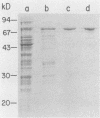
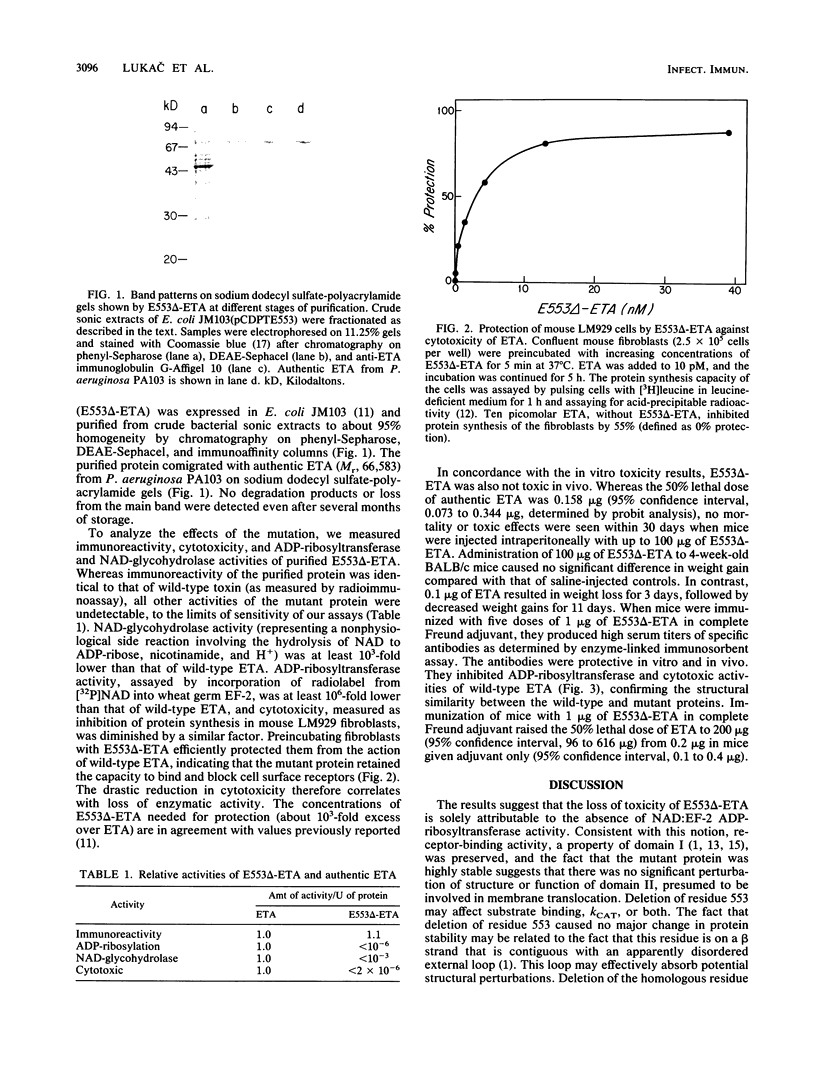
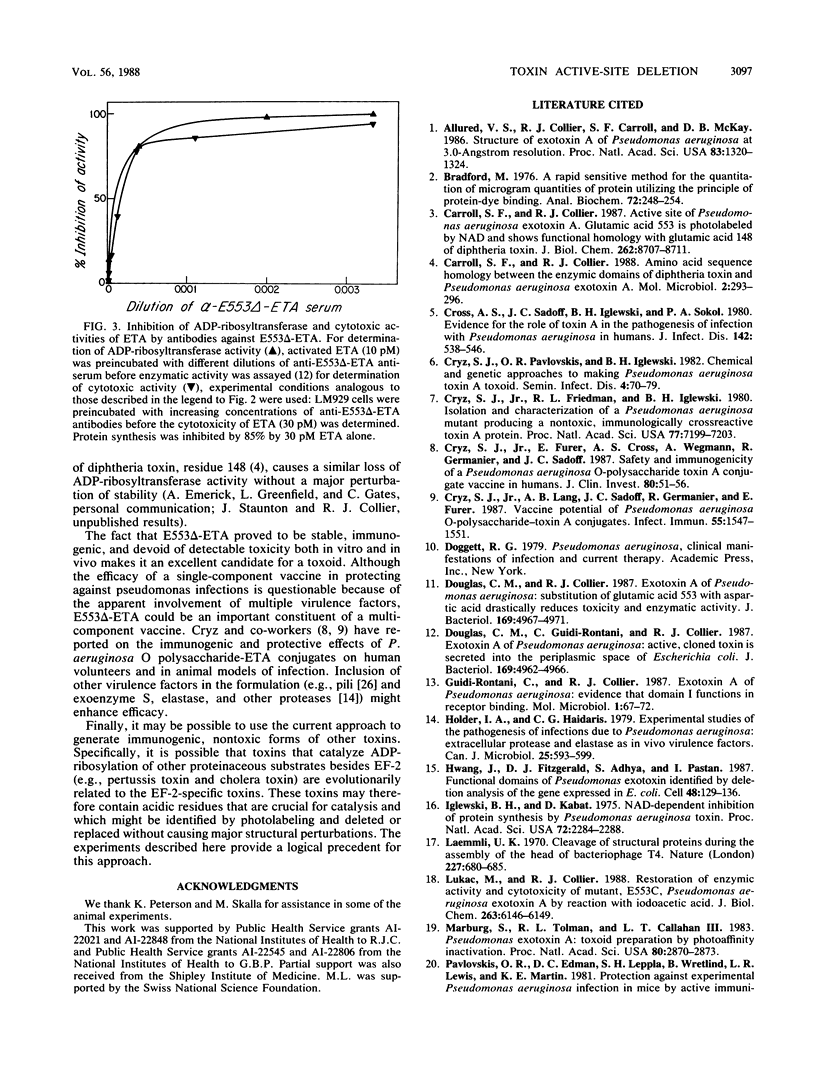
Abstract
Glutamic acid-553 of Pseudomonas aeruginosa exotoxin A (ETA), identified previously as an active-site residue, was deleted by oligonucleotide-directed mutagenesis of the cloned toxin gene in Escherichia coli. The purified mutant toxin was stable, fully immunoreactive, and capable of blocking toxin receptors. ADP-ribosyltransferase and cytotoxic activities were at least 10(6)-fold lower than those of wild-type ETA, and injection of mice with 50 micrograms (equivalent to 400 lethal doses of ETA) produced no ill effects. The mutant toxin elicited high levels of neutralizing anti-ETA antibodies in mice, which protected against a challenge with 100 micrograms of authentic ETA (greater than 600 lethal doses). The mutant protein has the attributes of a toxoid and may be useful as a component of vaccines for individuals at risk for infection by P. aeruginosa.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carroll S. F., Collier R. J. Active site of Pseudomonas aeruginosa exotoxin A. Glutamic acid 553 is photolabeled by NAD and shows functional homology with glutamic acid 148 of diphtheria toxin. J Biol Chem. 1987 Jun 25;262(18):8707–8711. [PubMed] [Google Scholar]
- Carroll S. F., Collier R. J. Amino acid sequence homology between the enzymic domains of diphtheria toxin and Pseudomonas aeruginosa exotoxin A. Mol Microbiol. 1988 Mar;2(2):293–296. doi: 10.1111/j.1365-2958.1988.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Sadoff J. C., Iglewski B. H., Sokol P. A. Evidence for the role of toxin A in the pathogenesis of infection with Pseudomonas aeruginosa in humans. J Infect Dis. 1980 Oct;142(4):538–546. doi: 10.1093/infdis/142.4.538. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Friedman R. L., Iglewski B. H. Isolation and characterization of a Pseudomonas aeruginosa mutant producing a nontoxic, immunologically crossreactive toxin A protein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7199–7203. doi: 10.1073/pnas.77.12.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Cross A. S., Wegmann A., Germanier R., Sadoff J. C. Safety and immunogenicity of a Pseudomonas aeruginosa O-polysaccharide toxin A conjugate vaccine in humans. J Clin Invest. 1987 Jul;80(1):51–56. doi: 10.1172/JCI113062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Lang A. B., Sadoff J. C., Germanier R., Fürer E. Vaccine potential of Pseudomonas aeruginosa O-polysaccharide-toxin A conjugates. Infect Immun. 