Abstract
In this study, we pharmacologically characterized gambierol, a marine polycyclic ether toxin which is produced by the dinoflagellate Gambierdiscus toxicus. Besides several other polycyclic ether toxins like ciguatoxins, this scarcely studied toxin is one of the compounds that may be responsible for ciguatera fish poisoning (CFP). Unfortunately, the biological target(s) that underlies CFP is still partly unknown. Today, ciguatoxins are described to specifically activate voltage-gated sodium channels by interacting with their receptor site 5. But some dispute about the role of gambierol in the CFP story shows up: some describe voltage-gated sodium channels as the target, while others pinpoint voltage-gated potassium channels as targets. Since gambierol was never tested on isolated ion channels before, it was subjected in this work to extensive screening on a panel of 17 ion channels: nine cloned voltage-gated ion channels (mammalian Nav1.1–Nav1.8 and insect Para) and eight cloned voltage-gated potassium channels (mammalian Kv1.1–Kv1.6, hERG and insect ShakerIR) expressed in Xenopus laevis oocytes using two-electrode voltage-clamp technique. All tested sodium channel subtypes are insensitive to gambierol concentrations up to 10 μM. In contrast, Kv1.2 is the most sensitive voltage-gated potassium channel subtype with almost full block (>97%) and an half maximal inhibitory concentration (IC50) of 34.5 nM. To the best of our knowledge, this is the first study where the selectivity of gambierol is tested on isolated voltage-gated ion channels. Therefore, these results lead to a better understanding of gambierol and its possible role in CFP and they may also be useful in the development of more effective treatments.
Keywords: Gambierol, Ciguatera fish poisoning, Voltage-gated potassium channels, Voltage-gated sodium channels
1. Introduction
Voltage-gated potassium channels (VGPC) are diverse and ubiquitous family of voltage-sensitive membrane proteins present in both excitable and nonexcitable cells. They selectively conduct potassium ions across the cell membrane and therefore play an important role in maintaining the electro-chemical gradient of these ions (Shieh et al., 2000). Today, the Shaker family counts eight different mammalian members (Kv1.1–Kv1.8) and an insect channel (Shaker). Members of this channel family play critical roles in cellular signaling processes regulating neurotransmitter release, heart rate, insulin secretion, neuronal excitability, smooth muscle contraction, cell volume regulation and are involved in cancer (Felipe et al., 2006). More specifically, Kv1.1 is found to be important in pain sensation (Beekwilder et al., 2003). Kv1.2 is important in the management of multiple sclerosis (Judge and Bever, 2006). Kv1.3 channels are widely exploited as pharmacological targets for immunosuppressive therapy since they play a critical role in the immune response via T-lymphocytes (Shieh et al., 2000; Triggle, 2006). They are also important in multiple sclerosis. Kv1.4 is important in the management of pain and diabetes (Nishiyama et al., 2001) while Kv1.5 is critical to cardiac excitability because it plays a fundamental role in repolarization of the action potential (Bertaso et al., 2002; Brendel and Peukert, 2003). The human ether-a-go-go-related gene (hERG) channel is a member of the VGPC family but shows a very unusual kinetic behavior which includes slow activation and deactivation but rapid and voltage-dependent inactivation kinetics. This channel is important in normal human cardiac electrical activity and any disturbance or mutation of the channel can lead to cardiac disorders (e.g. long QT syndrome) (Zunkler, 2006).
Voltage-gated sodium channels (VGSC) are transmembrane proteins which consist of a pore-forming α-subunit and auxiliary β-subunits which are important for the expression, localization and functional properties of the sodium channel. They are critical elements of action potential initiation and propagation in excitable cells. A variety of different sodium channels have been identified and cloned: 10 mammalian (Nav1.1–Nav1.9, Nax) and 3 insect sodium channels (Zlotkin, 1999; Yu and Catterall, 2003). Despite the diversity of VGSC, their functional properties are relatively similar (Catterall et al., 2003). The important role of VGSC is physiological and pharmacological, demonstrated in pain and neurological disorders like epilepsy and ataxia (Celesia, 2001; Kohling, 2002; Benarroch, 2007).
Gambierol, a marine polycyclic ether toxin which is produced by the dinoflagellate Gambierdiscus toxicus, is in addition to several other polycyclic ether toxins like ciguatoxin, one of the compounds that have been proposed to be responsible for ciguatera fish poisoning (CFP) (Yasumoto, 2001; Ito et al., 2003). CFP is a worldwide-spread food poisoning caused by the consumption of tropical reef fishes that includes both gastrointestinal disturbances (nausea, diarrhea, vomiting and abdominal pain) and neurological alterations (tingling lips, hands or feet and unusual temperature sensation) (Lewis, 2006). Severe cases of ciguatera can involve bradycardia, hypotension, paralysis, respiratory difficulties and are often associated with liver toxicity manifested by elevated serum ammonia levels (Sims, 1987). The duration of the illness is usually 1 or 2 days, but residual weakness and sensory changes can persist for weeks, even years in severe cases (Hung et al., 2005).
