Abstract
Pressor effects of the vasoconstrictor hormone arginine vasopressin (AVP), observed when systemic AVP concentrations are less than 100 pM, are important for the physiological maintenance of blood pressure, and they are also the basis for therapeutic use of vasopressin to restore blood pressure in hypotensive patients. However, the mechanisms by which circulating AVP induces arterial constriction are unclear. We examined the novel hypothesis that KCNQ potassium channels mediate the physiological vasoconstrictor actions of AVP. Reverse transcriptase polymerase chain reaction revealed expression of KCNQ1, KCNQ4, and KCNQ5 in rat mesenteric artery smooth muscle cells (MASMCs). Whole-cell perforated patch recordings of voltage-sensitive K+ (Kv) currents in freshly isolated MASMCs revealed 1,3-dihydro-1-phenyl-3,3-bis(4-pyridinylmethyl)-2H-indol-2-one (linopirdine)- and 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone (XE-991)-sensitive KCNQ currents that were electrophysiologically and phar-currents. Suppression of macologically distinct from other Kv KCNQ currents by AVP (100 pM) was associated with significant membrane depolarization, and it was abolished by the protein kinase C (PKC) inhibitor calphostin C (250 nM). The KCNQ channel blocker linopirdine (10 μM) inhibited KCNQ currents in MASMCs, and it induced constriction of isolated rat mesenteric arteries. The vasoconstrictor responses were not additive when combined with 30 pM AVP, and they were prevented by the L-type Ca2+ channel blocker verapamil. Ethyl-N-[2-amino-6-(4-fluorophenylmethylamino)pyridin-3-yl] carbamic acid (flupirtine) significantly enhanced KCNQ currents, and it reversed constrictor responses to 30 pM AVP. In vivo, i.v. administration of linopirdine induced a dose-dependent increase in mesenteric artery resistance and blood pressure, whereas flupirtine had the opposite effects. We conclude that physiological concentrations of AVP induce mesenteric artery constriction via PKC-dependent suppression of KCNQ currents and L-type Ca2+ channel activation in MASMCs.
Membrane voltage (Vm) determines the open probability of L-type Ca2+ channels in vascular smooth muscle cells (VSMCs), and K+ channels represent a primary effector for adjusting Vm. To the extent that K+ channels are open in resting VSMCs, the outward flux of K+ through these channels (measured as K+ current) will tend to stabilize the resting Vm at negative (hyperpolarized) voltages and prevent opening of voltage-sensitive Ca2+ channels. In contrast, reduction of outward K+ currents in VSMCs results in a shift to more positive Vm (membrane depolarization) leading to activation of L-type Ca2+ channels and entry of Ca2+ into the cell. Elevation of the cytosolic Ca2+ concentration in this manner can trigger VSMC contraction and vasoconstriction.
KCNQ channels (Kv7 family) are voltage-sensitive K+ (Kv) channels that have been recognized as mediators of neuronal “M-currents”: noninactivating, outwardly rectifying Kv currents. Their inhibition in response to muscarinic receptor activation results in increased neuronal excitation (Jentsch, 2000). Suppression of KCNQ channel-mediated neuronal M-currents is generally considered to involve phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis. PIP2 is thought to stabilize the open state of KCNQ channels; activation of phospholipase C (PLC), e.g., via m1 muscarinic receptor activation, leads to hydrolysis of PIP2 and suppression of channel activity (Delmas and Brown, 2005). In addition to this mechanism, bradykinin at high concentrations may suppress neuronal M-currents via PLC-mediated inositol trisphosphate formation and release of intracellular Ca2+ stores (Cruzblanca et al., 1998; Brown et al., 2007). Another proposed mechanism for suppression of M-currents is via protein kinase C (PKC) activation and potentially, direct PKC-mediated KCNQ channel phosphorylation (Hoshi et al., 2003; Nakajo and Kubo, 2005; Surti et al., 2005).
KCNQ channels have very recently been found to be expressed in VSMCs (Ohya et al., 2003; Yeung and Greenwood, 2005; Joshi et al., 2006; Brueggemann et al., 2007; Yeung et al., 2007), but little is known about their regulation or function in these cells. In A7r5 cells (a rat aortic smooth muscle cell line), we found that a physiological concentration of the vasoconstrictor hormone arginine vasopressin (AVP; 100 pM) can suppress native KCNQ5 currents via PKC activation, and this effect is sufficient to induce membrane depolarization and action potential firing (Brueggemann et al., 2007). We speculated that KCNQ channel suppression might represent the depolarizing mechanism responsible for the vasoconstrictor actions of physiological concentrations of AVP (Brueggemann et al., 2007).
The previous identification of KCNQ channels as regulators of neuronal excitation has led to the use of activators or blockers of KCNQ channels for treatment of epilepsy, neuropathic pain, and Alzheimer’s disease (Passmore et al., 2003; Surti and Jan, 2005; Rogawski, 2006). Although a reduction in systolic blood pressure and heart rate was noted in patients treated chronically with the KCNQ channel activator flupirtine (Herrmann et al., 1987), the effects of KCNQ channel modulators on arterial resistance have not been evaluated. Vasodilator/vasoconstrictor actions might have important implications for the use of KCNQ channel modulators in existing therapies as well as for their potential use in the treatment of cardiovascular diseases.
AVP-induced constriction of mesenteric arteries is thought to be essential for its physiological pressor effects (Altura, 1975; Banks et al., 1985). Mesenteric artery constriction also contributes to the clinical actions of AVP, which is increasingly used as a pressor agent to treat patients during cardio-pulmonary resuscitation, for septic/vasodilatory shock, or intraoperative hypotension (Altura, 1976; Holmes et al., 2001; Treschan and Peters, 2006; Barrett et al., 2007). In disease states such as hypertension, heart failure, and vasospasm, elevated circulating [AVP] may contribute to disease progression (Cowley et al., 1981; Delgado et al., 1988; Nakamura et al., 2006). Considering our previous findings that physiological concentrations of AVP regulate KCNQ channel function in cultured vascular smooth muscle cells, if these channels were present in mesenteric arteries they might represent a novel therapeutic target for blood pressure regulation or for treatment of cardiovascular diseases in which AVP levels are altered.
In the present study, we extend our previous findings by evaluating the expression and functional contribution of native mesenteric artery myocyte KCNQ channels. Our results indicate that the vasoconstrictor actions of vasopressin involve PKC-dependent suppression of mesenteric artery KCNQ currents. We also present evidence that isolated mesenteric arteries are sensitive to clinically used KCNQ channel blockers and activators (linopirdine and flupirtine, respectively) and that systemic administration of these KCNQ channel modulators influences mesenteric vascular resistance and systemic blood pressure in vivo.
Materials and Methods
All studies involving animal use were approved by the Institutional Animal Care and Use Committee of Loyola University Medical Center (Maywood, IL).
Reverse Transcription-Polymerase Chain Reaction
Primers for KCNQ mRNAs were adapted from Ohya et al. (KCNQ1–3 and KCNQ5; Ohya et al., 2002) and Yeung et al. (KCNQ4; Yeung et al., 2007) to correspond to the rat sequences. Preparation of RNA from freshly isolated mesenteric artery smooth muscle cells (MASMCs) (selected individually based on morphology) and PCR procedures have been published previously (Brueggemann et al., 2006, 2007). For rat brain positive controls and MASMC RNA, a second round of PCR was carried out using the same primers and 2 μl of PCR product. PCR products were confirmed for each primer pair by DNA sequencing. For KCNQ4, the second round of PCR was performed using a nested forward primer with the same reverse primer. Minus-reverse transcriptase controls using the same reaction conditions with MASMC RNA were negative for all KCNQ primer pairs (data not shown). PCR analysis of three different MASMC preparations produced similar results.