1987 Jul;55(7):1547–1551. doi: 10.1128/iai.55.7.1547-1551.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. M., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: substitution of glutamic acid 553 with aspartic acid drastically reduces toxicity and enzymatic activity. J Bacteriol. 1987 Nov;169(11):4967–4971. doi: 10.1128/jb.169.11.4967-4971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. M., Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: active, cloned toxin is secreted into the periplasmic space of Escherichia coli. J Bacteriol. 1987 Nov;169(11):4962–4966. doi: 10.1128/jb.169.11.4962-4966.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: evidence that domain I functions in receptor binding. Mol Microbiol. 1987 Jul;1(1):67–72. doi: 10.1111/j.1365-2958.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Holder I. A., Haidaris C. G. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: extracellular protease and elastase as in vivo virulence factors. Can J Microbiol. 1979 May;25(5):593–599. doi: 10.1139/m79-085. [DOI] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukac M., Collier R. J. Restoration of enzymic activity and cytotoxicity of mutant, E553C, Pseudomonas aeruginosa exotoxin A by reaction with iodoacetic acid. J Biol Chem. 1988 May 5;263(13):6146–6149. [PubMed] [Google Scholar]
- Marburg S., Tolman R. L., Callahan L. T., 3rd Pseudomonas exotoxin A: toxoid preparation by photoaffinity inactivation. Proc Natl Acad Sci U S A. 1983 May;80(10):2870–2873. doi: 10.1073/pnas.80.10.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Pollack M., Callahan L. T., 3rd, Iglewski B. H. Passive protection by antitoxin in experimental Pseudomonas aeruginosa burn infections. Infect Immun. 1977 Dec;18(3):596–602. doi: 10.1128/iai.18.3.596-602.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Longfield R. N., Karney W. W. Clinical significance of serum antibody responses to exotoxin A and type-specific lipopolysaccharides in patients with Pseudomonas aeruginosa infections. Am J Med. 1983 Jun;74(6):980–987. doi: 10.1016/0002-9343(83)90795-7. [DOI] [PubMed] [Google Scholar]
- Routman A., Van Manen W., Haddad R., Pollock B., Holmes B., Mogabgab W. J. Cefsulodin treatment for serious Pseudomonas aeruginosa infections. J Int Med Res. 1986;14(5):242–253. doi: 10.1177/030006058601400504. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Okinaga K. Role of pili in the adherence of Pseudomonas aeruginosa to mouse epidermal cells. Infect Immun. 1987 Aug;55(8):1774–1778. doi: 10.1128/iai.55.8.1774-1778.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]