Because of their limited availability, relatively little is known about the working mechanism of toxins producing ciguatera. Therefore, the development of a specific antidote has been severely hampered by the lack of information and it is only by supportive symptomatic treatment that ciguatera is currently treated. Today, at least 15 articles describe the effect of ciguatoxins on voltage-gated sodium and potassium channels (Lewis, 2006; Nicholson, 2006). For gambierol, there is some dispute about its ability to activate VGSCs. One manuscript (Louzao et al., 2006) describes the activation of VGSCs in human neuroblastoma cells by 30 μM gambierol while another (Lepage et al., 2007) describes gambierol as an antagonist of brevetoxin (PbTx-2) binding to VGSCs in CGN cells at 471 nM without channel activation.
On VGPC, one article describes an irreversible inhibition using patch-clamp technique on single taste cells isolated from the mouse vallate papilla (Ghiaroni et al., 2006). Very recently, we found that TRPV1 channels are a target of gambierol with an EC50 of 612 nM (Cuypers et al., 2007). Still, tests investigating the selectivity of gambierol on other isolated and cloned ion channels are missing. Despite the common polycyclic ether structure of gambierol and ciguatoxin and based on the aforementioned work, it is likely that they would show diverse biological activities and therefore may interact with different physiological targets. In this article, we tried to define the physiological target of gambierol by examining it on a wide number of isolated VGSC and Shaker-type VGPS and tried to couple them to the intoxication symptoms on the basis of which it would be possible to develop a more effective treatment in the future.
2. Methods
2.1. Compound synthesis
Gambierol was synthesized as previously described (Johnson et al., 2005, 2006; Majumder et al., 2006). Synthetic gambierol was identical spectroscopically (NMR 13C and 1H, MS, IR) to natural gambierol. For all the relevant spectral data see Johnson et al. (2005). The sample was dissolved in dimethyl sulfoxide (DMSO) and diluted with ND96. The total DMSO concentration in the test solution did not exceed 0.5%. Control experiments were performed to be sure that 0.5% DMSO has no effect on the tested voltage-gated ion channels.
2.2. Electrophysiological experiments
2.2.1. RNA preparation
Kv1.1−1.5 channels as well as hERG channels were prepared as previously described (Stuhmer et al., 1988; Huys et al., 2004). Kv1.6/pGEMHE and ShakerIR/pGEMHE was linearized with Nhe I and transcribed with T7 polymerase.
The Nav1.1/pLCT1, Nav1.2/pLCT1, Nav1.3/pNa3 T, Nav1.4/pUI-2 and Nav 1.6/pLCT1 were linearized with NotI and transcribed with the T7 mMESSAGEmMACHINE transcription kit (Ambion). Nav1.7/pBSTA.rPN1 were linearized with SacII and transcribed with the T7 mMESSAGEmMACHINE transcription kit. The para/pGH19−13−5 vector and tipE/pGH19 vector were linearized with NotI and transcribed with the T7 mMESSAGEmMACHINE transcription kit. Nav1.5 and β1 genes were previously subcloned into pSP64T. For in vitro transcription, Nav1.5/pSP64T and Nav1.8/pSP64T were first linearized with XbaI and b1/ pSP64T with EcoRI. Capped cRNAs were synthesized from the linearized plasmid using the SP6 mMESSAGEmMACHINE transcription kit.