Isolation of Myocytes and Patch-Clamp Recording
Segments of mesenteric artery were prepared as described previously (Henderson and Byron, 2007), and they were subjected to enzymatic digestion for isolation of MASMCs as described by Berra-Romani et al. (2005): MASMCs were kept on ice until use. For use, the cells were dispensed onto a glass coverslip base of the recording chamber, and they were allowed to adhere for at least 15 min at room temperature. Methods for recording isolated Kv currents in MASMCs were adapted from previous A7r5 cell studies (Brueggemann et al., 2007), and they are described in detail in Supplemental Material.
Pressure Myography
Methods used for isolated artery pressure myography have been described previously (Henderson and Byron, 2007).
In Vivo Cardiovascular Experiments
Adult male Sprague-Dawley rats were anesthetized with thiobutabarbital (100 mg/kg i.p.). After catheterization (unilateral femoral arterial and venous catheters for measurement of arterial pressure and drug injections), a blood flow probe (Transonic Systems Inc., Ithaca NY) was placed around the superior mesenteric artery through a mid-line laparotomy. Basal blood pressure/blood flow values were recorded (30 min) before drug administration. Vehicle for linopirdine and flupirtine was a 1:1 mixture of polyethylene glycol-400 and physiological saline. Vehicle responses (same sequence of infusion volumes as used for drug administration) were measured in each rat before administering the test drug. Each dose was administered over 5 s at 5-min intervals. Parameters measured during the last 120 s of each 5-min time period were averaged for the linopirdine responses, whereas peak values were averaged for flupirtine because of the more transient nature of the latter responses.
Statistics
SigmaStat (Systat Software, Inc., Point Richmond, CA) was used for all statistical analyses. Paired Student’s t test was used for comparisons of parameters measured before and after treatments. Comparisons among multiple treatment groups were evaluated by analysis of variance (ANOVA) followed by a Holm-Sidak post hoc test. Cumulative concentration-response data were analyzed by repeated measures ANOVA and post hoc Holm-Sidak test. Comparisons of constrictor responses to 10 nM AVP in the presence or absence of 10 μM linopirdine were evaluated using a Mann-Whitney rank sum test. Differences associated with p values <0.05 were considered statistically significant.
Materials
Linopirdine, flupirtine, glibenclamide, iberiotoxin, tetra-ethylammonium chloride, tetrodotoxin, collagenase, elastase, [Arg8]-vasopressin, and verapamil were from Sigma-Aldrich (St. Louis, MO). Calphostin C was from BIOMOL Research Laboratories (Plymouth Meeting, PA). 4β-Phorbol 12-myristate 13-acetate and amphotericin B were from Calbiochem (San Diego, CA). XE-991 was from Tocris Cookson, Inc. (Ellisville, MO).
Results
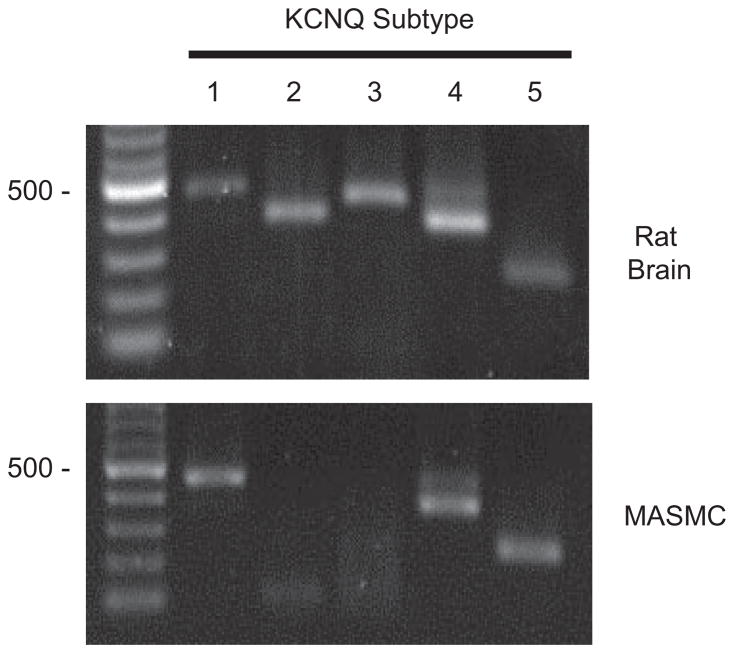
Using reverse transcriptase-PCR to evaluate gene expression of KCNQ channels in isolated rat MASMCs, we found that of the five known KCNQ channel subtypes, messages for KCNQ1, KCNQ4, and KCNQ5 were detected (Fig. 1).
Fig. 1.
KCNQ channel expression in MASMCs. Total RNA prepared from rat MASMCs or adult rat brain (as a positive control) was reverse transcribed and subjected to PCR using primers specific for rat KCNQ1 through KCNQ5. Molecular weight marker (M) is a 100-bp ladder (New England Biolabs, Ipswich, MA); 500 bp is indicated to the left of each panel. Expected sizes of each reaction product are KCNQ1, 453 bp; KCNQ2, 372 bp; KCNQ3, 424 bp; KCNQ4, 359 bp; and KCNQ5, 240 bp.
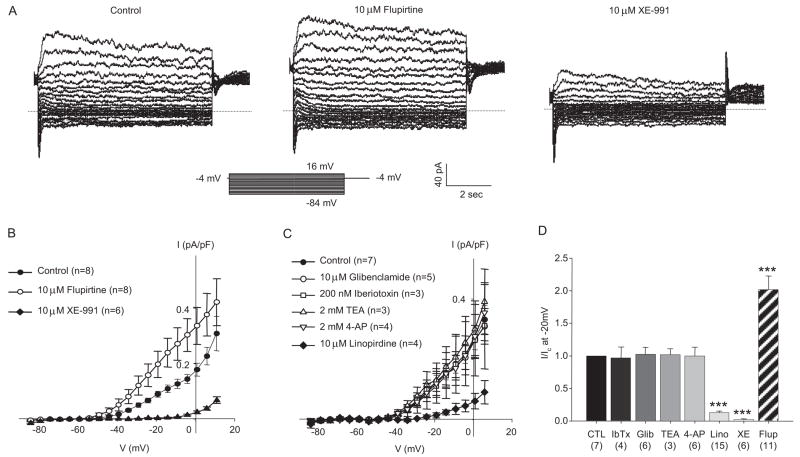
To evaluate the function of KCNQ channels in MASMCs, KCNQ currents were assessed using perforated patch whole-cell voltage-clamp techniques. As shown in Fig. 2A, a relatively small outwardly rectifying Kv current was measured during the last 500 ms of 5-s voltage steps from a −4-mV holding voltage. The voltage dependence of activation was well fit by a single Boltzmann distribution (V0.5 of −34.3 mV and slope factor of +6.5 mV; Fig. 3C). The selective KCNQ channel activator flupirtine (10 μM) enhanced the currents approximately 2-fold (mean current measured at −20 mV; Fig. 2, A, B, and D). Treatment with selective KCNQ channel blockers linopirdine (10 μM) or XE-991 (10 μM), almost abolished the isolated currents at all voltages negative to −15 mV (Figs. 2 and 3). Pharmacological studies using inhibitors of other classes of vascular K+ channels confirmed that the KCNQ currents are effectively isolated from other K+ currents under our recording conditions (Fig. 2, C and D).
Fig. 2.
