Abstract
Left-right patterning is a fascinating problem of morphogenesis, linking evolutionary and cellular signaling mechanisms across many levels of organization. In the last 15 years, enormous progress has been made in elucidating the molecular details of this process in embryos of several model species. While many outside the field seem to believe that the fundamental aspects of this pathway are now solved, workers on asymmetry are faced with considerable uncertainties over the details of specific mechanisms, a lack of conceptual unity of mechanisms across phyla, and important questions that are not being pursued in any of the popular model systems. Here, we suggest that data from clinical syndromes, cryptic asymmetries, and bilateral gynandromorphs, while not figuring prominently in the mainstream work on LR asymmetry, point to crucial and fundamental gaps of knowledge about asymmetry. We identify 12 big questions that provide exciting opportunities for fundamental new advances in this field.
Keywords: Left-right asymmetry
"The great field for new discoveries … is always the unclassified residuum. Round about the accredited and orderly facts of every science there ever floats a sort of dust-cloud of exceptional observations … Any one will renovate his science who will steadily look after the irregular phenomena."
--William James, 1890
Introduction
Left-right asymmetry is an important and puzzling feature of embryogenesis (Burdine and Schier, 2000; Levin, 2005), also having important implications for human health and development (Cohen, 2001; Ramsdell, 2005). There now seems to be a perception outside the field that the question of Left-Right (LR) patterning is largely solved. Cell biology textbooks and reviews now often summarize their view of the situation with one sentence, such as “… and ciliary movement also initiates Left-Right patterning”. This is unfortunate, because it leads young developmental biologists (and reviewers of grant proposals) to think that there is little interesting science left in this field. However, those working on left-right (LR) patterning know that there is considerable uncertainty about the early mechanisms of symmetry breaking, the evolutionary origins and degree of conservation of LR mechanisms at all levels, and the clinical implications of what we know and do not know about asymmetry (Tabin, 2005; Levin, 2006; Raya and Belmonte, 2006; Speder et al., 2007).
The study of LR patterning has largely been focused on the asymmetric gene cascades (Levin, 1998; Whitman and Mercola, 2001), understanding the dynamics of node cilia using technologically intricate approaches (Cartwright et al., 2004; Buceta et al., 2005), and modeling the properties of morphogen gradients of Nodal (Nakamura et al., 2006). However, a constellation of classical and recent observations suggests that, contrary to popular opinion, we are still missing major components of this field, and, in many ways, have been looking “where the light is good” (Levin, 2005).
Here, we briefly sketch 12 major open questions in an effort to catalyze work on these challenging and novel directions. We chose these specific issues to be distinct in nature from the many details that remain to be fleshed out regarding the known pathways. The major areas of ignorance are revealed because they are not currently being addressed, have no established powerful model for their investigation, and/or involve a complex phenomenon not centered on a specific gene product. While we highlight some of the most difficult issues facing the field, these are also the areas of the greatest opportunity for discovery of truly new biology in this field.
(1) What is the molecular mechanism of “concordance”
that determines whether a population is 50% situs inversus and 50% situs solitus vs. heterotaxic (random and independent sidedness of each organ)? Perturbations, like the spontaneous mouse mutants iv and lgl (Layton, 1976; McNeish et al., 1990; Singh et al., 1991), and patients with immotile cilia syndrome (Afzelius, 1976), exhibit 50% situs inversus and 50% situs solitus, while almost all of the known manipulations of early pathways (and mammalian mutations of Zic3, Lefty, cryptic/CFC, and ActRIIb) result in heterotaxia. None of the existing models (ciliary or intracellular) specifically address this; nor do they provide an explanation for how heterotaxia occurs. Thus, a more careful characterization of known mutations as to their degree of concordance (by scoring additional organ readouts and paying attention to subtle organ malformations as well as overall reversals), and the development of models that make predictions about the timing and nature of independent vs. linked situs decisions, will be essential.
(2) What is the base state of the LR axis?
Complete inversions are induced by partial deletion of inv (Mochizuki et al., 1998), overexpression of mature Vg1, (Hyatt and Yost, 1998), and inhibition of serotonin receptor 3 (albeit with a lower penetrance, (Fukumoto et al., 2005)). The inv mouse is especially curious, because its mutation results in the majority of its offspring exhibiting situs inversus (although there is a detectable level of heterotaxia) (Morgan et al., 1998; Otto et al., 2003). Unfortunately, the true genetic deletion has not been analyzed and all published experiments appear to have been carried out with the spontaneous mutant, in which a small part of the protein still exists (and hence may not be a true null). Thus, it remains to be determined whether this fragment is enough to bias asymmetry, refuting the bizarre possibility that the default state (without INV activity) is biased asymmetry, but in the reverse orientation, and that the INV gene product is needed to simply flip everything in the complete opposite direction). The inv mutation appears to affect a very early step, since a cross between an inv mouse and a Foxj1 knockout mouse (knockout Foxj1 mice are 50% situs inversus and 50% situs solitus (Chen et al., 1998; Brody et al., 2000; Zhang et al., 2004)) produce mice that more closely resemble the Foxj1 phenotype (Tamakoshi et al., 2006). An understanding of the definitive inv loss-of-function should be very informative.
Recent studies show that retinoic acid signaling shields somites from asymmetric cues (Vermot et al., 2005; Vermot and Pourquie, 2005). Symmetrical structures may inadvertently be susceptible to the effects of left-right signaling molecules, since many developmentally important factors are used in patterning programs that overlap in space and time. The advantages of symmetry, however, have selected for the evolution of processes that shield the developing symmetric structures from asymmetric cues (Vermot et al., 2005; Vermot and Pourquie, 2005). Along with the orientation, it should be determined whether the base state of symmetrical structures is symmetry or asymmetry (Jefferies et al., 1996; Palmer, 2004).
(3) Are the breaking and orientation steps truly distinct and sequential?
It is commonly thought that in order to pattern the LR axis, symmetry must first be broken and then oriented with respect to the other two axes (Brown and Wolpert, 1990). However, sometimes, perturbation of the same molecule in different locations can lead to predictable, differential laterality phenotypes. Ventral injection of chimeric BVg1 into the R3 cells causes almost complete situs inversus, while injection into the R1 cell causes heterotaxia, and injections into the left side do not cause any significant laterality defects (Hyatt and Yost, 1998). These data may indicate a close relationship between the mechanisms for the breaking of symmetry and the biasing of asymmetry, and could serve as an entrypoint into this process. Precedents for such a bistable switch include the Notch/Delta system (Raya et al., 2004). Regardless of whether the breaking of symmetry and biasing of asymmetry is one step or two steps, the iv mutant mouse, which exhibits random 50% situs inversus and 50% situs solitus, indicates that the next processes of amplification and restriction of the initial asymmetric signal may still be able to receive cues from, or act downstream of the symmetry breaking point, and that the orientation mechanism may not be necessary to bridge the symmetry breaking point with concordant downstream organ morphogenesis. This would make sense evolutionarily because the direction of asymmetry is not as important as concordance between the organs, since situs inversus individuals exhibit few clinical symptoms, but heterotaxic individuals can often have severe medical problems (Burn, 1991; Peeters and Devriendt, 2006).
A related aspect is the precise characterization of phenotypes reported for the various genetic and pharmacological perturbations. In order to arrive at a complete model for LR patterning mechanisms, detailed characterization of the phenotypes caused by each experimental system is essential. Indeed, both iv and inv mouse phenotypes are not purely 50%/50% and fully inverted respectively, but include a significant degree of heterotaxias, isomerisms, and cardiac malformations (Hummel and Chapman, 1959; Morgan et al., 1998; Morishima et al., 1998; McQuinn et al., 2001). Likewise, situs inversus individuals are not really normal with respect to cardiac function because of discordances of orientation of structures at different scales (Delhaas et al., 2004; Ramsdell, 2005). Thus, the distinctions between symmetry breaking, orientation, and concordance/morphogenesis may be more artifacts of our conceptual analysis of LR patterning phenotypes than a reflection of truly separate mechanisms during embryogenesis.
(4) Probably related is a set of puzzling inconsistencies about the universally-conserved Left determinant Nodal, other asymmetric genes, and downstream readouts
If Nodal is indeed a Left determinant, why does bilateral Nodal not lead to left isomerism, but to randomization (Levin et al., 1997; Sampath et al., 1997)? What kind of mechanism could explain cases where the upstream markers exhibit a higher level of incorrectly-sided expression then the downstream targets they induce in the LR cascade (Tsukui et al., 1999; Kitaguchi et al., 2000; Kelly et al., 2002; Levin et al., 2002; Morokuma et al., 2002), which may even become completely uncoupled from expression of Nodal (Cota et al., 2006)? Since the iv mouse exhibits 50% situs inversus and 50% situs solitus, we would expect nodal expression in the iv mouse population to be 50% left-sided, and 50% right-sided. Instead, Nodal in iv mice populations is expressed on the left-side, right-side, bilateral or absent, at statistically equal probabilities (Lowe et al., 1996). This indicates that in iv mice, bilateral or absent Nodal does not prevent organ concordance. Yet, when left-sided Nodal is experimentally removed, heterotaxia does result (Brennan et al., 2002)! In zebrafish, over 30% of cup mutant embryos show complete situs inversus, but only a small percentage of them show right-sided spaw expression (Schottenfeld et al., 2007), even though cup is a homolog of Pkd2 which is thought to be upstream of Nodal signaling. Another related puzzle: why do as many as 20% of siblings from a batch of embryos with 99% normal organ situs exhibit no expression of Nodal whatsoever (Levin and Mercola, 1998a; Takano et al., 2007)?