2.2.2. Oocyte isolation and ion channel expression
Stage-V oocytes, harvested from anaesthetized female Xenopus laevis frogs as previously described (Liman et al., 1992), were injected with 0.5−5 ng cRNA. One–seven days after injection, two-electrode voltage-clamp recording was performed in ND96 solution containing (in mM) 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES (4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid N-(2-hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid)) and pH 7.4. Potassium current–voltage (IV) relationships of potassium channels were taken in high-potassium solution (HK) containing (in mM) 2 NaCl, 96 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES and pH 7.4. Voltage and current electrodes were filled with 3 M KCl. Resistance of both electrodes was kept low (< 1 MΩ). Kv1.1−1.6 and ShakerIR currents were evoked by depolarization from a holding potential of −90 to a test potential of 0 mV and followed by a test potential of −50 mV. hERG currents were induced by depolarization to 40 mV, holding potential was −90 mV then clamping back to −120 mV. Current–voltage (IV) relationships were taken using a protocol starting from −90 to +50 mV in 5 mV steps using a holding potential of −90 mV. To obtain half maximal inhibitory concentration (IC50) values, the gambierol-induced block was normalized to the maximum current and plotted against the concentration of gambierol used and a fit with the Hill equation yielded the IC50. All sodium channel subtypes were measured as follows: Currents were evoked by depolarizations from a holding potential of −90 mV, then clamping at the voltage with maximum current (depending on the subtype). IV curves were taken using a protocol starting from −70 to +85 mV in 5 mV steps and using a holding potential of −70 mV. gV was calculated in HK conditions from IV as follows: g = IK/(Em–EK) with EK = (RT/zF)ln[K]0/[K]i = −3.1 mV. In these equations g is the conductance, IK is the potassium current, Em is the membrane potential, EK is the reversal potential for K+ ions, R is the gas constant (8.31 J/K.mol), T is the temperature, z is the charge of the ion (for K+ ions: z = 1), F is Faraday's constant (96,500 C/mol) and [K]0 and [K]i are respectively extracellular and intracellular concentrations of K+ ions. For all measurements, the leak currents were subtracted using a –P/4 protocol and each experiment was done on 3−5 different oocytes.
3. Results
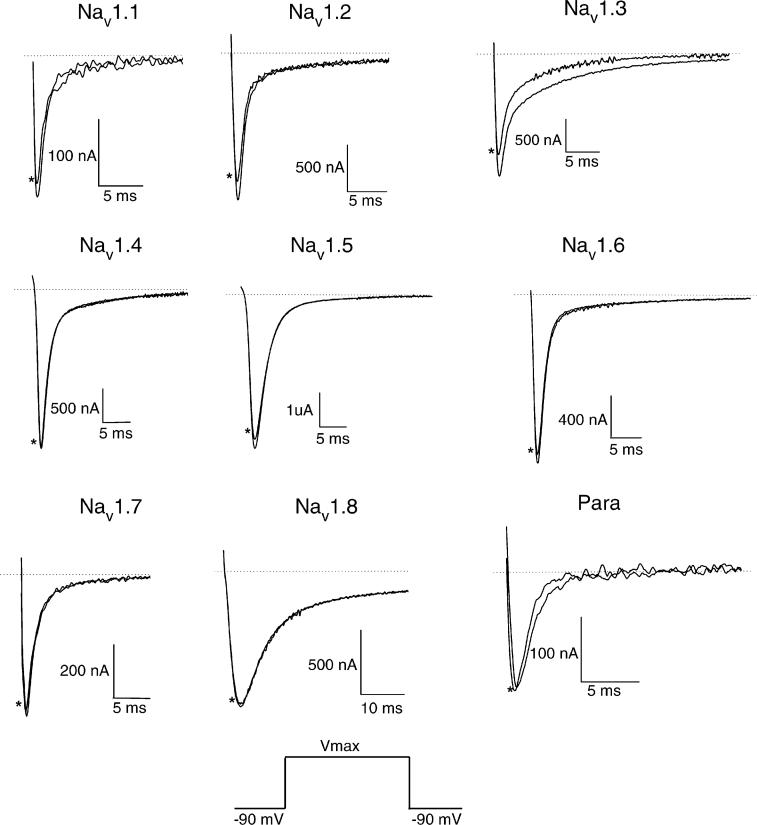
Gambierol (1 μM) was tested on nine subtypes of sodium channels including the insect sodium channel (Para). Current traces (Fig. 1) show no effect of 1 μM gambierol on all subtypes tested except Nav1.3. Higher concentrations up to 10 μM were tested and did not show any effect (data not shown). Gambierol (1 μM) has a small blocking effect (17.6%) on Nav1.3 but did not show more effect using higher concentrations up to 10 μM. The corresponding IV curve (Fig. 2A) reveals a change of reversal potential from +51 to +41 mV which indicates a change of ion selectivity. This observed shift was found not significant (P>0.05; n = 3) but can explain the small visible block effect in Fig. 1, since the driving force at any given voltage will be changed as compared to control conditions. The steady-state activation curve with a Boltzmann fit shown in Fig. 2B, using 1 μM gambierol, shows us that the V1/2 is not significantly different (P>0.05; n = 3) between control (V1/2 = −19.77 mV±2.05) and test conditions (V1/2 = −20.57 mV±1.38), which indicate that gambierol does not change the channel kinetics.
Fig. 1.
