Abstract
FLT3 is a receptor tyrosine kinase with important roles in hematopoietic stem/progenitor cell survival and proliferation. It is frequently overexpressed in acute leukemias and is frequently mutated in acute myeloid leukemia (AML). FLT3 internal tandem duplication (ITD) mutations in AML portend poor prognosis in both adult and pediatric patients. A number of small molecule tyrosine kinase inhibitors (TKIs) with activity against FLT3 have been discovered. Many of these are still in preclinical development, but several have entered clinical phase 1 and 2 trials as monotherapy in patients with relapsed AML. These trials have resulted in frequent but short-lived responses of peripheral blasts and less frequent responses of bone marrow blasts. This led to clinical testing of FLT3 TKIs in combination with conventional chemotherapy. Several combination trials are ongoing or planned in both relapsed and newly diagnosed FLT3-mutant AML patients. Anti-FLT3 antibodies may also prove to be an excellent way of targeting FLT3 in AML and acute lymphocytic leukemia (ALL) by inhibiting signaling and through antibody-dependent cell-mediated cytotoxicity.
Introduction
The human homologue of the murine fetal liver tyrosine kinase (FLT) gene was cloned by the Small laboratory at Johns Hopkins more than 15 years ago.1 Its product, FLT3, is a single transmembrane receptor with 5 immunoglobulin-like folds. The extracellular domain binds its growth factor, known as FLT3 ligand or FL. A single domain traverses the membrane, and then a kinase domain is split by the kinase insert. The kinase domain belongs to the type III receptor tyrosine kinase family, which includes KIT, FMS, and 2 genes for the platelet-derived growth factor receptors. Its ligand stimulates the proliferation of hematopoietic stem progenitor and dendritic cells. Studies have shown that FLT3 is highly expressed in most acute leukemias.2,3 In acute myeloid leukemia (AML) and acute lymphocytic leukemia (ALL), FLT3 is expressed at very high levels. FLT3 is also expressed in chronic myeloid leukemia (CML) in blast crisis but not in chronic phase. Overall, FLT3 is expressed in approximately 98% of pre-B ALL patients and in about 90% of AML patients.
The discovery of internal tandem duplication mutations (ITDs) in FLT3 was a major breakthrough in the understanding of FLT3’s important role in myeloid transformation.4 FLT3/ITD mutations are the most common type of FLT3 mutation in AML, and FLT3 mutations are the most frequent mutations in AML.5 The coding frame stays intact, so the protein is not truncated but gains new properties. These mutations constitutively activate the kinase activity of FLT3, analogous to a BCR/ABL fusion, which constitutively activates ABL kinase activity.
FLT3 in AML
Between 15% and 34% of AML patients show FLT3/ITD mutations, with the lower frequency in children and higher frequency in older adults. All of these mutations map to the negative regulatory juxtamembrane (JM) domain. The mutations change the amino acid sequence, which subsequently interrupts inhibition and constitutively activates the region. In addition, 8% to12% of AML patients have other types of FLT3 mutations that map to the activation loop, most frequently involving aspartic acid 835 or the immediately adjacent isoleucine 836.6-8 Both adult and pediatric AML patients with FLT3/ITD mutations have very poor prognosis.9,10 For example, in one study the cure rate with