Abstract
The thrombin receptor (PAR-1, Protease-Activated-Receptor-1) is over-expressed in highly metastatic melanoma cell lines and in patients with metastatic lesions. Activation of PAR-1 leads to cell signaling and upregulation of genes involved in adhesion, invasion and angiogenesis. Herein, we stably silence PAR-1 through the use of lentiviral shRNA and found significant decreases in both tumor growth (P<.01) and metastasis (P<.001) of highly metastatic melanoma cell lines in vivo. The use of viruses for therapy is not ideal as it can induce toxic immune responses and possible gene alterations following viral integration. Therefore, we also utilized systemic delivery of PAR-1 siRNA incorporated into neutral liposomes (DOPC) to decrease melanoma growth and metastasis in vivo. Significant decreases in tumor growth, weight and metastatic lung colonies (P<.001 for all) were found in mice treated with PAR-1-siRNA-DOPC. The in vivo effects of PAR-1 on invasion and angiogenesis were analyzed via immunohistochemistry. Concomitant decreases in VEGF, IL-8, and MMP-2 expression levels, as well as decreased blood-vessel density (CD31), were found in tumor samples from PAR-1 siRNA-treated mice, suggesting that PAR-1 is a regulator of melanoma cell growth and metastasis by affecting angiogenic and invasive factors. We propose that siRNA incorporated into DOPC nanoparticles could be delivered systemically and used as a new modality for melanoma treatment.
Keywords: Thrombin Receptor, Melanoma, Liposome, siRNA, shRNA
Introduction
The thrombin receptor is a 7-pass transmembrane G-protein coupled receptor. Unlike typical ligand-receptor interactions, thrombin does not activate PAR-1 upon N-terminus binding. Rather, it cleaves the N-terminus of PAR-1 at serine 42. Upon cleavage, the new amino terminal peptide acts as a tethered ligand that activates the receptor and initiates cellular signaling (1).
The pathway of cellular activation and induction of mitogenesis by thrombin involves increases in Ca2+ and activation of protein kinase C via the second messengers, inositol 1, 4,5-triphosphate and diacylglycerol (2). Activation of the PAR-1 can lead to cell signaling and upregulation of genes involved in adhesion (αIIb β3, αv5, αv β3 integrins) (3-5), invasion (MMP-2) (6), and angiogenesis (IL-8, VEGF) (7-10). This suggests that activation of PAR-1 may facilitate tumor invasion and metastasis through induction of cell adhesion molecules, matrix degrading proteases, and stimulating the secretion of angiogenic factors, thus contributing to the metastatic phenotype of melanoma.
PAR-1 can also be activated by ligands other than thrombin, such as factor Xa, granzyme A, trypsin and plasmin (11-13). Furthermore, studies suggest that PAR-1 in breast cancer cells can be activated by MMP-1 (14). PAR-1 was also described as a rate-limiting factor in thrombin-enhanced experimental pulmonary metastasis, attesting to the role of the thrombin receptor in melanoma metastasis (15). In addition to melanoma, over-expression of PAR-1 has been identified in various cancers, including breast (16, 17), lung (18), colon (19-21) and prostate (22, 23).
Our laboratory has previously demonstrated that PAR-1 is differentially expressed in melanoma cell lines with over-expression being found in highly metastatic cells compared to non-metastatic cell lines (22, 24). Over-expression of PAR-1 is predominantly seen in patients with malignant melanoma and in metastatic lesions compared to common melanocytic nevi and normal skin (25). Furthermore, our laboratory has found a significantly higher percentage of PAR-1 positive cells in metastatic melanoma than in both dysplastic nevi and primary melanoma (26). We also reported that the expression of PAR-1 in melanoma cells is regulated by the Activator Protein-2α (AP-2α) transcription factor and demonstrated an inverse correlation between the expression of AP-2α and PAR-1 in metastatic melanoma specimens (24, 26). Herein, we hypothesize that PAR-1 is a major contributor to the metastatic process of human melanoma. Therefore, we sought to silence PAR-1 in vivo by stably transfecting cells utilizing lentiviral-based shRNA as well as utilizing neutral liposomes to deliver PAR-1 siRNA systemically to decrease melanoma growth and metastasis.
Although lentiviral technology is a proven tool in the laboratory setting, the use of viruses for treatment has several adverse effects such as toxic immune responses and genetic alterations. Therefore, in the present study, we also utilized systemic delivery of liposomal incorporated siRNA, which is a much safer alternative to viral therapy for the treatment of melanoma.
Recently, siRNA incorporated into neutral 1,2 dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) liposomes has been utilized effectively in vivo (27-29). Evidence from these experiments suggests that there was higher tumor uptake of DOPC liposomes compared to cationic liposomes, and that neutral liposomes were less toxic. In fact, it is expected that liposomal packaging of siRNA will work best in solid tumors, such as melanoma, as there is increased vessel permeability and reduced lymphatic drainage, thereby allowing for increased accumulation of siRNA incorporated into liposomes (30). Here, we show for the first time that systemic treatment of melanoma-bearing mice with PAR-1 siRNA-DOPC resulted in inhibition of melanoma growth and metastasis.
Materials and Methods
Cell Lines and Culture Conditions
A375SM human melanoma cell line was established from pooled lung metastases produced by A375-P cells injected intravenously into nude mice (31). These cells were maintained in Eagle's minimal essential medium supplemented with 10% fetal bovine serum (FBS), as previously described (32). The C8161 is an aggressive amelanotic human melanoma cell line maintained in Dulbecco's modified minimal Eagle's medium and Ham's F-12 medium (DMEM-F12) supplemented with 5% FBS, as previously described (33). The 293FT cells (Invitrogen, Carlsbad, CA), utilized to make lentiviral shRNA, is a clonal isolate derived from transformed embryonal kidney cells containing the large T antigen for high-level expression of the packaging proteins, thereby resulting in higher viral titers. These cells were maintained in Dulbecco's modified minimal essential medium supplemented with 10% FBS, according to the manufacturers' instructions.
Transient Transfection of siRNA
PAR-1 siRNA purchased from Dharmacon (Lafayette, CO) was utilized to silence PAR-1 expression in melanoma cell lines (target sequence: AGAUUAGUCUCCAUCAAUA). Non-targeting siRNA (Qiagen, Santa Clarite, CA), with no sequence homology to any known human mRNA, was used as control (target sequence: UUCUCCGAACGUGUCACGU). A375SM cells were grown to 60% confluency in 6 well plates and transiently transfected with PAR-1 siRNA or non-targeting siRNA utilizing RNAifect Reagent (Qiagen, Santa Clarite, CA) according to the manufacturers' instructions. The cells were analyzed for PAR-1 expression 72 hours after transfection.