Current traces of all tested voltage-gated sodium channel subtypes. * marks the trace recorded in the presence of 1 μM gambierol. Currents were evoked by depolarizations from a holding potential of −90 mV, then maintained at the potential leading to maximum peak amplitude (depending on the subtype). Only a small effect is visible on Nav1.3 when 1 μM gambierol was administered. All other subtypes including Para did not show any visible effect with 1 μM gambierol.
Fig. 2.
(A) IV curve of Nav1.3. IV curve was taken using a protocol starting from −70 mV to +85 mV in 5 mV steps and using a holding potential of −70 mV. A small visible block in the maximum current is seen. Notice the change of reversal potential when 1 μM gambierol is administered. This shift was found not significant, but can explain the small visible block in this example. Higher concentrations up to 10 μM of gambierol did not show greater effect on Nav1.3. (B) The steady-state activation curve is shown using 1 μM gambierol. Notice that the V1/2 is not significantly different between control and test conditions which indicate that gambierol does not change the channel kinetics.
On VGPC (Kv1.1–Kv1.6, hERG and ShakerIR), gambierol shows almost full inhibition (>97%) of subtypes 1.2, 1.3 and 1.4 using 1 or 1.5 μM (Fig. 3). A strong inhibition is shown on Kv1.1 (maximum 85% block) and Kv1.5 (maximum 68% block) using 1 μM gambierol. No observable inhibition is found on Kv1.6, hERG and ShakerIR. Dose–response curves are shown in Fig. 4: Kv1.2 is the most sensitive subtype with an IC50 of 34.5 nM±1.5 (n = 3−5). Kv1.1 and Kv1.5 have a comparable sensitivity to gambierol with IC50 of respectively 64.2 nM±7.3 and 63.9 nM±5.4 (n = 3−5) but remarkable here is that both subtypes do not show full inhibition. Kv1.4 and Kv1.3 show the least sensitivity for gambierol with IC50 of respectively 108.3 nM±2.7 and 853.5 nM±35.0 (n = 3−5) but do show full inhibition.
Fig. 3.
Current traces of all tested voltage-gated potassium channel subtypes. * marks the traces were 1 or 1.5 μM gambierol was administered according to the maximum effect. Currents were evoked by depolarization from a holding potential of −90 to a test potential of 0 mV and followed by a test potential of −50 mV. hERG currents were induced by depolarization to 40 mV, holding potential was −90 mV then clamping back to −120 mV.
Fig. 4.
Dose–response of voltage-gated potassium channels. To obtain IC50 values, the gambierol-induced block was normalized to the maximum current (without gambierol) and plotted against the concentration of gambierol used and a fit with the Hill equation yielded the IC50.Kv1.2, 1.3 and 1.4 show almost full inhibition (>97%) with IC50 of respectively 34.5, 853.5 and 108.3 nM (n = 3 to 5). A strong inhibition is observed on Kv1.1 (maximum 85% block) and Kv1.5 (maximum 68% block) with IC50 of respectively 64.2 and 63.9 nM (n = 3 to 5). Note the remaining pedestal at high concentrations on Kv1.5. No observable inhibition is found on Kv1.6, Shaker IR and hERG.
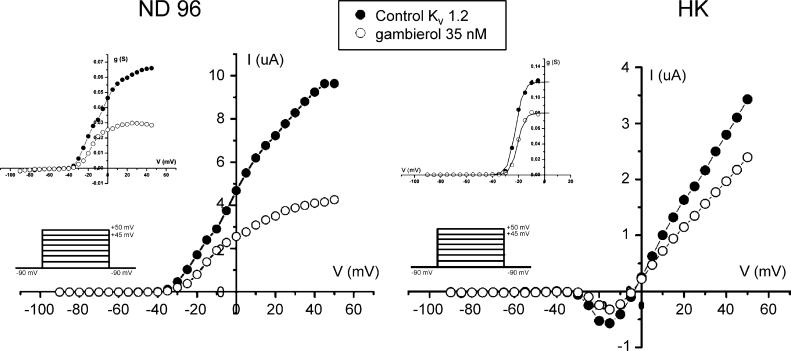
In order to test if the shown inhibitions are blocking or gating modifier effects, an IV curve in HK was taken (Fig. 5). Comparison of IV curves taken in ND96 and HK with the IC50 concentration (35 nM) of gambierol on Kv1.2 shows us respectively 52.6%±0.5 and 52.8%±5.3 block of the potassium current (n = 3). The observed effects were found irreversible. From this experiment where we tested the IV relationship in HK solution, we can conclude that at least the ion selectivity is not changed by gambierol since the reversal potential in control (−2.6 mV±2.3) and with 35 nM gambierol (−5.7 mV±2.8) is statistically not significantly different (P>0.05; n = 3) and in the range of the calculated reversal potential (EK = (RT/zF)ln [K]0/[K]i = −3.1 mV). Also, V1/2 is not significantly changed (P>0.05; n = 3) between the control (V1/2 = −23.3 mV±1.3) and 35 nM gambierol (V1/2 = −22.1 mV±0.9). gV curve taken in ND96 and HK and fitted with a Boltzmann curve shows there is no significant difference in both solutions (P>0.05; n = 3) between control and gambierol conditions. In ND96, V1/2 is respectively −10.06 mV±0.58 and −14.28 mV±1.85. In HK, V1/2 is respectively −25.60 mV±3.41 and −23.77 mV±3.27. These results indicate that the visible effect is not due to the change in ion channel kinetics.