chemotherapy for pediatric patients without a FLT3/ITD mutation was 44% compared to 7% for those with a mutation.9 Overall cure rates are between 10% and 20% in AML patients with a FLT3/ITD mutation.11 Patients with a high FLT3/ITD allelic ratio, those with a ratio of mutant gene to wild type allele greater than 0.4, have little chance for cure.12 A low allelic ratio suggests that the mutation occurred in a late progenitor cell rather than in a very immature stem or early precursor cell. These patients do as well as the nonFLT3-mutant patients.12
There are now some indications of improved outcome in FLT3/ITD patients with a matched, related donor transplant. Studies have shown improved survival of FLT3/ITD patients who received a matched, related donor transplant after complete response to initial therapy (CR1).13 A number of centers and cooperative groups are now including FLT3/ITD patients among those with very bad cytogenetics and are taking them to transplant in CR1 if a suitable donor is available.12,14
FLT3 Inhibition
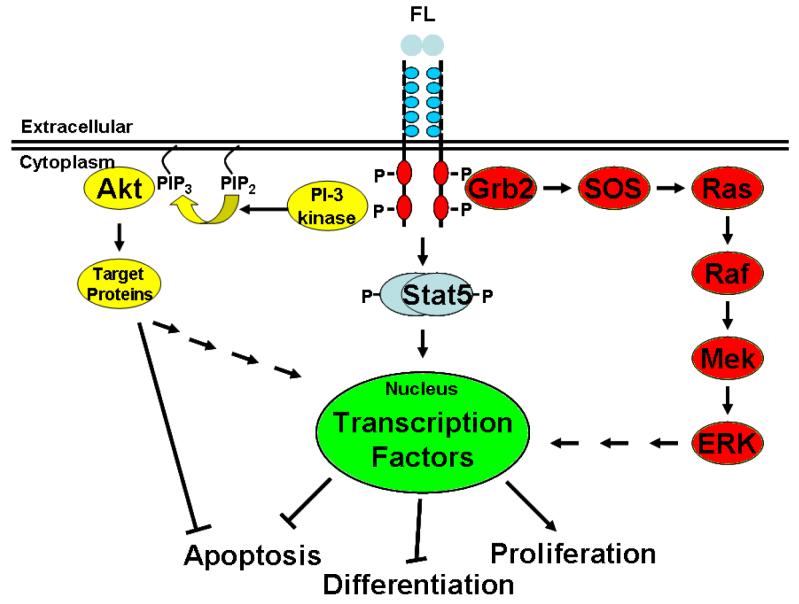
Mutated FLT3 signals via activation of multiple downstream pathways. The exploration of potential ways to reverse the consequences of FLT3 mutation in AML requires looking at these signal transduction pathways. Normally, FLT3 remains a monomeric protein on the cell surface. The binding of FLT3 ligand (FL) causes the FLT3 protein to dimerize, initiating kinase activity which includes autophosphorylation and phosphorylation of substrate proteins. In the case of constitutively activated FLT3 mutation, the kinase is always active, which in turn activates the PI3 kinase/AKT pathway, the RAS/MAP kinase pathway, and the STAT 5 phosphorylation pathway. Ultimately, all of these pathways impinge on the processes of apoptosis, differentiation, and proliferation (Figure 1).
Figure 1. Mutated FLT3 signals via activation of multiple downstream pathways.
The binding of FLT3 ligand causes the FLT3 protein to dimerize, initiating autophosphorylation and kinase activity. The kinase, which is always active in constitutively activated FLT3 mutation, activates numerous pathways, including the PI3 kinase/AKT pathway, the ras/MAP kinase pathway, and the STAT 5 phosphorylation pathway. All of these pathways interrupt the processes of apoptosis, differentiation, and proliferation
One way to interfere with FLT3 activity is to inhibit its kinase activity. PDGFR is a member of the same family of receptors as FLT3 and the first FLT3 inhibitors, AG1295/6, were discovered by screening a number of compounds active against PDGFR. The tyrosine kinase inhibitors compete with ATP for binding to the active pocket. Though the protein is still being expressed, it is unable to phosphorylate itself or substrate proteins by transferring the terminal phosphate of ATP. This inability interrupts signal transduction produced by the mutated receptor. In cell lines dependent on FLT3/ITD signaling, this process induces a cytotoxic and/or differentiative response.
The FLT3 tyrosine kinase inhibitor AG1295 was used in 23 consecutive samples from the Johns Hopkins AML bank.15 Samples containing FLT3/ITD mutations were preferentially sensitive to killing by FLT3 inhibition. This is an interesting demonstration of Robert Weinberg’s oncogene addiction hypothesis,16 in which cells with active oncogenes tend to be dependent on, or addicted to, the mutant signaling pathways for their survival.