(5) How conserved are the early, intermediate, and late mechanisms in LR patterning?
The various intracellular, biophysical, and ciliary mechanisms have each only been probed in a small number of representative model systems (Palmer, 2004; Levin, 2006; Levin and Palmer, 2007). While cilia appear to be involved in mouse (Nonaka et al., 1998; McGrath et al., 2003), zebrafish (Otto et al., 2003; Amack and Yost, 2004), and Xenopus (Schweickert et al., 2007), numerous other mechanisms such as cytoskeletal polarity (Thitamadee et al., 2002; Qiu et al., 2005), serotonergic signaling (Fukumoto et al., 2005), gap junctions (Levin and Mercola, 1998a; Levin and Mercola, 1999; Chuang et al., 2007), and ion flows (Adams et al., 2006) are conserved in LR patterning among other phyla, including Arabidopsis, Drosophila, C. elegans, and ciliates (Levin and Palmer, 2007; Oviedo and Levin, 2007). The recent demonstration that individual cells can align the LR axis from intracellular polarity cues (Xu et al., 2007) suggests that subcellular and ancient chiral components of all cells may underlie an origin of LR patterning existing prior to the evolution of multi-cellularity (Levin, 2006; Levin and Palmer, 2007). This powerful idea was presciently suggested in 1977, prior to the identification of any of the molecular aspects of asymmetry: “one may begin to look for a relationship between the bilateral symmetry of organisms and the mirror symmetry between daughter cells at an early state of the embryo” (Albrecht-Buehler, 1977).
The question of which, if any, of the early physiological mechanisms are used in mouse asymmetry is still open (see (Levin and Palmer, 2007) for a discussion of alternative interpretations of the genetic data vis-à-vis the proposal of cilia as originators of asymmetry). Because of the ubiquitous compensation and redundancy that exists among ion transport machinery, a truly satisfactory approach to answering this would require knock-in of dominant negative and constitutively active transporter mutants that will change cell membrane potential in pre-streak embryos in well-defined ways, rather than knockout of individual channel/pump genes. Related to this is the need to reexamine roles of “ciliary” proteins in mammals, as they are likely to have other functions in addition to ciliary roles, and hence, knockouts do not cleanly implicate a specific function of ciliary proteins in LR patterning. For example, LRD is expressed in a random manner in head folds (Supp et al., 1997), and, intriguingly, has been shown to be important for biased chromatid segregation (Armakolas and Klar, 2007).
(6) What explains the relatively low “penetrance” of some laterality-perturbing reagents and mutations?
In a population where the background of defects is ~1%, a 25% incidence of heterotaxia in 1000 embryos is unquestionably significant (Levin and Mercola, 1998a; Levin et al., 2002; Bunney et al., 2003). But, are the remaining embryos insensitive to the reagent? Are they somehow regulating to normalize asymmetry despite the effect of the reagent? Or are they truly part of an “affected” group of which 75% are developmentally determined to be situs solitus? With respect to pharmacological inhibition of ion channels and transporters, a possible reason for this may relate to the existence of robust physiological homeostasis mechanisms that can compensate for the loss of channel function, or even that the precise repertoire of transporters expressed in a given individual may be a complex function of heredity, diet, and culture conditions that remain to be worked out. For example (unpublished observations in our lab), a pharmacological reagent from the same aliquot, on the same day, when applied to embryos of two different mothers, can cause heterotaxia rates of 3% to one group, and 55% in the other! This type of result reveals the complexity underlying the simple question of whether a particular pathway is or is not “involved in LR patterning”.
One possible explanation for the variability in phenotypes may involve the early embryonic fate-map. Some organisms (e.g., C. elegans) have a very mosaic and stereotypical development; others (e.g., Xenopus) use a basic scheme in which blastomeres give rise to which tissues, but the exact cell boundaries vary considerably among individuals of normal egg batches. This is clearly seen in the original datasets of fate mapping studies (Dale and Slack, 1987; Moody and Kline, 1990; Chalmers and Slack, 2000). One prediction of this model is that a meta-analysis of LR perturbations among phyla should show more variability in phenotypic outcome in model species whose development is less stereotypical. If true, this would also imply the existence of endogenous robustness mechanisms compensating for high levels of plasticity in embryonic cell fate (Palmer, 2004). Importantly, an understanding of these robustness mechanisms will also improve our ability to investigate LR patterning, since it is still not known why laboratory culture of rodent and flatfish embryos automatically results in a significant background of LR defects (Fujinaga et al., 1990; Fujinaga and Baden, 1991; Schreiber, 2006). The understanding of stability against, and susceptibility to, environmental factors in LR patterning will be a key component of a complete appreciation of the stochasticity underlying consistent asymmetry.
(7) What is the nature of “subtle” asymmetries?
The field is almost entirely focused on pathways determining situs of heart and visceral organs, with some attention now being paid to laterality of the brain (Toga and Thompson, 2003; Halpern et al., 2005; Sun and Walsh, 2006; Hendricks and Jesuthasan, 2007). CNS asymmetry may be patterned differently from that of body asymmetry, along pathways that may either be unrelated but parallel or, more likely, divergent from a common early step: although humans with situs inversus exhibit reversal of some anatomical brain asymmetries, they do not show reversals in functional asymmetries, and establish language dominance on the left cerebral hemisphere and a strong bias for right-handedness, just like in the general population (Kennedy et al., 1999; Tanaka et al., 1999; McManus et al., 2004). The zebrafish model system has not provided an ideal model for this phenomenon because changes in canonical visceral situs pathways do seem to randomize fish brain asymmetry (Bisgrove et al., 2000; Concha et al., 2000; Barth et al., 2005; Essner et al., 2005), although point mutations of cyclops (the zebrafish Nodal homolog) result in normal heart looping and visceral situs but perhaps anatomical randomization in the brain since asymmetric gene expression is altered (Saude et al., 2005). However, the clinical data indicate that numerous other body structures and processes pay attention to LR cues, in pathways not predicted by or included in any of the current models (ciliary or intracellular).
There are several human syndromes that affect symmetrical or paired structures like the limbs (Smith et al., 1979), face or hips (Paulozzi and Lary, 1999), that consistently present more strongly on one side than the other. Facial clefting presents a consistent asymmetry (Delaney and Boyd, 2007), and this has recently become experimentally-tractable, with the identification of molecular markers of asymmetry in symmetrical embryonic structures (somites, (Golding et al., 2004a; Golding et al., 2004b)), and the discovery of a genetic entrypoint into zebrafish craniofacial asymmetry (Albertson and Yelick, 2005). Hemihyperplasia (Fraumeni et al., 1967; Clericuzio, 1993; Leung et al., 2002), a rare phenomenon where one side of the body to begins to grow in adulthood, is right-biased (Hoyme et al., 1998), and not only suggests that symmetric structures (knee joints, long bones of the legs) have LR identity, but that, astonishingly, they are able to retain that information for decades after embryogenesis.
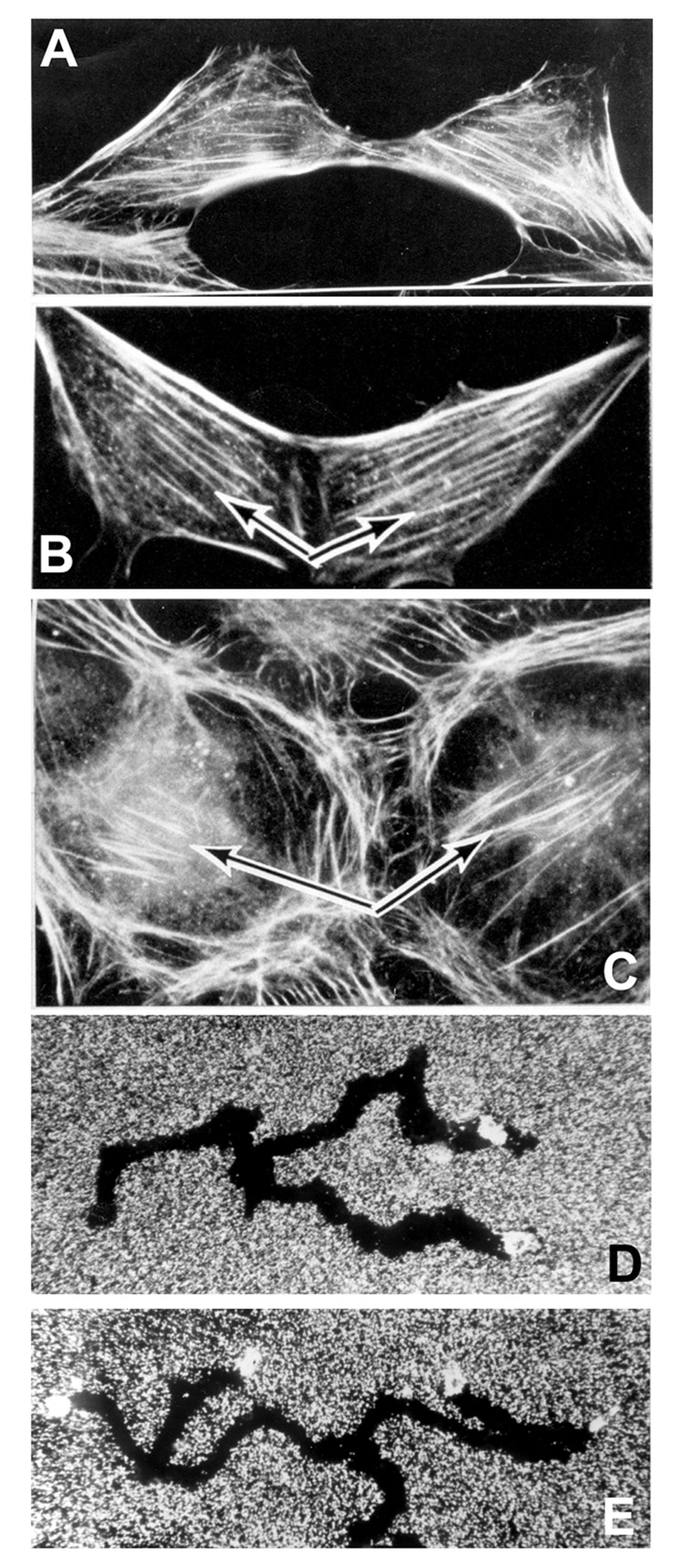
Monozygotic twins are discordant for hemihypertrophy (West et al., 2003), and they display opposite-sided hair whorl direction, unilateral tooth defects, and other “bookending” phenomena (reviewed in (Levin, 1999)), strengthening the link between very early chiral decisions and subtle asymmetries in man. Is this related to the mirroring (Fig. 1) of cytoskeletal structures and migration paths observed when cells split (Albrecht-Buehler, 1977)? What is the molecular nature of the asymmetrical information that exists at early cleavages of vertebrate embryos and that results in asymmetry defects in one of the twins when they are separated early? Why does this separation result in opposite sidedness of subtle characteristics such as hair whorls in man (Golbin et al., 1993b; Golbin et al., 1993a; Morison et al., 1994) but also affect visceral and cardiac situs in some vertebrates (Mangold, 1921; Takano et al., 2007)? An entrypoint into the mechanisms of these subtle asymmetries may be provided by the study of hair whorls on the face of cattle produced by egg splitting (in the context of ongoing in vitro production of valuable animals (Ozil et al., 1982; Gatica et al., 1984; Lanier et al., 2001; Meola et al., 2004)). Does the fact that these subtle asymmetries in human monozygotic twins exist almost entirely above the neck suggest a separate LR-organizer for the head and brain, distinct from that for the body?