Fig. 5.
IV curves of Kv1.2 taken in respectively ND96 and HK solutions. Gambierol was tested at IC50 concentration (35 nM) and IV curves were taken using a protocol starting from −90 to +50 mV in 5 mV steps using a holding potential of −90 mV. gV was calculated in both solutions using the equation described in the methods part. In both solutions, there is no statistically significant shift in V1/2 (P>0.05; n = 3).
4. Discussion
Until today, relatively little is known about the mechanism of action of CFP. The major toxins involved in ciguatera are sodium channel activator toxins and this fits in the symptomology. Compounds such as gambierol are described to be produced by G. toxicus dinoflagellates, but relatively little is known about their targets. Although sodium and potassium channels have been proposed in the literature as possible targets (Inoue et al., 2003; Ghiaroni et al., 2005; Louzao et al., 2006), very few papers can couple these targets to the intoxication symptoms (Cameron, 1993). In this article, we define VGPCs as a physiological target of gambierol, examine its selectivity and speculate on gambierol's role in CFS intoxications.
Kv1.2 was the most sensitive channel to gambierol with an IC50 of 34.5 nM±1.5 (n = 3−5). This is, besides dendrotoxin (DTX), one of the most potent toxins known on Kv1.2 (Harvey and Robertson, 2004). Since Kv1.2 is an important player in multiple sclerosis, gambierol might be considered as a lead compound in further study of the use of toxins in the treatment of pathogenic conditions.
It has been suggested that the highly lipophilic gambierol molecules are expected to enter the lipid bilayer of the cell membranes and disrupt the normal lipid environment that surrounds the potassium channels and which would likely have effect on the channel operation (Ghiaroni et al., 2005). However, from our experiment where we tested the IV relationship in HK solution, we can conclude that at least the ion selectivity is not changed by gambierol since the reversal potential in control (−2.6 mV±2.3; n = 5) and with 35 nM gambierol (−5.7 mV±2.8; n = 3) is statistically not significantly different (P>0.05; n = 3) and in the range of the calculated reversal potential (EK = (RT/zF)ln[K]0/[K]i = −3.1 mV). Also, V1/2 is not significantly changed (P>0.05; n = 3) between the control (V1/2 = −23.3 mV±1.3; n = 3 and 35nM gambierol (V1/2 = −22.1 mV±0.9; n = 3). Furthermore, when we compare the percentage of block induced by gambierol in either ND96 or HK solution, we can conclude that the extracellular potassium concentration did not influence the percentage block induced by gambierol, and hence, the block of Kv channels by gambierol does not seem to be dependent on the direction of the flux of potassium current. This observation contrasts with the finding that peptidyl toxins from scorpion venom can block more efficaciously inward potassium current in comparison with outward potassium current in Shaker-type channels (Srinivasan et al., 2002). In the light of the foregoing, it can be speculated to suggest that the toxin actually binds with high potency (IC50 = 34.5 nM) to some specific epitope in one or more transmembrane segments of the channel protein as it is described for ciguatoxins and VGSC (Wang and Wang, 2003). Taken into consideration that V1/2, calculated from the gV curve taken in ND96 and HK, shows no significant difference in ND96 nor in HK (P>0.05; n = 3) between control and gambierol, we might conclude that the visible effect is not due to the change in ion channel kinetics.
Although gambierol can be considered as a potent ligand interacting with Shaker-type VGPCs in the submicromolar range, the compound is not selective between Kv1.1−1.5. However, Kv1.5 was the only isoform where the efficacy of block was significantly less as illustrated by the remaining pedestal at high concentrations (Fig. 4).