Western Blot Analysis
PAR-1 was detected in cell extracts by Western blot, as previously described (22). Briefly, proteins of total cell extracts (40 μg) were separated by SDS-polyacrylamide gel electrophoresis and transferred to Immobilon P transfer membrane (Millipore Corp, Bedford, MA). The membranes were washed in Tris-HCl-buffered saline (10 mM Tris-HCl, pH 8, containing 150 mM NaCl) and blocked with 5% nonfat milk in TBS for 1 hour at room temperature. The blots were then probed overnight in Tris-HCl-buffered saline with the monoclonal antibody for PAR-1 (WEDE15; Immunotech-Coulter, Miami, FL) at a dilution of 1:2000. α-actin antibody (Sigma, St. Louis, MO) was used as a loading control.
Semiquantitative Reverse Transcriptase-PCR
One microgram of total RNA was reverse-primed with an oligo (dT) primer and extended with Maloney murine leukemia virus reverse transcriptase (Clontech, Mountain View, CA). The PCR was performed, using the Clontech Advantage cDNA PCR kit (Mountain View, CA), in a 50μl reaction mixture containing 1X PCR buffer, 5 μl cDNA, 0.2 mM dNTP, and 2.5 units =Taq polymerase. For PAR-1 quantification, specific primers (5′-GCAGAGCCCGGGACAATGGGG-3′ and 5′-AGATGGCCAGACAAGTGAGG-3′) were used. GAPDH cDNAs were amplified by PCR in the same reaction mixture and carried out by an initial denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 63°C for 1 min, and extension at 72°C for 1 min. A final elongation step was carried out at 72°C for 10 min.
Lentiviral shRNA to PAR-1
Sense and antisense oligonucleotides from the PAR-1 siRNA target sequence and the NT sequence were designed with a hairpin and sticky ends (Cla1 and Mlu1) for use with the lentiviral system developed and kindly provided by Didier Trono (34). The oligonucleotides were annealed into the lentiviral gene transfer vector, pLVTHM, using the Cla1 and Mlu1 restriction enzyme sites. Competent E.coli bacteria were transformed with the annealed lentiviral vector and grown overnight. Several bacterial colonies were isolated and grown in Luria Broth overnight. DNA was isolated from the bacteria utilizing a Maxi plasmid DNA purification kit (Qiagen, Santa Clarite, CA). The DNA was sequenced to test for proper insertion and length of the inserts. Lentivirus was then produced by transfecting human embryonic kidney cells (293FT, Invitrogen, Carlsbad, CA) with the sequenced-verified PLVTHM vector containing the PAR-1 shRNA sequence, the packaging plasmid (MD2G), and envelope plasmid (PAX2) required for viral production. Three days later, the viral supernatant was collected and filtered to remove cellular debris. The highly metastatic and PAR-1 positive A375SM and C8161 cell lines plated at 70% confluency in 6 well plates were transduced with the virus. After 16 hours, the virus containing media was removed and replaced with normal growth media.
Fluorescence-Activated Cell Sorter (FACS) Analysis
These data were obtained in a BD FACS ARIA flow cytometer (BD Biosciences, San Jose, CA). A375SM and C8161 cells transduced with lentiviral constructs were washed with PBS and detached with 1% EDTA. Cells were centrifuged at 1000 rpm, the supernatant removed and cells were incubated on a rotating shaker with PAR-1 Ab (ATAP2, Santa Cruz Biotechnology, Santa Cruz, CA) at 1:50 dilution in PBS with 2% FBS (PBS-FBS) for 45 min at 4°C. Cells were washed with PBS-FBS, centrifuged and incubated on a rotating shaker with a PE conjugated secondary antibody (PE anti-mouse; Jackson ImmunoResearch, West Grove, PA) at a 1:100 dilution in PBS-FBS for 30 minutes at 4°C. Cells were washed three times and analyzed.
In vivo Experiments
Female athymic nude mice (NCr-nu) were purchased from the National Cancer Institute-Frederick Cancer Research and Development Center. All studies were approved and supervised by The University of Texas M.D. Anderson Cancer Center IACUC. For experiments utilizing stably transduced cells with PAR-1 or NT shRNA, 5 × 105 transduced cells were injected subcutaneously into the right flank of mice (n=10/group) to analyze tumor growth. Tumors were measured twice weekly in two dimensions with a caliper and mice were sacrificed after 6 weeks. Tumor volumes for all tumor growth experiments were calculated using the formula V= a × b2/2, as described previously (35), where a is the largest diameter and b is the smallest. To analyze for metastatic potential of lentiviral transduced cells, 1 × 106 PAR-1 or shRNA transduced cells were injected intravenously into the tail vein of mice (n=10/group). The mice were sacrificed after 6 weeks at which time the lungs were fixed in Bouin's solution and the metastatic colonies were counted as we previously described (36). To determine whether siRNA delivered by neutral liposomes could reach the subcutaneous tumors, a NT siRNA sequence was tagged with Alexa-555 and incorporated into DOPC liposomes as described previously (28). A375SM cells were implanted subcutaneously into mice. After the tumors reached a size of 5mm3, the mice were administered a single injection of 5μg of Alexa-555 siRNA incorporated in DOPC liposomes into the tail vein. Mice (n= 5 /time point) were sacrificed 2, 4 and 6 days after injection of siRNA. To determine the amount and frequency of injections of PAR-1 siRNA-DOPC to silence PAR-1 in vivo, mice were subcutaneously injected with A375SM cells and after the tumors grew to a size of 5mm3, a single administration of PAR-1 siRNA-DOPC (5μg or 10μg) or NT siRNA-DOPC (5μg or 10μg) was administered into the tail vein of nude mice. Five mice per day were then sacrificed at 2, 4, and 6 days after treatment for each treatment group. To determine the effects of liposomal delivered PAR-1 siRNA on tumor growth, 1 × 106 A375SM cells were injected subcutaneously into nude mice (n=10/group). After the tumors reached a size of 3-5mm3, mice were randomized into the two treatment groups and administered 10μg of PAR-1 siRNA-DOPC or NT siRNA-DOPC into the tail vein. Systemic administration of DOPC-incorporated siRNA was performed twice per week for 4 weeks. Mice were measured in two dimensions with a caliper twice per week. Mice were sacrificed after 4 weeks at which time subcutaneous tumors were harvested. To determine metastatic potential 1×106 A375SM cells were injected into the tail vein of nude mice (n=10/group). As with the tumor growth experiment, mice were randomized into 2 treatment groups and after 7 days, treatments began with 10μg of either NT or PAR-1 siRNA-DOPC. After 4 weeks of biweekly treatments, the lungs were fixed in Bouin's solution and metastatic colonies counted.
Real-time PCR
RNA (20ng/μl) from subcutaneous tumors was harvested using RNAqueous kit (Ambion, Austin, TX) according to manufacturers' instructions. The RNA was then made into cDNA using TaqMan reverse transcriptase reagents (Applied Biosystems, Foster City, CA). The primers and fluorescence probes were obtained from Applied Biosystems (PAR-1: HS00189258, Foster City, CA) specific for human PAR-1. Reaction components for reverse transcription PCR included TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA), 20x Assay Mix (Applied Biosystems, Foster City, CA), RNAse-free water, and the diluted cDNA (1:5). The amplifications were carried out in an Applied Biosystems 7700 Prism reverse transcription PCR device using the following temperature profile: denaturation at 95°C for 15 seconds, annealing and extension at 60°C for 1 minute for 40 cycles. Amplifications were run in triplicates and averages obtained after normalization with 18s rRNA (Applied Biosystems, Foster City, CA).
