Abstract
Retinoids are known to have potent effects on hematopoietic stem cell integrity, and our objective was to learn if they influence cells destined to replenish the immune system. Total CD19+ B lineage cells increased substantially in marrow and spleens of ATRA treated C57BL6 mice, while lymphoid progenitors were reduced. All B lymphoid progenitors were targets of ATRA in culture and overall cell yields declined without reductions in proliferation. Remarkably, ATRA shortened the time required for primitive progenitors to generate CD19+ cells. PCR analysis and a panel of RAR/RXR agonist treatments suggested that RARα mediates these responses. The transcription factors EBF1 and Pax-5 were elevated during treatment and ATRA had similar effects on human B cell differentiation. That is, it inhibited the expansion of human progenitor cells and accelerated their differentiation to B lineage cells. There may be previously unsuspected side effects of ATRA therapy, and the new findings suggest retinoids can normally contribute to the lymphopoietic environment in bone marrow.
Introduction
Metabolites of vitamin A (retinol) have diverse biological activities that include roles in vision, embryonic development, cell proliferation, differentiation and survival(1). All-trans retinoic acid (ATRA) has been particularly well studied as a developmental morphogen, a regulator of gene transcription and a substance that may normally regulate hematopoietic stem cells (HSC). Evidence for the latter initially came from findings that ATRA promotes retention of stem cell properties in culture (2, 3). More recently, it was discovered that numbers of HSC were low in mice with a targeted retinoid acid receptor gamma (RARγ) gene, while hematopoietic progenitors were abnormally increased (4). Furthermore, the HSC in these animals were defective with respect to long-term reconstitution ability and appeared to exhaust with age.
ATRA has long been known to promote progression in the myeloid lineage, and this property has been effectively exploited for therapy in acute promyelocytic leukemia (1). Furthermore, both innate and adaptive arms of the immune system are compromised in vitamin A deficient animals (5). ATRA and 9-cis- retinoic acid have been shown to block activation induced apoptosis in thymocytes while two other metabolites, 14-hydroxy-retro-retinol and anhydroretinol compete with each other in supporting lymphocyte viability and expansion (6). There is also a report that retinoids inhibit IL-7 driven growth of murine pre-B cells (7).
There are hundreds of retinoid regulated genes, and only some of their promoters contain retinoic acid response elements (8). Furthermore, HSC and other potential targets of retinoid action are rare in bone marrow. Consequently, it has been difficult to describe mechanisms that account for retinoic acid effects on hematopoiesis. Genes that have been considered in connection with this issue include c-Myc, Stra-13, C/EBPε, Hoxb4, p21Cip1/Waf1, p27Kip1 and Notch 1 (1, 4). The pre-B cell leukemic homeobox1 (PBX1) gene is up-regulated, and the half life of the PBX1 protein is extended by ATRA (9). The PBX1 gene is translocated in 23% of pediatric pre-B leukemias such that a PBX1-E2A fusion protein is made (10). Importantly, a role for PBX1 in normal B lymphopoiesis has just been discovered (11). Thymic stromal lymphopoietin (TSLP) was originally discovered as a cytokine that accelerated B lymphopoiesis (12). Although it is now attracting interest as a master regulator of atopic dermatitis and asthma, TSLP drives production of the first wave of B cells, and especially B1 progenitors (13-15). It is therefore interesting that TSLP expression is induced by retinoids (16).
Collectively, these findings suggest possible roles for retinoids in development and maintenance of the immune system. However, information is needed concerning target cells and molecular mechanisms. We have now determined that B lymphopoiesis was accelerated when several types of progenitors were exposed to ATRA.
Materials and Methods
Reagents and Treatments
All trans retinoic acid, 9-cis-Retinoic acid, 13-cis-retinoic acid and AC-41848 were purchased from Sigma Chemical Co. (St. Louis, MO). TTNPB and AM-580 were from Biomol (Plymouth Meeting, PA). RAG-1/GFP knock-in mice have been described (17). Heterozygous F1 RAG-1/GFP mice were generated at the OMRF Laboratory Animal Resource Center (LARC). C57BL6 mice used in CFSE assays (CD45.2 alloantigen) were bred and maintained in the LARC. Twenty one day time-release pellets of all trans retinoic acid (10 mg/pellet) were purchased from Innovative Research of America (Sarasota, FL) and implanted subcutaneously with a 10-gauge precision trochar. Assuming constant delivery, this would correspond to 15mg/kg/day or 45 mg/m2 (calculated according to: http://www.fda.gov/cder/cancer/animalframe.htm). This dose is used for human therapy in acute promyelocytic leukemia (18). After 1 week, mice were killed and bone marrow, splenocytes and thymocytes were isolated and analyzed by flow cytometry. All animal protocols and use of human umbilical cord blood were approved by appropriate institutional committees.
Antibodies
Regarding mAbs for murine antigens, anti-CD3, anti-CD8, anti-CD19 (1D3), anti-CD45RA (14.8), anti-FcRγ (2.4G2), anti-TER119 mAbs were purified from the cultured supernatant of hybridoma cells grown in our laboratory. Purified anti-Ly-6G (Gr-1) mAbs, Biotin-conjugated anti-CD3 (145-2C11), anti-CD8 (53-6.7), anti-CD19 (1D3), anti-CD45R/B220 (RA3/6B2), anti-Mac-1 (M1/70), anti-Gr1 (RB6/8C5), anti-NK1.1 (PK136), anti-Ter119 (ly-76), and anti-CD43 (S7) mAbs, allophycocyanin-conjugated anti-c-Kit (2B8), and anti-NK1.1 (PK136), APC-Cy7 conjugated anti-CD19 (1D3), anti-B220 (RA3-6B2), anti-CD4 (GK1.5) and anti-CD25 (PC61), PE-Cy5-confugated-anti CD45RA/B220, PerCp-CY5.5-conjugated anti-CD45.2 (104), and anti-IgM (R6-60.2),PE-conjugated anti-IL7Rα (SB199), anti-CD11c (HL3), and anti-CD3 (145-2c11) mAbs were all purchased from BD PharMingen (San Diego, CA). PE-Cy5-conjugated anti-Sca1 (D7), anti-CD24 (M1/169), anti-Gr-1 (RB6-8C5), anti-CD44 (IM7), Allophycocyaninconjugated anti-CD93 (AA4.1), PE-conjugated anti-IgM (eB121-15F9), anti-F4/80 (BM8), anti-CD21 (eBio8D9), and anti-CD11b (M1/70) were obtained from eBioscience (San Diego, CA). Allophycocyanin-conjugated anti-CD23 (2G8) was obtained from Southern Biotech (Birmingham, AL). PE-conjugated anti-CD23 (B3B4) was obtained from Pharmingen. PE-Texas red tandem-conjugated streptavidin, allophycocyaninconjugated streptavidin, PE-Cy5.5-conjugated streptavidin were purchased from Caltag Laboratories (Burlingame, CA). Regarding mAbs for human antigens, FITC-conjugated anti-CD38 and APC-conjugated anti-CD34 were obtained from BD PharMingen, FITC conjugated anti-CD14 and PE-conjugated anti-CD19, anti-CD14, anti-CD33, anti-CD56, anti-CD64 and anti-Glycophorin A mAbs were purchased from Caltag Laboratories (Burlingame, CA).
Immunofluorescence Staining and Cell Sorting
Cells recovered from animals or cultures were treated with anti-FCRγ (Fc gamma RIII/II, 2.4G2) antibody to minimize nonspecific binding. Cells were stained with antibodies, and flow cytometry analyses were performed on a LSR II using the BD FACSDiva Software (BD Biosciences, San Jose, CA).