KCNQ currents in MASMCs. A, representative current traces recorded from a cluster of four to five MASMCs (capacitance, 208.5 pF): control (untreated, left), 10 μM flupirtine (middle), and 10 μM XE-991 (right). B, summarized I–V relationship from six to eight recordings from cells treated as described in A. Currents measured at all membrane potentials between −34 to +1 mV after XE-991 and flupirtine treatment are significantly different from control treatment (p < 0.05, one-way repeated measures ANOVA and post hoc Holm-Sidak method). C, summarized I–V relationships recorded in the presence of conventional VSMC K+ channel blockers (at the concentrations indicated on the figure; 10-min treatment followed by a 10-min washout) and after subsequent exposure for 10 min to 10 μM linopirdine. Currents measured at all membrane potentials positive to −34 mV after linopirdine treatment are significantly different from control treatment (n = 4; p < 0.05, one-way repeated measures ANOVA and post hoc Holm-Sidak method). D, mean currents recorded at −20 mV normalized to control currents (CTL). Numbers below treatment indicate n for each group. ***, p < 0.001, significant difference from control, one-way ANOVA and Holm Sidak post hoc analysis.
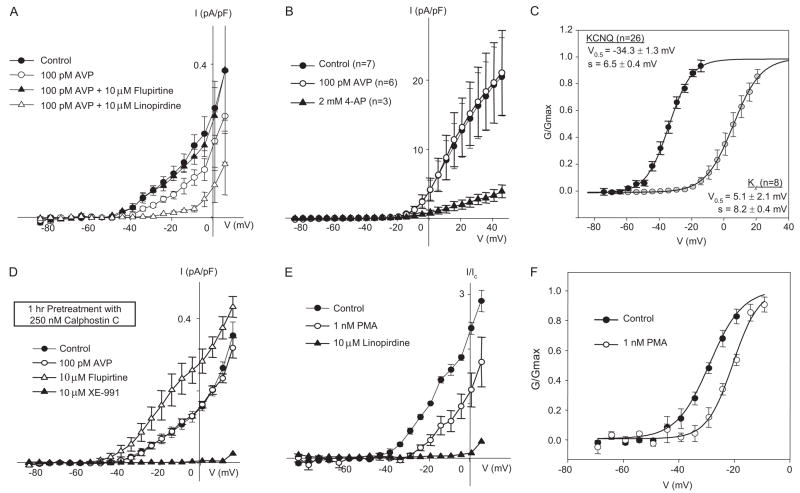
Fig. 3.
AVP suppresses KCNQ currents in a PKC-dependent manner, but it does not influence 4-AP-sensitive Kv currents. A, summarized I–V relationships for KCNQ currents recorded from MASMCs. The currents are decreased after treatment for 15 min with 100 pM AVP. This effect is reversed by 10-min treatment with 10 μM flupirtine. Subsequent exposure to 10 μM linopirdine for 10 min significantly reduces the current. Currents measured at all membrane potentials between −34 to +1 mV after AVP and linopirdine treatment are significantly different from control and flupirtine treatment (n = 4; p < 0.05, one-way repeated measures ANOVA and post hoc Holm-Sidak method). B, 4-AP-sensitive Kv currents were recorded as described in the Supplemental Material. Treatment for 15 min with 100 pM AVP had no significant effect, whereas the currents were significantly reduced after 10-min treatment with 2 mM 4-AP (n = 3). C, steady-state activation curves fitted by single Boltzmann functions for KCNQ currents and 4-AP-sensitive Kv currents. D, summarized I–V relationships for KCNQ currents in MASMCs pretreated with 250 nM calphostin C (PKC inhibitor) for 1 h at room temperature. Pretreatment with calphostin C abolished the negative regulation by 100 pM AVP. However, subsequent application of 10 μM flupirtine and 10 μM XE-991 enhanced and suppressed KCNQ currents, respectively (n = 4). E, summarized I–V relationships for KCNQ currents normalized to control currents measured at −20 mV. Application of 1 nM PMA for 15 min significantly reduced KCNQ current at all voltages positive to −39 mV (n = 4; paired Student’s t test, p < 0.05). Subsequent application of 10 μM linopirdine suppressed the remaining current. F, steady-state activation curves fitted by single Boltzmann functions. Treatment with 1 nM PMA induced a significant positive shift of the activation curve (V0.5 = −27.5 ± 1.7 mV versus −20.8 ± 0.3 mV for control and 1 nM PMA treatment, respectively; n = 4; p < 0.05 using paired Student’s t test).
Treatment of freshly isolated MASMCs with a physiological concentration of AVP (100 pM) led to a reduction of the KCNQ current amplitude by ~50% (Fig. 3A) without a significant shift in the conductance-voltage curve (data not shown). Subsequent addition of the KCNQ channel activator flupirtine (10 μM) completely restored the currents. Much larger voltage-sensitive K+ currents can be detected using a different voltage step protocol and omitting GdCl3 from the external solution (Fig. 3B). These currents were activated at more positive voltages (V0.5 = +5.1 mV; Fig. 3C) than KCNQ currents, and they were inhibited by 4-aminopyridine (4-AP; 2 mM), but they were not sensitive to treatment with 100 pM AVP (Fig. 3B) or 10 μM linopirdine (Supplemental Fig. 1A).
Pretreatment of MASMCs with the selective PKC inhibitor calphostin C (250 nM) prevented the suppression of KCNQ currents by 100 pM AVP (Fig. 3D). There was no significant difference in resting current density between untreated and calphostin C-treated MASMCs. Direct activation of PKC with phorbol 12-myristate 13-acetate (PMA; 1 nM) was sufficient to significantly suppress KCNQ currents (Fig. 3E). This effect was manifested as both a significant decrease in current amplitude (by 70 ± 7% at −20 mV) and a significant positive shift of the conductance-voltage curve (by 7 ± 2 mV; Fig. 3F).
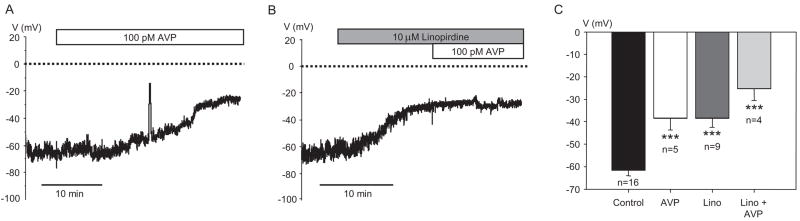
If KCNQ channels are active in resting MASMCs, then suppression of KCNQ currents is expected to induce membrane depolarization. We measured membrane voltage in isolated myocytes using whole-cell current clamp. AVP (100 pM) significantly depolarized the membrane of freshly isolated MASMCs from an average resting voltage of −61.5 ± 2.4 mV to −38.4 ± 5.2 mV (Fig. 4, A and C). Treatment with linopirdine (10 μM) also significantly depolarized the resting membrane of MASMCs to −38.5 ± 3.9 mV, but this effect was not significantly enhanced by subsequent addition of 100 pM AVP (Fig. 4, B and C).
Fig. 4.
Membrane depolarization by AVP and the KCNQ channel blocker linopirdine. A, representative time course of membrane depolarization in response to 100 pM AVP recorded from a single MASMC. B, representative time course of membrane depolarization in response to 10 μM linopirdine followed by additional application of 100 pM AVP. C, average membrane voltage values in MASMCs measured before treatment and after stabilization in the presence of 100 pM AVP, 10 μM linopirdine, or both 10 μM linopirdine and 100 pM AVP. All treatments were significantly different from control (p < 0.001), but differences among treatment groups were not statistically significant.