Although voltage-gated potassium and sodium channels were previously proposed as targets for gambierol, no effect was visible on Kv1.6, hERG, ShakerIR (1.5 μM) and on all tested sodium channels except Nav1.3 (1−10 μM). The discrepancy between the lack of effect on most cloned Nav channels as observed in our oocyte experiments with the effect described on sodium channels in human neuroblastoma cells and cerebellar granule neurons (Louzao et al., 2006) might be explained as follows: the block of Kv channels most likely contributes to a depolarization of the membrane. This gives a lowering of the action potential threshold and leads to a more easy activation of Nav channels. Since in our oocyte expression system only one type of channel is overexpressed at once, it is possible that we did not find any effect since the above-described coupling is not possible. If this is correct, it can be concluded from our study that gambierol has only a direct effect on Kv channels while the effect on Nav channels is most probably secondary. The voltage-clamp condition of our oocyte expression system is a point which we should take into consideration when comparing these data with previous studies.
The observed effects can also explain pain: block of Kv1.1 and Kv1.4 increases the excitability of central circuits which can cause pain. The high lipophilic nature of gambierol suggests that the compound might stay in the lipid bilayer of the cell membrane for several days or weeks, which can explain the fact that the illness can last for several weeks. Some symptoms like hypotension and respiratory difficulties cannot be explained by these targets. Therefore, we suggest three theories: (1) Other ion channels might be involved (2) Ciguatera poisoning does not only involve gambierol, but a mixture of substances like ciguatera toxin, brevetoxin and many other polycyclic ether toxins which also have an influence on the symptomology (Ito et al., 2003). (3) Keeping in mind the example of Kv1.3 and Kv1.5 heteromers forming a voltage-gated potassium current in macrophages (Vicente et al., 2006), synergistic effect of different isoforms, possibly forming heteromers, might play a role in the development of some symptoms.
In conclusion, since only few articles were published about gambierol and since the methods used were variable and not always channel specific, it was not very clear what gambierol's physiological targets were. Therefore, in this study we have attempted to unravel the physiological target and selectivity of gambierol and lead to a better understanding of gambierol and its role in CFP. In this way, these results may also be useful in the development of more effective treatments. Nevertheless, some more research is necessary in order to check other ion channels (e.g. Kv2.x and Kv3.x subfamilies) and to learn more about the precise mechanism of action on the studied VGPS.
Acknowledgments
We thank O. Pongs for the Kv1.2, Kv1.4, Kv1.5 and Kv1.6 cDNA. The Kv1.3 clone was kindly provided by M.L. Garcia. We are grateful to G. Yellen for sharing the ShakerIR clone and to M. Keating for sharing hERG. We thank John N. Wood for sharing Nav1.8, A.L. Goldin for sharing Nav1.1, Nav1.2, Nav1.3 and Nav1.6, G. Mandel for sharing Nav1.4, R.G. Kallen for sharing Nav1.5, S.H. Heinemann for sharing the β1 subunit and Martin S. Williamson for providing the Para and tipE clone. We are grateful to Roche (Palo Alto, CA) for sharing Nav1.7. The authors would like to acknowledge the skillful technical assistance of S. Debaveye and thank B. Billen and T. Vandendriessche for the useful discussions. This work was supported in part by the K.U. Leuven (Grant OT-05-64), G.0330.06 (F.W.O.-Vlaanderen) and P6/31 (Interuniversity attraction Poles Programme-Belgian State-Belgian Science Policy), and the National Institute of Health and General Medical Sciences (GM56677). Y. Abdel-Motalleb is a doctoral student supported by IRO KULeuven Scholarship.
Abbreviations
- VGSC
Voltage-gated sodium channels
- VGPC
Voltage-gated potassium channels
- CFP
Ciguatera fish poisoning
- HERG
Human ether-a-go-go-related gene
- DMSO
Dimethyl sulfoxide
- HEPES
4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)
- IC50
Half maximal inhibitory concentration
- HK
High-potassium solution (96 mM K+)
- DTX
Dendrotoxin
Footnotes
Ethical statement: The authors declare that this work has not been published elsewhere and that the guidelines for animal welfare have been followed.