Follicular B cells were defined as B220+CD24intCD21int, T1 were defined as B220+CD24hiCD21−, T2 were defined as B220+CD24hiCD21hiCD23+ and marginal zone cells were defined as B220+CD24hiCD21hiCD23−, respectively (19). In another type of staining, mouse follicular B cells were defined as B220+AA4.1−sIgMlowCD23+, T1 were defined as B220+AA4.1+ sIgM hi CD23−, T2 were defined as B220+AA4.1+ sIgM hi CD23+, T3 were defined as B220+AA4.1+ sIgM low CD23+, and marginal zone cells were defined as B220+AA4.1−sIgMhiCD23−, respectively (20).
Mouse bone marrow cells collected from 6-12 week old mice were suspended in PBS buffer supplemented with 3% FCS. Cells were incubated with mAbs to lineage markers (Gr-1 and Mac-1 for myeloid cells, CD19 and CD45RA for B lineage cells, TER-119 for erythroid cells, CD3 and CD8 for T lineage cells, and NK1.1 for NK cells), followed by incubation with goat anti-rat IgG-coated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Lineage cells attached to beads were removed with a magnetic separator. The lineage-depleted bone marrow cells were then incubated with a mixture of labeled Abs to the lineage markers, PE-Cy5-anti-Sca-1 and APC- anti-c-Kit. Cells were sorted on a Moflo Cell Sorter (Dako, Fort Collins, CO) or FACSAria (BD Biosciences, San Jose, CA). The LSK fraction was defined as Lin−GFP− Scal-1+c-KitHi, ELP were defined as Lin−GFP+Sca1+c-KitHi, and Pro-L were defined as Lin−Sca1+c-KitLo. Purity of each sorted population was confirmed by post-sorting analyses.
Human cord blood mononuclear cells were separated over Ficoll/Hypaque (Lymphocyte Separation Medium; Cellgro-Mediatech, Herndon, VA). Enrichment for CD34+ cells was performed using the Direct CD34 Isolation kit (Miltenyi Biotec). Enriched CD34+ cells were stained with anti-Lin-PE (CD33, CD14 and CD64 for monocyte, CD19 for B lineage, CD56 for NK, CD3 and CD8 for T, and Glycophorin for erythrocyte), anti-CD34-APC and anti-CD38-FITC for 20 min at 4°C. One hundred CD34+CD38− cells were sorted directly into 96-well flat-bottom plates containing pre-established MS-5 stromal cells using the Automated Cell Sorting System of the MoFlo Cell Sorter.
Murine Stromal Cell Co-cultures
Double or triple sorted progenitor cells were co-cultured with OP9 stromal cells in 96 well plates. The α-MEM medium (Cellgro-Mediatech) contained 10% FCS, rm SCF (20 ng/ml), rm Flt3 ligand (FL;100 ng/ml), rm IL-7 (1 ng/ml), 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. One hundred progenitor cells were co-cultured with two hundred stromal cells up to 11 days without replating the stromal cell. At the end of culture, hematopoietic cells were counted excluding stromal cells and then subjected to flow cytometry. We also used anti-CD45.2 mAb to exclude potential contamination of stromal cells.
Human Stromal Cell Co-cultures
Human cell cultures were performed as described (21) with some minor modifications. Briefly, one thousand cells per well of MS-5 stromal cells were plated in 96-well plates one day prior to the seeding of hematopoietic progenitor cells at a concentration of one hundred cells per well. Cells were cultured in α-MEM medium and a combination of rhSCF (20 ng ml−1), rhG-CSF (10 ng ml−1), and rh FL (10 ng ml−1) with or without the presence of ATRA. Dup-697 (Cayman Chemical Co., Ann Arbor, MI) was always included in human stromal cell co-culture with a final concentration of 1 × 10−7 M to improve the production efficiency of the culture (22). The cultures were maintained for 4 weeks and fed weekly by removing half of the medium and replacing it with fresh medium. Cytokines and ATRA were added with each feeding. Flow cytometry was performed at the indicated intervals.
Stromal Cell Free, Serum Free Cultures
Double or triple sorted progenitor cells were put in the X-Vivo medium containing 1% detoxified bovine serum albumin, 20 ng/ml SCF, 100 ng/ml FL, 1ng/ml IL7, 5 × 10−5 M 2-mercaptoethanol, 2mM L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin as described (23). Cells were analyzed by flow cytometry or Pre-B colony assay 4 to 11 days later.
ProB Colony Assays
Progenitor cells derived from co-cultured ELP were put in Iscove's MDM-based methylcellulose medium (Methocult GF 3630; StemCell Technologies, Vancouver, Canada) with additional supplement of 20 ng/ml of rm SCF and 100 ng/ml of rm FL. After 7 days, colonies were enumerated under an inverted microscope. In some experiments, flow cytometry was performed to confirm the CD19+ expression, and May-Grunwald/Giemsa staining was used to verify the B lineage lymphoid cell morphology.
Real-Time PCR Analysis of Gene Expression
Double sorted progenitor cells including LSK, ELP and Pro-L were lysed, and total RNA was isolated using RNeasy Mini Kit (Qiagen). To analyze the gene expression pattern affected by ATRA, sorted LSK cells were treated with vehicle (0.1% ethanol) or ATRA in stromal cell free culture for 1, 3, 5, or 7 days. The treated cells were washed, and total RNA was isolated. Total RNA was treated with DNase I (Invitrogen) to remove contaminating genomic DNA, and cDNA was made using random primers (Invitrogen) and moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA). The 20 µl real-time PCR amplification mixture contained template cDNA, 1 µl of pre-designed Taqman PCR primers (Applied Biosystems, Foster City, CA) and 10 µl 2x Taqman PCR Master Mix (ABI). Reactions were 50°C 2 min, 95°C 10 min followed by 40 cycles of 95°C 15 Sec and 60°C 1min. Relative gene expression was calculated as 2−ΔΔCT values with eukaryotic 18sRNA used as an endogenous control. Real-time PCR was performed on an ABI PRISM 7500 (Applied Biosystems, Foster City, CA). Three repeats were run for each sample, and each experiment was performed three times. Taqman pre-designed PCR primers were purchased from ABI.
CFSE Labeling
LSK Sorted from C57BL6 mice (1 × 106 cells/ml) were labeled with 10 µM CFSE (Invitrogen, Cat number: C34554) from a 5 mM stock solution (in DMSO) for 10 min at 37°C. Cells were washed and put back in stromal cell free culture, and intensity of CFSE signal and the proliferation status of cultured cells were monitored at different times by flow cytometry.
Results
All trans retinoic acid increases B cell numbers in vivo
As a first approach, time-release pellets containing ATRA or placebo were implanted into RAG-1/GFP knock-in mice. One week later, spleens in the ATRA treated mice were notably increased in size, and total nucleated cells were elevated two fold (Fig. 1A). Flow cytometry revealed that this resulted primarily from increases in B lymphocyte numbers while T cells and NK cell numbers were within the normal range. CD11b+ myeloid cells were not substantially changed, but normally rare B220− CD11c+ CD11b+ myeloid dendritic cells were elevated in response to ATRA treatment.
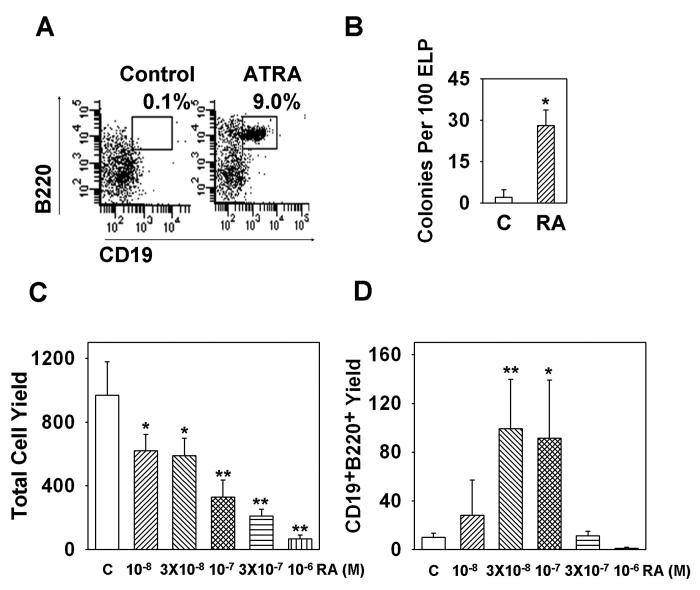
Figure 1. All trans retinoic acid depletes primitive lymphoid progenitors in bone marrow and increases B cells in the spleen.
