table 2. The 2 patients with AMI had peak cTNI of 3 and 19 ng·mL-1, respectively.
Table 2.
Events in All Patients Based on Contrast Echocardiographic Findings
| Variable | Normal MCE (n=523) | Abnormal RF Normal MP (n=164) | Abnormal RF Abnormal MP (n=270) |
|---|---|---|---|
| Acute Myocardial Infarction | 2 (0.4%) | 16 (9.8%) | 45 (16.0%) |
| Death | 1 (0.2%) | 0 (0%) | 1 (0.4%) |
| Unstable Angina Pectoris | 8 (1.5%) | 9 (5.5%) | 31 (11.0%) |
| Percutaneous Coronary Intervention | 7 (1.3%) | 1 (0.6%) | 2 (0.7%) |
| Congestive Heart Failure | 0 (0%) | 3 (1.8%) | 9 (3.3%) |
| Coronary Artery Bypass Surgery | 1 (0.2%) | 0 (0%) | 2 (0.7%) |
There were 434 patients (45%) who had an abnormal MCE and all of these would have theoretically been admitted to the hospital for further care as shown in Figure 1. Of these, 270 had both abnormal RF and MP, and 164 had abnormal RF but normal MP. Patients with abnormal RF but normal MP, as well as those with both abnormal RF and abnormal MP, had significantly higher primary and secondary event rates within 48 hours compared to those with normal RF and normal MP (Table 2).
Overall, a MCE-guided approach would have prevented 207 admissions to hospital with a DRG of CP, and significantly decreased the period of observation for the other 316 patients with normal MCE. The average cost per patient using a MCE-guided approach was found to be $4,100 despite the added cost ($269 including the cost of the contrast agent) of performing MCE studies in all patients. This results in savings of $900 per patient (or $861,300 for the entire cohort) using the MCE-guided approach compared to the Usual Care arm.
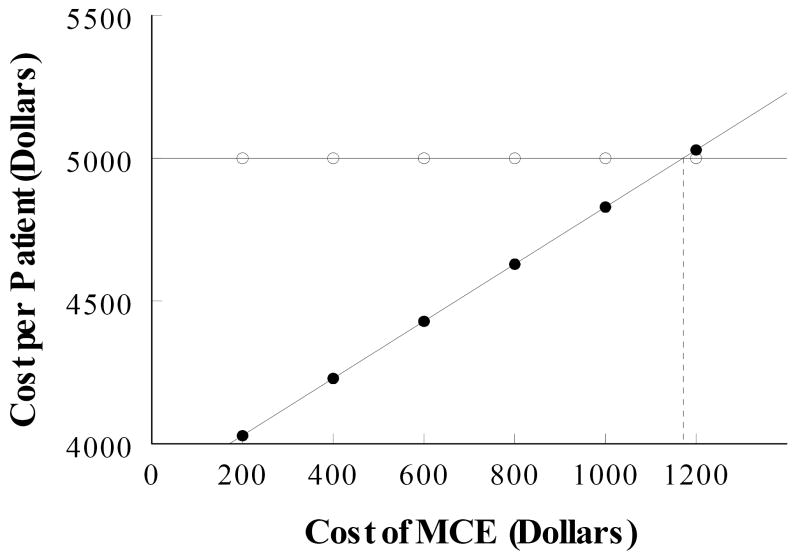
The cost of MCE was varied and the total cost per patient for each value was recalculated using the decision-analysis software to determine the maximum cost of MCE that would still be cost-efficient compared to Usual Care. As shown in Figure 2, the cost of MCE can increase up to $1,151 before the cost of Usual Care would be exceeded.
Figure 2.
Sensitivity analysis for MCE. The cost of Usual Care is shown in open circles. As MCE cost increases, there is a proportional increase in the cost of MCE-guided Care (closed circles). See text for details.
Discussion
Only a minority of patients presenting to the ED with CP have a serious underlying cause. Although clinical variables such as the history, physical examination, EKG6, and cardiac serum markers7,8 can identify patients at increased risk for early cardiac events, they are inadequate for identifying those who can be discharged early. In this study, we have shown that despite the added cost of performing MCE in all patients, the strong negative predictive value of MCE can identify low-risk patients with non-cardiac CP, thereby potentially reducing unnecessary hospitalizations. By decreasing downstream resource utilization in patients with normal studies, MCE is a cost-efficient tool that can also accelerate appropriate triage of CP patients. Because echocardiography is portable and the results are immediately available, it could be performed early in the management pathway and potentially decrease the treatment/observation time of these patients in EDs and CP centers.