Lestaurtinib (CEP701) is a small-molecule inhibitor that produces dose-dependent inhibition of FLT3 phosphorylation with a half maximal inhibitory concentration (IC50) of 2 to 3 nanomolar (nM) range.17 No significant inhibition of PDGFR, FMS, or KIT, the most closely related receptor tyrosine kinases in the FLT3 family, was observed at less than 500 nM.
When lestaurtinib was used to treat leukemic cell samples from pediatric AML patients, it caused the greatest cytotoxicity in samples that had a FLT3/ITD mutation.18 Samples containing point mutant or wild-type FLT3 had lower cytotoxicity.
Both ITD and point mutations of FLT3 appear to constitutively activate FLT3, even in the absence of growth factor binding. In the case of a normal hematopoietic stem progenitor cell where FLT3 is normally expressed, very low levels of the receptor are expressed on the surface, and the growth factor is made by the surrounding bone marrow stromal cells. However, leukemic cells frequently overexpress FLT3 and coexpress the growth factor, setting up a positive feedback autocrine signaling loop.
FLT3 Inhibition in AML
As FLT3 activating mutations are the most common mutations in AML, FLT3 is a promising target for therapeutics in leukemia. As noted above, expression of FLT3/ITD mutations portends poor prognosis for AML patients, FLT3 inhibition induces cytotoxicity in AML cells with FLT3/ITD mutations, and cytotoxic responses occur, albeit at a much lower rate, when wild-type FLT3 is activated by ligand coexpression. This begs the question: Could FLT3 inhibitors be as effective as imatinib (Gleevec®, Novartis) for AML? There are a number of potential problems that make this unlikely. The acute leukemias require multiple “hits” to fully transform hematopoietic cells and FLT3 inhibitors will only take care of one of these mutations. Also, because of the genetic plasticity of cancer, these cells can accumulate additional mutations that render FLT3 signaling dispensable. FLT3 mutations can also occur, as noted above, at a stage later than the leukemic stem cell. When this is the case, a FLT3 inhibitor would not affect the leukemic stem cell, which must happen to achieve a cure. In addition, the development of resistance is likely, as has been seen in CML, through mutations within the FLT3 molecule itself, which render the drugs unable to bind and thus unable to competitively inhibit the binding of ATP to the active pocket.
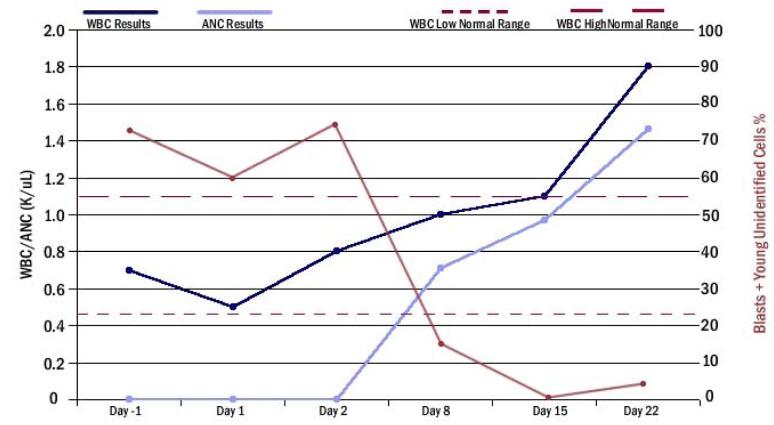
Nevertheless, lestaurtinib showed activity as a single agent in patients with relapsed or refractory AML.19 Peripheral and bone marrow blasts were reduced, and, unlike the usual effect of chemotherapy in AML, the absolute neutrophil count (ANC) rose after administration of lestaurtinib. Figure 2 shows a patient response to lestaurtinib.
Figure 2. Patient response to CEP701 (lestaurtinib).