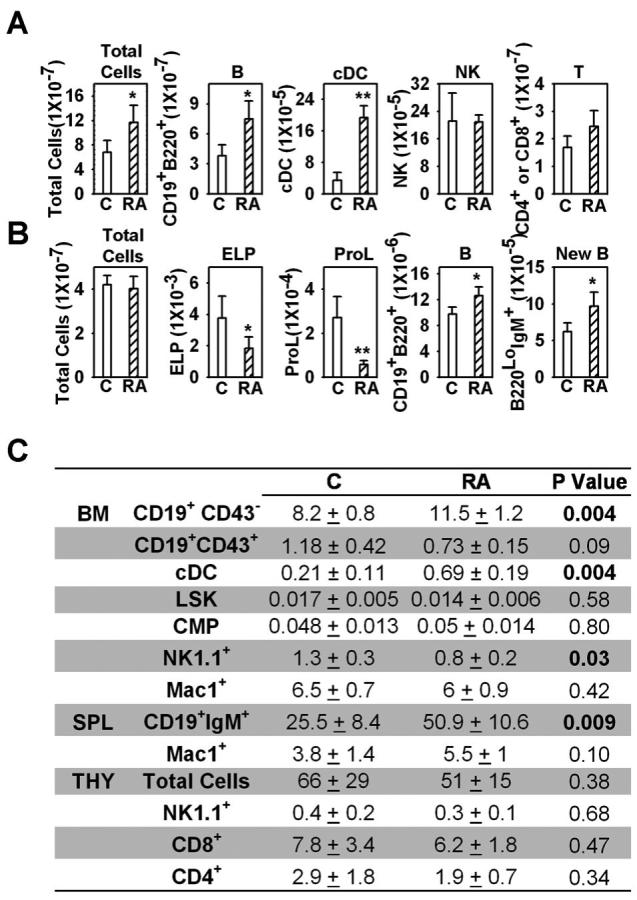
RAG-1/GFP mice were treated with ATRA or placebo pellets for seven days. Bone marrow, splenocytes and thymocytes were isolated, dead cells were excluded by trypan blue staining, flow cytometry was performed and absolute numbers of cells in the spleen (A or C), one femur (B or C) or thymus (C) were calculated. cDC were defined as B220−CD11b+CD11c+; NK cells were defined as NK1.1+; ELP (early lymphoid progenitor) were defined as Lin− RAG-1+ Scal-1+c-KitHi; Pro-L were defined as Lin−RAG-1+Sca-1+c-KitLo; LSK was defined as Lin−GFP−Sca1+c-KitHi; CMP (common lymphoid progenitor) were defined as Lin−GFP−Sca1−c-KitHi. All data are reported as mean ± S.D for n= 4. *, p < 0.05, and **, p < 0.01 (Student's t-test). All numbers are given as millions of cells, and P values were bolded when p < 0.05 in Fig. 1C. The results shown are representative of five independent experiments.
Two staining protocols were used to determine if particular B cell subsets were preferentially increased in spleens of ATRA treated mice. Follicular B cells, T1, T2 and marginal zone cells increased by 55%, 52%, 89% and 82%, respectively (19). Follicular B cells, T1, T2, T3 and marginal zone B cells were elevated by 70%, 60%, 65%, 53 and 59% with another type of analysis (20).
We then conducted a thorough analysis of hematopoietic cells in bone marrow and were surprised to find that numbers of total nucleated cells were within the normal range (Fig. 1B). Further scrutiny of the B lineage showed that numbers of Lin− RAG-1/GFP+ Sca-1+ c-KitHi early lymphoid progenitors (ELP) and Lin− Sca-1+ c-KitLo RAG-1/GFP+ pro-lymphocytes (Pro-L) were significantly reduced, while CD19+ CD43+ ProB/large Pre-B cells were slightly decreased (Figure 1C and data not shown). In contrast, numbers of CD19+ B220+ lymphocytes and CD45R/B220Lo IgM+ newly formed B cells were increased. These changes were both stage and lineage specific, because the larger Lin− Sca-1+ c-KitHi RAG-1/GFP− (LSK) category of stem/progenitors, Lin− Sca-1− c-KitHi RAG-1/GFP− common myeloid progenitors (CMP) and CD11b+ /Mac1+ myeloid cells were all unchanged (Fig. 1C). As with the spleen, there was an increase in CD11c+ CD11b+ myeloid dendritic cells (cDC), while NK1.1+ NK/IKDC cells were somewhat decreased in bone marrow. In contrast to the spleen, numbers of thymocytes were equivalent to those in placebo controls (Fig. 1C). These results demonstrate that retinoids can selectively increase numbers of peripheral B lymphocytes. Reductions in primitive B lineage progenitors in bone marrow, together with increased numbers of B cells might suggest accelerated differentiation or emigration from that site.
ATRA accelerates differentiation of B lineage progenitors in vitro
The in vivo results indicated that retinoids may normally contribute to regulation of B lymphopoiesis, and culture experiments were performed to identify retinoid responsive target cells. ELP were sorted to high purity and cultured for four days on OP9 stromal cells with or without 10−7M ATRA. The cells were then harvested and examined by flow cytometry. ELP normally require 10 days to generate CD19+ cells in culture (17). Therefore, we were surprised to find that CD45R/B220+ CD19+ progenitors were produced from ELP in 7 day co-cultures when the retinoid was present (Fig. 2A). As another indication of maturity, pre-cultured cells were tested for their ability to proliferate in response to IL-7 in Methocel cultures (Fig. 2B). This clonal assay normally measures competence of CD19+ lymphoid progenitors to respond to IL-7, and many colony-forming cells were generated in suspensions that had been pretreated for only 4 days with ATRA.
Figure 2. All trans retinoic acid accelerates B lineage potential in culture.
One hundred highly purified ELP were co-cultured with OP9 stromal cells in 96 well flat bottom plates for four or seven days with ATRA or 0.1% ethanol diluents, and then the four day cultured cells were transferred to Pre-B colony medium (A) and the seven day cultured cells were subject to flow cytometry to examine the production of CD19+B220+ cells (B). Total Pre-B colony numbers were scored after 7 days of culture. Sorted ELP were treated with different concentrations of ATRA ranging from 10−8 to 10−6 M for 7 days and subject to flow cytometry, total cell yields (C) and B lineage lymphoid cell yields (D) were calculated. All treated groups were then compared with the control group using student t test. Data are reported as mean ± S.D for n= 4-6. *, p < 0.05, and **, p < 0.01. The results shown are representative of five independent experiments.
Experiments were then conducted to learn if lymphopoiesis is affected by physiological concentrations of ATRA. As little as 10−8 M accelerated the differentiation of pro-lymphocytes (data not shown) and 3 × 10−8 M concentrations optimally stimulated B lineage differentiation from ELP (Fig. 2D). It should be noted that numbers of total recovered cells were suppressed in a dose dependent fashion (Fig. 2C).