Three patients with normal MCE developed a primary event (2 had uncomplicated AMI and 1 suffered a non-cardiac death). Using the MCE-guided approach these patients would have theoretically been discharged from the ED. That is, a total of 3/523 (0.6%) primary events would have been missed and these patients sent home using a MCE-guided approach, which is an extremely low false negative rate. Other studies have reported that between 2-8% of patients with AMI who present to the ED with chest pain are sent home inadvertently using the standard care approach9-12.
Incremental cost-effective ratio is another way to look at cost-effectiveness13. It represents the ratio of the difference in cost between two approaches to the difference in outcomes between them. Thus, the incremental cost-effectiveness ratio summarizes the additional cost per unit of health benefit gained in switching from one approach to another. Because the usual care cost in patients with normal RF is $900 more than the MCE-guided approach, the cost-effectiveness ratio is $450 per patient or $235,350 per missed AMI using the normal approach. Using $75,000 per event as an acceptable benchmark for cost14, the MCE-guided approach, therefore, is more cost-effective.
Theoretical discharge in this analysis was based solely on MCE findings which were analyzed outside of the clinical context. In reality, it is unlikely that patients with normal MCE who developed events would have been discharged, since all had a history of prolonged or stuttering chest pain, multiple cardiovascular risk factors, and 7 of the 19 had previously documented CAD.
Stress testing prior to discharge has been advocated in patients with a low to intermediate clinical risk. The rationale for performing these studies is to detect occult CAD and for risk stratification. The incidence of positive tests in low-risk patients after evaluation in a CP unit was 13% in one study15. According to current guidelines, however, stress testing is only recommended after clinical assessment including serial resting EKG’s and cardiac serum marker determinations have been performed to rule out an ACS - delaying stress testing for 8 to 12 h in most cases16. An accelerated protocol with early dobutamine echocardiography has been reported to be safe, but even then the testing was delayed for up to 6 hours17.
In our study, 83% of patients were monitored for a minimum of 12 h, while the median time to MCE was only 3 h. The incidence of events in patients with normal resting MCE who were clinically low or intermediate risk was negligible. Thus, using MCE-guided care, it may be possible to further expedite these tests prior to discharge. Such an approach still requires further confirmation.
There are some limitations to our study. Our patients were entered in the study based on specific selection criteria and were also evaluated by cardiologists before it was decided that the cause of CP could be cardiac. The potential cost-savings generated by our analysis have, therefore, to be interpreted in this context. If MCE were to be used indiscriminately in all CP patients presenting to the ED then cost savings may not be realized.
Our results imply that we could cut down the ED stay of patients with a normal MCE by several hours. This could also entail additional cost savings. Unfortunately the practice of medicine can be driven by reimbursement rather than cost-savings and if the re-imbursement is higher for a lengthier ED stay, then the results of the MCE will be ignored. In that case adding MCE may increase cost further.
Finally, in this large single center study of patients with suspected cardiac CP, we had no serious adverse events with Optison. In a continuation of this study an additional ~1000 similar patients received Definity, again with no serious side effects. We, therefore, believe that the use of ultrasound contrast agents is also safe in patients with suspected ACS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tong KL, Kaul S, Wang XQ, Rinkevich D, Kalvaitis S, Belcik T, Lepper W, Foster WA, Wei K. Myocardial Contrast Echocardiography versus TIMI Score in Patients Presenting to the Emergency Department with Chest Pain and a Non-Diagnostic Electrocardiogram. J Am Coll Cardiol. 2005;46:920–927. doi: 10.1016/j.jacc.2005.03.076. [DOI] [PubMed] [Google Scholar]
