The marine ladder toxin class of natural products has generated considerable interest from the synthetic and biomedical communities because of their fascinating structures and biological activities.1,2 This group includes a target that has recently become of interest to us, adriatoxin (Scheme 1), a polyether toxin isolated by Fattorusso and coworkers from the digestive gland of the Adriatic mussel Mytilus galloprovincialis.3-5 Synthetically, our fascination with adriatoxin came out of an interest in determining whether our recently described coupling protocol, which leads to the pairing of two subunits and the generation of two additional rings, would enable us to carry out a convergent synthesis of the ten-ring system from three bicyclic precursors representing the AB ring (1), the EF ring (2), and the IJ ring (3).6 Biologically, that adriatoxin had been implicated in diarrhetic shellfish poisoning would allow us to continue our collaborative studies which have been focused on the ability of natural and non-natural polyethers to bind to ion channels.7 In addition to this, that Shimizu has identified structurally related yessotoxin analogues as having exceptional cytotoxic activity against human cancer cell lines was also intriguing.8
Scheme 1.
Retrosynthetic analysis of adriatoxin. P = protecting group.
We had hoped to utilize iterative C-glycoside technology to access the adriatoxin AB-ring subunit, starting from 2-deoxy-d-ribose (Scheme 2).9 Conversion of this precursor into olefinic alcohol 5 required four steps and was carried out in a 64 % overall yield.10 Esterification with butanoic acid derivative 6 and then olefinic ester cyclization, under our recently disclosed reduced-titanium reaction conditions, gave 8 in 70 % yield.11 In contrast, the use of a more conventional two-step enol ether–olefin ring-closing metathesis sequence gave 8 in a 50 % overall yield from 7.
Scheme 2.
Synthesis of glycal 8. Reagents and conditions: a) Ph3PMeBr, THF, tBuOK; b) PhCH(OMe)2, CSA (80% over two steps); c) (COCl)2, DMSO, Et3N, −78 °C; d) MeMgBr, Et2O, −78 °C (80% over two steps); e) HO2CCH2CH2CH(OMe)2 (6), DCC, DMAP, CH2Cl2 (80%); f) TiCl4, TMEDA, Zn, PbCl2, THF, CH2Cl2,CH3CH2Br2 (70%). CSA = (+)-camphorsulfonic acid, DMSO = dimethylsulfoxide; DCC = dicyclohexylcarbodiimide; DMAP = 4-(dimethylamino)pyridine; TMEDA = N,N,N′,N′-tetramethyl-1,2-ethanediamine.
Next, we needed to introduce an oxygen atom at C6 and to reduce C7 of 8. In principle, these goals could be accomplished in a single flask by either subjecting the enol ether to a hydroboration/oxidation reaction or by the use of a 2,2-dimethyldioxirane (DMDO)/diisobutylaluminum hydride protocol.12 However, because of the presence of the C3 angular methyl group in 8 we felt that both of these procedures would generate the undesired stereochemistry at both C6 and C7.13 Whereas we believed that this problem could be overcome by oxidizing the alcohol to the corresponding ketone, equilibrating the C7 stereocenter to the desired thermodynamically more stable isomer, and subsequently reducing the ketone, we sought a more direct solution. We realized that we might be able to take advantage of an epoxide rearrangement reaction that had, in the past, been problematic for us.14 The reaction is outlined in Scheme 3 for 9 and involves a Lewis acid catalyzed pinacol-type rearrangement to give 10. As illustrated, when the rearrangement was induced with allyl Grignard, it resulted in the generation of the corresponding tertiary alcohol (11). Of importance to our use of this reaction for the adriatoxin AB subunit was that 11 was isolated as a single diastereomer, implying that the rearrangement of 9 was stereoselective.
Scheme 3.
Glycal epoxide rearrangement of 9. Reagents and conditions: a) propenyl magnesium chloride, THF, 0 °C to RT (70%).