Immunohistochemistry and Immunofluorescence
Formalin-fixed, paraffin-embedded sections were deparaffinized by sequential washing with xylene, graded ethanol and PBS. Antigen retrieval was done by heating in steam cooker in 1X Target Retrieval Solution (Dako, Carpinteria, CA) for 30 minutes. After cooling and washing with PBS, endogenous peroxide was blocked with 3% hydrogen peroxidase inhibitor in PBS for 12 minutes. Non-specific proteins were blocked in 5% horse serum and 1% goat serum for 20 minutes. Slides were incubated with the following antibodies: VEGF (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), IL-8 (1:25, Biosource International), MMP-2 (1:400, Chemicon, Temecula, CA), overnight at 4°C followed by a peroxidase labeled anti-rabbit antibody (1:500, Jackson Immunoresearch, West Grove, PA) for 1 hour at room temperature. Signal was detected with 3,3V-diaminobenzidine (DAB; Phoenix Biotechnologies, San Antonio, TX) substrate for 6 minutes, and counterstained with Gill's no. 3 hematoxylin (Sigma, St. Louis, MO) for 20 seconds. IHC for PAR-1 (1:20, Immunotech Coulter, Miami, FL) also included an overnight blocking step with an anti-mouse IgG (1:10, Jackson Immunoresearch, West Grove, PA) prior to incubating with primary antibody overnight. For CD31 staining, frozen sections were fixed with cold acetone, followed by a 1:1 mixture of acetone:chloroform, again followed by cold acetone each for 5 minutes. Non-specific proteins were blocked in 5% horse serum and 1% goat serum for 20 minutes. Overnight incubation at 4°C with anti-mouse CD31 Ab (1:800, Pharmingen) was performed followed by a 1 hour incubation with peroxidase labeled anti-rat antibody (1:200, Jackson Immunoresearch, West Grove, PA) incubation at room temperature. Signal was detected with DAB substrate for 5 minutes, and counterstained with Gill's no. 3 hematoxylin for 20 seconds. For detection of scavenging macrophages, frozen slides were fixed and non-specific proteins blocked as per CD31 protocol. Overnight incubation with anti-mouse F4/80 (1:600, Serotec, Raleigh, NC) at 4°C (with no exposure to light as the slides had fluorescence from the Alexa-555 tagged siRNA) was followed by anti-rat Alexa 488 antibody (1:1000, Molecular Probes, Carlsbad, CA) for 1 hour at room temperature. After washing, Hoechst (1:10,000, Molecular Probes, Carlsbad, CA) was added for visualization of cell nuclei.
Statistics
Statistical analysis on animal studies was performed using the Mann-Whitney tests. All statistical tests were two-sided.
Results
Development of PAR-1 shRNA
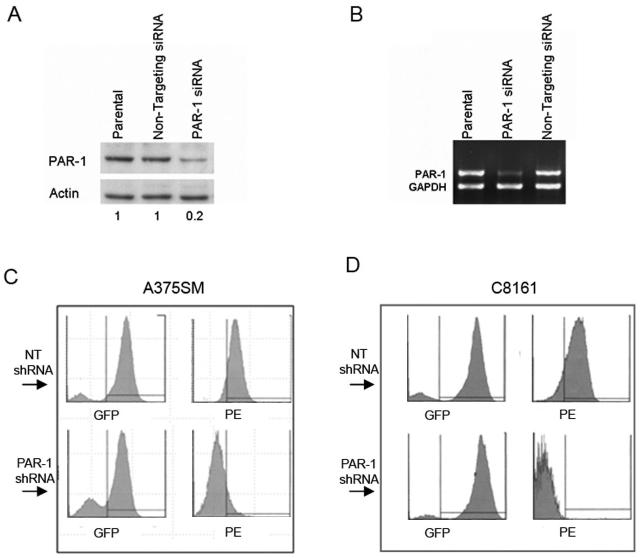
We have previously shown that metastatic melanoma cells express high levels of PAR-1 compared to non-metastatic melanoma cells (24). PAR-1 siRNA constructs targeting the human PAR-1 gene were purchased from Dharmacon (Lafayette, CO) and tested to determine effective gene silencing. A non-targeting (NT) construct with no homology to any known human gene was also utilized as a negative control (28). We observed downregulation of both mRNA and protein levels of PAR-1 by ∼80%, 72 hours after transfection of one of the PAR-1 siRNA constructs (Figure 1A and B).
Figure 1. Silencing of PAR-1 by siRNA and shRNA.
A) Western blot depicting silencing of PAR-1 in A375SM transfected with PAR-1 siRNA compared to parental and non-targeting control. α-actin is used as loading control. Approximately 80% PAR-1 downregulation was observed in PAR-1 siRNA transfected cells compared to NT siRNA as determined by densitometry (0.2 and 1, respectively). B) Semi-quantitative RT-PCR depicting a decrease of PAR-1 (by ∼90%) in A375SM. C) A375SM and D) C8161 cells were transduced with lentivirus containing either NT shRNA or PAR-1 shRNA. The lentiviral vectors have GFP expression and therefore transduced cells will have green fluorescence. Cells were incubated with a PAR-1 antibody and a secondary PE-tagged antibody (red fluorescence). Top panels depict cells transduced with NT shRNA showing GFP positive/PE positive (presence of PAR-1). Bottom panels depict cells transduced with PAR-1 shRNA showing GFP positive/PE negative (decreased PAR-1 expression)
After obtaining a functional PAR-1 siRNA construct, the targeting sequence was used to develop lentiviral shRNA to stably silence PAR-1 utilizing the system developed and kindly provided by Didier Trono (34). Fluorescence activated cell sorter (FACS) analysis was utilized after transduction of the two most highly metastatic and aggressive melanoma cell lines we have available (A375SM and C8161). Cells transduced with PAR-1 shRNA are GFP-positive and PE-negative (after using the PAR-1 antibody and a PE-tagged secondary antibody), while those transduced with a non-targeting sequence control are GFP positive and PE positive. In both A375SM (Figure 1C) and C8161 (Figure 1D) metastatic melanoma cells, there is almost complete silencing of PAR-1 after transduction with lentiviral PAR-1 shRNA. Next, we sought to determine tumorigenicity and metastatic potential of these cells with silenced PAR-1 expression in an in vivo setting.