Figure 1. mirroring among single cells.
(A–C) Actin cytoskeletal elements of daughter cells in culture showing a mirroring of the angle of filament orientation. (D,E) Sample migration tracks of cells in culture showing mirroring of cell paths after splitting. These data indicate the presence of chirality in single cells that is preserved at splitting, possibly providing a model for understanding enantiomer (bookending) of symmetry properties in monozygotic twins derived from early splitting of the egg. Images are used with permission of Albrecht Guenther-Buehler (Albrecht-Buehler, 1977).
The link to sex determination pathways (which have no obvious asymmetry) is fascinating, revealed by the consistently-sided presence of testes vs. ovaries in hermaphrodites (van Niekerk and Retief, 1981; Krob et al., 1994; Mittwoch, 2000; Mittwoch, 2001; Mittwoch, 2008). There is a cryptic asymmetry aspect to sex determination; for example, the consistent asymmetry in foot size is opposite between women and men (Levy and Levy, 1978). A model system may be available, since, in the Zic3 mutant, all affected males are heterotaxic while females are wild-type or situs inversus (Gebbia et al., 1997). Finally, there are associations between asymmetry and the immune response (Renoux et al., 1983; Fride et al., 1990; Betancur et al., 1991; Neveu, 2002; Fu et al., 2003; Meador et al., 2004; Quaranta et al., 2004; Shen et al., 2005), and cancer (McManus, 1992; Sandson et al., 1992). The origin and implications of these links remain completely mysterious.
(8) “What is randomization”?
This is a term often used in the field but attempting to construct a logical, mechanistic model of randomization quickly reveals our lack of conceptual clarity. For example, the normal chick embryo has a depolarization of cells on the left side of the streak followed by expression of Sonic hedgehog on the left side of the node. The simple way of modeling this would be to hypothesize that depolarization induces Shh expression. However, experimental induction of bilateral depolarization (Levin et al., 2002) does not result in bilateral Shh, but instead gives a spectrum of left-sided, right-sided, bilateral, and absent Shh expression. A mechanistic understanding of randomization would not only have to explain this bizarre result (which also occurs for other LR components), but would also have to show how cells are synchronized (so that the L and R domains of Hensen’s node act as a unit, and do not exhibit a salt-and-pepper pattern on each side, reflecting cells’ individual randomization). It would also need to predict the non-equiprobable outcomes (right-sided Shh is less frequent than bilateral Shh for example).
(9) What happens upstream of asymmetric gene expression in the chick?
Prior to the right-sided expression of Activin Receptor IIa in the early chick streak (Levin et al., 1995; Stern et al., 1995), there are no nodal cilia, and the subsequent serotonin localization does not match the asymmetric pattern described in Xenopus (Fukumoto et al., 2005). Yet, many of the same molecular components are involved – serotonin and its receptors, gap junctions, and the H,K-ATPase/V-ATPase system (Levin, 2005). How are the same molecular signaling elements implemented in a body-plan with a completely different large-scale architecture and cell size? Is the differential timing of similar physiological mechanisms among Xenopus and chick an instance of evolutionary heterochrony? Might the temporal sliding of molecular LR components relative to other embryonic events explain the evolutionary differences and similarities observed in asymmetry across phyla? Finally, what is the role of molecular chirality in amniotes, given that application of non-biological stereoisomers of simple molecules induces heterotaxia in chick (Gray et al., 1942)?
(10) Is there a LR coordinator or organizer?
In many types of embryos, LR patterning first takes place in a sheet of cells; it has been suggested that the information originates locally – in a kind of organizer or LR coordinator that is a single cell or small group of cells. This organizer is suggested to be in early blastomeres in Xenopus (Hyatt and Yost, 1998), in the node in mice (Pagan-Westphal and Tabin, 1998) and in the base of the primitive streak in chick (Levin and Mercola, 1998b). One proposal has been that LR patterning is a kind of planar cell polarity (Aw et al., 2008), as occurs in Drosophila wings and hair cells of the inner ear in mammals (Axelrod and McNeill, 2002; Zallen, 2007), and is a mechanism by which an organizer imposes a coherent LR directionality on the rest of the blastoderm. However, the ability of individual mammalian cells to set up a consistent LR polarity within a single cell (Xu et al., 2007), and the long-known ability of ciliates to epigenetically establish handedness (Nelsen et al., 1989) suggest that perhaps cells can independently orient their intracellular directionality along the LR axis. It will be crucial to determine whether LR information (including direction of the L and R sides, and position with respect to the midline) is imposed from without or generated internally in embryonic cells, or maybe even an interplay of both.
(11) Why can LR phenotypes be dissociated from essential housekeeping functions for numerous pathways?
Several pathways that have been implicated in asymmetry also have important and profound roles in basic cell physiology. These include the V-ATPase H+ pump (Adams et al., 2006), which acidifies vacuoles, gap junctions (Levin and Mercola, 1998a; Chuang et al., 2007), which regulate numerous aspects of small metabolites between cells, tight junctions (Brizuela et al., 2001; Eckert et al., 2005; Vanhoven et al., 2006), which provide epithelial integrity, K+ channels (Levin et al., 2002), that regulate transmembrane potential, microtubules and actin filaments (Thitamadee et al., 2002; Abe et al., 2004; Qiu et al., 2005; Adams et al., 2006; Aw et al., 2008), which are required for cell division and intracellular transport, and Ca++ signaling (McGrath et al., 2003; Raya et al., 2004), which regulates numerous second-messenger pathways. Despite the pleiotropic nature of these mechanisms, it has proven possible to analyze clean left-right asymmetry phenotypes after loss-of-function perturbations of key members of these pathways. A priori, this is highly unexpected; blocking all K+ channels with barium chloride or disrupting actin organization with Latrunculin would be expected to result in massive defects or toxicity, not the observed heterotaxia with normal dorso-anterior development. While this is not always possible, in the above cases a moderate level of abrogation could be identified and achieved at which normal development and cell health was maintained, but the LR pathway was specifically disrupted. What signaling mechanisms or network properties in development allow LR patterning to be intimately dependent upon, and yet separable from, effectors of fundamental cell functions?
(12) When is the midline really determined?
The left-right axis is defined with respect to the midline – a reflection line across which overall symmetry is preserved. There are many open questions about the mechanisms of midline determination, and this is clearly central to the question of LR patterning. In Xenopus, while the plane of first cleavage can be experimentally repositioned (Black and Vincent, 1988; Danilchik and Black, 1988), in normal embryos the cleavage furrow usually corresponds to the future midline of the embryo (Klein, 1987; Masho, 1990). This makes it a natural candidate for LR mechanisms that rely on chirality (Danilchik et al., 2006) or redistribution of maternal intracellular components to the prospective L and R sides (Esser et al., 2006; Aw et al., 2008; Morokuma et al., 2008) during early cleavages. In contrast, the midline is thought to be established quite late in amniotes, when thousands of small cells exist and none have the advantage of spanning the midline of the whole embryo. If midline (and thus position with respect to the LR axis) is not determined until cells are too small to enable transport to the whole embryo’s L or R side, then initiation models based on multicellular signaling must be sought. Thus, knowing when the midline is truly set up is crucial for being able to gauge the plausibility of intracellular transport and planar cell polarity models in embryos of zebrafish, chick, and mouse.