We recognized that if we were able harness the rearrangement in a synthetic context, that it might prove to be a useful solution for the adriatoxin A-ring problem and allow us to avoid the equilibration sequence mentioned above. That is, if the epoxidation of 8 was directed by the C3 angular methyl group and if the subsequent rearrangement was stereoselective per our previous results, the reaction would deliver the desired C7 stereochemistry along with a C6 ketone.
The oxidation of 8 using “acetone free” DMDO presumably gave 12 (not isolated; Scheme 4),15 which was then directly subjected to Lewis acids in an attempt to induce the rearrangement. We focused on Mg salts and examined MgBr2·Et2O, an “aged” bottle of MeMgCl, and reagent grade MgCl2. Pleasingly, because it was the simplest to use, the MgCl2 worked best; upon exposure to MgCl2, 12 rearranged to ketone 13 in 82 % yield. Extensive nOe experiments indicated that 13 had the desired adriatoxin C7 stereochemistry, thus confirming our earlier hypothesis regarding the stereoselectivity of the rearrangement.
Scheme 4.
Pinacol rearrangement of 12. Reagents and conditions: a) DMDO, CH2Cl2, −60 °C to 0 °C; MgCl2, −60 °C to RT (82%).
Having successfully generated 13 we next focused our attention on the C6 center and the B ring. To accomplish these goals the ketone in 13 was reduced with NaBH4 to obtain the desired equatorial alcohol (Scheme 5), which then underwent acid-mediated cyclization and elimination to give the adriatoxin B ring (14).16 DMDO oxidation of 14 and propenyl magnesium chloride addition to the resulting epoxide provided a mixture of secondary alcohols 15 and 16. The undesired diastereomer (16) was converted into 15 by using the three-step protocol mentioned earlier involving: 1) oxidation of the secondary alcohol, 2) equilibration of the C10 stereocenter, and 3) reduction of the ketone. The synthesis of AB subunit 15 was reasonably efficient in that it required 13 steps (15 % overall yield) and utilized d-ribose as the sole source of chirality.
Scheme 5.
Synthesis of adriatoxin AB-precursor 15. Reagents and conditions: a) NaBH4, MeOH, −65 °C to RT (95%); b) PPTS, PhCl, pyridine, 135 °C (86%); c) DMDO, CH2Cl2, −60 °C to RT; propenyl magnesium bromide, THF, −60 °C to RT (75%, 2.3:1 ratio of 15/16); d) SO3·pyridine, DMSO, CH2Cl2,0 °C to RT; e) DBU, PhCH3, 110 °C; f) NaBH4, MeOH, −65 °C to RT (60% over three steps). PPTS = pyridinium para-toluenesulfonate; DBU = 1,8-diazabicycloundec-7-ene.
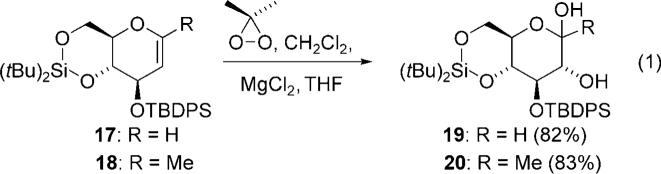
Intrigued by the reaction to access ketone 13, we examined the scope of the rearrangement reaction by exploring the effect of substitution in d-glucal model substrates [Eq. (1); TBDPS = tert-butyldiphenylsilyl]. In these studies we were somewhat disappointed to find that the epoxides from 17 and 18 gave no rearranged products, but instead gave only diols 19 and 20.
In contrast to the results from 17 and 18, epoxides lacking substitution at the allylic position gave the ketones in high yield. Thus, when unsubstituted glycals 21 and 22 were subjected to DMDO and subsequently reacted with MgCl2 we isolated ketones 23 and 24, respectively [Eq. (2)]. As with the rearrangement to 13, each of these compounds was isolated as a single diastereomer. Although additional experiments on a broader range of substrates are needed, our results imply that less sterically hindered glycal epoxides are more likely to undergo the rearrangement reaction, and are consistent with previous results from our lab which described an inverse dependence between epoxide reactivity and the steric bulk of the allylic substituent.17
Seven-membered ring ketones can also be synthesized by using the epoxide rearrangement reaction. When 25 was subjected to DMDO and MgCl2 we isolated enol 26 as a single diastereomer in 75% yield [Eq. (3)].18
Our efforts to access the adriatoxin EF ring began with d-glyceraldehyde acetonide as a precursor to the E ring (Scheme 6). The addition of homoallyl grignard in the
presence of ZnCl2 gave alcohol 27.19 Methanolysis of the acetonide and benzylidene acetal formation gave cyclization precursor 30 after esterification with acetal 29. Olefinic ester cyclization gave the adriatoxin E-ring subunit (31)in 65% yield.