- 2.Rinkevich D, Kaul S, Lepper W, Wang XQ, Tong KL, Belcik T, Kalvitis S, Lepper W, Dent JM, Wei K. Regional Left ventricular Perfusion and Function in Patients Presenting to the Emergency Department with Chest Pain and no ST Segment Elevation. Eur Heart J. 2005;26:1606–1611. doi: 10.1093/eurheartj/ehi335. [DOI] [PubMed] [Google Scholar]
- 3.Kalvaitis S, Kaul S, Rinkevich D, Tong KL, Belcik T, Wei K. Effect of Time Delay on the Diagnostic and Prognostic Utility of Myocardial Contrast Echocardiography in Patients Presenting with Suspected Cardiac Chest Pain to the Emergency Department. J Am Soc Echocardiogr. 2006;19:1488–1493. doi: 10.1016/j.echo.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Dawson D, Rinkevich D, Belcik T, Jayaweera AR, Rafter P, Kaul S, Wei K. Measurement of Myocardial Blood Flow Velocity Reserve with Myocardial Contrast Echocardiography in Patients with Suspected Coronary Artery Disease: Comparison with Quantitative gated 99mTc Sestamibi SPECT. J Am Soc Echocardiogr. 2003;16:1171–1177. doi: 10.1067/S0894-7317(03)00646-1. [DOI] [PubMed] [Google Scholar]
- 5.TreeAge Pro 2006 User’s Manual. Williamstown, MA: TreeAge SoftwareInc; 2006. pp. 16–25. [Google Scholar]
- 6.Villanueva FS, Sabia PJ, Afrookteh A, Pollock SG, Hwang LJ, Kaul S. Value and limitations of current methods of evaluating patients presenting to the emergency room with cardiac-related symptoms for determining long-term prognosis. Am J Cardiol. 1992;69:746–750. doi: 10.1016/0002-9149(92)90499-o. [DOI] [PubMed] [Google Scholar]
- 7.Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335:1342–1349. doi: 10.1056/NEJM199610313351802. [DOI] [PubMed] [Google Scholar]
- 8.Luscher MS, Thygesen K, Ravkilde J, Heickendorff L, for the TRIM Study Group Applicability of cardiac troponin T and I for early risk stratification in unstable coronary artery disease. Circulation. 1997;96:2578–2585. doi: 10.1161/01.cir.96.8.2578. [DOI] [PubMed] [Google Scholar]
- 9.Lee TH, Cook EF, Weisberg M, Sargent RK, Wilson C, Goldman L. Acute chest pain in the emergency room. Identification and examination of low-risk patients. Arch Intern Med. 1985;145:65–69. [PubMed] [Google Scholar]
- 10.McCarthy BD, Beshansky JR, D’Agostino RB, Selker HP. Missed diagnoses of acute myocardial infarction in the emergency department: results from a multicenter study. Ann Emerg Med. 1993;22:579–82. doi: 10.1016/s0196-0644(05)81945-6. [DOI] [PubMed] [Google Scholar]
- 11.Pope JH, Ruthazer R, Beshansky JR, Griffith JL, Selker HP. The clinical presentation of patients with acute cardiac ischemia in the emergency department: a multicenter controlled clinical trial. J Thromb Thrombolysis. 1998;6:63–74. doi: 10.1023/A:1008876322599. [DOI] [PubMed] [Google Scholar]
- 12.Lee TH, Rouan GW, Weisberg MC, Brand DA, Acampora D, Stasiulewicz C, Walshon J, Terranova G, Gottlieb L, Goldstein-Wayne B. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol. 1987;60:219–24. doi: 10.1016/0002-9149(87)90217-7. [DOI] [PubMed] [Google Scholar]
- 13.Bambha K, Kim WR. Cost-effectiveness analysis and incremental cost-effectiveness ratios: uses and pitfalls. Eur J Gatroen Hepat. 2004;16:519–526. doi: 10.1097/00042737-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein MC, Fineberg HV, Ebstein AS, Frazier HS, Heuhauser D, Neutra RR, McNeil BS. Clinical Decision Analysis. Philadelphia, Pennsylvania: WB Saunders Co.; 1998. Clinical decision and limited resources; pp. 228–265. [Google Scholar]
- 15.Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed S. Immediate exercise testing to evaluate low risk patients presenting to the emergency department with chest pain. J Am Coll Cardiol. 2002;40:251–256. doi: 10.1016/s0735-1097(02)01968-x. [DOI] [PubMed] [Google Scholar]
- 16.Stein RA, Chaitman BR, Balady GJ, Fleg JL, Limacher MC, Pina IL, Williams MA, Bazzarre T. Safety and utility of exercise testing in emergency room chest pain centers. An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association. Circulation. 2000;102:1463–1467. doi: 10.1161/01.cir.102.12.1463. [DOI] [PubMed] [Google Scholar]
- 17.Nucifora G, Badano LP, Sarraf-Zadegan N, Karavidas A, Trocino G, Scaffidi G, Pettinati G, Astarita C, Vysniauskas V, Gregori D, Ilerigelen B, Marinigh R, Fioretti PM. Comparison of Early Dobutamine Stress Echocardiography and Exercise Electrocardiographic Testing for Management of Patients Presenting to the Emergency Department With Chest Pain. Am J Cardiol. 2007;100:1068–1073. doi: 10.1016/j.amjcard.2007.05.027. [DOI] [PubMed] [Google Scholar]