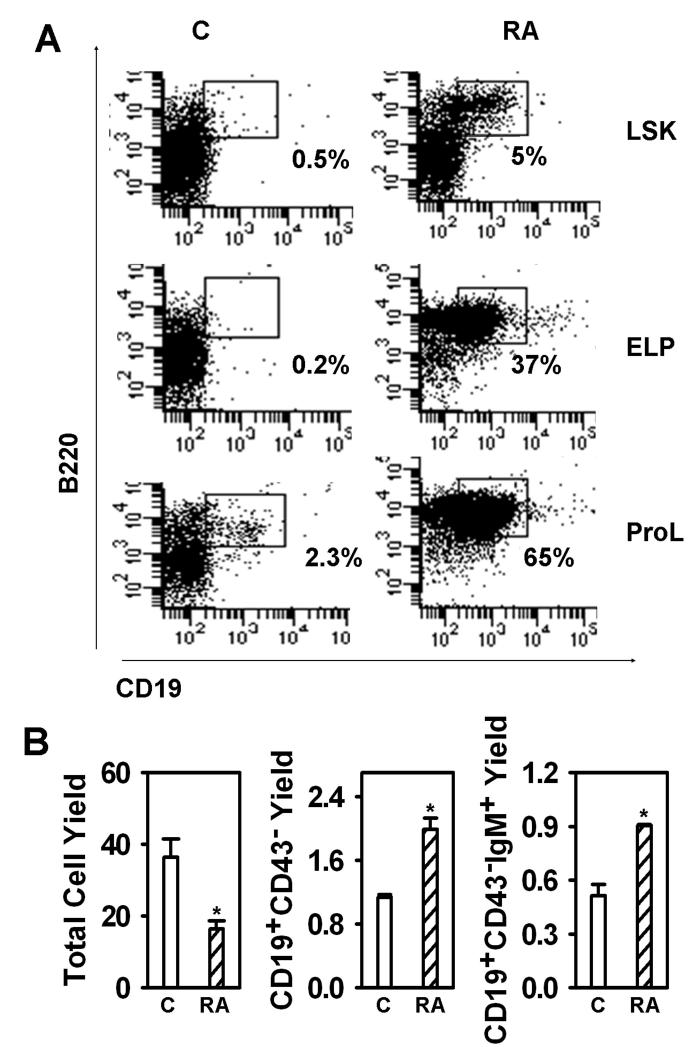
Stromal cells could have been influenced by retinoids in the co-culture experiments, and we sought additional information concerning target cells. Therefore, progenitors representing three successive stages of differentiation were used to initiate stromal cell-free, serum-free cultures (Fig. 3A). Stem cells, multipotent progenitors and ELP are contained within the rare LSK fraction of bone marrow, and LSK generated CD19+ cells in seven day cultures containing ATRA. Even more CD19+ progenitors arose in cultures initiated with highly purified ELP. Pro-lymphocytes, a subset that includes common lymphoid progenitors (CLP) generate CD19+ cells in one week cultures, and this was augmented by inclusion of ATRA.
Figure 3. ATRA accelerates differentiation of four types of lymphoid progenitors in defined culture.
(A) LSK, ELP and Pro-L were sorted and put in serum free, stromal cell free culture for 7 days. Recovered cells were then counted and subject to flow cytometry. Data shown are percentages of B lineage lymphoid cells recovered from LSK (top panel), ELP (middle panel) and Pro-L (bottom panel). (B) Pre-B/Pro-B cells (Lin−CD19+CD43+) were sorted and were put in stromal cell free culture for 7 days. Recovered cells were then counted and subject to flow cytometry. Data shown are total cell yields (left panel), CD19+CD43−(middle panel) cell yields, and D19+CD43−IgM+ cell yields (right panel). The results shown are representative of three independent experiments.
The analysis was then extended to later stages of B lineage differentiation by initiation of cultures with CD19+ CD43+ progenitors, a fraction that contains pro-B and large pre-B cells (Fig. 3B). Again, numbers of total nucleated cells were depressed by the retinoid while there were marked increases in CD19+ CD43− pre-B cells as well as progression to CD19+ CD43− sIgM+ B cells.
These results show that most, if not all stages of B lymphopoiesis are influenced by ATRA and that lymphoid progenitors are direct targets of the compound. At least some cells in each population responded by rapidly differentiating.
Time required for acceleration of B lymphopoiesis by ATRA
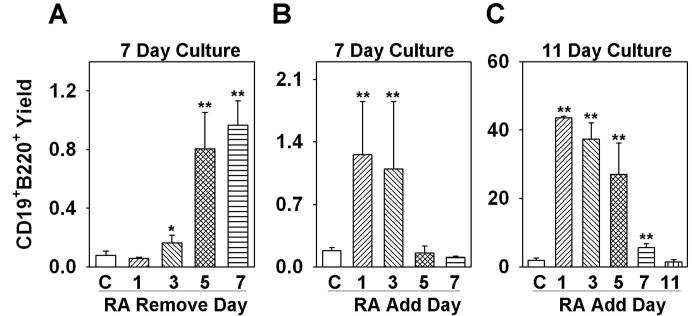
Additional information about target cells was then sought with a series of time course experiments. Defined, stromal cell-free conditions were again used for seven day cultures initiated with RAG-1/GFP− LSK. When ATRA was washed out after five days, the yield of CD19+ cells was almost as high as when the drug remained in culture for the whole time (Fig. 4A). Differentiation was significantly augmented when ATRA was present for three or more days of the seven day culture, but there were no changes with just one day of exposure.
Figure 4. Time required for ATRA effects on B lymphopoiesis.
(A) Sorted LSK were treated with ATRA in stromal cell free culture for 1, 3, 5, or 7 days, and the treated cells were washed and put back in culture. All the cells were recovered on day 7 and flow cytometry was performed to examine B lineage lymphoid cell (CD19+B220+) production. (B) Sorted LSK cells were put in stromal cell free culture and ATRA was added at day 0, day 1, day 3 or day 5, and was kept in the culture until day 7. All of the cells were then recovered and flow cytometry was performed to examine the B lineage lymphoid cell (CD19+B220+) production. (C) Sorted LSK were put in stromal cell free culture and ATRA was added on day 0, day 1, day 3, day 5 or day 7, and was kept in the culture until day 11. All of the cells were recovered and flow cytometry was performed to examine the B lineage lymphoid cell (CD19+B220+) production. All data are reported as means ± S.D. for n= 3.*, p < 0.05, and **, p < 0.01 (Student's t-test). Different Y axis scales were used to reflect production of B lineage cells over time, and the results shown are representative of three independent experiments.
The experimental design was then modified slightly to determine the minimum time required for retinoid responsiveness. Again, a seven day culture interval was used, but with ATRA added on different days. Delay until the next day of culture was as effective as when the compound was present for all seven days (Fig. 4B). However, there was no measurable influence when ATRA was added on day three or day five.
The total culture interval was then extended to 11 days (Fig. 4C) to allow more time for progenitors to differentiate. Total yields of CD19+ lymphocytes were now very high when the drug was added at the beginning of culture (day 0), on day one or on day three. A smaller but still significant increment was even seen when ATRA was added on day five for a total period of 11 days in culture.
These results complement those obtained when cultures were initiated with various types of progenitors (Fig. 3 above). That is, the short time required for a biological response suggests that primitive lymphoid progenitors are direct targets of ATRA. Exposure during the first three days was needed to achieve maximum responses.
Retinoids augment critical events in lymphopoiesis by ligating RARα receptor and do not inhibit proliferation
Ebf and Pax-5 transcription factors are needed to initiate and sustain B lymphopoiesis, respectively (24-27). Expression of these transcription factors in LSK was below levels detectable by quantitative real-time PCR during the first day of culture (Fig. 5A and B). However, both were measurable on day two and significantly increased in response to ATRA (Fig. 5 and data not shown). Levels of Ebf1 mRNA rose with duration of culture, and the ratio of ATRA treated to controls increased from 3 on days 3 and 5 to 10 by day 7. The magnitude of change in Pax-5 transcripts progressively increased with time such that ATRA containing cultures were 34, 133 and 190 fold greater than control values on days 3, 5 and 7, respectively (Fig. 5B).