Tumor growth and metastatic potential of PAR-1 shRNA transduced melanoma cells
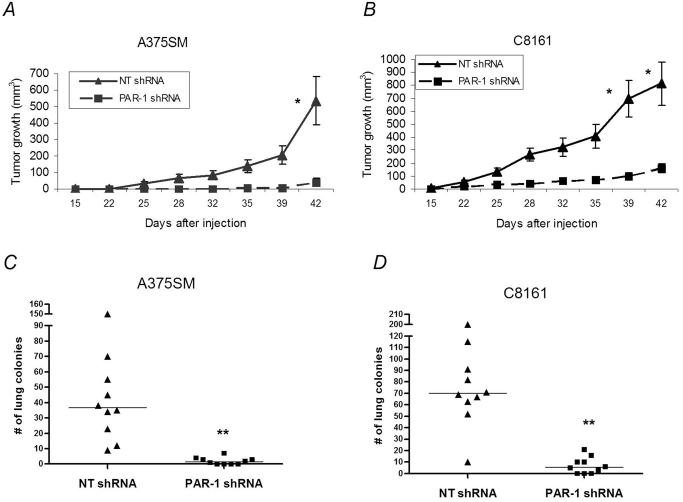
To test the effect of PAR-1 on tumor growth, stably transduced highly metastatic cells were injected subcutaneously into nude mice (n=10). Figure 2A shows significant inhibition of tumor growth in A375SM cells stably transduced with PAR-1 shRNA compared to NT shRNA transduced cells (41.1 mm3 and 534.3 mm3, respectively; P<0.01). This difference was also observed in C8161 cells transduced with PAR-1 shRNA as compared to NT shRNA (159.7 mm3 and 813.5 mm3, respectively; P< 0.01, Figure 2B). In order to ascertain that the changes seen in tumor growth were not due to differences in cell proliferation, thiazolyl blue (MTT) proliferation assays were performed on both cell lines transduced with PAR-1 or NT shRNA in vitro. No difference in proliferation rates was observed (data not shown).
Figure 2. Effects of PAR-1 shRNA on melanoma growth and metastasis.
A) In vivo tumor growth was determined on the PAR-1-positive highly metastatic melanoma cell lines, A375SM and (B) C8161 after stable silencing of PAR-1 with shRNA. Mice (n=10/group) were measured with a caliper in two dimensions for 6 weeks. Silencing of PAR-1 resulted in significant inhibition of tumor growth in both A375SM (PAR-1 shRNA- 41.1 mm3 and NT shRNA 534.3 mm3) and C8161 melanoma cells (PAR-1 shRNA- 159.7 mm3 and NT shRNA 813.5 mm3). * P<0.01 for both A375SM and C8161. C) A375SM melanoma cells stably transduced with NT lentiviral shRNA or PAR-1 shRNA were injected intravenously into nude mice (n=10/group). The mice were sacrificed after 6 weeks, the lungs were removed, fixed in Bouin's solution and the number of lung colonies was counted. Each symbol represents one mouse. There is a significant decrease in the number of lung metastases in PAR-1 shRNA transduced A375SM cells as compared to NT shRNA transduced cells (median 1 and 37, respectively. D) Decreased metastases also occurred in PAR-1 shRNA transduced C8161 cells compared to NT shRNA transduced cells (median 6 and 70 respectively). ** P<0.001 for both A375SM and C8161.
We sought to determine if silencing of PAR-1 would also limit experimental lung metastasis of these transduced cell lines. Nude mice (n=10) were intravenously injected with A375SM or C8161 cells transduced with either PAR-1 or NT shRNA. As with tumor growth, there was also significant inhibition of lung metastasis in both A375SM (Figure 2C) (median: NT shRNA - 37, PAR-1 shRNA - 1; P<0.001) and C8161 (Figure 2D) (median: NT shRNA – 70, PAR-1 siRNA – 6; P<0.001) 6 weeks after the challenge. Taken together, we herein present data for the first time that silencing of PAR-1 utilizing shRNA results in inhibition of tumor growth and metastatic potential of human melanoma cells, thereby strengthening the hypothesis that PAR-1 is a major contributor to the acquisition of the metastatic phenotype of human melanoma.
Systemic delivery of PAR-1 siRNA utilizing neutral liposomes
Next, we determined whether delivery of PAR-1 siRNA was feasible with the use of neutral liposomes in our experimental melanoma mouse model. As a proof of principle, we chose the most metastatic melanoma cell line, A375SM, to continue our in vivo therapeutic experiments.
Utilizing liposomes to deliver therapeutic agents is safer than using viruses. Recent evidence demonstrates the usefulness of neutral liposomes in delivering siRNA to intraperitoneal tumors in vivo in various cancers, such as ovarian, colorectal carcinomas and lymphomas (27-29, 37, 38). By utilizing this liposomal method to package PAR-1 specific siRNAs, we sought to achieve similar results in human melanoma. However, it has not been previously established if intravenous delivery of DOPC liposomes is able to effectively reach subcutaneous tumors.
Therefore, we sought first to determine if intravenous injection of siRNA tagged with Alexa-555 incorporated in DOPC liposomes could reach subcutaneous tumors. Results indicate that DOPC-siRNAs were reaching the subcutaneous tumors at Day 2 (Figure 3A). Even at Day 6 after a single intravenous injection, there were siRNA particles (red) in a perinuclear orientation within the subcutaneous tumor cells (blue Hoechst nuclear staining) almost imperceptible to scavenging macrophages (green) (Figure 3A) as observed previously in studies with intraperitoneal delivery of DOPC-siRNA (28). We concluded that siRNAs will be able to reach subcutaneous tumors effectively by systemic administration of DOPC incorporated siRNA.
Figure 3. Localization of siRNA-DOPC particles in subcutaneous tumors after single i.v. injection.
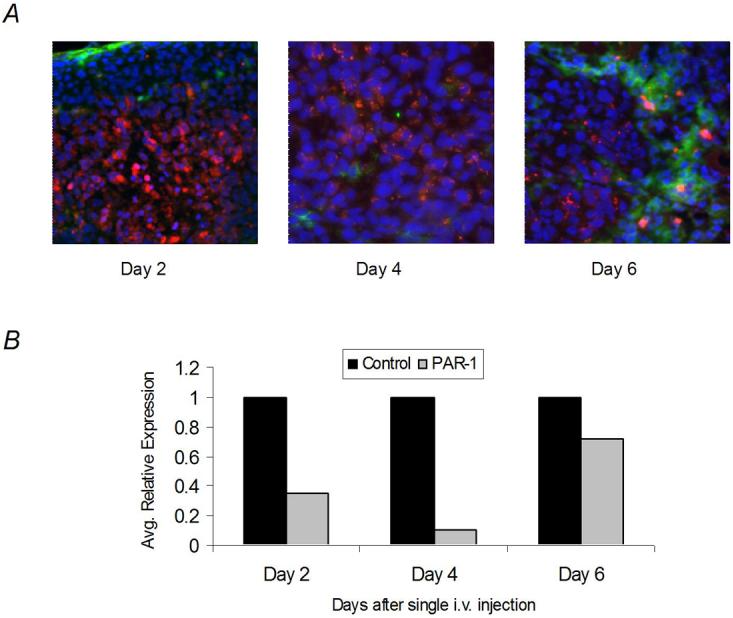
A) NT siRNA tagged with Alexa-555 (red) incorporated into DOPC liposomes were administered at Day 0 to melanoma-bearing nude mice (5mm3) (n=5/day). Mice were sacrificed on Days 2, 4, and 6 after single intravenous. administration. The siRNA was found within the tumor cells at all time-points. Even at Day 6 after intravenous injection, there were siRNA (red) particles within the subcutaneous tumor (blue Hoechst nuclear staining) in a perinuclear orientation almost imperceptible to scavenging macrophages (green). B) In vivo silencing of PAR-1 after single intravenous injection of PAR-1 siRNA-DOPC as assessed by real-time PCR. Data depict optimal decrease of PAR-1 expression in subcutaneous melanoma tumors 4 days after single intravenous injection of PAR-1 siRNA-DOPC (10μg) compared to NT siRNA-DOPC treatment. All tumors (n=5/group) were run in triplicates and averages obtained after normalization with 18s rRNA. Significant downregulation of PAR-1 was observed 4 days but not 6 days after injection.