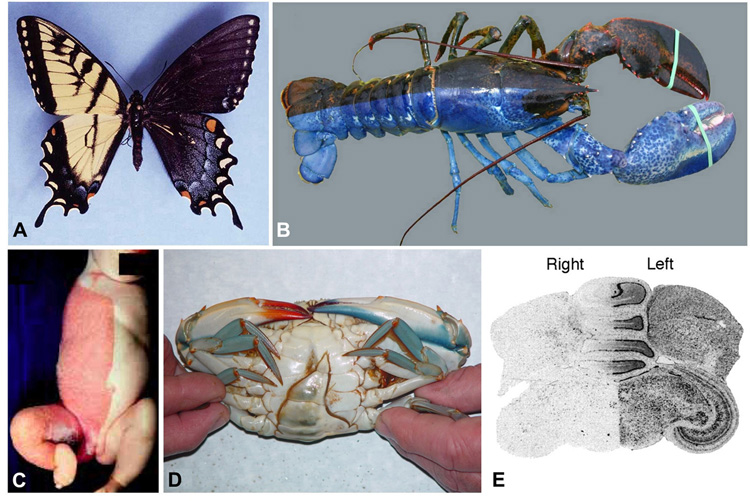
The remarkable phenomenon of gynandromorphy throws doubt onto the certainty of late establishment of the midline in a wide range of phyla. In orthoptera, bilateral gynandromorphs are thought to result when one of the X chromosomes in an XX zygote is eliminated at the first cleavage division (Barranco et al., 1995), resulting in an animal that is part male and part female. The human cases revealed by X-linked genetic pigmentation syndromes (Happle et al., 1995) resemble examples of gynandromorphs found throughout phyla (Fig. 2), including butterflies, ants, crabs, crustaceans, and chickens (Farmer, 1972; Dang and Peterson, 1979; Homsher and Yunker, 1981; Sivaradjam and Bierne, 1981; Mey, 1982; Taber and Francke, 1986; Taylor, 1986; Micheli, 1991; Stevens and Munk, 1991; Barranco et al., 1995; Sagi et al., 1996;Moriyasu et al., 1998; Zou and Fingerman, 2000; Sagi et al., 2002). What is truly remarkable is that the male-female division takes place precisely at the LR midline of the animal.
Figure 2. bilateral gynandromorphy.
(A) Papilio glaucus, used with permission from James K. Adams, showing boundary between male and female cells exactly down the midline.
(B) A bilateral gynandromorph lobster, used with permission of the Bangor Daily News; photo by Abigail Curtis.
(C) A CHILD syndrome patient with harlequin pigmentation, used with permission of John Wiley and Sons from the American Journal of Medical Genetics, 2000, 90: 340.
(D) A gynandromophic blue crab, Callinectes sapidus (ventral view; left side of the body exhibiting the narrower abdomen typical of males), with permission from Rom Lipcius (VIMS); taken from (Kleps et al., 2007).
(E) A section of the brain of a gynandromorphic finch processed for in situ hybridization with probes to sex-specific transcripts (dark signal = female chromosome, no signal = male chromosome), where expression of a molecular marker in the brain of a gynandromorphic finch reveals that the separation between male and female cells is exactly down the midline. Image taken from Fig. 6 of (Agate et al., 2003), copyright held by National Academy of Sciences of the United States.
This is most strikingly seen in bird embryos, where the midline was always thought to be determined in a blastodisc of many thousands of cells. When failure of polar body extrusion occurs during meiosis (producing a binucleate ovum with Z and W nuclei), the resulting birds are chimeras of male and female cells (Lillie, 1931). Amazingly, the separation (revealed by the sex-specific pigmentation pattern) is exactly down the midline, and in roosters, is respected even by the comb on top of the head of the male half. One recent example is shown in Fig. 2E, where expression analysis of a molecular marker in the brain of a gynandromorphic finch reveals that the separation between male and female cells is exactly down the midline (Agate et al., 2003). A late definition of the midline would predict a random, mosaic distribution of male/female regions with respect to the axes of the adult. In contrast, the sharp midline separation suggests that the first cell cleavage may give rise to the L and R halves of the animal that can then inherit differential chiral information from the egg.
The main axes of rabbit embryos are known to be established prior to the appearance of the streak (Viebahn et al., 1995) and a similar situation has been proposed in the case of mouse development (Gardner, 2001; Piotrowska and Zernicka-Goetz, 2001; Plusa et al., 2002). Indeed, bilateral symmetry is said to be discernible in untreated living eggs and early embryos of the rat ((Jones-Seaton, 1950), cited in (Gardner, 1996)). In human embryogenesis, the sharply unilateral pigmentation patterns that occur in humans with X-linked diseases such as CHILD syndrome requires unilateral X-inactivation (or the asymmetric activation or accumulation of some factor responsible for subsequent X-inactivation) to occur at 2-or 4-cell stage in such patients. This also suggests that the midplane is defined quite early in human embryos – a possibility consistent with the “bookending” phenomenon observed in monozygotic twinning (Levin, 1999). More research is clearly needed, to determine whether gynandromorphy is always bilateral, and to understand the mechanisms of midline determination in different amniote embryos. Mice also exhibit the strong sidedness of hermaphroditic organs observed in human cases, (Eicher and Washburn, 1983; Ward et al., 1987; Biddle et al., 1994), although the consistent laterality of testes’ versus ovaries’ placement is opposite of that observed in humans (van Niekerk and Retief, 1981; Krob et al., 1994).
If the chick embryo indeed sets up its midline at first cleavage, some of the puzzles regarding early mechanisms in the chick may need to be reformulated (part 9); the induction of situs inversus by division of the pre-incubation chick blastoderm (Lepori, 1967) is consistent with this model. However, even if set up very early, the midline can be experimentally re-specified at later stages in mice and birds (Mintz, 1971; Eyal-Giladi and Fabian, 1980). A detailed study of the LR asymmetry phenotype of such animals has not been performed, and it remains to be determined exactly how heavily LR initiation relies on alignment between blastomere cleavage and the prospective midline in various model species. Indeed, even though Xenopus normally sets midline and asymmetry by the 2nd cleavage, frog embryos can initiate correct LR patterning in organizers induced during blastula stages (Nascone and Mercola, 1997). Thus, while it is possible that some embryos establish a midline far earlier than previously thought, the models relying on intracellular transport across the prospective midline may need to be extended, to explain how LR patterning still occurs correctly when a new primary axis is initiated in fields of many small cells.
Conclusion
Left-right patterning is a truly fascinating aspect of developmental biology. Its implications extend to behavior (Klar, 1999; Crow, 2002; Bisazza and de Santi, 2003), immunology (Wise et al., 1993), and human culture (McManus, 2002). While tremendous progress has been made over the last 15 years, it is clear that major questions and fundamental areas of ignorance remain (especially with respect to subtle asymmetries and the timing of midline definition). There will be important and far-reaching discoveries to be made here for decades to come, and we hope that young scientists from physics, engineering, and cell biology enter the field, bringing fresh approaches to the contradictory data and brittle models existing today.
At the turn of the last century, Lord Kelvin identified “two small dark clouds on the horizon of classical physics” – at the time, minor observations that did not fit neatly into the powerful, unified paradigm of classical physics. Investigations of these anomalies gave rise to quantum theory and relativity. We hope that these open questions indeed contain several clouds of this type, leading to new discoveries, better questions, and perhaps a profound unification across scales of organization from quantum parity violations (Kondepudi, 1987; Mason, 1991) to large-scale patterning of a whole embryonic axis.
Acknowledgements
We gratefully acknowledge the many helpful discussions with Richard Palmer, other members of the Levin lab, and the LR asymmetry community. M.L. was supported by NIH grants R01-GM077425 and R01-GM067227, and American Heart Association Established Investigator Grant #0740088N. S.A. was supported by the A*STAR overseas National Science Scholarship from the Agency for Science, Technology, and Research (Singapore). This article was written in a Forsyth Institute facility renovated with support from Research Facilities Improvement Grant Number CO6RR11244 from the National Center for Research Resources, National Institutes of Health.