Figure 5. ATRA dramatically increase EBF1 and Pax-5 expression.
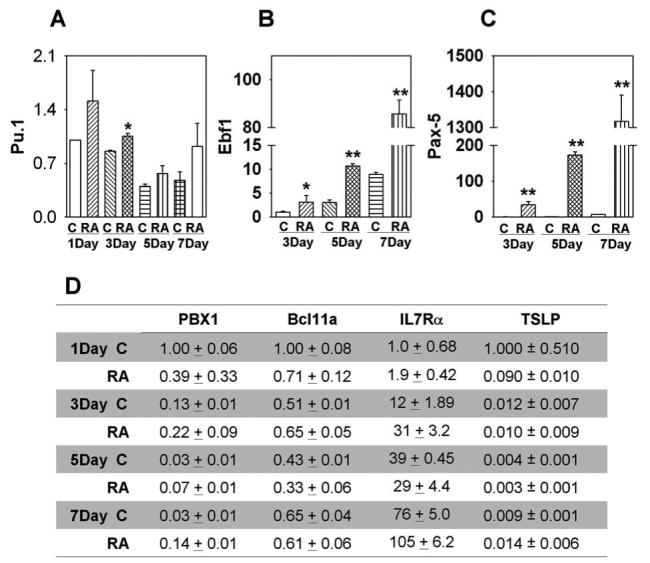
Double sorted LSK were treated with ATRA in stromal cell free culture for 1, 3, 5, or 7 days and the treated cells were washed and total RNA was isolated, RT-PCR was then performed to examine the mRNA expression of EBF1 (A), Pax-5 (B), Pu.1, PBX1, Bcl11a, IL7Rα and TSLP (C). Expression was normalized to 18sRNA mRNA in each sample and given as 2−ΔΔCT values on Y axes. EBF1 and Pax-5 results were calculated relative to the 3 day control group, and the expressions of other genes were determined by comparison to the 1 day control group. All data are reported as means ± S.D. for n= 3.*, p < 0.05, and **, p < 0.01 compared with vehicle (0.1% ethanol) treated cells within the same period of time. The results shown are representative of three independent experiments.
Critical levels of the PU.1 transcription factor are also needed for B lymphopoiesis and the EBF1 gene is a potential target (28). Real-time PCR analyses revealed elevations in PU.1 transcripts at all time points in retinoid treated cultures (Figure 5C). PBX1 was of special interest because of its known responsiveness to retinoic acid and Bcl11a is also important for early stages of lymphopoiesis (11, 29). However, no consistent trends were found with PBX1 or Bcl11a transcripts in ATRA treated cultures (Fig. 5C). IL-7 is essential for expansion of pro-B and pre-B populations in adult mice (30), and levels of IL-7Rα steadily increased with time in culture (Fig. 5C). This was slightly augmented by ATRA at some time points. TSLP is an IL-7 like cytokine and, like ATRA, can induce the production of IgM+ cells (12). Furthermore, one report suggested ATRA may induce TSLP in the skin (16). It is interesting that TLSP was detectable by quantitative real time PCR in hematopoietic cells because it might be involved in autocrine regulation of lymphopoiesis (Fig. 5C). However, expression of TSLP was not consistently induced by ATRA and levels were even reduced when ATRA was present for only 1 day.
Combinations of three RAR and three RXR receptor subunits are utilized by cells to recognize retinoids (31, 32). To determine what RAR subunits participate in B cell differentiation, we first determined the mRNA expression patterns of RARα, RARβ and RARγ on all three lymphoid progenitor subsets including LSK, ELP and Pro-L. Real-time PCR quantification indicated that LSK express all three isoforms of RAR, and this agrees with a recent report about RAR expression patterns on LSK (4). Both ELP and Pro-L express RARα and RARγ, but neither of them expresses RARβ. RARα was the most abundant subtype expressed on all three progenitors (Fig. 6A). To further explore mechanisms, the results of ATRA analyses were extended to related compounds. We compared five well studied retinoids to ATRA for their potential influence on B lymphopoiesis (Fig. 6B and C). With the exception of AC 41848, a known RARγ specific ligand, all were effective. All other substances depressed total nucleated cell numbers and accelerated generation of CD19+ lymphocytes comparable to ATRA. Patterns of receptor utilization of these compounds are given in the Materials and Methods.
Figure 6. Retinoid receptors involved in B lymphopoiesis.
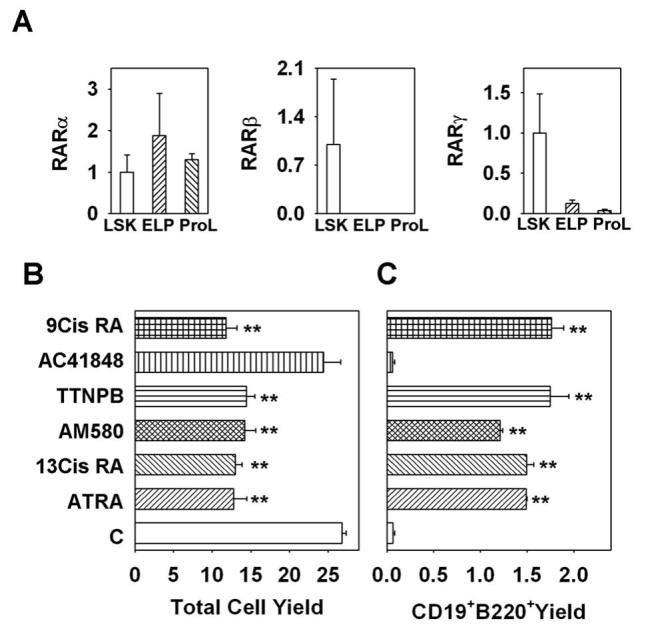
(A) Expression patterns of different RAR isoforms on lymphoid progenitors. Double sorted LSK, ELP and Pro-L were subjected to real-time PCR. Results obtained for LSK with each primer set were artificially defined as 1, and numbers given on the Y axes were calculated as 2−ΔΔCT values. Sorted LSK cells were put in stromal cell free culture and treated with different retinoids for 7 days and subject to flow cytometry, total cell yield (B) and B lineage lymphoid cell yield (C) were calculated. ATRA is all trans retinoic acid, a ligand for RARα, RARβ and RARγ; 13Cis RA is 13-cis- retinoic acid, a ligand specific for RARα and RARγ; AM580 is a ligand specific for RARα; TTNPB is a ligand for RARα, RARβ and RARγ; AC 41848 is a specific ligand for RARγ; 9Cis RA is 9-cis-retinoic acid, a ligand for RARα, RARβ, RARγ, RXRα, RXRβ and RXRγ. All data are reported as means ± S.D. for n= 3. **, p < 0.01 (Student's t-test). The results shown are representative of three independent experiments.
It has been reported that retinoids inhibit proliferation of lymphoid precursors (7, 33). However, analysis of CFSE label dilution from ATRA treated cells suggests that was not obvious for cells that survived in our cultures (Fig. 7A and B). Indeed, CD19+ lymphocytes had an even higher history of proliferation than other cells in the culture (Fig. 7C).
Figure 7. ATRA does not inhibit proliferation of B lineage cells.