Next, a kinetics experiment was performed to determine the amount of siRNA incorporated into DOPC-liposomes that needs to be delivered for effective silencing of PAR-1 in vivo. Based on this kinetics experiments, we found that downregulation of PAR-1, as measured by real-time PCR, peaked 4 days after liposomal injection, but recovered by Day 6 (Figure 3B). Therefore, we concluded that the PAR-1 siRNA-DOPC should be administrated twice weekly at a dosage of 10μg per treatment.
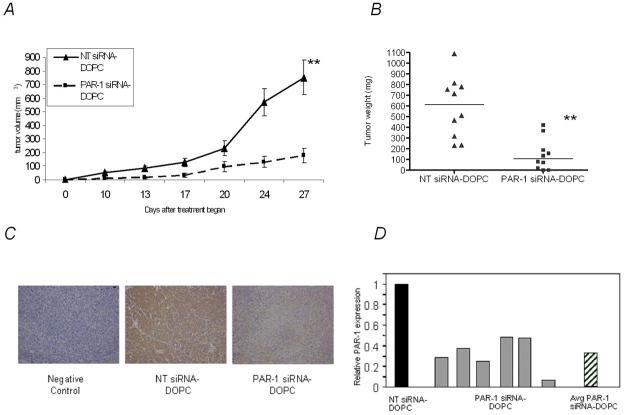
Then, we performed the first in vivo experiments using PAR-1 siRNA-DOPC to inhibit both tumor growth and metastasis of A375SM melanoma cells in nude mice. Mice (n=10) injected subcutaneously with A375SM cells were given twice weekly intravenous injections of 10μg of PAR-1 siRNA-DOPC or NT siRNA-DOPC after tumors grew to a size of 3-5 mm3. As can be seen in Figure 4, there is significant inhibition of both tumor growth (NT siRNA-DOPC – 749.9 mm3, PAR-1 siRNA-DOPC – 176.2 mm3; P<0.001) (Figure 4A) and tumor weight (median NT siRNA-DOPC – 614 mg, PAR-1 siRNA-DOPC – 106.5 mg; P<0.001) (Figure 4B). These tumors were analyzed for expression of PAR-1 via immunohistochemistry (IHC) and quantitative real-time PCR and found that indeed, after treatment with liposomal-incorporated PAR-1 siRNA, there was a decrease in PAR-1 expression at the end of treatment (Day 27) (Figure 4C and D). These results show that in vivo PAR-1 expression was reduced in PAR-1 siRNA-DOPC treated tumors 4 weeks after twice weekly intravenous treatments.
Figure 4. Effects of PAR-1 silencing after twice weekly systemic administration of PAR-1 siRNA-DOPC.
A) Nude mice (n=10/group) were subcutaneously injected with 5×105 A375SM cells. After tumors grew to a size of 3-5mm3, mice were then intravenously treated with 10μg of liposomal incorporated siRNA twice per week for 4 weeks. There is a significant decrease in tumor growth in mice treated with PAR-1 siRNA-DOPC compared to mice treated with NT siRNA-DOPC (749.9 mm3 compared to176.2 mm3, respectively). ** P<0.001 B) Individual subcutaneous tumor weights were measured after 4 weeks of twice weekly intravenous treatment of 10μg of liposomal incorporated siRNA. There is significant inhibition of tumor weight between NT siRNA-treated mice (median 614 mg) and PAR-1 siRNA-treated mice (median 107 mg). ** P<0.001. C) Representative slides from immunohistochemistry analysis for PAR-1 in tumors from mice treated with liposomal PAR-1 and NT siRNA. PAR-1 silencing was observed at the end of treatment by Day 27. As a negative control, the tumors were incubated without primary antibody. D) Real-time PCR for PAR-1 expression of individual tumors from PAR-1 siRNA-DOPC-treated mice compared to the average expression of NT siRNA-DOPC-treated mice (n=10). All tumors were run in triplicates and averages obtained after normalization with 18s rRNA. Striped bar depicts the average expression of PAR-1 from PAR-1 siRNA-DOPC-treated tumors and reveals a significant reduction in expression in vivo by Day 27. Four of the tumors from the PAR-1 siRNA-DOPC-treated mice were too small to obtain sufficient RNA and were not included for this analysis.
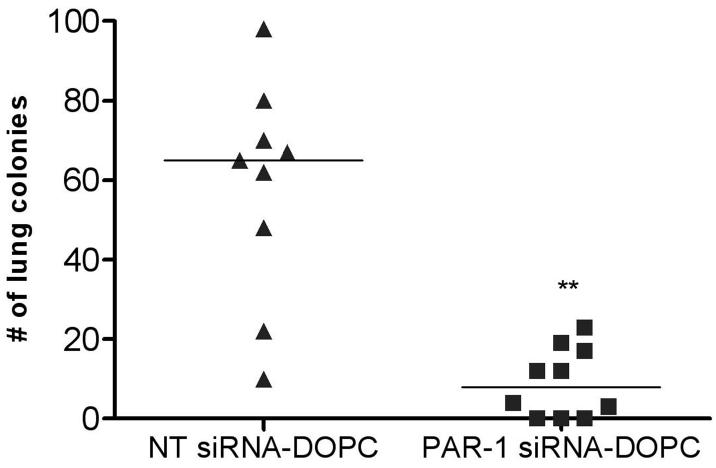
Not only was there a significant inhibition of tumor growth with siRNA delivered via neutral liposomes, but also there was a robust and significant inhibition in the ability of these cells to form metastatic lung colonies. Figure 5 shows the effects of PAR-1 siRNA treatment on lung metastasis, as mice treated with NT siRNA had a median of 62 lung colonies, while PAR-1 siRNA-treated mice had a median of only 12 lung colonies (P<0.001). There were no obvious toxicities observed as assessed by mobility and feeding behavior in any of the mice utilized for systemic liposomal delivery of PAR-1 siRNA. Taken together, we now present data that administration of PAR-1 siRNA delivered systemically via neutral liposomes is feasible for in vivo treatment of melanoma.