A typical individual patient response after CEP701 monotherapy. Peripheral blasts were 75% initially, by day 8 were reduced to about 15%, and by day 15 were undetectable.
There are several other FLT3 inhibitors currently in clinical development, such as PKC412 (midostaurin), BAY-93006 (sorafinib), and SU11248 (sunitinib). Many other FLT3 inhibitors are currently being tested in clinical or preclinical trials. To date, early clinical trials have been completed with lestaurtinib,20 midostaurin,21 sunitinib,22,23 and MLN518 (tandutinib).24,25 All of these trials involved oral administration of single agents to relapsed or refractory AML patients including at least some with FLT3 mutations. Dosages and regimens were mostly well-tolerated. FLT3 phosphorylation was shown to be inhibited in a sustained fashion in these trials. Clinical activity was seen with all of them, with clearance of peripheral blasts typically seen in 30% to 50% of patients. Bone marrow blasts were reduced 2- to 3-fold or more in some patients, but that was less common than clearance of peripheral blasts. A few patients could be said to have had a CR in these trials, but the responses have been transient, lasting anywhere from weeks to months.
Combination Therapy
The monotherapy experience with FLT3 inhibitors demonstrates that, until targeted therapies are available for other mutations that contribute to transformation in AML, the best approach at the present time is to combine these agents with conventional chemotherapy. However, cell-cycle dynamics must be carefully considered. FLT3 has a very strong proliferative signal, and many chemotherapeutic agents require cells to be actively proliferating. FLT3 inhibitors used before chemotherapy can decrease the percentage of cells in S/G2/M phase, which will antagonize the effect of chemotherapy. In contrast, use of FLT3 inhibitors either with chemotherapy or after chemotherapy has induced DNA damage, resulted in synergistic killing of actively dividing cells.26,27
A trial is underway using lestaurtinib in combination with conventional chemotherapy in relapsed FLT3-mutant AML patients. The trial has shown a correlation of CRs if FLT3 is more than 85% inhibited and if the blasts from a patient are sensitive to FLT3 inhibition in vitro.28 Midostaurin has been used in a combination trial in newly diagnosed AML patients regardless of their FLT3 status.29 The subset of patients with FLT3 mutations showed a CR rate of 92%. Tandutinib has also been used with standard induction chemotherapy in newly diagnosed and secondary AML patients, regardless of FLT3 status, and the CR rate was about 50% for the FLT3-mutant patients.24 Based on the results with lestaurtinib, a new British Medical Research Council trial has begun randomizing FLT3-mutant patients to receive lestaurtinib.30
FLT3 signaling may also play an important role in ALL. Samples from ALL patients expressing high levels of FLT3 are preferentially susceptible to the induction of a cytotoxic response when treated with FLT3 tyrosine kinase inhibitors and synergize nicely with cytotoxics in these cells when compared to those with low FLT3 expression.31,32
Antibody Approaches to FLT3 Mutation Inhibition
FLT3 can also be targeted via an antibody approach for therapy of both AML and ALL. Antibodies that bind to FLT3 and inhibit FL binding to the receptor have been developed. The anti-FLT3 antibody IMC-EB10 can inhibit FLT3 signaling in some leukemic samples as well as decrease the engraftment of primary AML FLT3/ITD cells and improve survival of NOD/SCID mice injected with MOLM-14 human leukemia cells.33 The antibody inhibits FLT3 autophosphorylation and decreases cell engraftment from greater than 80% to very low levels in this mouse model. In another set of experiments, SEM K2 cells, which have extreme overexpression of constitutively activated wtFLT3, were transformed with a luciferase reporter.34 Using this tag, the cells can be identified on a scan in real time after injecting the mice with luciferin. Eleven days after injection of the cells, the mice showed activity, and locations of proliferation were identified. The mice were then treated with either erbitux, an anti-EGFR antibody, or EB10, an anti-FLT3 antibody. A scan performed 3 days after a single dose of antibody revealed that the mice treated with erbitux had evidence of increasing SEM K2 activity. In contrast, the mice treated with EB10 showed less leukemic activity. These monoclonal antibody approaches may soon be ready to enter testing in the clinic.