References
- Abe T, Thitamadee S, Hashimoto T. Microtubule defects and cell morphogenesis Nin the lefty1lefty2 tubulin mutant of Arabidopsis thaliana. Plant Cell Physiol. 2004;45:211–220. doi: 10.1093/pcp/pch026. [DOI] [PubMed] [Google Scholar]
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–319. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc Natl Acad Sci U S A. 2003;100:4873–4878. doi: 10.1073/pnas.0636925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson RC, Yelick PC. Roles for fgf8 signaling in left-right patterning of the visceral organs and craniofacial skeleton. Dev Biol. 2005 doi: 10.1016/j.ydbio.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Albrecht-Buehler G. Daughter 3T3 cells. Are they mirror images of each other? J Cell Biol. 1977;72:595–603. doi: 10.1083/jcb.72.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amack JD, Yost HJ. The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Curr Biol. 2004;14:685–690. doi: 10.1016/j.cub.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Armakolas A, Klar AJ. Left-right dynein motor implicated in selective chromatid segregation in mouse cells. Science. 2007;315:100–101. doi: 10.1126/science.1129429. [DOI] [PubMed] [Google Scholar]
- Aw S, Adams DS, Qiu D, Levin M. H,K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech Dev. 2008;125:353–372. doi: 10.1016/j.mod.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, McNeill H. Coupling planar cell polarity signaling to morphogenesis. ScientificWorldJournal. 2002;2:434–454. doi: 10.1100/tsw.2002.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barranco P, Cabrero J, Camacho JPM, Pascual F. Chromosomal basis for a bilateral gynandromorph in Pycnogaster Inermis (Rambur, 1838) (Orthoptera, Tettigoniidae) Contrib Zool. 1995;65:123–127. [Google Scholar]
- Barth KA, Miklosi A, Watkins J, Bianco IH, Wilson SW, Andrew RJ. fsi Zebrafish Show Concordant Reversal of Laterality of Viscera, Neuroanatomy, and a Subset of Behavioral Responses. Curr Biol. 2005;15:844–850. doi: 10.1016/j.cub.2005.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur C, Neveu PJ, Vitiello S, Le Moal M. Natural killer cell activity is associated with brain asymmetry in male mice. Brain Behav Immun. 1991;5:162–169. doi: 10.1016/0889-1591(91)90014-2. [DOI] [PubMed] [Google Scholar]
- Biddle FG, Eisner JR, Eales BA. The testis-determining autosomal trait, Tda-1, of C57BL/6J is determined by more than a single autosomal gene when compared with DBA/2J mice. Genome. 1994;37:296–304. doi: 10.1139/g94-041. [DOI] [PubMed] [Google Scholar]
- Bisazza A, de Santi A. Lateralization of aggression in fish. Behav Brain Res. 2003;141:131–136. doi: 10.1016/s0166-4328(02)00344-3. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Essner JJ, Yost HJ. Multiple pathways in the midline regulate concordant brain, heart and gut left-right asymmetry. Development. 2000;127:3567–3579. doi: 10.1242/dev.127.16.3567. [DOI] [PubMed] [Google Scholar]
- Black SD, Vincent JP. The first cleavage plane and the embryonic axis are determined by separate mechanisms in Xenopus laevis. II. Experimental dissociation by lateral compression of the egg. Developmental Biology. 1988;128:65–71. doi: 10.1016/0012-1606(88)90267-9. [DOI] [PubMed] [Google Scholar]
- Brennan J, Norris DP, Robertson EJ. Nodal activity in the node governs left-right asymmetry. Genes Dev. 2002;16:2339–2344. doi: 10.1101/gad.1016202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizuela BJ, Wessely O, De Robertis EM. Overexpression of the Xenopus tight-junction protein claudin causes randomization of the left-right body axis. Developmental Biology. 2001;230:217–229. doi: 10.1006/dbio.2000.0116. [DOI] [PubMed] [Google Scholar]
- Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol. 2000;23:45–51. doi: 10.1165/ajrcmb.23.1.4070. [DOI] [PubMed] [Google Scholar]
- Brown N, Wolpert L. The development of handedness in left/right asymmetry. Development. 1990;109:1–9. doi: 10.1242/dev.109.1.1. [DOI] [PubMed] [Google Scholar]
- Buceta J, Ibanes M, Rasskin-Gutman D, Okada Y, Hirokawa N, Izpisua-Belmonte JC. Nodal cilia dynamics and the specification of the left/right axis in early vertebrate embryo development. Biophys J. 2005;89:2199–2209. doi: 10.1529/biophysj.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney TD, De Boer AH, Levin M. Fusicoccin signaling reveals 14-3-3 protein function as a novel step in left-right patterning during amphibian embryogenesis. Development. 2003;130:4847–4858. doi: 10.1242/dev.00698. [DOI] [PubMed] [Google Scholar]
- Burdine R, Schier A. Conserved and divergent mechanisms in left-right axis formation. Genes & Development. 2000;14:763–776. [PubMed] [Google Scholar]
- Burn J. Disturbance of morphological laterality in humans. CIBA Found Symp. 1991;162:282–296. doi: 10.1002/9780470514160.ch16. [DOI] [PubMed] [Google Scholar]
- Cartwright JH, Piro O, Tuval I. Fluid-dynamical basis of the embryonic development of left-right asymmetry in vertebrates. Proc Natl Acad Sci U S A. 2004;101:7234–7239. doi: 10.1073/pnas.0402001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers AD, Slack JM. The Xenopus tadpole gut: fate maps and morphogenetic movements. Development - Supplement. 2000;127:381–392. doi: 10.1242/dev.127.2.381. [DOI] [PubMed] [Google Scholar]
- Chen J, Knowles HJ, Hebert JL, Hackett BP. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J Clin Invest. 1998;102:1077–1082. doi: 10.1172/JCI4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CF, Vanhoven MK, Fetter RD, Verselis VK, Bargmann CI. An Innexin- Dependent Cell Network Establishes Left-Right Neuronal Asymmetry in C. elegans. Cell. 2007;129:787–799. doi: 10.1016/j.cell.2007.02.052. [DOI] [PubMed] [Google Scholar]
- Clericuzio CL. Clinical phenotypes and Wilms tumor. Med Pediatr Oncol. 1993;21:182–187. doi: 10.1002/mpo.2950210306. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr. Asymmetry: molecular, biologic, embryopathic, and clinical perspectives. Am J Med Genet. 2001;101:292–314. doi: 10.1002/ajmg.1217. [DOI] [PubMed] [Google Scholar]
- Concha ML, Burdine RD, Russell C, Schier AF, Wilson SW. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron. 2000;28:399–409. doi: 10.1016/s0896-6273(00)00120-3. [DOI] [PubMed] [Google Scholar]
- Cota CD, Bagher P, Pelc P, Smith CO, Bodner CR, Gunn TM. Mice with mutations in Mahogunin ring finger-1 (Mgrn1) exhibit abnormal patterning of the left-right axis. Dev Dyn. 2006;235:3438–3447. doi: 10.1002/dvdy.20992. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Handedness, language lateralisation and anatomical asymmetry: relevance of protocadherin XY to hominid speciation and the aetiology of psychosis - Point of view [Review] British Journal of Psychiatry. 2002;181:295–297. doi: 10.1192/bjp.181.4.295. [DOI] [PubMed] [Google Scholar]
- Dale L, Slack J. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987;100:279–296. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- Dang PT, Peterson BV. A case of bilateral gynandromorphism in Simulioum soubrense Vajime and Dunbar (Diptera: Simuliidae) Tropenmed Parasitol. 1979;30:548–550. [PubMed] [Google Scholar]
- Danilchik MV, Black SD. The first cleavage plane and the embryonic axis are determined by separate mechanisms in Xenopus laevis. I. Independence in undisturbed embryos. Developmental Biology. 1988;128:58–64. doi: 10.1016/0012-1606(88)90266-7. [DOI] [PubMed] [Google Scholar]
- Danilchik MV, Brown EE, Riegert K. Intrinsic chiral properties of the Xenopus egg cortex: an early indicator of left-right asymmetry? Development. 2006;133:4517–4526. doi: 10.1242/dev.02642. [DOI] [PubMed] [Google Scholar]
- Delaney M, Boyd TK. Case report of unilateral clefting: is sonic hedgehog to blame? Pediatr Dev Pathol. 2007;10:117–120. doi: 10.2350/06-03-0063.1. [DOI] [PubMed] [Google Scholar]
- Delhaas T, Decaluwe W, Rubbens M, Kerckhoffs R, Arts T. Cardiac fiber orientation and the left-right asymmetry determining mechanism. Annals of the New York Academy of Sciences. 2004;1015:190–201. doi: 10.1196/annals.1302.016. [DOI] [PubMed] [Google Scholar]
- Eckert JJ, McCallum A, Mears A, Rumsby MG, Cameron IT, Fleming TP. Relative contribution of cell contact pattern, specific PKC isoforms and gap junctional communication in tight junction assembly in the mouse early embryo. Dev Biol. 2005;288:234–247. doi: 10.1016/j.ydbio.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL. Inherited sex reversal in mice: identification of a new primary sex-determining gene. J Exp Zool. 1983;228:297–304. doi: 10.1002/jez.1402280213. [DOI] [PubMed] [Google Scholar]
- Esser AT, Smith KC, Weaver JC, Levin M. Mathematical model of morphogen electrophoresis through gap junctions. Dev Dyn. 2006;235:2144–2159. doi: 10.1002/dvdy.20870. [DOI] [PubMed] [Google Scholar]
- Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–1260. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- Eyal-Giladi H, Fabian BC. Axis determination in uterine chick blastodiscs under changing spatial positions during the sensitive period for polarity. Dev Biol. 1980;77:228–232. doi: 10.1016/0012-1606(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Farmer A. A bilateral gynandromorph of Nephrops norvegicus. Marine Biology. 1972;15:344–349. [Google Scholar]