Figure 5. Inhibition of lung metastases by melanoma cells after twice weekly systemic administration of PAR-1 siRNA-DOPC.
Nude mice were intravenously injected with 1×106 A375SM. Mice were subsequently treated intravenously with 10μg of either liposomal incorporated PAR-1 siRNA or NT siRNA twice weekly for 5 weeks. Lungs were harvested, fixed in Bouin's solution and individual tumor nodules counted. There is a significant decrease in the number of lung colonies in PAR-1-siRNA treated mice (median 12) compared to those treated with NT siRNA (median 62). One mouse died in the NT group two weeks after treatment began. ** P<0.001.
Effects of PAR-1 siRNA-DOPC therapy
To further analyze the effects of PAR-1 silencing on melanoma growth and metastasis, we performed IHC analysis on tumor samples to determine the expression levels of several invasive and angiogenic factors (MMP-2, IL-8, VEGF) known to be upregulated with the activation of PAR-1 (6, 7, 9). There is decrease expression of MMP-2, IL-8, and VEGF after treatment with PAR-1 siRNA-DOPC compared to NT treated mice (Figure 6A) attesting to the important role PAR-1 plays in the progression of melanoma. Since these factors are major players in angiogenesis, we wanted to determine if there was an overall decrease in angiogenesis with PAR-1 siRNA-DOPC treatment. Indeed, there is an overall decrease in blood vessels (CD31) in PAR-1 siRNA-treated mice (Figure 6A) allowing us to conclude that PAR-1 siRNA affects tumor angiogenesis.
Figure 6. Effects of PAR-1 silencing on angiogenic and invasive factors.
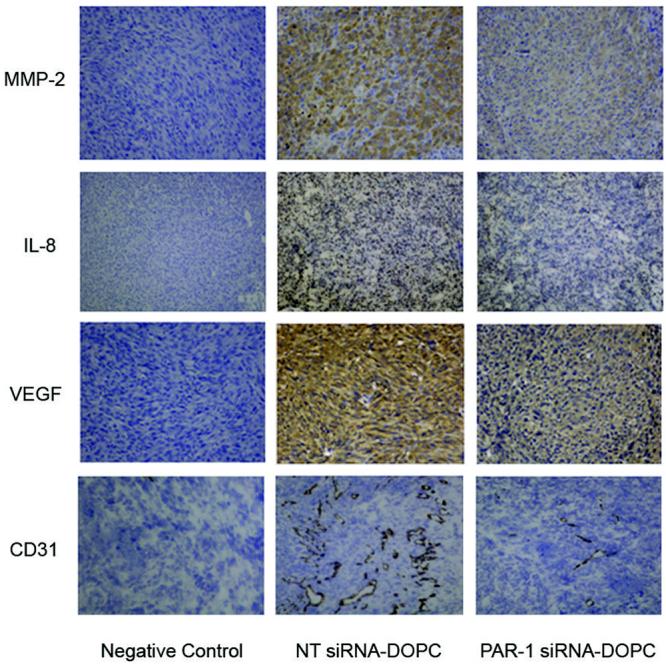
IHC analysis was performed on tumors from systemically treated mice with PAR-1 siRNA-DOPC or NT siRNA-DOPC. Representative images demonstrate that silencing of PAR-1 affects angiogenic (IL-8, VEGF) and invasive (MMP-2) factors. There is also a reduction in the number of blood vessels (CD31; microvessel density) in tumors treated with PAR-1 siRNA. As a negative control, the tumor samples were incubated without primary antibody. All images are at 10X magnification.
Our results show that silencing of PAR-1 in vivo by shRNA or by systemic treatment of liposomal incorporated PAR-1 siRNA, reduces melanoma growth and metastasis. We further show that silencing of PAR-1 expression reduces angiogenesis (IL-8 and VEGF) and invasive (MMPs) factors involved in tumor progression.
Discussion
We have previously reported that the loss of transcription factor AP-2α leads to the progression of metastatic melanoma (39-41). Furthermore, there exists an inverse correlation between AP-2α and PAR-1 with an increase in PAR-1 expression in highly metastatic melanoma cells and in melanoma patient samples (24, 26). Thrombin is a potent inducer of angiogenesis via PAR-1 activation. PAR-1 is a regulator of several genes and molecules involved in tumor growth and metastatic progression such as VEGF, IL-8 and MMPs (6-10). Thus, PAR-1 is critical in the transition from radial growth phase to the vertical growth phase of human melanoma. In this study, we sought to determine whether PAR-1 silencing could reduce melanoma growth and metastasis via shRNA and via systemic lipsomal delivery of PAR-1 siRNA.
PAR-1 was stably silenced utilizing a lentiviral construct that allowed for the use of transduced cells in long-term in vivo experiments to determine the effects of PAR-1 in melanoma. After injecting PAR-1 shRNA transduced melanoma cells, both subcutaneously and intravenously, we determined that PAR-1 expression in highly metastatic melanoma leads to increased experimental tumor growth and lung metastasis. Transduction of cells with PAR-1 shRNA was able to reduce these effects in vivo.
After determining that PAR-1 contributes to the acquisition of the metastatic phenotype we utilized a liposomal system that allowed delivery of siRNA as a possible therapeutic modality for melanoma treatment. Lentiviral technology works well in the laboratory setting for stable silencing of genes. The use of viruses in clinical applications, however, is hindered by their induction of toxic immune responses and the possibility of gene expression changes following random integration into the host genome (42). Therefore, siRNA has the potential to become a safer and effective therapeutic alternative to viral therapy. However, the major limitations of siRNA systemic administration include degradation, poor bioavailability and intracellular delivery. In fact, one of the main challenges that scientists have come upon in employing siRNAs for human therapeutics has been the development of suitable delivery agents (28, 43, 44). Potential carriers, such as cationic liposomes, have numerous limitations, such as toxicity, rapid elimination from blood, entrapment by the reticulo-endothelial system, association with negatively charged serum proteins, colloidal instability, and failure to release incorporated materials (43). Furthermore, macrophages seem to preferentially take up charged liposomes (28, 45). Although addition of ligands to the cationic liposomes or inclusion of packaging elements within these liposomes have improved delivery and more specific targeting of the siRNA, undesired immune reactions have been reported (44).
In the present study, we utilized a more effective and less toxic neutral liposome that has previously been utilized effectively to deliver siRNA for the treatment of lymphoma and ovarian cancer as well as colorectal carcinoma ((27-29). Liposomes, the first nanotechnology to benefit cancer patients, are continuing to evolve as tools for delivering potentially useful therapies to tumors. Therefore, we wanted to determine whether systemic delivery of PAR-1 siRNA incorporated into DOPC liposomes was feasible and whether this treatment could successfully affect melanoama growth and metastasis.