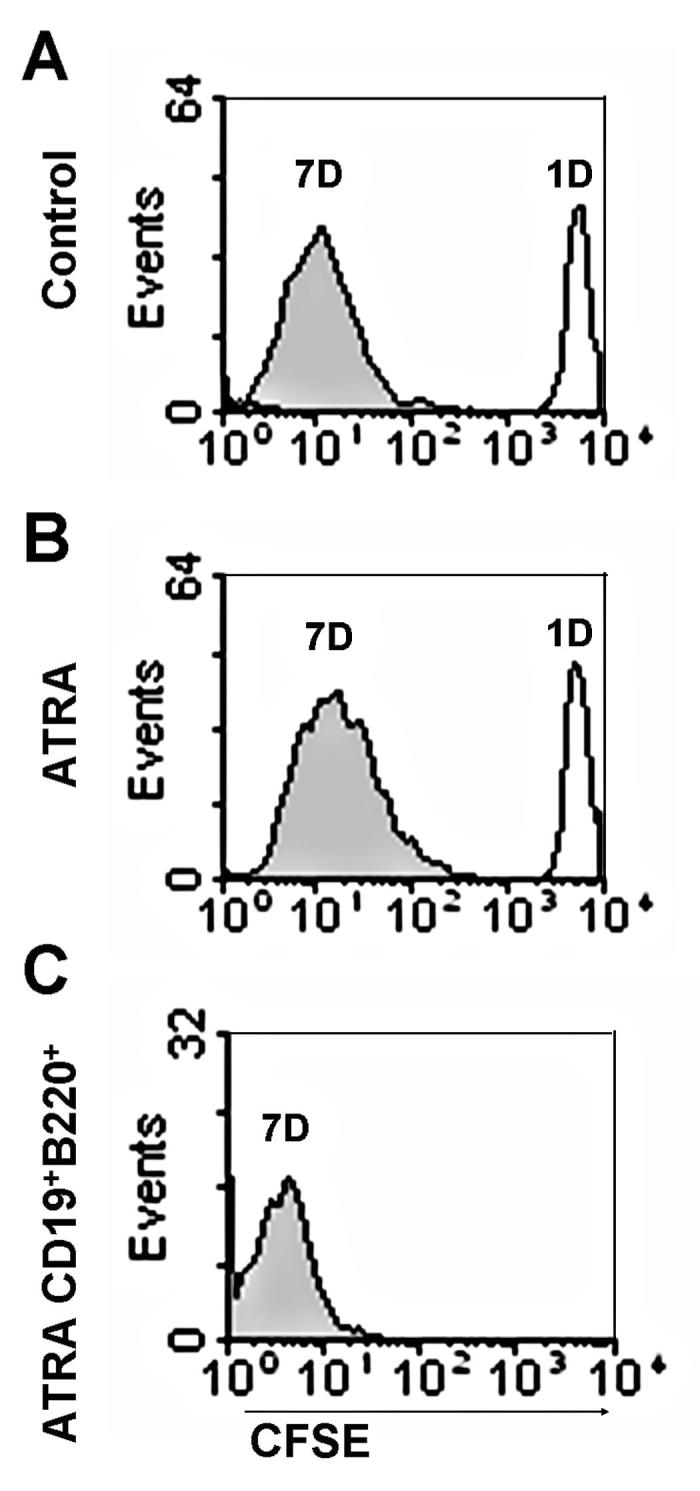
Sorted LSK were treated with 10 µM CFSE and the treated cells were put back in stromal cell free cultures with or without the presence of ATRA. The intensities of CFSE signals were then monitored at day 1 and day 7 by flow cytometry. (A) Recovered total cell CFSE intensity of control group on day 1 (dark line) and day 7(grey area). (B) Recovered total cell CFSE intensity of ATRA treated group at day 1 (dark line) and day 7 (grey area). (C) CFSE intensity of gated CD19+B220+ cells (as Fig. 3A top panel indicated) derived from 7 day stromal cell free culture with ATRA. The results shown are representative of three independent experiments.
We conclude from these experiments that expression of two critical transcription factors is augmented by ATRA. PCR analysis indicates all lymphoid progenitors express high levels of RARα and low levels of RARγ. The primitive LSK subset can recognize at least five retinoids. Absence of responsiveness to the drug AC 41848 suggests RARγ is not important, so it is possible that RARα mediates the ATRA effect in B lineage cell differentiation. Contrary to previous reports, proliferation of B lineage progenitors was not inhibited.
Human lymphoid progenitors are also responsive to retinoic acid
Human B lineage differentiation in culture is much less efficient than with murine progenitors, and it has not been achieved under defined conditions (34). However, human umbilical cord blood CD34+CD38− cells differentiate into CD19+ B lineage lymphocytes when cultured with MS-5 mouse stromal cells (35). Therefore, we used this model to determine if retinoids influence B lymphopoiesis.
Similar to our experience with murine cells, addition of ATRA reduced overall cell yields in two week cultures, but the substance actually improved cell recoveries when the culture interval was extended to four weeks (Fig. 8). ATRA was replenished at each weekly feeding, and the cells were not sub-cultured during this time. More importantly, numbers of CD19+ CD14− lymphocytes were significantly increased in ATRA containing cultures maintained for three or more weeks. Although there was experiment to experiment variability, improved differentiation was seen in each of three independent experiments, as well as in another conducted with total CD34+ cells.
Figure 8. ATRA has a similar effect on human B cell development.
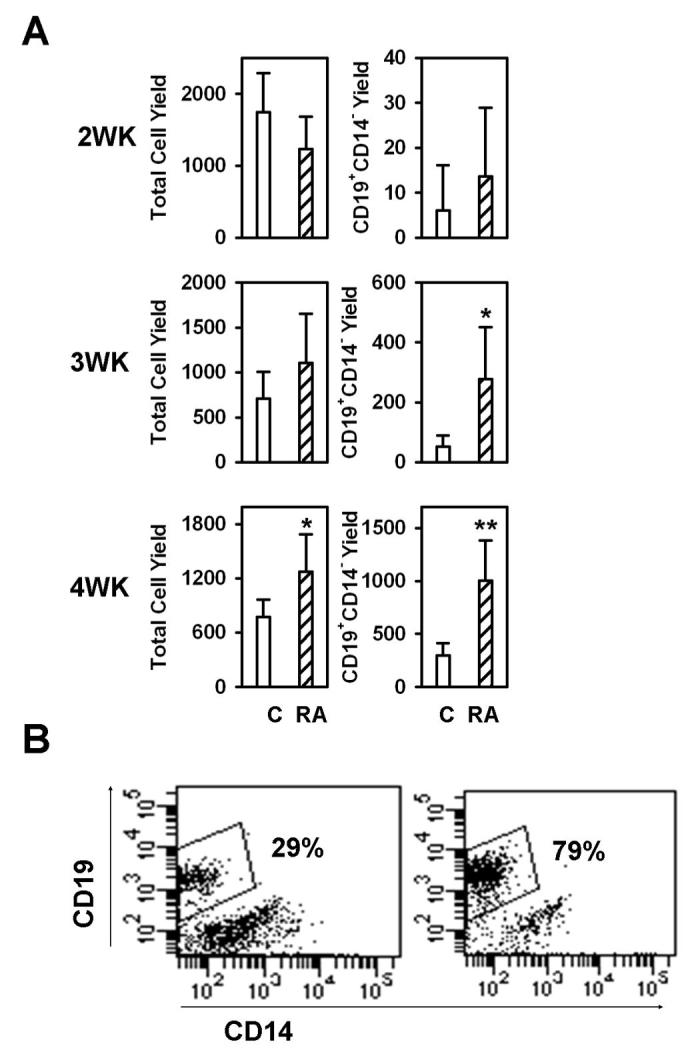
CD34+CD38− cells sorted from human cord blood were co-cultured with MS-5 stromal cells with or without the presence of ATRA for two weeks (top panel), three weeks (middle panel) or four weeks (bottom panel) and subjected to flow cytometry. (A) Total cell yields and B lineage lymphoid cell yields (CD19+CD14−) were calculated. All data are reported as means ± S.D. for n= 5-20. *, p < 0.05, and **, p < 0.01 (Student's t-test). Different Y axis scales were used in the right panels to reflect production of B lineage cells over time, and the results shown are representative of three independent experiments. (B) An example of flow cytometry results for four week cultures is shown.