Scheme 6.
Synthesis of adriatoxin E-ring precursor 31. Reagents and conditions: a) butenyl magnesium bromide, ZnCl2, Et2O, −90 °C (87%, 6:1); b) PPTS, MeOH, 65 °C (89%); c) PhCH(OMe)2, CSA (82%); 29, DCC, DMAP, CH2Cl2 (85%); d) TiCl4, TMEDA, Zn, PbCl2, THF, CH2Cl2, CH3CHBr2, 60 °C (65%).
Oxidation and reduction of the enol ether in 31 was carried out using DMDO and iBu2AlH (Scheme 7). Additional oxidation of the resulting secondary alcohol gave ketone 32. Notably, the reaction of 31 with DMDO and iBu2AlH was stereoselective and ketone 32 existed as its keto tautomer. In contrast, diastereomeric substrate 26 existed as the enol tautomer [Eq. (3)]. Conversion of 32 into the corresponding tertiary alcohol and cyclization/elimination gave 33 in good yield.16
Scheme 7.

Synthesis of adriatoxin EF-precursor 35. Reagents and conditions: a) DMDO, iBu2AlH, CH2Cl2, −78 °C (80%); b) SO3·pyridine, Et3N, DMSO (85%); c) MeMgBr, −78 °C (92%, 5:1); d) PPTS, PhCl, pyridine, 135 °C (75%); e) mCPBA, MeOH, −78 °C to RT (88%); f) 3-bromo-1-propene, nBu4NI, DMF, 0 °C to 65 °C (85%). mCPBA = 3-chloroperbenzoic acid.
Two comments on the generation of 33 are worth noting. First, we had hoped that the C19 angular methyl group would ultimately dictate the stereochemistry at C23 (see below). Thus, we were relieved that the formation of the C19 tertiary alcohol was stereoselective. Second, whereas we have examined a number of related cyclization/elimination reactions,16 the cyclization to obtain 33 was the first in which a ketal was employed, and we were pleased that the expected endocyclic enol ether was the only isomer obtained from this reaction.
For the generation of the C23 stereocenter and the completion of the adriatoxin F ring, we planned to employ a Claisen rearrangement from an in situ generated allyl enol ether (see 36, Scheme 8). Whereas Claisen rearrangements to give C-glycosides are well precedented,20 the proposed reaction is somewhat unique in that the oxygen atom linking the two alkenes is not in the allylic position on the pyran ring. To the best of our knowledge there is only one other related reaction and it was accomplished by us during our gambierol work.21 Oxidation of 33 with mCPBA in methanol gave 34 in 88% yield and then allyl ether formation gave rearrangement precursor 35.
Scheme 8.

Synthesis of adriatoxin EF-precursor 38. Reagents and conditions: a) PPTS, PhCH3, pyridine, 100 °C to 120 °C (92%, > 10:1); b) NaBH4, MeOH −65 °C to RT (94%).
With the stage set for the rearrangement to the C23 stereocenter, we subjected 35 to PPTS, pyridine, and heat, and isolated ketone 37 in 92% yield and as a greater than 10:1 mixture of diastereomers (Scheme 8). Presumably, the path to 37 proceeds through allyl enol ether 36 and, as mentioned above, a stereoselective Claisen rearrangement. Having successfully generated 37, all that remained to obtain EF-ring precursor 38 was the reduction of the ketone, and this was accomplished in 94% yield using NaBH4. Our synthesis of the EF-ring precursor required 13 steps (11% overall yield) utilizing d-glyceraldehyde acetonide as the precursor to the remaining chiral centers.