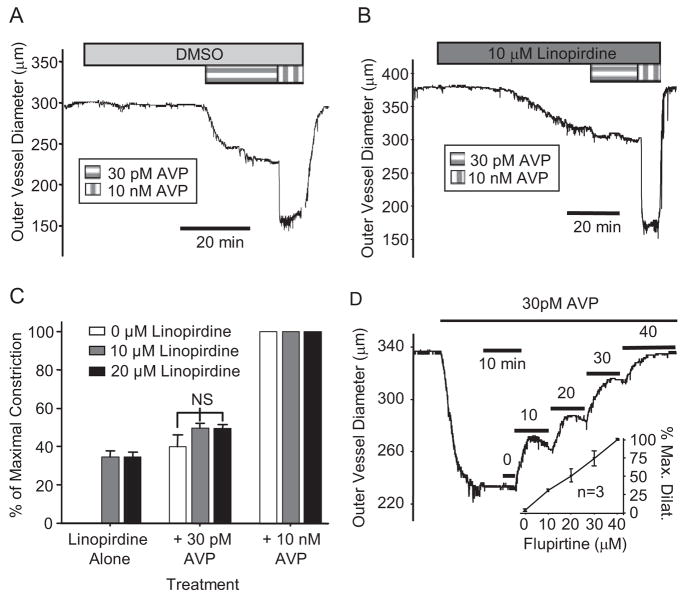
Results presented above indicate that KCNQ channels are present and functional in freshly isolated MASMCs and that treatment with a physiological concentration of AVP (100 pM) leads to suppression of KCNQ currents and membrane depolarization. To investigate the physiological relevance of these responses, we measured constriction/dilation of isolated mesenteric arterial (MA) segments maintained at 37°C and pressurized to a physiological pressure of 80 mm Hg (Fenger-Gron et al., 1995). The KCNQ channel blocker linopirdine (10 μM) caused significant constriction of MAs (Fig. 5B) compared with vehicle control [dimethyl sulfoxide (DMSO)], which had no effect (Fig. 5A). Doubling the linopirdine concentration to 20 μM did not induce greater constriction (35 ± 3% of maximal constriction compared with 35 ± 2% induced by 10 μM linopirdine, n = 6 for each) (Fig. 5C).
Fig. 5.
Constriction of mesenteric arteries: effects of KCNQ channels blockers or activators. A and B, representative recordings of MA constriction in response to 30 pM AVP and 10 nM AVP in an artery pretreated with DMSO (vehicle control; A) or KCNQ channel blocker linopirdine (10 μM) followed by addition of 30 pM AVP and then 10 nM AVP (B). C, summarized results (mean ± S.E.) expressed as percentage of maximal constriction. Significant constriction in response to 10 or 20 μM linopirdine is not significantly augmented by 30 pM AVP. Total occlusion of the vessel lumen (100% maximal constriction) was induced by 10 nM AVP under all conditions. DMSO alone (0 μM linopirdine) failed to induce any detectable constriction such that the error bars are not visible. C, concentration-dependent vasodilation in response to the KCNQ channel activator flupirtine (10–40 μM) after preconstriction of MAs with 30 pM AVP. Inset, mean concentration-response data expressed as percentage of maximal dilation (n = 3).
The maximal response to linopirdine (~35% constriction) was achieved at a concentration of 10 μM, and we have previously shown that 30 pM AVP induces a similar degree of constriction of rat MAs (Henderson and Byron, 2007). As shown in Fig. 5, A to C, the constrictions induced by 30 pM AVP and 10 μM linopirdine were not additive. The inability of 30 pM AVP to significantly augment the constrictor responses to 10 μM linopirdine was not due to a limitation of L-type Ca2+ channel availability, because 60 mM external K+ elicited a maximal constrictor response when applied in the presence of linopirdine (Supplemental Fig. 2, A and B).
If physiological concentrations of AVP constrict MAs via suppression of KCNQ currents, then the KCNQ channel activator flupirtine, which we found could reverse the AVP-induced suppression of KCNQ currents in isolated myocytes (Fig. 3A), would be expected to reverse the constrictor effects of AVP. This prediction was borne out by results demonstrating that flupirtine reproducibly exerted a concentration-dependent vasodilatory effect on MAs preconstricted with 30 pM AVP (Fig. 5D).
A supraphysiological concentration of AVP (10 nM) induced a maximal constrictor response (i.e., complete occlusion of the vessel lumen) regardless of the absence or presence of 10 μM linopirdine (outer vessel diameters were 156 ± 11 versus 158 ± 5 μm, respectively; p = 0.589, Mann-Whitney rank sum test). This is consistent with our previous finding that the acute constrictor responses of MAs to 10 nM AVP involve different signal transduction mechanisms compared with responses activated by 30 pM AVP (Henderson and Byron, 2007).
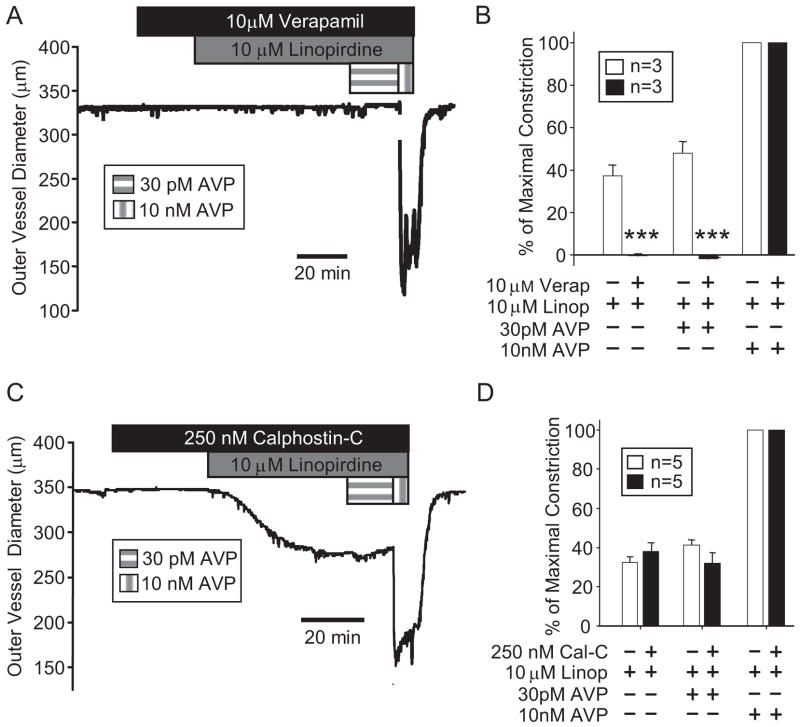
The L-type Ca2+ channel blocker verapamil (10 μM) abolished constrictor responses of mesenteric arteries to 10 μM linopirdine and 30 pM AVP, but it had no impact on the maximal constriction induced by supraphysiological AVP (10 nM) (Fig. 6, A and B). Pretreatment of mesenteric arteries with 250 nM calphostin C, which was shown previously to prevent MA constriction in response to 10 nM PMA or 30 pM AVP (Henderson and Byron, 2007), did not significantly affect the constrictor responses to 10 μM linopirdine (Fig. 6, C and D). In agreement with our previous report (Henderson and Byron, 2007), acute responses to 10 nM AVP were not altered by calphostin C treatment.
Fig. 6.