Conclusion
FLT3/ITD mutations in AML portend poor prognosis in both adult and pediatric patients, but kinase point mutations do not appear to have prognostic significance. It is not yet clear why signaling through the point mutants does not also give poor prognosis. Constitutively activated FLT3 signals through pathways that include ras/MAP kinase, STAT5, and PI3 kinase/AKT, contributing to blocks in apoptosis and differentiation and stimulating proliferation. Smallmolecule tyrosine kinase inhibitors have been developed and have been shown to successfully inhibit FLT3 signaling both in vitro and in vivo. Clinical trials of FLT3 inhibitors as monotherapy have resulted in frequent responses in peripheral blasts, but less frequent significant responses in bone marrow blasts. The responses also tend to be short-lived. Trials of these agents in combination with chemotherapy are ongoing and show very encouraging responses, but clinical responses appear to correlate with in vitro sensitivity of the blasts and the achievement of adequate levels of FLT3 inhibition in vivo. The pharmacodynamic studies associated with these trials are thus very important. Anti-FLT3 antibodies may also prove to be an excellent way to target FLT3 in both AML and ALL by inhibiting signaling and through antibody-dependent cellular-mediated cytotoxicity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Small D, Levenstein M, Kim E, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci USA. 1994;91:459–463. doi: 10.1073/pnas.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carow CE, Levenstein M, Kaufmann SH, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood. 1996;87:1089–1096. [PubMed] [Google Scholar]
- 3.Brown P, Small D.FLT3 inhibitors: a paradigm for the development of targeted therapeutics for paediatric cancer Eur J Cancer, discussion, 200440707–21. [DOI] [PubMed] [Google Scholar]
- 4.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- 5.Gilliland DG, Griffin JD. Role of FLT3 in leukemia. Curr Opin Hematol. 2002;9:274–281. doi: 10.1097/00062752-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 7.Bao L, Wang X, Ryder J, et al. Prospective study of 174 de novo acute myelogenous leukemias according to the WHO classification: subtypes, cytogenetic features and FLT3 mutations. Eur J Haematol. 2006;77:35–45. doi: 10.1111/j.1600-0609.2006.00660.x. [DOI] [PubMed] [Google Scholar]
- 8.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 9.Meshinchi S, Woods WG, Stirewalt DL, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97:89–94. doi: 10.1182/blood.v97.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Rombouts WJ, Blokland I, Lowenberg B, Ploemacher RE. Biological characteristics and prognosis of adult acute myeloid leukemia with internal tandem duplications in the Flt3 gene. Leukemia. 2000;14:675–683. doi: 10.1038/sj.leu.2401731. [DOI] [PubMed] [Google Scholar]
- 11.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 12.Meshinchi S, Alonzo TA, Stirewalt DL, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–3661. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bornhauser M, Illmer T, Schaich M, Soucek S, Ehninger G, Thiede C. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109:2264–2265. doi: 10.1182/blood-2006-09-047225. [DOI] [PubMed] [Google Scholar]
- 14.Gale RE, Hills R, Kottaridis PD, Srirangan S, Wheatley K, Burnett AK, Linch DC. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106:3658–3665. doi: 10.1182/blood-2005-03-1323. [DOI] [PubMed] [Google Scholar]
- 15.Levis M, Tse KF, Smith BD, Garrett E, Small D. A FLT3 tyrosine kinase inhibitor is selectively cytotoxic to acute myeloid leukemia blasts harboring FLT3 internal tandem duplication mutations. Blood. 2001;98:885–887. doi: 10.1182/blood.v98.3.885. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 17.Levis M, Allebach J, Tse KF, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99:3885–3891. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 18.Brown P, Meshinchi S, Levis M, et al. Pediatric AML primary samples with FLT3/ITD mutations are preferentially killed by FLT3 inhibition. Blood. 2004;104:1841–1849. doi: 10.1182/blood-2004-03-1034. [DOI] [PubMed] [Google Scholar]