References
- Beekwilder JP, O'Leary ME, van den Broek LP, van Kempen GT, Ypey DL, van den Berg RJ. Kv1.1 channels of dorsal root ganglion neurons are inhibited by n-butyl-p-aminobenzoate, a promising anesthetic for the treatment of chronic pain. J. Pharmacol. Exp. Ther. 2003;304:531–538. doi: 10.1124/jpet.102.042135. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Sodium channels and pain. Neurology. 2007;68:233–236. doi: 10.1212/01.wnl.0000252951.48745.a1. [DOI] [PubMed] [Google Scholar]
- Bertaso F, Sharpe CC, Hendry BM, James AF. Expression of voltage-gated K+ channels in human atrium. Basic Res. Cardiol. 2002;97:424–433. doi: 10.1007/s00395-002-0377-4. [DOI] [PubMed] [Google Scholar]
- Brendel J, Peukert S. Blockers of the Kv1.5 channel for the treatment of atrial arrhythmias. Curr. Med. Chem. Cardiovasc. Hematol. Agents. 2003;1:273–287. doi: 10.2174/1568016033477441. [DOI] [PubMed] [Google Scholar]
- Cameron J. The basis of the paradoxical disturbance of temperature perception in ciguatera poisoning. Clin.Toxicol. 1993;31:571–579. doi: 10.3109/15563659309025762. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International union of pharmacology. XXXIX. Compendium of voltage-gated ion channels: sodium channels. Pharmacol. Rev. 2003;55:575–578. doi: 10.1124/pr.55.4.7. [DOI] [PubMed] [Google Scholar]
- Celesia GG. Disorders of membrane channels or channelopathies. Clin. Neurophysiol. 2001;112:2–18. doi: 10.1016/s1388-2457(00)00496-x. [DOI] [PubMed] [Google Scholar]
- Cuypers E, Yanagihara A, et al. TRPV1 as a key determinant in ciguatera and neurotoxic shellfish poisoning. Biochem. Biophys. Res. Commun. 2007;361(1):214–217. doi: 10.1016/j.bbrc.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felipe A, Vicente R, Villalonga N, Roura-Ferrer M, Martinez-Marmol R, Sole L, Ferreres JC, Condom E. Potassium channels: new targets in cancer therapy. Cancer Detect. Prev. 2006;30:375–385. doi: 10.1016/j.cdp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Ghiaroni V, Sasaki M, Fuwa H, Rossini GP, Scalera G, Yasumoto T, Pietra P, Bigiani A. Inhibition of voltage-gated potassium currents by gambierol in mouse taste cells. Toxicol. Sci. 2005;85:657–665. doi: 10.1093/toxsci/kfi097. [DOI] [PubMed] [Google Scholar]
- Ghiaroni V, Fuwa H, Inoue M, Sasaki M, Miyazaki K, Hirama M, Yasumoto T, Rossini GP, Scalera G, Bigiani A. Effect of ciguatoxin 3C on voltage-gated Na+ and K+ currents in mouse taste cells. Chem. Senses. 2006;31:673–680. doi: 10.1093/chemse/bjl008. [DOI] [PubMed] [Google Scholar]
- Harvey AL, Robertson B. Dendrotoxins: structure–activity relationships and effects on potassium ion channels. Curr. Med. Chem. 2004;11:3065–3072. doi: 10.2174/0929867043363820. [DOI] [PubMed] [Google Scholar]
- Hung YM, Hung SY, Chou KJ, Huang NC, Tung CN, Hwang DF, Chung HM. Short report: persistent bradycardia caused by ciguatoxin poisoning after barracuda fish eggs ingestion in southern Taiwan. Am. J. Trop. Med. Hyg. 2005;73:1026–1027. [PubMed] [Google Scholar]
- Huys I, Xu CQ, Wang CZ, Vacher H, Martin-Eauclaire MF, Chi CW, Tytgat J. BmTx3, a scorpion toxin with two putative functional faces separately active on A-type K+ and HERG currents. Biochem. J. 2004;378:745–752. doi: 10.1042/BJ20031324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Hirama M, Satake M, Sugiyama K, Yasumoto T. Inhibition of brevetoxin binding to the voltage-gated sodium channel by gambierol and gambieric acid-A. Toxicon. 2003;41:469–474. doi: 10.1016/s0041-0101(02)00369-0. [DOI] [PubMed] [Google Scholar]
- Ito E, Suzuki-Toyota F, Toshimori K, Fuwa H, Tachibana K, Satake M, Sasaki M. Pathological effects on mice by gambierol, possibly one of the ciguatera toxins. Toxicon. 2003;42:733–740. doi: 10.1016/j.toxicon.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Johnson HW, Majumder U, Rainier JD. The total synthesis of gambierol. J. Am. Chem. Soc. 2005;127:848–849. doi: 10.1021/ja043396d. [DOI] [PubMed] [Google Scholar]
- Johnson HW, Majumder U, Rainier JD. Total synthesis of gambierol: subunit coupling and completion. Chemistry. 2006;12:1747–1753. doi: 10.1002/chem.200500994. [DOI] [PubMed] [Google Scholar]