Constriction of mesenteric arteries by linopirdine depends on L-type Ca2+ channels but not protein kinase C activation. A, representative recording of mesenteric arterial constriction in response to the sequential addition of the L-type Ca2+ channel blocker verapamil (10 μM; 20 min), 10 μM linopirdine, 30 pM AVP, and 10 nM AVP. B, summarized results from MAs treated with or without the L-type Ca2+ channel blocker verapamil (10 μM) followed by additions of linopirdine (10 μM), 30 pM AVP, and 10 nM AVP. ***, p < 0.001, significant difference between treated and untreated groups. Full arterial occlusion was induced by 10 nM AVP in the presence and absence of verapamil. C, representative recording of mesenteric arterial constriction in response to the sequential addition of the PKC inhibitor calphostin C (250 nM; 30 min), 10 μM linopirdine, 30 pM AVP, and 10 nM AVP. D, summarized results from MAs pretreated with or without the PKC inhibitor calphostin C (250 nM) followed by exposures to 10 μM linopirdine, 30 pM AVP, and 10 nM AVP. Responses to linopirdine with or without 30 pM or 10 nM AVP were not significantly affected by calphostin C (p > 0.1 for each). Statistical comparisons are based on Student’s t test.
Our voltage-clamp results (Figs. 2 and 3) distinguish linopirdine-sensitive Kv currents (detected at voltages negative to −40 mV and suppressed in response to physiological concentrations of AVP) from 4-AP-sensitive Kv currents (activated at more positive voltages and not inhibited by 100 pM AVP). In pressurized MAs, we found that 2 mM 4-AP was ineffective as a vasoconstrictor, but the same arteries constricted robustly when, in the continued presence of 4-AP, the KCNQ channel blocker linopirdine was added (Supplemental Fig. 1, B and C). Arteries constricted more rapidly (Supplemental Fig. 1, D and E), and to a significantly greater extent (approximately 10% greater constriction) when linopirdine was added in the presence of 4-AP (Supplemental Fig. 1C).
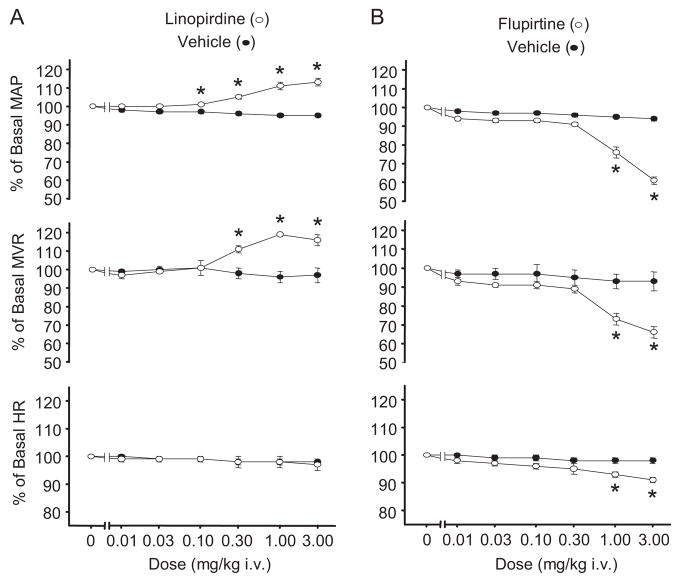
To assess the role of KCNQ channels in mesenteric vascular resistance in vivo, we measured superior mesenteric artery blood flow and systemic blood pressure in anesthetized rats. Mesenteric vascular resistance (MVR) was determined by dividing perfusion pressure [estimated as mean arterial blood pressure (MAP)] by superior mesenteric artery blood flow. As seen in Fig. 7, linopirdine (0.01–3 mg/kg i.v.) produced a concentration-dependent increase in both MAP and MVR. In contrast, the KCNQ channel activator flupirtine (0.01–3 mg/kg i.v.) produced a dose-dependent decrease in both MAP and MVR. Flupirtine also induced a modest dose-dependent decrease in heart rate, whereas linopirdine failed to significantly alter heart rate.
Fig. 7.
Effects of KCNQ channel modulators on blood pressure, mesenteric vascular resistance, and heart rate (HR) measured in vivo. Dose-dependent MAP, MVR, and HR responses (expressed as a percentage of basal value ± S.E.) to linopirdine (open symbols; n = 3) (A) and flupirtine (open symbols; n = 3) (B). Effects of injecting vehicle alone are shown for comparison (closed symbols; n = 6). Average pretreatment values for each variable were as follows: MAP, 90.3 ± 2.7 mm Hg; MVR, 7.8 ± 0.8 mm Hg·min·ml−1; and HR, 276.3 ± 7.3 beats/min. Asterisk (*) indicates significant differences from control (p < 0.05), two-way repeated measures ANOVA and Holm-Sidak post hoc test.
Discussion
Mesenteric arteries are highly sensitive to the vasoconstrictor actions of AVP, and they are important targets for its pressor effects (Altura, 1975; Banks et al., 1985). Our results suggest the following: 1) KCNQ channels expressed in mesenteric artery myocytes are active at resting membrane potentials and that they are negatively regulated by physiological concentrations of AVP; 2) suppression of KCNQ currents is sufficient to induce membrane depolarization and L-type Ca2+ channel-dependent arterial constriction; 3) regulation of KCNQ channels is downstream of PKC activation in the signaling cascade activated by AVP; and 4) the effects of KCNQ channel modulation in arterial myocytes are apparent at the level of the single cell, isolated artery, and intact vasculature of a live animal. These findings are significant for the elucidation of the biochemical mechanisms by which physiological concentrations of AVP exert their pressor effects, and also because they implicate KCNQ channels, which until very recently were not considered among the cohort of vascular potassium channels.
Other classes of Kv channels have been postulated to play important roles in vasoconstrictor actions. Several Kv1-Kv4 subtypes, which are inhibited to varying extents by 4-AP (Xu et al., 1999; Cox, 2005), have been implicated as mediators of VSMC Kv currents. Evidence suggests that inhibition of 4-AP-sensitive Kv currents by circulating vasoconstrictor hormones may contribute to vasoconstrictor actions (Nelson and Quayle, 1995; Cole et al., 1996; Jackson, 2005), but the link between inhibition of these currents and activation of L-type Ca2+ channels is not clearly established.
The threshold for voltage-dependent activation of L-type Ca2+ channels and 4-AP-sensitive Kv channels in VSMCs is positive to −40 mV (Rubart et al., 1996; Xu et al., 1999). However, Vm measured in VSMCs in arteries subjected to normal intravascular pressures in vitro or from arteries in vivo are more negative, generally between −40 and −60 mV (Nelson and Quayle, 1995). 4-AP-sensitive currents were undetectable in MASMCs at voltages negative to approximately −30 mV (Fig. 3, B and C), making them unlikely to contribute appreciably at the resting Vm (−61.5 ± 2.4 mV) of isolated MASMCs. Their insensitivity to 100 pM AVP further suggests that suppression of these 4-AP-sensitive currents cannot mediate the membrane depolarization required for the vasoconstrictor responses to physiological concentrations of AVP. On the other hand, KCNQ channels are active at voltages negative to −40 mV and might therefore be effective targets for vasoconstrictor signaling.
In addition to Kv channels, Ca 2+-activated, inward rectifier, and ATP-sensitive K+ (KATP) channels are also known to be expressed in VSMCs (Nelson and Quayle, 1995). Relevant to our findings, AVP has been shown to negatively regulate KATP channel activity (Martin et al., 1989; Wakatsuki et al., 1992; Shi et al., 2007). This effect has been measured experimentally in VSMCs when KATP channels are preactivated pharmacologically, and it has been posited as a potential mechanism by which AVP raises blood pressure in patients with prolonged vasodilatory shock (Holmes et al., 2001; Barrett et al., 2007). It is noteworthy that the concentrations of AVP needed to detectably inhibit KATP currents in these experimental systems (Martin et al., 1989; Wakatsuki et al., 1992; Shi et al., 2007) exceed, by at least an order of magnitude, the plasma concentrations of AVP achieved with effective clinical therapy (approximately 100 pM; Holmes et al., 2001). Our finding that glibenclamide (a KATP channel blocker) did not affect AVP-sensitive Kv currents in MASMCs (Fig. 2, C and D) and that it did not induce constriction of rat MAs (Supplemental Fig. 2, C and D), suggests that KATP channels do not contribute significantly to the AVP effect in MASMCs from healthy rats (in agreement with previous studies of rat arteries (Dumont and Lamontagne, 1995; Sanz et al., 2003). Instead, we attribute the effects of physiological [AVP] to the suppression of KCNQ channel activity.