Our synthesis of the IJ subunit is illustrated in Scheme 9. From d-glucal derived C-glycoside 39,22 vinyl ether formation and enol ether–olefin ring-closing metathesis, using the second generation Grubbs catalyst (40), gave 41. The mCPBA oxidation of the enol ether resulted in a 5.5:1 mixture of anomeric acetals, both of which had the desired stereochemistry at C36. Here, we opted to invert the C32 stereocenter to that required for the synthesis of adriatoxin.23 This inversion was accomplished in three steps: hydrogenolysis of 42 and then oxidation of the resulting alcohol gave 43; reduction of the ketone in 42 with L-Selectride gave the IJ subunit to adriatoxin as 44.24 The synthesis of IJ-precursor 44 was efficient in that it required 12 steps (17% overall yield) from d-glucal.
Scheme 9.
Synthesis of adriatoxin IJ-precursor 44. Reagents and conditions: a) Hg(OC(O)CF3)2, ethyl vinyl ether; b) 40 (20 mol%), PhH (60% over 2 steps); c) m-CPBA, MeOH, −63 °C to RT (75%, 5.5:1); d) Ac2O, DMAP, NEt3, CH2Cl2 (96%); e) H2, Pd(OH)2/C (90%); f) SO3·pyridine, DMSO, CH2Cl2, NEt3 (90%); g) L-Selectride, THF, −78 °C (90%). Mes = 2,4,6-trimethylphenyl; Ac2O = acetic anhydride; L-Selectride = lithium tri-sec-butylborohydride.
In summary, described herein is our synthetic strategy toward the diarrhetic shellfish poison, adriatoxin, which includes the use of a glycal-epoxide rearrangement to obtain the AB subunit, a Claisen rearrangement to access the EF subunit, and the use of C-glycoside-forming chemistry to obtain the IJ subunit. This work expands the scope of glycal epoxide chemistry by introducing epoxide rearrangements as a viable synthetic reaction. Our future efforts will be focused on the coupling of the subunits, the completion of the synthesis of adriatoxin, and the study of its ability to interact with biological receptors.
Footnotes
We are grateful to the National Institutes of Health, General Medical Sciences (GM56677) for support of this work. C.O.A. acknowledges Pfizer for a graduate fellowship. We would like to thank the support staff at the University of Utah and especially Dr. Charles Mayne (NMR) and Dr. Jim Muller (MS) for their help in obtaining data.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200803791.
References
- 1.For a recent review on the synthesis of polycyclic ethers, see: Nakata T. Chem. Rev. 2005;105:4314. doi: 10.1021/cr040627q.
- 2.For a recent review that discusses ion channel binding of ladder toxins, see: Nicholson GM, Lewis RJ. Mar. Drugs. 2006;4:82.
- 3.Ciminiellow P, Fattorusso E, Forino M, Magno S, Poletti R, Viviani R. Tetrahedron Lett. 1998;39:8897. [Google Scholar]
- 4.With the exception of the terminal K ring, adriatoxin shares a core skeleton with yessotoxin, see: Murata M, Kumagai M, Lee JS, Yasumoto T. Tetrahedron Lett. 1987;28:5869.
- 5.For synthetic efforts to adriatoxin, see: Hiramatsu N, Mori Y. Heterocycles. 2006;69:437.Kadota I, Ueno H, Yamamoto Y. Tetrahedron Lett. 2003;44:8935.Mori Y, Takase T, Noyori R. Tetrahedron Lett. 2003;44:2319.Mori Y, Nogami K, Hayashi H, Noyori R. J. Org. Chem. 2003;68:9050. doi: 10.1021/jo035145d.Suzuki K, Nakata T. Org. Lett. 2002;4:3943. doi: 10.1021/ol026804w.