- Judge SI, Bever CT., Jr. Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment. Pharmacol. Ther. 2006;111:224–259. doi: 10.1016/j.pharmthera.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Kohling R. Voltage-gated sodium channels in epilepsy. Epilepsia. 2002;43:1278–1295. doi: 10.1046/j.1528-1157.2002.40501.x. [DOI] [PubMed] [Google Scholar]
- LePage KT, Rainier JD, et al. Gambierol acts as a functional antagonist of neurotoxin site 5 on voltage-gated sodium channels in cerebellar granule neurons. J. Pharmacol. Exp. Ther. 2007;323(1):174–179. doi: 10.1124/jpet.107.124271. [DOI] [PubMed] [Google Scholar]
- Lewis RJ. Ciguatera: Australian perspectives on a global problem. Toxicon. 2006;48:799–809. doi: 10.1016/j.toxicon.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Louzao MC, Cagide E, Vieytes MR, Sasaki M, Fuwa H, Yasumoto T, Botana LM. The sodium channel of human excitable cells is a target for gambierol. Cell. Physiol. Biochem. 2006;17:257–268. doi: 10.1159/000094138. [DOI] [PubMed] [Google Scholar]
- Majumder U, Cox JM, Johnson HW, Rainier JD. Total synthesis of gambierol: the generation of the A–C and F–H subunits by using a C-glycoside centered strategy. Chemistry. 2006;12:1736–1746. doi: 10.1002/chem.200500993. [DOI] [PubMed] [Google Scholar]
- Nicholson GM, Lewis R. Ciguatoxins: Cyclic Polyether Modulators of Voltage-gated ion Channel Function. Mar. Drugs. 2006;4:82–118. [Google Scholar]
- Nishiyama A, Ishii DN, Backx PH, Pulford BE, Birks BR, Tamkun MM. Altered K(+) channel gene expression in diabetic rat ventricle: isoform switching between Kv4.2 and Kv1.4. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1800–H1807. doi: 10.1152/ajpheart.2001.281.4.H1800. [DOI] [PubMed] [Google Scholar]
- Shieh CC, Coghlan M, Sullivan JP, Gopalakrishnan M. Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacol. Rev. 2000;52:557–594. [PubMed] [Google Scholar]
- Sims JK. A theoretical discourse on the pharmacology of toxic marine ingestions. Ann. Emerg. Med. 1987;16:1006–1015. doi: 10.1016/s0196-0644(87)80750-3. [DOI] [PubMed] [Google Scholar]
- Srinivasan KN, Sivaraja V, Huys I, Sasaki T, Cheng B, Kumar TK, Sato K, Tytgat J, Yu C, San BC, Ranganathan S, Bowie HJ, Kini RM, Gopalakrishnakone P. kappa-Hefutoxin1, a novel toxin from the scorpion Heterometrus fulvipes with unique structure and function. Importance of the functional diad in potassium channel selectivity. J. Biol. Chem. 2002;277:30040–30047. doi: 10.1074/jbc.M111258200. [DOI] [PubMed] [Google Scholar]
- Stuhmer W, Stocker M, Sakmann B, Seeburg P, Baumann A, Grupe A, Pongs O. Potassium channels expressed from rat brain cDNA have delayed rectifier properties. FEBS Lett. 1988;242:199–206. doi: 10.1016/0014-5793(88)81015-9. [DOI] [PubMed] [Google Scholar]
- Triggle D. Voltage-gated ion channels as drug targets. 2006 [Google Scholar]
- Vicente R, Escalada A, Villalonga N, Texido L, Roura-Ferrer M, Martin-Satue M, Lopez-Iglesias C, Soler C, Solsona C, Tamkun MM, Felipe A. Association of Kv1.5 and Kv1.3 contributes to the major voltage-dependent K+ channel in macrophages. J. Biol. Chem. 2006;281:37675–37685. doi: 10.1074/jbc.M605617200. [DOI] [PubMed] [Google Scholar]
- Wang SY, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell Signal. 2003;15:151–159. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Yasumoto T. The chemistry and biological function of natural marine toxins. Chem. Rec. 2001;1:228–242. doi: 10.1002/tcr.1010. [DOI] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4:207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotkin E. The insect voltage-gated sodium channel as target of insecticides. Annu. Rev. Entomol. 1999;44:429–455. doi: 10.1146/annurev.ento.44.1.429. [DOI] [PubMed] [Google Scholar]
- Zunkler BJ. Human ether-a-go-go-related (HERG) gene and ATP-sensitive potassium channels as targets for adverse drug effects. Pharmacol. Ther. 2006;112:12–37. doi: 10.1016/j.pharmthera.2006.03.002. [DOI] [PubMed] [Google Scholar]