- Fraumeni JF, Jr., Geiser CF, Manning MD. Wilms' tumor and congenital hemihypertrophy: report of five new cases and review of literature. Pediatrics. 1967;40:886–899. [PubMed] [Google Scholar]
- Fride E, Collins RL, Skolnick P, Arora PK. Immune function in lines of mice selected for high or low degrees of behavioral asymmetry. Brain Behav Immun. 1990;4:129–138. doi: 10.1016/0889-1591(90)90015-i. [DOI] [PubMed] [Google Scholar]
- Fu QL, Shen YQ, Gao MX, Dong J, Neveu PJ, Li KS. Brain interleukin asymmetries and paw preference in mice. Neuroscience. 2003;116:639–647. doi: 10.1016/s0306-4522(02)00746-7. [DOI] [PubMed] [Google Scholar]
- Fujinaga M, Baden JM. Evidence for an adrenergic mechanism in the control of body asymmetry. Dev Biol. 1991;143:203–205. doi: 10.1016/0012-1606(91)90067-d. [DOI] [PubMed] [Google Scholar]
- Fujinaga M, Baden JM, Shepard TH, Mazze RI. Nitrous oxide alters body laterality in rats. Teratology. 1990;41:131–135. doi: 10.1002/tera.1420410202. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol. 2005;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Gardner RL. Can developmentally significant spatial patterning of the egg be discounted in mammals? Human Reproduction Update. 1996;2:3–27. doi: 10.1093/humupd/2.1.3. [DOI] [PubMed] [Google Scholar]
- Gardner RL. Specification of embryonic axes begins before cleavage in normal mouse development. Development - Supplement. 2001;128:839–847. doi: 10.1242/dev.128.6.839. [DOI] [PubMed] [Google Scholar]
- Gatica R, Boland MP, Crosby TF, Gordon I. Micromanipulation of sheep morulae to produce monozygotic twins. Theriogenology. 1984;21:555–560. doi: 10.1016/0093-691x(84)90440-0. [DOI] [PubMed] [Google Scholar]
- Gebbia M, Ferrero GB, Pilia G, Bassi MT, Aylsworth A, Penman-Splitt M, Bird LM, Bamforth JS, Burn J, Schlessinger D, Nelson DL, Casey B. X-linked situs abnormalities result from mutations in ZIC3. Nat Genet. 1997;17:305–308. doi: 10.1038/ng1197-305. [DOI] [PubMed] [Google Scholar]
- Golbin A, Golbin Y, Keith L, Keith D. Mirror imaging in twins: biological polarization--an evolving hypothesis. Acta Genet Med Gemellol (Roma) 1993a;42:237–243. doi: 10.1017/s0001566000003238. [DOI] [PubMed] [Google Scholar]
- Golbin A, Golbin Y, Keith L, Keith D. Mirror imagiong in twins: biological polarization. Acta Genet Med Gemellol. 1993b;42:237–243. doi: 10.1017/s0001566000003238. [DOI] [PubMed] [Google Scholar]
- Golding JP, Partridge TA, Beauchamp JR, King T, Brown NA, Gassmann M, Zammit PS. Mouse myotomes pairs exhibit left-right asymmetric expression of MLC3F and alpha-skeletal actin. Developmental Dynamics. 2004a;231:795–800. doi: 10.1002/dvdy.20176. [DOI] [PubMed] [Google Scholar]
- Golding JP, Tsoni S, Dixon M, Yee KT, Partridge TA, Beauchamp JR, Gassmann M, Zammit PS. Heparin-binding EGF-like growth factor shows transient left-right asymmetrical expression in mouse myotome pairs. Mechanisms of Development: Gene Expression Patterns. 2004b;5:3–9. doi: 10.1016/j.modgep.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Gray P, Cox GJ, Worthing H, Everhart WH, MacDonald E. The effect of stereo-isomeric substances on the heterotaxic rate in chick embryo. Anatomical Record. 1942;82:416. [Google Scholar]
- Halpern ME, Gunturkun O, Hopkins WD, Rogers LJ. Lateralization of the vertebrate brain: taking the side of model systems. J Neurosci. 2005;25:10351–10357. doi: 10.1523/JNEUROSCI.3439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happle R, Mittag H, Kuster W. The CHILD nevus: a distinct skin disorder. Dermatology. 1995;191:210–216. doi: 10.1159/000246548. [DOI] [PubMed] [Google Scholar]
- Hendricks M, Jesuthasan S. Asymmetric innervation of the habenula in zebrafish. J Comp Neurol. 2007;502:611–619. doi: 10.1002/cne.21339. [DOI] [PubMed] [Google Scholar]
- Homsher PJ, Yunker CE. Bilateral gynandromorphism in Dermacentor andersoni (Acari: Ixodidae): morphologic and cytogenetic analysis. J Med Entomol. 1981;18:89–91. doi: 10.1093/jmedent/18.1.89. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, Seaver LH, Jones KL, Procopio F, Crooks W, Feingold M. Isolated hemihyperplasia (hemihypertrophy): report of a prospective multicenter study of the incidence of neoplasia and review. Am J Med Genet. 1998;79:274–278. [PubMed] [Google Scholar]
- Hummel KP, Chapman DB. Visceral inversion and associated anomalies in the mouse. J Hered. 1959;50:9–23. [Google Scholar]
- Hyatt BA, Yost HJ. The left-right coordinator: the role of Vg1 in organizing left-right axis formation. Cell. 1998;93:37–46. doi: 10.1016/s0092-8674(00)81144-7. [DOI] [PubMed] [Google Scholar]
- Jefferies RPS, Brown NA, Daley PEJ. The early phylogeny of chordates and echinoderms and the origin of chordate left-right asymmetry and bilateral symmetry. Acta Zoologica (Stockholm) 1996;77:101–122. [Google Scholar]
- Jones-Seaton A. [Study of the cytoplasmic organization of the ovum of rodents, principally in regard to the ribonucleic basophilia.] Arch Biol (Liege) 1950;61:291–444. [PubMed] [Google Scholar]
- Kelly KA, Wei Y, Mikawa T. Cell death along the embryo midline regulates left-right sidedness. Dev Dyn. 2002;224:238–244. doi: 10.1002/dvdy.10098. [DOI] [PubMed] [Google Scholar]
- Kennedy D, O'Craven K, Ticho B, Goldstein A, Makris N, Henson J. Structural and functional brain asymmetries in human situs inversus totalis. Neurology. 1999;53:1260–1265. doi: 10.1212/wnl.53.6.1260. [DOI] [PubMed] [Google Scholar]
- Kitaguchi T, Nagai T, Nakata K, Aruga J, Mikoshiba K. Zic3 is involved in the left-right specification of the Xenopus embryo. Development - Supplement. 2000;127:4787–4795. doi: 10.1242/dev.127.22.4787. [DOI] [PubMed] [Google Scholar]
- Klar AJ. Genetic models for handedness, brain lateralization, schizophrenia, and manic-depression. Schizophr Res. 1999;39:207–218. doi: 10.1016/s0920-9964(99)00075-4. [DOI] [PubMed] [Google Scholar]
- Klein SL. The first cleavage furrow demarcates the dorsal-ventral axis in Xenopus embryos. Developmental Biology. 1987;120:299–304. doi: 10.1016/0012-1606(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Kleps RA, Myers TC, Lipcius RN, Henderson TO. A sex-specific metabolite identified in a marine invertebrate utilizing phosphorus-31 nuclear magnetic resonance. PLoS ONE. 2007;2:e780. doi: 10.1371/journal.pone.0000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondepudi DK. Selection of molecular chirality by extremely weak chiral interactions under far-from-equilibrium conditions. Biosystems. 1987;20:75–83. doi: 10.1016/0303-2647(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Krob G, Braun A, Kuhnle U. True hermaphroditism: geographical distribution, clinical findings, chromosomes and gonadal histology. Eur J Pediatr. 1994;153:2–10. doi: 10.1007/BF02000779. [DOI] [PubMed] [Google Scholar]
- Lanier JL, Grandin T, Green R, Avery D, McGee K. A note on hair whorl position and cattle temperament in the auction ring. Appl Anim Behav Sci. 2001;73:93–101. doi: 10.1016/s0168-1591(01)00132-0. [DOI] [PubMed] [Google Scholar]
- Layton WM., Jr. Random determination of a developmental process: reversal of normal visceral asymmetry in the mouse. J Hered. 1976;67:336–338. doi: 10.1093/oxfordjournals.jhered.a108749. [DOI] [PubMed] [Google Scholar]
- Lepori N. Orientation et situs viscerium et cordis chez les embryons jumeaux de cane. Monitore Zool Ital. 1967:1. [Google Scholar]
- Leung AK, Fong JH, Leong AG. Hemihypertrophy. J R Soc Health. 2002;122:24–27. doi: 10.1177/146642400212200111. [DOI] [PubMed] [Google Scholar]
- Levin M. Left-right asymmetry and the chick embryo. Seminars in Cell & Developmental Biology. 1998;9:67–76. doi: 10.1006/scdb.1997.0192. [DOI] [PubMed] [Google Scholar]
- Levin M. Twinning and embryonic left-right asymmetry. Laterality. 1999;4:197–208. doi: 10.1080/713754338. [DOI] [PubMed] [Google Scholar]
- Levin M. Left-right asymmetry in embryonic development: a comprehensive review. Mech Dev. 2005;122:3–25. doi: 10.1016/j.mod.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Levin M. Is the early left-right axis like a plant, a kidney, or a neuron? The integration of physiological signals in embryonic asymmetry. Birth Defects Res C Embryo Today. 2006;78:191–223. doi: 10.1002/bdrc.20078. [DOI] [PubMed] [Google Scholar]
- Levin M, Johnson R, Stern C, Kuehn M, Tabin C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell. 1995;82:803–814. doi: 10.1016/0092-8674(95)90477-8. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junctions are involved in the early generation of left-right asymmetry. Dev Biol. 1998a;203:90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. The compulsion of chirality: toward an understanding of left- right asymmetry. Genes Dev. 1998b;12:763–769. doi: 10.1101/gad.12.6.763. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junction-mediated transfer of left-right patterning signals in the early chick blastoderm is upstream of Shh asymmetry in the node. Development. 1999;126:4703–4714. doi: 10.1242/dev.126.21.4703. [DOI] [PubMed] [Google Scholar]