Different vascular beds express different complements of the five known KCNQ subtypes. KCNQ1 and KCNQ3 genes were found previously to be expressed in vascular smooth muscle of murine portal vein and rat pulmonary arteries (Ohya et al., 2003; Yeung and Greenwood, 2005; Joshi et al., 2006), and a more quantitative analysis recently revealed KCNQ4 and KCNQ5 to be the most abundantly expressed subtypes in mouse carotid and femoral arteries (Yeung et al., 2007). We previously reported that rat aortic smooth muscle cells express KCNQ1 and KCNQ5 (Brueggemann et al., 2007), and we determined in the present study that KCNQ1, KCNQ4, and KCNQ5 are expressed in rat MASMCs (Fig. 1). Although each of the five KCNQ subtypes can form functional Kv channels, it is unknown whether vascular KCNQ channels form homo- or heteromeric tetramers of the individual gene products (Jentsch, 2000; Schwake et al., 2003). It also remains to be determined whether the heterogeneity of expression patterns among different vascular beds is related to differences in resting tone, autoregulatory mechanisms, or in reactivity to vasoactive agents.
KCNQ channel blockers (linopirdine and XE-991) were shown to constrict both rat and mouse intrapulmonary arteries (Joshi et al., 2006), but they were without effect on mesenteric arteries from the same animals. The reasons for this discrepancy with our current findings are not immediately apparent. One notable difference was that Joshi et al. (2006) measured constriction using a wire myograph as opposed to the pressurized artery preparation used in our experiments. Constrictor responses of pulmonary arteries to linopirdine were similar to the responses we observed with pressurized mesenteric arteries: maximal responses were obtained with 10 μM linopirdine, and these responses were abolished by a blocker of L-type Ca2+ channels (nifedipine; 1 μM) (Joshi et al., 2006).
Our in vivo studies suggest that vascular KCNQ channels contribute to MVR, in agreement with our in vitro electro-physiological and functional studies. Dose-dependent increases in MAP and MVR in response to the KCNQ channel blocker linopirdine may be expected considering our in vitro results demonstrating that linopirdine depolarizes the membrane of isolated MASMCs and constricts mesenteric arteries. The opposite effects of flupirtine on both MAP and MVR are in accordance with its hyperpolarizing influence on MASMCs and its concentration-dependent vasodilatory effects on mesenteric arteries (Fig. 5D). A modest decrease in heart rate in response to flupirtine may be a consequence of nonvascular KCNQ channel activation (e.g., a reduction of sympathetic ganglionic nerve activity). In contrast, linopirdine did not affect heart rate and the in vitro constriction of artery segments in response to linopirdine was not affected by treatment with the neuronal voltage-sensitive Na+ channel blocker tetrodotoxin (100 nM; data not shown). Although we cannot rule out some contribution of nonvascular KCNQ channels to the systemic effects of linopirdine or flupirtine, our findings are consistent with our hypothesis that modulation of vascular KCNQ channel activity (by physiological agonists or pharmacological agents) will affect mesenteric vascular resistance and systemic blood pressure in vivo. These findings suggest that direct pharmacological modulation of vascular KCNQ channels may prove useful in clinical settings where acute blood pressure regulation is required.
KCNQ channels are targeted by neurotransmitters to regulate neuronal excitation, but signal transduction pathways for the regulation of neuronal versus vascular KCNQ channels may be different. Suppression of KCNQ channel-mediated neuronal M-currents in response to receptor-mediated signal transduction is not generally considered to involve PKC (Bosma and Hille, 1989; Cruzblanca et al., 1998; Ma et al., 2006). Instead, evidence favors hydrolysis of PIP2 as an essential mechanism (Delmas and Brown, 2005). This mechanism is robustly activated during cholinergic synaptic transmission, where release of acetylcholine results in very high local concentrations that probably saturate muscarinic receptors and maximally activate PLC to deplete PIP2. Bradykinin-induced suppression of neuronal M-currents may involve PLC-mediated inositol trisphosphate formation and release of intracellular Ca2+ stores, but this mechanism has also only been observed at very high agonist concentrations (Cruzblanca et al., 1998; Brown et al., 2007). In contrast, the endocrine actions of AVP are evident at very low concentrations that will occupy only a small fraction of the V1a vasopressin receptors. These concentrations are at least an order of magnitude below the EC50 for AVP-stimulated inositol trisphosphate formation or release of intracellular Ca2+ stores (Doyle and Ruegg, 1985; Byron, 1996). We speculate that physiological vasoconstrictor concentrations of AVP will not induce significant depletion of PIP2 and that the observed suppression of KCNQ channel-mediated Kv currents at these low concentrations of AVP must be attributed to other mechanisms (e.g., PKC-mediated channel phosphorylation (Hoshi et al., 2003; Nakajo and Kubo, 2005; Surti et al., 2005). Our findings (Fig. 3, D and E) suggest that PKC activation is both sufficient to reduce KCNQ currents in MASMCs and necessary for suppression of KCNQ current by physiological concentrations of AVP.
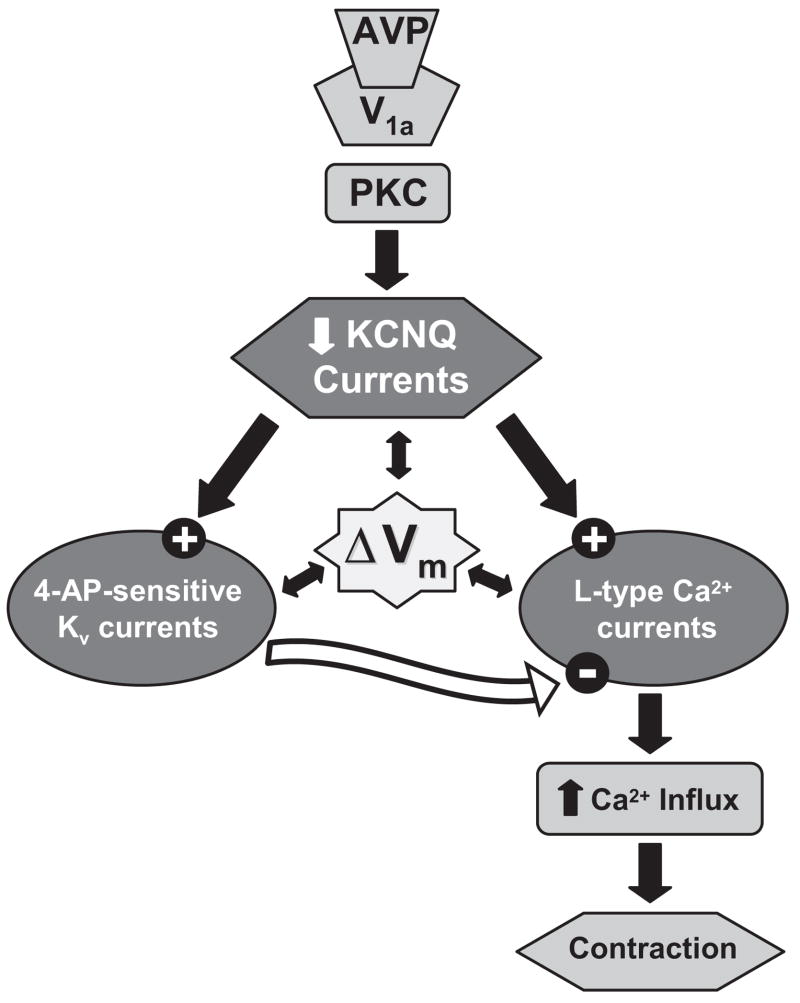
In summary, we have demonstrated expression of KCNQ channels in rat MASMCs and revealed noninactivating Kv currents that contribute to resting Vm and exhibit pharmacological and electrophysiological characteristics of KCNQ currents. These vascular KCNQ currents display an exquisite sensitivity to AVP at concentrations that are both physiologically and clinically relevant for regulation of MVR and systemic blood pressure. We propose a novel physiological mechanism for AVP-induced constriction of mesenteric arteries that involves PKC-dependent suppression of KCNQ channel activity at resting voltages and that results in membrane depolarization, activation of L-type Ca2+ channels, and myocyte contraction (Fig. 8). We also demonstrate in vivo effects of KCNQ channel modulators on systemic blood pressure and MVR that may have important implications for the clinical use of these drugs.