Our first challenge was to determine if systemic delivery of these neutral liposomes would reach the subcutaneous tumors as direct intra-tumor injections would cause an acute inflammatory response and alter the tumor microenvironment thereby affecting the results. Furthermore, the use of DOPC liposomes had only been utilized effectively in vivo for intraperitoneal tumors administered into the peritoneal cavity. After intravenously injecting a single dose of DOPC-incorporated Alexa-555-tagged siRNA, we determined that the siRNA was found within the subcutaneous tumor cells almost imperceptible to scavenging macrophages. This demonstrates that delivery of siRNA would not be sequestered by macrophages and that sufficient levels of siRNA could reach the target tissues. Previously, Landen et al. utilized these DOPC liposomes to treat ovarian cancer (28). They observed that the siRNA delivery in DOPC was not restricted to the vasculature and was efficiently delivered deep into the tumor parenchyma. Furthermore, they found that delivery of siRNA with DOPC liposomes did reach other organs such as liver, lungs and kidneys. However, they saw robust silencing of their target gene in intraperitoneal tumors. These observations as well as our preliminary studies allowed us to conclude that using these neutral liposomes systemically to deliver siRNA was feasible.
We determined that twice weekly injections of 10μg of siRNA would be sufficient to silence PAR-1 in vivo. Indeed, the results of this experiment were dramatic as decreases in both melanoma growth and tumor weight was seen in mice treated with PAR-1 siRNA-DOPC compared to the NT control. These tumors were harvested and assayed for PAR-1 expression via IHC and quantitative real-time PCR. Both assays showed a significant decrease in PAR-1 expression following treatment, which demonstrates that the liposomal delivered PAR-1 siRNA reached its target and effectively decreased the expression levels of PAR-1 in vivo. Furthermore, experimental lung metastasis was also significantly decreased in PAR-1 siRNA-DOPC-treated mice, again attesting to the functionality of delivering siRNA in vivo with these neutral liposomes.
The strong link between PAR-1 activation and angiogenesis led us to investigate whether silencing of PAR-1 would affect factors involved in angiogenesis, such as IL-8 and VEGF as well as factors involved in invasion (MMPs). It was likely that the effects seen in vivo after PAR-1 silencing could be explained by decreases in angiogenesis and invasion, which minimized tumor growth and metastasis. Thus, we analyzed the tumors obtained from our in vivo studies by IHC for MMP-2, IL-8, and VEGF expression and observed a decrease in all of these angiogenic factors in PAR-1 siRNA-DOPC-treated mice. Moreover, staining with CD31 clearly demonstrated a reduction in the number of blood vessels within the tumors, and thus, inhibition of angiogenesis in the PAR-1 siRNA-DOPC-treated mice.
Taken together, we show that PAR-1 is a regulator of melanoma cell growth and metastasis. Furthermore, we demonstrate that delivery of PAR-1 siRNA via neutral liposomes in vivo is feasible and were able to reduce melanoma growth and metastasis by decreasing the expression of genes involved in angiogenesis and invasion. This further establishes the link between PAR-1 and the progression of melanoma. Our future experiments will focus on determining novel downstream genes affected by PAR-1 silencing in human melanoma. These might lead to genes that could be further targeted by siRNA-DOPC therapies.
Acknowledgments
This work was supported by NIH RO1 grant CA76098 (MBE). Work was also supported in part by a Program Project Development Grant from the Ovarian Cancer Research Fund, Inc. (AKS). We would like to thank Didier Trono for kindly providing the lentiviral backbone vectors used to incorporate PAR-1 shRNA, and Donna Reynolds for her expertise in immunohistochemical techniques.
References
- 1.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–68. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 2.Vouret-Craviari V, Van Obberghen-Schilling E, Rasmussen UB, Pavirani A, Lecocq JP, Pouyssegur J. Synthetic alpha-thrombin receptor peptides activate G protein-coupled signaling pathways but are unable to induce mitogenesis. Mol Biol Cell. 1992;3:95–102. doi: 10.1091/mbc.3.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Even-Ram SC, Maoz M, Pokroy E, et al. Tumor cell invasion is promoted by activation of protease activated receptor-1 in cooperation with the alpha vbeta 5 integrin. J Biol Chem. 2001;276:10952–62. doi: 10.1074/jbc.M007027200. [DOI] [PubMed] [Google Scholar]
- 4.Wojtukiewicz MZ, Tang DG, Nelson KK, Walz DA, Diglio CA, Honn KV. Thrombin enhances tumor cell adhesive and metastatic properties via increased alpha IIb beta 3 expression on the cell surface. Thromb Res. 1992;68:233–45. doi: 10.1016/0049-3848(92)90081-k. [DOI] [PubMed] [Google Scholar]
- 5.Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- 6.Zucker S, Conner C, DiMassmo BI, et al. Thrombin induces the activation of progelatinase A in vascular endothelial cells. Physiologic regulation of angiogenesis. J Biol Chem. 1995;270:23730–8. doi: 10.1074/jbc.270.40.23730. [DOI] [PubMed] [Google Scholar]
- 7.Ueno A, Murakami K, Yamanouchi K, Watanabe M, Kondo T. Thrombin stimulates production of interleukin-8 in human umbilical vein endothelial cells. Immunology. 1996;88:76–81. doi: 10.1046/j.1365-2567.1996.d01-635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu S, Gabazza EC, Hayashi T, Ido M, Adachi Y, Suzuki K. Thrombin stimulates the expression of PDGF in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L503–10. doi: 10.1152/ajplung.2000.279.3.L503. [DOI] [PubMed] [Google Scholar]
- 9.Huang YQ, Li JJ, Hu L, Lee M, Karpatkin S. Thrombin induces increased expression and secretion of VEGF from human FS4 fibroblasts, DU145 prostate cells and CHRF megakaryocytes. Thromb Haemost. 2001;86:1094–8. [PubMed] [Google Scholar]
- 10.Cucina A, Borrelli V, Di Carlo A, et al. Thrombin induces production of growth factors from aortic smooth muscle cells. J Surg Res. 1999;82:61–6. doi: 10.1006/jsre.1998.5514. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien PJ, Molino M, Kahn M, Brass LF. Protease activated receptors: theme and variations. Oncogene. 2001;20:1570–81. doi: 10.1038/sj.onc.1204194. [DOI] [PubMed] [Google Scholar]
- 12.Ruf W, Mueller BM. Thrombin generation and the pathogenesis of cancer. Semin Thromb Hemost. 2006;32(Suppl 1):61–8. doi: 10.1055/s-2006-939555. [DOI] [PubMed] [Google Scholar]