- Levin M, Pagan S, Roberts DJ, Cooke J, Kuehn MR, Tabin CJ. Left/right patterning signals and the independent regulation of different aspects of Situs in the chick embryo. Developmental Biology. 1997;189:57–67. doi: 10.1006/dbio.1997.8662. [DOI] [PubMed] [Google Scholar]
- Levin M, Palmer AR. Left-right patterning from the inside out: widespread evidence for intracellular control. Bioessays. 2007;29:271–287. doi: 10.1002/bies.20545. [DOI] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+- ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Levy J, Levy JM. Human lateralization from head to foot: sex-related factors. Science. 1978;200:1291–1292. doi: 10.1126/science.663611. [DOI] [PubMed] [Google Scholar]
- Lillie FR. Bilateral Gynandromorphism and Lateral Hemihypertrophy in Birds. Science. 1931;74:387–390. doi: 10.1126/science.74.1920.387. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Supp DM, Sampath K, Yokoyama T, Wright CV, Potter SS, Overbeek P, Kuehn MR. Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature. 1996;381:158–161. doi: 10.1038/381158a0. [DOI] [PubMed] [Google Scholar]
- Mangold O. Situs inversus bei Triton. Arch. Entwickl.-Mech. Org. 1921;48:505–516. [Google Scholar]
- Masho R. Close Correlation between the 1st Cleavage Plane and the Body Axis in Early Xenopus Embryos. Development Growth & Differentiation. 1990;32:57–64. doi: 10.1111/j.1440-169X.1990.00057.x. [DOI] [PubMed] [Google Scholar]
- Mason SF. Origins of the handedness of biological molecules. Ciba Found Symp. 1991;162:3–10. doi: 10.1002/9780470514160.ch2. discussion 10–15. [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- McManus C. Right Hand, Left Hand : The Origins of Asymmetry in Brains, Bodies, Atoms and Cultures. London: Weidenfeld and Nicolson; 2002. [Google Scholar]
- McManus IC. Reversed cerebral asymmetry and breast cancer. Lancet. 1992;339:1055. [PubMed] [Google Scholar]
- McManus IC, Martin N, Stubbings GF, Chung EM, Mitchison HM. Handedness and situs inversus in primary ciliary dyskinesia. Proc R Soc Lond B Biol Sci. 2004;271:2579–2582. doi: 10.1098/rspb.2004.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeish JD, Thayer J, Walling K, Sulik KK, Potter SS, Scott WJ. Phenotypic characterization of the transgenic mouse insertional mutation, legless. J Exp Zool. 1990;253:151–162. doi: 10.1002/jez.1402530205. [DOI] [PubMed] [Google Scholar]
- McQuinn TC, Miga DE, Mjaatvedt CH, Phelps AL, Wessels A. Cardiopulmonary malformations in the inv/inv mouse. Anat Rec. 2001;263:62–71. doi: 10.1002/ar.1077. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Loring DW, Ray PG, Helman SW, Vazquez BR, Neveu PJ. Role of cerebral lateralization in control of immune processes in humans. Ann Neurol. 2004;55:840–844. doi: 10.1002/ana.20105. [DOI] [PubMed] [Google Scholar]
- Meola MG, Grandin T, Burns P, Deesing M. Hair whorl patterns on the bovine forehead may be related to breeding soundness measures. Theriogenology. 2004;62:450–457. doi: 10.1016/j.theriogenology.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Mey W. A bilateral gynandromorph of Anabolia-Furcata Brauer (Insecta, Trichoptera) Zool. Anz. 1982;209:394–396. [Google Scholar]
- Micheli F. Bilateral Gynandromorph of the Fresh-Water Crab Potamon- Fluviatile Herbst (Decapoda, Brachyura) Journal of Crustacean Biology. 1991;11:561–568. [Google Scholar]
- Mintz B. Genetic mosaicism in vivo: development and disease in allophenic mice. Fed Proc. 1971;30:935–943. [PubMed] [Google Scholar]
- Mittwoch U. Genetics of sex determination: exceptions that prove the rule. Mol Genet Metab. 2000;71:405–410. doi: 10.1006/mgme.2000.3075. [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Genetics of mammalian sex determination: some unloved exceptions. J Exp Zool. 2001;290:484–489. doi: 10.1002/jez.1091. [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Different gene expressions on the left and the right: a genotype/phenotype mismatch in need of attention. Ann Hum Genet. 2008;72:2–9. doi: 10.1111/j.1469-1809.2007.00402.x. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Saijoh Y, Tsuchiya K, Shirayoshi Y, Takai S, Taya C, Yonekawa H, Yamada K, Nihei H, Nakatsuji N, Overbeek PA, Hamada H, Yokoyama T. Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature. 1998;395:177–181. doi: 10.1038/26006. [DOI] [PubMed] [Google Scholar]
- Moody SA, Kline MJ. Segregation of fate during cleavage of frog (Xenopus laevis) blastomeres. Anatomy & Embryology. 1990;182:347–362. doi: 10.1007/BF02433495. [DOI] [PubMed] [Google Scholar]
- Morgan D, Turnpenny L, Goodship J, Dai W, Majumder K, Matthews L, Gardner A, Schuster G, Vien L, Harrison W, Elder FF, Penman-Splitt M, Overbeek P, Strachan T. Inversin, a novel gene in the vertebrate left-right axis pathway, is partially deleted in the inv mouse. Nat Genet. 1998;20:149–156. doi: 10.1038/2450. [DOI] [PubMed] [Google Scholar]
- Morishima M, Yasui H, Nakazawa M, Ando M, Ishibashi M, Takao A. Situs variation and cardiovascular anomalies in the transgenic mouse insertional mutation, inv. Teratology. 1998;57:302–309. doi: 10.1002/(SICI)1096-9926(199806)57:6<302::AID-TERA3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Morison D, Reyes C, Skorodin M. Mirror-image tumors in mirror-image twins. Chest. 1994;106:608–610. doi: 10.1378/chest.106.2.608. [DOI] [PubMed] [Google Scholar]
- Moriyasu M, Mallet P, Comeau M, Benhalima K, Ghica O. Occurrence of pseudohermaphroditism in the rock crab, Cancer irroratus Say, 1817, in the southern Gulf of St. Lawrence, Canada (Decapoda, Brachyura) Crustaceana. 1998;71:655–662. [Google Scholar]
- Morokuma J, Blackiston D, Levin M. KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos. Cell Biochem Biophys. 2008 doi: 10.1159/000129628. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morokuma J, Ueno M, Kawanishi H, Saiga H, Nishida H. HrNodal, the ascidian nodal-related gene, is expressed in the left side of the epidermis, and lies upstream of HrPitx. Dev Genes Evol. 2002;212:439–446. doi: 10.1007/s00427-002-0242-3. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Mine N, Nakaguchi E, Mochizuki A, Yamamoto M, Yashiro K, Meno C, Hamada H. Generation of robust left-right asymmetry in the mouse embryo requires a self-enhancement and lateral-inhibition system. Dev Cell. 2006;11:495–504. doi: 10.1016/j.devcel.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Nascone N, Mercola M. Organizer induction determines left-right asymmetry in Xenopus. Dev Biol. 1997;189:68–78. doi: 10.1006/dbio.1997.8635. [DOI] [PubMed] [Google Scholar]
- Nelsen EM, Frankel J, Jenkins LM. Non-genic inheritance of cellular handedness. Development. 1989;105:447–456. doi: 10.1242/dev.105.3.447. [DOI] [PubMed] [Google Scholar]
- Neveu PJ. Cerebral lateralization and the immune system. Int Rev Neurobiol. 2002;52:303–323. doi: 10.1016/s0074-7742(02)52014-6. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, O'Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo NJ, Levin M. Gap junctions provide new links in left-right patterning. Cell. 2007;129:645–647. doi: 10.1016/j.cell.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Ozil JP, Heyman Y, Renard JP. Production of monozygotic twins by micromanipulation and cervical transfer in the cow. Vet Rec. 1982;110:126–127. doi: 10.1136/vr.110.6.126. [DOI] [PubMed] [Google Scholar]
- Pagan-Westphal S, Tabin C. The transfer of left-right positional information during chick embryogenesis. Cell. 1998;93:25–35. doi: 10.1016/s0092-8674(00)81143-5. [DOI] [PubMed] [Google Scholar]
- Palmer AR. Symmetry breaking and the evolution of development. Science. 2004;306:828–833. doi: 10.1126/science.1103707. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ, Lary JM. Laterality patterns in infants with external birth defects. Teratology. 1999;60:265–271. doi: 10.1002/(SICI)1096-9926(199911)60:5<265::AID-TERA7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Peeters H, Devriendt K. Human laterality disorders. Eur J Med Genet. 2006;49:349–362. doi: 10.1016/j.ejmg.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Piotrowska K, Zernicka-Goetz M. Role for sperm in spatial patterning of the early mouse embryo. Nature. 2001;409:517–521. doi: 10.1038/35054069. [DOI] [PubMed] [Google Scholar]
- Plusa B, Piotrowska K, Zernicka-Goetz M. Sperm entry position provides a surface marker for the first cleavage plane of the mouse zygote. Genesis: the Journal of Genetics & Development. 2002;32:193–198. doi: 10.1002/gene.10027. [DOI] [PubMed] [Google Scholar]
- Qiu D, Cheng SM, Wozniak L, McSweeney M, Perrone E, Levin M. Localization and loss-of-function implicates ciliary proteins in early, cytoplasmic roles in left-right asymmetry. Dev Dyn. 2005;234:176–189. doi: 10.1002/dvdy.20509. [DOI] [PubMed] [Google Scholar]