Fig. 8.
Working model for AVP-induced MA constriction. Coordinated regulation of three types of voltage-gated ion channels in MASMCs determines contractile responses to physiological concentrations of AVP. Our working model attempts to explain our present findings, along with our previous observation that constrictor responses of MAs to physiological concentrations of AVP are prevented by pretreatment with PKC inhibitors or by treatment with verapamil, a blocker of L-type Ca2+ channels (Henderson and Byron, 2007). We propose that AVP induces PKC-dependent suppression of KCNQ channel activity at resting membrane voltages (typically negative to −50 mV), resulting in membrane depolarization (ΔVm) and activation of L-type Ca2+ channels. Ca2+ influx via L-type channels then provides the contractile stimulus for the vasoconstrictor effects of AVP. 4-AP-sensitive Kv channels will also activate at membrane voltages positive to −30 mV, and their activity will tend to limit Ca2+ influx and oppose constriction (see Supplemental Fig. 1). Our finding that verapamil, but not calphostin C, abolished constrictor responses to linopirdine supports our working model and suggests that by blocking KCNQ channels directly, linopirdine by-passes the PKC-dependent signal transduction step.
Acknowledgments
This work was supported by the National Heart Lung and Blood Institute Grant R01 HL070670 (to K.L.B.) and the American Heart Association Grant 0715618Z (to A.R.M.).
We gratefully acknowledge the technical support of Chris Hill and Eric Formeister and expert assistance of Dr. Ruslan Tiniakov.
ABBREVIATIONS
- Vm
membrane voltage
- VSMC
vascular smooth muscle cell
- Kv
voltage-sensitive K+
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PLC
phospholipase C
- AVP
arginine vasopressin
- PKC
protein kinase C
- MASMC
mesenteric artery smooth muscle cell
- PCR
polymerase chain reaction
- ANOVA
analysis of variance
- 4-AP
4-aminopyridine
- PMA
phorbol 12-myristate 13-acetate
- MA
mesenteric artery
- DMSO
dimethyl sulfoxide
- MVR
mesenteric vascular resistance
- MAP
mean arterial pressure
- HR
heart rate
- KATP
ATP-sensitive K+
- I–V
current-voltage
- XE-991
10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone
- linopirdine
1,3-dihydro-1-phenyl-3,3-bis(4-pyridinylmethyl)-2H-indol-2-one
- flupirtine
ethyl-N-[2-amino-6-(4-fluorophenylmethylamino)pyridin-3-yl] carbamic acid
Footnotes
The chemical structures for the KCNQ channel modulators (linopirdine, XE-991, and flupirtine) are provided in Munro and Dalby-Brown (2007).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
References
- Altura BM. Dose-response relationships for arginine vasopressin and synthetic analogs on three types of rat blood vessels: possible evidence for regional differences in vasopressin receptor sites within a mammal. J Pharmacol Exp Ther. 1975;193:413–423. [PubMed] [Google Scholar]
- Altura BM. Microcirculatory approach to the treatment of circulatory shock with a new analog of vasopressin, (2-phenylalanine, 8-ornithine)vasopressin. J Pharmacol Exp Ther. 1976;198:187–196. [PubMed] [Google Scholar]
- Banks RO, Gallavan RH, Jr, Zinner MH, Bulkley GB, Harper SL, Granger DN, Jacobson ED. Vasoactive agents in control of the mesenteric circulation. Fed Proc. 1985;44:2743–2749. [PubMed] [Google Scholar]
- Barrett LK, Singer M, Clapp LH. Vasopressin: mechanisms of action on the vasculature in health and in septic shock. Crit Care Med. 2007;35:33–40. doi: 10.1097/01.CCM.0000251127.45385.CD. [DOI] [PubMed] [Google Scholar]
- Berra-Romani R, Blaustein MP, Matteson DR. TTX-sensitive voltage-gated Na+ channels are expressed in mesenteric artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;289:H137–H145. doi: 10.1152/ajpheart.01156.2004. [DOI] [PubMed] [Google Scholar]
- Bosma MM, Hille B. Protein kinase C is not necessary for peptide-induced suppression of M current or for desensitization of the peptide receptors. Proc Natl Acad Sci U S A. 1989;86:2943–2947. doi: 10.1073/pnas.86.8.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Hughes SA, Marsh SJ, Tinker A. Regulation of M(Kv7.2/7.3) channels in neurons by PIP2 and products of PIP2 hydrolysis: significance for receptor-mediated inhibition. J Physiol. 2007;582:917–925. doi: 10.1113/jphysiol.2007.132498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Markun DR, Henderson KK, Cribbs LL, Byron KL. Pharmacological and electrophysiological characterization of store-operated currents and capacitative Ca2+ entry in vascular smooth muscle cells. J Pharmacol Exp Ther. 2006;317:488–499. doi: 10.1124/jpet.105.095067. [DOI] [PubMed] [Google Scholar]
- Brueggemann LI, Moran CJ, Barakat JA, Yeh JZ, Cribbs LL, Byron KL. Vasopressin stimulates action potential firing by protein kinase C-dependent inhibition of KCNQ5 in A7r5 rat aortic smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H1352–H1363. doi: 10.1152/ajpheart.00065.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byron KL. Vasopressin stimulates Ca2+ spiking activity in A7r5 vascular smooth muscle cells via activation of phospholipase A2. Circ Res. 1996;78:813–820. doi: 10.1161/01.res.78.5.813. [DOI] [PubMed] [Google Scholar]
- Cole WC, Clement-Chomienne O, Aiello EA. Regulation of 4-aminopyridine-sensitive, delayed rectifier K+ channels in vascular smooth muscle by phosphorylation. Biochem Cell Biol. 1996;74:439–447. doi: 10.1139/o96-048. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Jr, Cushman WC, Quillen EW, Jr, Skelton MM, Langford HG. Vasopressin elevation in essential hypertension and increased responsiveness to sodium intake. Hypertension. 1981;3:I93–I100. doi: 10.1161/01.hyp.3.3_pt_2.i93. [DOI] [PubMed] [Google Scholar]
- Cox RH. Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophys. 2005;42:167–195. doi: 10.1385/CBB:42:2:167. [DOI] [PubMed] [Google Scholar]
- Cruzblanca H, Koh DS, Hille B. Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1998;95:7151–7156. doi: 10.1073/pnas.95.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado TJ, Arbab MA, Warberg J, Svendgaard NA. The role of vasopressin in acute cerebral vasospasm. Effect on spasm of a vasopressin antagonist or vasopressin antiserum. J Neurosurg. 1988;68:266–273. doi: 10.3171/jns.1988.68.2.0266. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Doyle VM, Ruegg UT. Vasopressin induced production of inositol trisphosphate and calcium efflux in a smooth muscle cell line. Biochem Biophys Res Commun. 1985;131:469–476. doi: 10.1016/0006-291x(85)91826-1. [DOI] [PubMed] [Google Scholar]