- 6.Johnson HWB, Majumder U, Rainier JD. J. Am. Chem. Soc. 2005;127:848. doi: 10.1021/ja043396d. [DOI] [PubMed] [Google Scholar]
- 7.a Cuypers E, Abdel-Mottaleb Y, Kopljar I, Rainier JD, Raes AL, Snyders DJ, Tytgat J. Toxicon. 2008;51:974. doi: 10.1016/j.toxicon.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cuypers E, Yanagihara A, Rainier JD, Tytgat J. Biochem. Biophys. Res. Commun. 2007;361:214. doi: 10.1016/j.bbrc.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; c LePage KT, Rainier JD, Johnson HWB, Baden DG, Murray TF. J. Pharmacol. Exp. Ther. 2007;361:214. [Google Scholar]; d Cao Z, George J, Gerwick WH, Baden DG, Rainier JD, Murray TF. J. Pharmacol. Exp. Ther. 2008;326:604. doi: 10.1124/jpet.108.138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konishi M, Yang X, Li B, Fairchild CR, Shimizu Y. J. Nat. Prod. 2004;67:1309. doi: 10.1021/np040008c. [DOI] [PubMed] [Google Scholar]
- 9.Allwein SP, Cox JM, Howard BE, Johnson HWB, Rainier JD. Tetrahedron. 2002;58:1997. [Google Scholar]
- 10.Nicolaou KC, Nugiel DA, Couladouros E, Hwang C-K. Tetrahedron. 1990;46:4517. [Google Scholar]
- 11.Iyer K, Rainier JD. J. Am. Chem. Soc. 2007;129:12604. doi: 10.1021/ja073880r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majumder U, Cox JM, Johnson HWB, Rainier JD. Chem. Eur. J. 2006;12:1736. doi: 10.1002/chem.200500993. [DOI] [PubMed] [Google Scholar]
- 13.Alternatively, superhydride reduction of the glycal epoxide from 8 would potentially deliver the desired stereochemistry at C(7), see: Inoue M, Yamashita S, Tatami A, Miyazaki K, Hirama M. J. Org. Chem. 2004;69:2797. doi: 10.1021/jo049877x.
- 14.Rainier JD, Allwein SP, Cox JM. J. Org. Chem. 2001;66:1380. doi: 10.1021/jo001514j. [DOI] [PubMed] [Google Scholar]
- 15.Ferrer M, Gibert M, Sánchez-Baeza F, Messeguer A. Tetrahedron Lett. 1996;37:3585. [Google Scholar]
- 16.Rainier JD, Allwein SP. Tetrahedron Lett. 1998;39:9601. [Google Scholar]
- 17.Roberts SW, Rainier JD. Org. Lett. 2005;7:1141. doi: 10.1021/ol0501469. [DOI] [PubMed] [Google Scholar]
- 18.That 23, 24, and 26 were isolated as single diastereomers implies a stereoselective epoxidation. For a discussion of the reaction of DMDO with cyclic enol ethers, see: Orendt AM, Roberts SW, Rainier JD. J. Org. Chem. 2006;71:5565. doi: 10.1021/jo060502g.
- 19.Clark JS, Kettle JG. Tetrahedron Lett. 1997;38:127. [Google Scholar]
- 20.For reviews, see: Arjona O, Gomez AM, Lopez JC, Plumet J. Chem. Rev. 2007;107:1919. doi: 10.1021/cr0203701.Martin Castro AM. Chem. Rev. 2004;104:2939. doi: 10.1021/cr020703u.
- 21.Cox JM, Rainier JD. Org. Lett. 2001;3:2919. [PubMed] [Google Scholar]
- 22.Clark JS, Kimber MC, Robertson J, McErlean CSP, Wilson C. Angew. Chem. 2005;117:6313. doi: 10.1002/anie.200501925. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:6157. [Google Scholar]
- 23.The stereochemistry at C32 was instrumental in setting the C33 and C34 stereocenters, see: reference [17] and Majumder U, Cox JM, Rainier JD. Org. Lett. 2003;5:913. doi: 10.1021/ol034100w.
- 24.Mitsunobu inversion of the C32 alcohol from 42 was slow and gave low yields of the desired alcohol.