- 19.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 20.Knapper S, Burnett AK, Littlewood T, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 21.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 22.O’Farrell AM, Foran JM, Fiedler W, et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9:5465–5476. [PubMed] [Google Scholar]
- 23.Fiedler W, Serve H, Dohner H, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105:986–993. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 24.DeAngelo DJ, Amrein PC, Kovacsocics TJ, et al. Phase 1/2 study of tandutinib (MLN518) plus standard induction chemotherapy in newly diagnosed acute myelogenous leukemia (AML) Blood (abstr 158), 200610851a [Google Scholar]
- 25.DeAngelo DJ, Stone RM, Heaney ML, et al. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics. Blood. 2006;108:3674–3681. doi: 10.1182/blood-2006-02-005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104:1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 27.Brown P, Levis M, McIntyre E, Griesemer M, Small D. Combinations of the FLT3 inhibitor CEP-701 and chemotherapy synergistically kill infant and childhood MLL-rearranged ALL cells in a sequence-dependent manner. Leukemia. 2006;20:1368–1376. doi: 10.1038/sj.leu.2404277. [DOI] [PubMed] [Google Scholar]
- 28.Levis M, Smith D, Beran M, et al. A randomized, open-label study of lestaurtinib (CEP-701), an oral FLT3 inhibitor, administered in sequence with chemotherapy in patients with relapsed AML harboring FLT3 activating mutations: clinical response correlates with successful FLT3 inhibition Blood (abstr 403), 2005106121a [Google Scholar]
- 29.Stone RM, Fischer T, Paquette R, et al. Phase IB study of PKC412, an oral FLT3 kinase inhibitor, in sequential and simultaneous combinations with daunorubicin and cytarabine (DA) induction and high-dose cytarabine consolidation in newly diagnosed adult patients (pts) with acute myeloid leukemia (AML) under age 61 Blood (abstr 157), 200610850a [Google Scholar]
- 30.Burnett A, et al. AML17: A programme of treatment development in younger patients with acute myeloid leukaemia and high risk myelodysplastic syndrome http://www.controlled-trials.com/cctspringview2/mrct/showTrial.html?mrid=274435&srch= Accessed 2-25-2008
- 31.Brown P, Levis M, McIntyre E, Griesemer M, Small D. Combinations of the FLT3 inhibitor CEP-701 and chemotherapy synergistically kill infant and childhood MLL-rearranged ALL cells in a sequence-dependent manner. Leukemia. 2006;20:1368–1376. doi: 10.1038/sj.leu.2404277. [DOI] [PubMed] [Google Scholar]
- 32.Brown P, Levis M, Shurtleff S, Campana D, Downing J, Small D. FLT3 inhibition selectively kills childhood acute lymphoblastic leukemia cells with high levels of FLT3 expression. Blood. 2005;105:812–820. doi: 10.1182/blood-2004-06-2498. [DOI] [PubMed] [Google Scholar]
- 33.Piloto O, Levis M, Huso D, et al. Inhibitory anti-FLT3 antibodies are capable of mediating antibody-dependent cell-mediated cytotoxicity and reducing engraftment of acute myelogenous leukemia blasts in nonobese diabetic/severe combined immunodeficient mice. Cancer Res. 2005;65:1514–1522. doi: 10.1158/0008-5472.CAN-04-3081. [DOI] [PubMed] [Google Scholar]
- 34.Piloto O, Nguyen B, Huso D, et al. IMC-EB10, an anti-FLT3 monoclonal antibody, prolongs survival and reduces nonobese diabetic/severe combined immunodeficient engraftment of some acute lymphoblastic leukemia cell lines and primary leukemic samples. Cancer Res. 2006;66:4843–4851. doi: 10.1158/0008-5472.CAN-06-0018. [DOI] [PubMed] [Google Scholar]