- Dumont E, Lamontagne D. No role of ATP-sensitive potassium channels in the vasoconstriction produced by vasopressin. J Vasc Res. 1995;32:138–142. doi: 10.1159/000159088. [DOI] [PubMed] [Google Scholar]
- Fenger-Gron J, Mulvany MJ, Christensen KL. Mesenteric blood pressure profile of conscious, freely moving rats. J Physiol. 1995;488:753–760. doi: 10.1113/jphysiol.1995.sp021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson KK, Byron KL. Vasopressin-induced vasoconstriction: two concentration-dependent signaling pathways. J Appl Physiol. 2007;102:1402–1409. doi: 10.1152/japplphysiol.00825.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann WM, Kern U, Aigner M. On the adverse reactions and efficacy of long-term treatment with flupirtine: preliminary results of an ongoing twelvemonth study with 200 patients suffering from chronic pain states in arthrosis or arthritis. Postgrad Med J. 1987;63(Suppl 3):87–103. [PubMed] [Google Scholar]
- Holmes CL, Patel BM, Russell JA, Walley KR. Physiology of vasopressin relevant to management of septic shock. Chest. 2001;120:989–1002. doi: 10.1378/chest.120.3.989. [DOI] [PubMed] [Google Scholar]
- Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N, Langeberg LK, Yoneda Y, Scott JD, Brown DA, et al. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci. 2003;6:564–571. doi: 10.1038/nn1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Joshi S, Balan P, Gurney AM. Pulmonary vasoconstrictor action of KCNQ potassium channel blockers. Respir Res. 2006;7:31. doi: 10.1186/1465-9921-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Bielefeldt K, Tan ZY, Whiteis CA, Snitsarev V, Abboud FM, Chapleau MW. Dual mechanisms of angiotensin-induced activation of mouse sympathetic neurones. J Physiol. 2006;573:45–63. doi: 10.1113/jphysiol.2006.106716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SC, Yule DI, Dunne MJ, Gallacher DV, Petersen OH. Vasopressin directly closes ATP-sensitive potassium channels evoking membrane depolarization and an increase in the free intracellular Ca2+ concentration in insulin-secreting cells. EMBO J. 1989;8:3595–3599. doi: 10.1002/j.1460-2075.1989.tb08532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro G, Dalby-Brown W. K(v)7 (KCNQ) channel modulators and neuropathic pain. J Med Chem. 2007;50:2576–2582. doi: 10.1021/jm060989l. [DOI] [PubMed] [Google Scholar]
- Nakajo K, Kubo Y. Protein kinase C shifts the voltage dependence of KCNQ/M channels expressed in Xenopus oocytes. J Physiol. 2005;569:59–74. doi: 10.1113/jphysiol.2005.094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Funayama H, Yoshimura A, Tsuruya Y, Saito M, Kawakami M, Ishikawa SE. Possible vascular role of increased plasma arginine vasopressin in congestive heart failure. Int J Cardiol. 2006;106:191–195. doi: 10.1016/j.ijcard.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Ohya S, Asakura K, Muraki K, Watanabe M, Imaizumi Y. Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2002;282:G277–G287. doi: 10.1152/ajpgi.00200.2001. [DOI] [PubMed] [Google Scholar]
- Ohya S, Sergeant GP, Greenwood IA, Horowitz B. Molecular variants of KCNQ channels expressed in murine portal vein myocytes: a role in delayed rectifier current. Circ Res. 2003;92:1016–1023. doi: 10.1161/01.RES.0000070880.20955.F4. [DOI] [PubMed] [Google Scholar]
- Passmore GM, Selyanko AA, Mistry M, Al-Qatari M, Marsh SJ, Matthews EA, Dickenson AH, Brown TA, Burbidge SA, Main M, et al. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci. 2003;23:7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA. Diverse mechanisms of antiepileptic drugs in the development pipeline. Epilepsy Res. 2006;69:273–294. doi: 10.1016/j.eplepsyres.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubart M, Patlak JB, Nelson MT. Ca2+ currents in cerebral artery smooth muscle cells of rat at physiological Ca2+ concentrations. J Gen Physiol. 1996;107:459–472. doi: 10.1085/jgp.107.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz E, Fernandez N, Monge L, Climent B, Dieguez G, Garcia-Villalon AL. Role of K+ channels in the coronary and renal vascular reactivity to vasopressin in diabetic rats. Eur J Pharmacol. 2003;471:35–40. doi: 10.1016/s0014-2999(03)01815-6. [DOI] [PubMed] [Google Scholar]
- Schwake M, Jentsch TJ, Friedrich T. A carboxy-terminal domain determines the subunit specificity of KCNQ K+ channel assembly. EMBO Rep. 2003;4:76–81. doi: 10.1038/sj.embor.embor715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Cui N, Shi Y, Zhang X, Yang Y, Jiang C. Arginine vasopressin inhibits Kir6.1/SUR2B channel and constricts the mesenteric artery via V1a receptor and protein kinase C. Am J Physiol Regul Integr Comp Physiol. 2007;293:R191–R199. doi: 10.1152/ajpregu.00047.2007. [DOI] [PubMed] [Google Scholar]
- Surti TS, Huang L, Jan YN, Jan LY, Cooper EC. Identification by mass spectrometry and functional characterization of two phosphorylation sites of KCNQ2/KCNQ3 channels. Proc Natl Acad Sci U S A. 2005;102:17828–17833. doi: 10.1073/pnas.0509122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surti TS, Jan LY. A potassium channel, the M-channel, as a therapeutic target. Curr Opin Investig Drugs. 2005;6:704–711. [PubMed] [Google Scholar]
- Treschan TA, Peters J. The vasopressin system: physiology and clinical strategies. Anesthesiology. 2006;105:599–612. doi: 10.1097/00000542-200609000-00026. [DOI] [PubMed] [Google Scholar]
- Wakatsuki T, Nakaya Y, Inoue I. Vasopressin modulates K+-channel activities of cultured smooth muscle cells from porcine coronary artery. Am J Physiol Heart Circ Physiol. 1992;263:H491–H496. doi: 10.1152/ajpheart.1992.263.2.H491. [DOI] [PubMed] [Google Scholar]
- Xu C, Lu Y, Tang G, Wang R. Expression of voltage-dependent K+ channel genes in mesenteric artery smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 1999;277:G1055–G1063. doi: 10.1152/ajpgi.1999.277.5.G1055. [DOI] [PubMed] [Google Scholar]
- Yeung SY, Greenwood IA. Electrophysiological and functional effects of the KCNQ channel blocker XE991 on murine portal vein smooth muscle cells. Br J Pharmacol. 2005;146:585–595. doi: 10.1038/sj.bjp.0706342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung SY, Pucovsky V, Moffatt JD, Saldanha L, Schwake M, Ohya S, Greenwood IA. Molecular expression and pharmacological identification of a role for K(v)7 channels in murine vascular reactivity. Br J Pharmacol. 2007;151:758–770. doi: 10.1038/sj.bjp.0707284. [DOI] [PMC free article] [PubMed] [Google Scholar]