- Quaranta A, Siniscalchi M, Frate A, Vallortigara G. Paw preference in dogs: relations between lateralised behaviour and immunity. Behav Brain Res. 2004;153:521–525. doi: 10.1016/j.bbr.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Ramsdell AF. Left-right asymmetry and congenital cardiac defects: getting to the heart of the matter in vertebrate left-right axis determination. Dev Biol. 2005;288:1–20. doi: 10.1016/j.ydbio.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Raya A, Belmonte JC. Left-right asymmetry in the vertebrate embryo: from early information to higher-level integration. Nat Rev Genet. 2006;7:283–293. doi: 10.1038/nrg1830. [DOI] [PubMed] [Google Scholar]
- Raya A, Kawakami Y, Rodriguez-Esteban C, Ibanes M, Rasskin-Gutman D, Rodriguez-Leon J, Buscher D, Feijo JA, Izpisua Belmonte JC. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature. 2004;427:121–128. doi: 10.1038/nature02190. [DOI] [PubMed] [Google Scholar]
- Renoux G, Biziere K, Renoux M, Guillaumin JM, Degenne D. A balanced brain asymmetry modulates T cell-mediated events. J Neuroimmunol. 1983;5:227–238. doi: 10.1016/0165-5728(83)90043-7. [DOI] [PubMed] [Google Scholar]
- Sagi A, Khalaila I, Barki A, Hulata G, Karplus I. Intersex red claw crayfish, Cherax quadricarinatus (von Martens): Functional males with pre-vitellogenic ovaries. Biol Bull. 1996;190:16–23. doi: 10.2307/1542672. [DOI] [PubMed] [Google Scholar]
- Sagi A, Manor R, Segall C, Davis C, Khalaila I. On intersexuality in the crayfish Cherax quadricarinatus: an inducible sexual plasticity model. Invertebrate Reproduction & Development. 2002;41:27–33. [Google Scholar]
- Sampath K, Cheng AM, Frisch A, Wright CV. Functional differences among Xenopus nodal-related genes in left-right axis determination. Development - Supplement. 1997;124:3293–3302. doi: 10.1242/dev.124.17.3293. [DOI] [PubMed] [Google Scholar]
- Sandson TA, Wen PY, LeMay M. Reversed cerebral asymmetry in women with breast cancer. Lancet. 1992;339:523–524. doi: 10.1016/0140-6736(92)90341-y. [DOI] [PubMed] [Google Scholar]
- Saude L, Lourenco R, Goncalves A, Palmeirim I. terra is a left-right asymmetrygene required for left-right synchronization of the segmentation clock. Nat Cell Biol. 2005;7:918–920. doi: 10.1038/ncb1294. [DOI] [PubMed] [Google Scholar]
- Schottenfeld J, Sullivan-Brown J, Burdine RD. Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development. 2007;134:1605–1615. doi: 10.1242/dev.02827. [DOI] [PubMed] [Google Scholar]
- Schreiber AM. Asymmetric craniofacial remodeling and lateralized behavior in larval flatfish. J Exp Biol. 2006;209:610–621. doi: 10.1242/jeb.02056. [DOI] [PubMed] [Google Scholar]
- Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol. 2007;17:60–66. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- Shen YQ, Hebert G, Moze E, Li KS, Neveu PJ. Asymmetrical distribution of brain interleukin-6 depends on lateralization in mice. Neuroimmunomodulation. 2005;12:189–194. doi: 10.1159/000084852. [DOI] [PubMed] [Google Scholar]
- Singh G, Supp DM, Schreiner C, McNeish J, Merker HJ, Copeland NG, Jenkins NA, Potter SS, Scott W. legless insertional mutation: morphological, molecular, and genetic characterization. Genes Dev. 1991;5:2245–2255. doi: 10.1101/gad.5.12a.2245. [DOI] [PubMed] [Google Scholar]
- Sivaradjam S, Bierne J. Sex differentiation in bilaterally allophenic animals produced by cloning of two bipartite male/female chimaeras of Lineus sanguineus. J Embryol Exp Morphol. 1981;65:173–184. [PubMed] [Google Scholar]
- Smith AT, Sack GH, Jr., Taylor GJ. Holt-Oram syndrome. J Pediatr. 1979;95:538–543. doi: 10.1016/s0022-3476(79)80758-1. [DOI] [PubMed] [Google Scholar]
- Speder P, Petzoldt A, Suzanne M, Noselli S. Strategies to establish left/right asymmetry in vertebrates and invertebrates. Curr Opin Genet Dev. 2007 doi: 10.1016/j.gde.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Stern C, Yu R, Kakizuka A, Kintner C, Mathews L, Vale W, Evans R, Umesono K. Activin and its receptors during gastrulation and the later phases of mesoderm development in the chick embryo. Developmental Biology. 1995;172:192–205. doi: 10.1006/dbio.1995.0015. [DOI] [PubMed] [Google Scholar]
- Stevens BG, Munk JE. Lateral Asymmetry in the Thoracic Segmentation of a King Crab, Paralithodes-Camtschaticus (Tilesius, 1815) (Decapoda, Anomura), from Kodiak, Alaska. Crustaceana. 1991;61:317–320. [Google Scholar]
- Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci. 2006;7:655–662. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- Supp DM, Witte DP, Potter SS, Brueckner M. Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature. 1997;389:963–966. doi: 10.1038/40140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber SW, Francke OF. A bilateral gynandromorph of the Western harvester ant, Pogonomyrmex-Occidentalis (Hymenoptera, Formicidae). Southw. Natural. 1986;31:274–276. [Google Scholar]
- Tabin C. Do we know anything about how left-right asymmetry is first established in the vertebrate embryo? J Mol Histol. 2005:1–7. doi: 10.1007/s10735-005-9000-y. [DOI] [PubMed] [Google Scholar]
- Takano K, Ito Y, Obata S, Oinuma T, Komazaki S, Nakamura H, Asashima M. Heart formation and left-right asymmetry in separated right and left embryos of a newt. Int J Dev Biol. 2007;51:265–272. doi: 10.1387/ijdb.072270kt. [DOI] [PubMed] [Google Scholar]
- Tamakoshi T, Itakura T, Chandra A, Uezato T, Yang Z, Xue XD, Wang B, Hackett BP, Yokoyama T, Miura N. Roles of the Foxj1 and Inv genes in the left-right determination of internal organs in mice. Biochem Biophys Res Commun. 2006;339:932–938. doi: 10.1016/j.bbrc.2005.11.097. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kanzaki R, Yoshibayashi M, Kamiya T, Sugishita M. Dichotic listening in patients with situs inversus: brain asymmetry and situs asymmetry. Neuropsychologia. 1999;37:869–874. doi: 10.1016/s0028-3932(98)00144-4. [DOI] [PubMed] [Google Scholar]
- Taylor DM. A Bilateral Gynandromorph of the Snow Crab, Chionoecetes-Opilio, from Newfoundland, Canada. Crustaceana. 1986;51:309–312. [Google Scholar]
- Thitamadee S, Tuchihara K, Hashimoto T. Microtubule basis for left-handed helical growth in Arabidopsis. Nature. 2002;417:193–196. doi: 10.1038/417193a. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Tsukui T, Capdevila J, Tamura K, Ruiz-Lozano P, Rodriguez-Esteban C, Yonei-Tamura S, Magallon J, Chandraratna RA, Chien K, Blumberg B, Evans RM, Belmonte JC. Multiple left-right asymmetry defects in Shh(−/−) mutant mice unveil a convergence of the shh and retinoic acid pathways in the control of Lefty-1. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:11376–11381. doi: 10.1073/pnas.96.20.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niekerk WA, Retief AE. The gonads of human true hermaphrodites. Hum Genet. 1981;58:117–122. doi: 10.1007/BF00284158. [DOI] [PubMed] [Google Scholar]
- Vanhoven MK, Bauer Huang SL, Albin SD, Bargmann CI. The claudin superfamily protein nsy-4 biases lateral signaling to generate left-right asymmetry in C. elegans olfactory neurons. Neuron. 2006;51:291–302. doi: 10.1016/j.neuron.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Vermot J, Gallego Llamas J, Fraulob V, Niederreither K, Chambon P, Dolle P. Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science. 2005;308:563–566. doi: 10.1126/science.1108363. [DOI] [PubMed] [Google Scholar]
- Vermot J, Pourquie O. Retinoic acid coordinates somitogenesis and left-right patterning in vertebrate embryos. Nature. 2005;435:215–220. doi: 10.1038/nature03488. [DOI] [PubMed] [Google Scholar]
- Viebahn C, Mayer B, Hrabe de Angelis M. Signs of the principle body axes prior to primitive streak formation in the rabbit embryo. Anat Embryol (Berl) 1995;192:159–169. doi: 10.1007/BF00186004. [DOI] [PubMed] [Google Scholar]
- Ward HB, McLaren A, Baker TG. Gonadal development in T16H/XSxr hermaphrodite mice. J Reprod Fertil. 1987;81:295–300. doi: 10.1530/jrf.0.0810295. [DOI] [PubMed] [Google Scholar]
- West PM, Love DR, Stapleton PM, Winship IM. Paternal uniparental disomy in monozygotic twins discordant for hemihypertrophy. J Med Genet. 2003;40:223–226. doi: 10.1136/jmg.40.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M, Mercola M. Science's STKE [Electronic Resource]: Signal Transduction Knowledge Environment 2001:RE1. 2001. TGF-beta superfamily signaling and left-right asymmetry. [DOI] [PubMed] [Google Scholar]
- Wise SL, Meador KJ, Thompson WO, Avery SS, Loring DW, Wray BB. Cerebral lateralization and histamine skin test asymmetries in humans. Ann Allergy. 1993;70:328–332. [PubMed] [Google Scholar]
- Xu J, Van Keymeulen A, Wakida NM, Carlton P, Berns MW, Bourne HR. Polarity reveals intrinsic cell chirality. Proc Natl Acad Sci U S A. 2007;104:9296–9300. doi: 10.1073/pnas.0703153104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Zhang M, Bolfing MF, Knowles HJ, Karnes H, Hackett BP. Foxj1 regulates asymmetric gene expression during left-right axis patterning in mice. Biochem Biophys Res Commun. 2004;324:1413–1420. doi: 10.1016/j.bbrc.2004.09.207. [DOI] [PubMed] [Google Scholar]
- Zou EM, Fingerman M. External features of an intersex fiddler crab, Uca pugilator (Bosc, 1802) (Decapoda, Brachyura) Crustaceana. 2000;73:417–423. [Google Scholar]