Discussion
Retinoids are widely used as therapeutics, and there is evidence that Vitamin A deprivation can cause immunodeficiency (5). Furthermore, there are numerous reports of an influence on hematopoiesis (1), and recent findings suggest the RARγ receptor subunit is important for maintaining stem cell integrity (4). However, much less is known concerning roles in B lymphocyte formation. Our study began with the finding that ATRA treatment of mice caused profound changes in spleen B cell numbers. Subsequent analyses were designed to learn whether lymphoid progenitors or other cells are direct retinoid targets. We conclude that retinoids affect expression of two key transcription factors and accelerate progression through the B lymphocyte lineage. Therefore, retinoid gradients may normally provide rate limiting signals for B cell production.
The availability of reporter strains of mice, multi-parameter flow cytometry and cell culture innovations has permitted considerable progress to be made in identification of lympho-hematopoietic cells in bone marrow. For example, we can isolate extremely rare RAG-1+ Early lymphoid progenitors (ELP) and know that it will take them approximately 10 days to yield CD19+ lymphocytes in culture. Addition of ATRA significantly shortens this interval, and we found large numbers of CD19+ cells within one week in treated cultures. Oral treatment of humans with 1 mg/kg/day is said to result in a mean plasma concentration of 400 ng/ml or 1.3 × 10−6 M. Importantly, we observed significant effects on lymphoid progenitors in culture with as little as 3 × 10−8 M concentrations. We consider this good evidence that retinoids can accelerate B lymphopoiesis.
Cultures initiated with the stem cell enriched Lin− Sca-1+ c-KitHi (LSK) fraction of bone marrow was similar to ELP in response to ATRA. That is, ATRA increased numbers of CD19+ cells generated in one week cultures. Cultures started with more differentiated Lin− c-KitLo RAG-1+ pro-lymphocytes normally generate lymphocytes in just one week, and this was augmented by retinoid treatment. CD43+ CD19+ pro-B/large pre-B cells gave rise to more CD43− CD19+ cells, including sIgM+ B cells when treated with this drug. Our experimental design utilized stromal cells for culture of LSK and ELP, but no stromal cells were present when we assessed retinoid effects on pro-lymphocytes or pro-B cells. In that case, it was clear that numbers of sIgM+ B cells were substantially increased by retinoids. Therefore, these findings suggest that multiples stages in the B lineage are affected and at least the more mature progenitors are responsive.
Alterations in our treatment protocols were then used to obtain further information about retinoid responsive cells. As little as three days of ATRA exposure altered B lymphopoiesis, and five days were required for maximum effect. The kinetic information again suggests that retinoids may work across multiple stages of B lymphopoiesis. It also provided a basis for initiating further investigation concerning mechanisms.
Expression of many genes is altered by ATRA, even when the relevant promoters lack a retinoid acid response element (8). Also, cultures containing more CD19+ cells would be expected to have more lymphocyte related transcripts. Therefore, it is difficult to identify proximal events that determine retinoid effects on B lymphopoiesis and we narrowed our search in two ways. Most of our measurements were not begun before three days of treatment because that interval is required for biological activity. In addition, we focused on genes that are essential for lymphopoiesis and/or are known to be retinoid regulated.
The PU.1 transcription factor is needed for B lymphopoiesis, it can act in dose dependent fashion and levels were significantly higher at an early time point in cultures containing ATRA. In contrast, there was no consistent trend with Bcl11a. PBX1 levels fluctuated in our experiments even though the abundance and stability of its transcripts are increased by retinoids and conditional targeting in early progenitors selectively arrested the lineage (11). IL-7 is essential for expansion of pro-B and pre-B populations in adult mice (30), but levels of IL-7Rα transcripts were only higher at some intervals of treatment. TSLP is an IL-7 like cytokine that can induce the production of IgM+ cells (12) and we were surprised to consistently find evidence for its production in hematopoietic cells. Also, there was a report that ATRA may induce TSLP in the skin (16). However, the PCR data do not suggest that TSLP accounted for augmentation of B lymphopoiesis by ATRA in culture.
Detectable levels of EBF1 and Pax-5 transcripts were present within three days of culture. Quantitative real-time PCR analyses suggest that while both were elevated with ATRA treatment, the Pax-5 response was particularly impressive. EBF1 controls Pax-5 transcription in a feed forward mechanism whereby Pax-5 augments EBF1 transcription (36). Additionally, promoters of PU.1, Ebf1 and Pax-5 genes have candidate retinoic acid response elements (data not shown). Direct regulation of these genes would represent a powerful rheostat for controlling lymphocyte production. On the other hand, slight changes in cell surface markers were evident as early as four days after drug treatment, and transcript levels might just reflect the increased abundance of differentiating lymphocytes. Further study will be required to learn if any of these critical genes are directly or indirectly regulated by retinoids.
It is possible that retinoids control events other than the time required for progenitors to differentiate in bone marrow. For example, the reductions in primitive lymphoid cells, together with increases in all spleen B cell subsets of ATRA treated mice could have resulted from increased proliferation, survival and/or replenishment by B cells exported from bone marrow. It has been reported that retinoic acid can increase the expression of a gut homing receptor and modulate both B and T migration in mucosal immunity (37, 38). If that is the case in the hematopoietic system, ATRA may contribute to B lineage cell exit from the bone marrow. It is easier to draw conclusions from experiments involving highly purified progenitors under defined conditions of culture. Then at least altered migration is excluded from consideration. It has been reported that ATRA inhibits proliferation of B cell precursors (7, 33) and we always recovered fewer total cells from drug containing cultures. However, CFSE labeling revealed that a subset of cells rapidly differentiated under the influence of ATRA and had a history of extensive proliferation. In addition, there was evidence for some proliferation by cells that lacked CD19 at the end of culture. Preliminary experiments with Bcl-2 transgenic mice and staining with Anexin V did not suggest that retinoids increased apoptosis, so we are left with no clear explanation for the reductions in numbers of total nucleated cells. The findings would be compatible with heterogeneity in progenitor populations or changes in proliferation rates at particular times.
Our studies were inspired in part by a report that RARγ has a critical role in stem cell maintenance (4). However, one agonist that is specific for this receptor subunit had no effect on B lymphopoiesis in culture. Testing of additional compounds and analysis of knockout mice might be informative in this regard.
In two preliminary experiments, murine LSK were held with ATRA for 24 hours before transplantation. Numbers of donor type CD19+ lymphocytes in bone marrow were increased (data not shown). Furthermore, human umbilical cord blood CD34+ CD38− progenitors were responsive to ATRA (Fig. 8). Consequently, one could imagine augmenting B cell production in some clinical settings.
In summary, our observations indicate the widely used drug ATRA has a previously undisclosed effect on B lymphopoiesis. The ability of retinoids to accelerate B lineage cell differentiation may be applied to improve humeral immune recovery following chemotherapy or bone marrow transplantation. Given the widespread therapeutic use of ATRA, it is important to learn more about effects on human B cell development.
Acknowledgments
The authors are grateful to Jacob Bass and Diana Hamilton for cell sorting, Tara Khamphanthala for technical assistance, Beverly Hurt for graphics assistance, and Shelli Wasson for editorial assistance.
This work was supported by grants AI20069 and AI58162 from the National Institutes of Health and grant N01-A1-30070 from the US Immunodeficiency Network. P.W.K. holds the William H. and Rita Bell Endowed Chair in Biomedical Research.
Abbreviations
- ATRA
all trans retinoic acid
- CFSE
carboxyfluorescein succinimidyl ester
- CMP
Lin− Sca-1− c-KitHi common myeloid progenitor
- EBF1
early B cell factor 1
- ELP
Lin− RAG-1/GFP+ Sca-1+ c-KitHi early lymphoid progenitors
- HSC
hematopoietic stem cell
- LSK
Lin− Sca-1+ c-KitHi
- Pax-5
paired box gene 5
- PBX1
Pre-B cell leukemic homeobox1
- Pro-L
Lin− Sca-1+ c-KitLo pro-lymphocytes
- RAR
retinoic acid receptor
- RXR
retinoic X receptor
- TSLP
thymic stromal lymphopoietin
Footnotes
Disclosures The authors have no financial conflict of interest.