- 13.Hansen KK, Saifeddine M, Hollenberg MD. Tethered ligand-derived peptides of proteinase-activated receptor 3 (PAR3) activate PAR1 and PAR2 in Jurkat T cells. Immunology. 2004;112:183–90. doi: 10.1111/j.1365-2567.2004.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–13. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Nierodzik ML, Chen K, Takeshita K, et al. Protease-activated receptor 1 (PAR-1) is required and rate-limiting for thrombin-enhanced experimental pulmonary metastasis. Blood. 1998;92:3694–700. [PubMed] [Google Scholar]
- 16.Even-Ram S, Uziely B, Cohen P, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4:909–14. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- 17.Henrikson KP, Salazar SL, Fenton JW, Pentecost BT. Role of thrombin receptor in breast cancer invasiveness. Br J Cancer. 1999;79:401–6. doi: 10.1038/sj.bjc.6690063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin E, Fujiwara M, Pan X, et al. Protease-activated receptor (PAR)-1 and PAR-2 participate in the cell growth of alveolar capillary endothelium in primary lung adenocarcinomas. Cancer. 2003;97:703–13. doi: 10.1002/cncr.11087. [DOI] [PubMed] [Google Scholar]
- 19.Heider I, Schulze B, Oswald E, Henklein P, Scheele J, Kaufmann R. PAR1-type thrombin receptor stimulates migration and matrix adhesion of human colon carcinoma cells by a PKCepsilon-dependent mechanism. Oncol Res. 2004;14:475–82. doi: 10.3727/0965040042380496. [DOI] [PubMed] [Google Scholar]
- 20.Rudroff C, Schafberg H, Nowak G, Weinel R, Scheele J, Kaufmann R. Characterization of functional thrombin receptors in human pancreatic tumor cells (MIA PACA-2) Pancreas. 1998;16:189–94. doi: 10.1097/00006676-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Wojtukiewicz MZ, Tang DG, Ben-Josef E, Renaud C, Walz DA, Honn KV. Solid tumor cells express functional “tethered ligand” thrombin receptor. Cancer Res. 1995;55:698–704. [PubMed] [Google Scholar]
- 22.Tellez C, Bar-Eli M. Role and regulation of the thrombin receptor (PAR-1) in human melanoma. Oncogene. 2003;22:3130–7. doi: 10.1038/sj.onc.1206453. [DOI] [PubMed] [Google Scholar]
- 23.Kaushal V, Kohli M, Dennis RA, Siegel ER, Chiles WW, Mukunyadzi P. Thrombin receptor expression is upregulated in prostate cancer. Prostate. 2006;66:273–82. doi: 10.1002/pros.20326. [DOI] [PubMed] [Google Scholar]
- 24.Tellez C, McCarty M, Ruiz M, Bar-Eli M. Loss of activator protein-2alpha results in overexpression of protease-activated receptor-1 and correlates with the malignant phenotype of human melanoma. J Biol Chem. 2003;278:46632–42. doi: 10.1074/jbc.M309159200. [DOI] [PubMed] [Google Scholar]
- 25.Massi D, Naldini A, Ardinghi C, et al. Expression of protease-activated receptors 1 and 2 in melanocytic nevi and malignant melanoma. Hum Pathol. 2005;36:676–85. doi: 10.1016/j.humpath.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Tellez CS, Davis DW, Prieto VG, et al. Quantitative analysis of melanocytic tissue array reveals inverse correlation between activator protein-2alpha and protease-activated receptor-1 expression during melanoma progression. J Invest Dermatol. 2007;127:387–93. doi: 10.1038/sj.jid.5700539. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez-Puente Y, Tari AM, Stephens C, Rosenblum M, Guerra RT, Lopez-Berestein G. Safety, pharmacokinetics, and tissue distribution of liposomal P-ethoxy antisense oligonucleotides targeted to Bcl-2. J Pharmacol Exp Ther. 1999;291:865–9. [PubMed] [Google Scholar]
- 28.Landen CN, Jr., Chavez-Reyes A, Bucana C, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–8. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 29.Gray MJ, Van Buren G, Dallas NA, et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J Natl Cancer Inst. 2008;100:109–20. doi: 10.1093/jnci/djm279. [DOI] [PubMed] [Google Scholar]
- 30.Fang J, Sawa T, Maeda H. Factors and mechanism of “EPR” effect and the enhanced antitumor effects of macromolecular drugs including SMANCS. Adv Exp Med Biol. 2003;519:29–49. doi: 10.1007/0-306-47932-X_2. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Price JE, Fan D, Zhang RD, Bucana CD, Fidler IJ. Correlation of growth capacity of human tumor cells in hard agarose with their in vivo proliferative capacity at specific metastatic sites. J Natl Cancer Inst. 1989;81:1406–12. doi: 10.1093/jnci/81.18.1406. [DOI] [PubMed] [Google Scholar]
- 32.Huang S, Jean D, Luca M, Tainsky MA, Bar-Eli M. Loss of AP-2 results in downregulation of c-KIT and enhancement of melanoma tumorigenicity and metastasis. Embo J. 1998;17:4358–69. doi: 10.1093/emboj/17.15.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welch DR, Bisi JE, Miller BE, et al. Characterization of a highly invasive and spontaneously metastatic human malignant melanoma cell line. Int J Cancer. 1991;47:227–37. doi: 10.1002/ijc.2910470211. [DOI] [PubMed] [Google Scholar]
- 34.Wiznerowicz M, Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol. 2003;77:8957–61. doi: 10.1128/JVI.77.16.8957-8961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlsson G, Gullberg B, Hafstrom L. Estimation of liver tumor volume using different formulas - an experimental study in rats. J Cancer Res Clin Oncol. 1983;105:20–3. doi: 10.1007/BF00391826. [DOI] [PubMed] [Google Scholar]
- 36.Luca M, Hunt B, Bucana CD, Johnson JP, Fidler IJ, Bar-Eli M. Direct correlation between MUC18 expression and metastatic potential of human melanoma cells. Melanoma Res. 1993;3:35–41. doi: 10.1097/00008390-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Landen CN, Merritt WM, Mangala LS, et al. Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol Ther. 2006;5:1708–13. doi: 10.4161/cbt.5.12.3468. [DOI] [PubMed] [Google Scholar]
- 38.Halder J, Kamat AA, Landen CN, Jr., et al. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2006;12:4916–24. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bar-Eli M. Molecular mechanisms of melanoma metastasis. J Cell Physiol. 1997;173:275–8. doi: 10.1002/(SICI)1097-4652(199711)173:2<275::AID-JCP35>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 40.Bar-Eli M. Role of AP-2 in tumor growth and metastasis of human melanoma. Cancer Metastasis Rev. 1999;18:377–85. doi: 10.1023/a:1006377309524. [DOI] [PubMed] [Google Scholar]
- 41.Gershenwald JE, Sumner W, Calderone T, Wang Z, Huang S, Bar-Eli M. Dominant-negative transcription factor AP-2 augments SB-2 melanoma tumor growth in vivo. Oncogene. 2001;20:3363–75. doi: 10.1038/sj.onc.1204450. [DOI] [PubMed] [Google Scholar]
- 42.Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–32. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sioud M. On the delivery of small interfering RNAs into mammalian cells. Expert Opin Drug Deliv. 2005;2:639–51. doi: 10.1517/17425247.2.4.639. [DOI] [PubMed] [Google Scholar]
- 44.Szoka F. Molecular biology. The art of assembly. Science. 2008;319:578–9. doi: 10.1126/science.1154253. [DOI] [PubMed] [Google Scholar]
- 45.Miller CR, Bondurant B, McLean SD, McGovern KA, O'Brien DF. Liposome-cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry. 1998;37:12875–83. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]