References
- 1.Collins SJ. The role of retinoids and retinoic acid receptors in normal hematopoiesis. Leukemia. 2002;16:1896–1905. doi: 10.1038/sj.leu.2402718. [DOI] [PubMed] [Google Scholar]
- 2.Purton LE, Bernstein ID, Collins SJ. All-trans retinoic acid enhances the long-term repopulating activity of cultured hematopoietic stem cells. Blood. 2000;95:470–477. [PubMed] [Google Scholar]
- 3.Leung AY, Verfaillie CM. All-trans retinoic acid (ATRA) enhances maintenance of primitive human hematopoietic progenitors and skews them towards myeloid differentiation in a stroma-noncontact culture system. Exp. Hematol. 2005;33:422–427. doi: 10.1016/j.exphem.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Purton LE, Dworkin S, Olsen GH, Walkley CR, Fabb SA, Collins SJ, Chambon P. RARγ is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J. Exp. Med. 2006;203:1283–1293. doi: 10.1084/jem.20052105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephensen CB. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 6.O'Connell MJ, Chua R, Hoyos B, Buck J, Chen Y, Derguini F, Hammerling U. Retro-retinoids in regulated cell growth and death. J. Exp. Med. 1996;184:549–555. doi: 10.1084/jem.184.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobsen SEW, Fahlman C, Blomhoff HK, Okkenhaug C, Rusten LS, Smeland EB. All-Trans- and 9-Cis-Retinoic acid: Potent direct inhibitor of primitive murine hematopoietic progenitors in vitro. J. Exp. Med. 1994;179:1665–1670. doi: 10.1084/jem.179.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J. Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 9.Qin P, Haberbusch JM, Soprano KJ, Soprano DR. Retinoic acid regulates the expression of PBX1, PBX2, and PBX3 in P19 cells both transcriptionally and post-translationally. J. Cell Biochem. 2004;92:147–163. doi: 10.1002/jcb.20057. [DOI] [PubMed] [Google Scholar]
- 10.Aspland SE, Bendall HH, Murre C. The role of E2A-PBX1 in leukemogenesis. Oncogene. 2001;20:5708–5717. doi: 10.1038/sj.onc.1204592. [DOI] [PubMed] [Google Scholar]
- 11.Sanyal M, Tung JW, Karsunky H, Zeng H, Selleri L, Weissman IL, Herzenberg LA, Cleary ML. B-cell development fails in the absence of the Pbx1 proto-oncogene. Blood. 2007;109:4191–4199. doi: 10.1182/blood-2006-10-054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin SD, Koelling RM, Friend SL, Isaksen DE, Ziegler SF, Perlmutter RM, Farr AG. Thymic stromal lymphopoietin: A cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J. Immunol. 1999;162:677–683. [PubMed] [Google Scholar]
- 13.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J. Exp. Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nat. Immunol. 2003;4:773–779. doi: 10.1038/ni956. [DOI] [PubMed] [Google Scholar]
- 15.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. USA. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 18.Castaigne S, Lefebvre P, Chomienne C, Suc E, Rigal-Huguet F, Gardin C, Delmer A, Archimbaud E, Tilly H, Janvier M, Isnard F, Travade P, Montfort L, Delannoy A, Rapp MJ, Christian B, Montastruc M, Weh H, Fenaux P, Dombret H, Gourmel B, Degos L. Effectiveness and pharmacokinetics of low-dose all-trans retinoic acid (25 mg/m2) in acute promyelocytic leukemia. Blood. 1993;82:3560–3563. [PubMed] [Google Scholar]
- 19.Astrakhan A, Omori M, Nguyen T, Becker-Herman S, Iseki M, Aye T, Hudkins K, Dooley J, Farr A, Alpers CE, Ziegler SF, Rawlings DJ. Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat. Immunol. 2007;8:522–531. doi: 10.1038/ni1452. [DOI] [PubMed] [Google Scholar]
- 20.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 21.Igarashi H, Medina KL, Yokota T, Rossi MI, Sakaguchi N, Comp PC, Kincade PW. Early lymphoid progenitors in mouse and man are highly sensitive to glucocorticoids. Int. Immunol. 2005;17:501–511. doi: 10.1093/intimm/dxh230. [DOI] [PubMed] [Google Scholar]
- 22.Yokota T, Meka CS, Medina KL, Igarashi H, Comp PC, Takahashi M, Nishida M, Oritani K, Miyagawa J, Funahashi T, Tomiyama Y, Matsuzawa Y, Kincade PW. Paracrine regulation of fat cell formation in bone marrow cultures via adiponectin and prostaglandins. J. Clin. Invest. 2002;109:1303–1310. doi: 10.1172/JCI14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouro T, Medina KL, Oritani K, Kincade PW. Characteristics of early murine B lymphocyte precursors and their direct sensitivity to negative regulators. Blood. 2001;97:2708–2715. doi: 10.1182/blood.v97.9.2708. [DOI] [PubMed] [Google Scholar]
- 24.Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–562. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- 25.Nutt SL, Urbánek P, Rolink A, Busslinger M. Essential functions of Pax5 (BSAP) in pro-B cell development: Difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev. 1997;11:476–491. doi: 10.1101/gad.11.4.476. [DOI] [PubMed] [Google Scholar]
- 26.Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]
- 27.Urbánek P, Wang Z-Q, Fetka I, Wagner EF, Busslinger M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell. 1994;79:901–912. doi: 10.1016/0092-8674(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 28.Zou GM, Chen JJ, Yoder MC, Wu W, Rowley JD. Knockdown of Pu.1 by small interfering RNA in CD34+ embryoid body cells derived from mouse ES cells turns cell fate determination to pro-B cells. Proc. Natl. Acad. Sci. USA. 2005;102:13236–13241. doi: 10.1073/pnas.0506218102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu P, Keller JR, Ortiz M, Tessarollo L, Rachel RA, Nakamura T, Jenkins NA, Copeland NG. Bcl11a is essential for normal lymphoid development. Nat. Immunol. 2003;4:525–532. doi: 10.1038/ni925. [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J. Exp. Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangelsdorf DJ, Umesono K, Kliewer SA, Borgmeyer U, Ong ES, Evans RM. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991;66:555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- 32.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 33.Fahlman C, Jacobsen SE, Smeland EB, Lomo J, Næss CE, Funderud S, Blomhoff HK. All-trans- and 9-cis-retinoic acid inhibit growth of normal human and murine B cell precursors. J. Immunol. 1995;155:58–65. [PubMed] [Google Scholar]
- 34.Lebien TW. B-cell lymphopoiesis in mouse and man. Curr. Opin. Immunol. 1998;10:188–195. doi: 10.1016/s0952-7915(98)80248-3. [DOI] [PubMed] [Google Scholar]
- 35.Nishihara M, Wada Y, Ogami K, Ebihara Y, Ishii T, Tsuji K, Ueno H, Asano S, Nakahata T, Maekawa T. A combination of stem cell factor and granulocyte colony- stimulating factor enhances the growth of human progenitor B cells supported by murine stromal cell line MS-5. Eur. J. Immunol. 1998;28:855–864. doi: 10.1002/(SICI)1521-4141(199803)28:03<855::AID-IMMU855>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 36.Roessler S, Gyory I, Imhof S, Spivakov M, Williams RR, Busslinger M, Fisher AG, Grosschedl R. Distinct promoters mediate the regulation of Ebf1 gene expression by interleukin-7 and Pax5. Mol. Cell Biol. 2007;27:579–594. doi: 10.1128/MCB.01192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, Von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]