Abstract
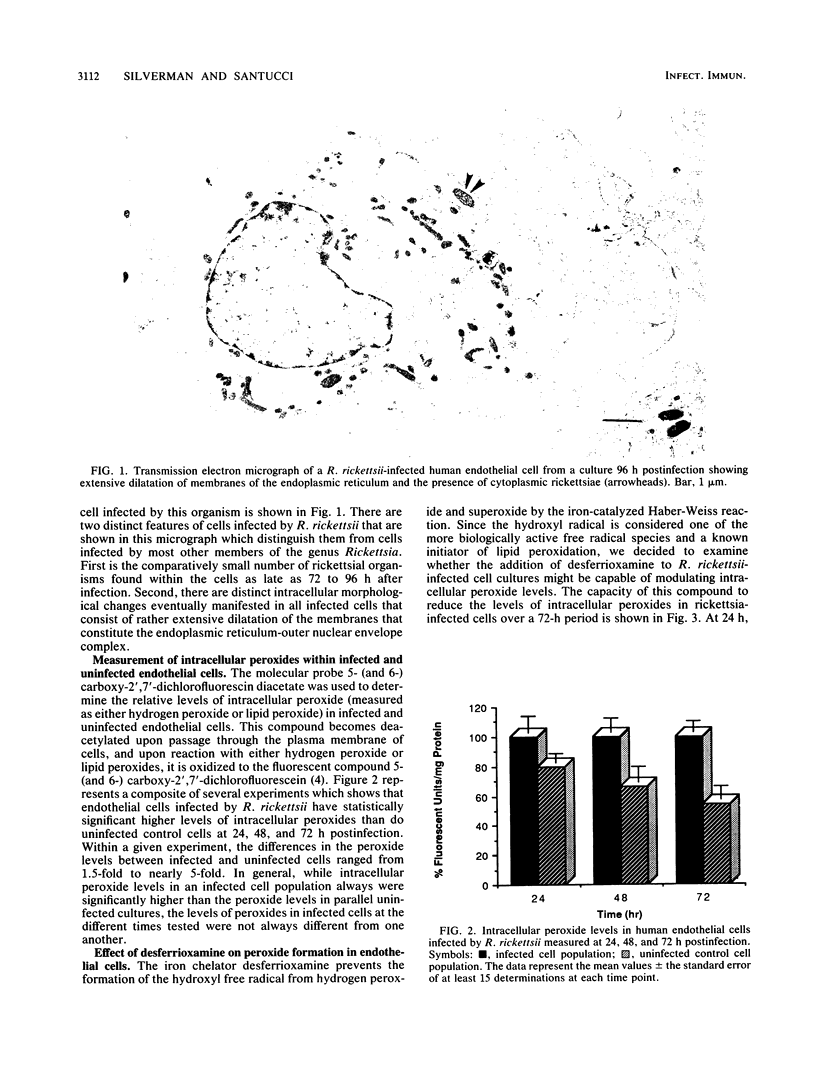
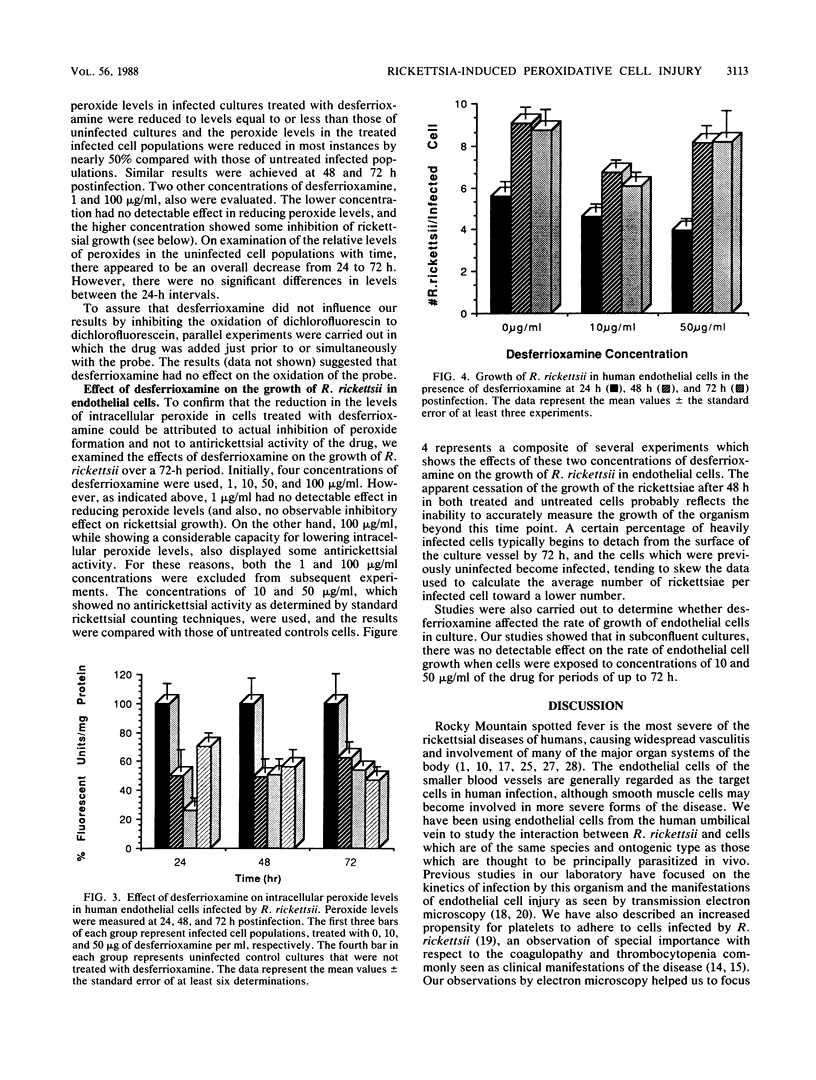
Cells infected by Rickettsia rickettsii, the causative agent of Rocky Mountain spotted fever, display unusual intracellular morphological changes characterized by dilatation of the membranes of the endoplasmic reticulum and outer nuclear envelope. These changes are consistent with those that might be expected to occur following peroxidation of membrane lipids initiated by oxygen radical species, such as the hydroxyl radical or a variety of organic radicals. Using a fluorescent probe, we have found significantly increased levels of peroxides in human endothelial cells infected by R. rickettsii. Studies with desferrioxamine, an iron chelator effective in preventing formation of the hydroxyl radical from hydrogen peroxide and the superoxide free radical, reduced peroxide levels in infected cells to those found in uninfected cells. This observation suggests that the increased peroxides in infected cells may be lipid peroxides, degradation products of free radical attack on polyenoic fatty acids. The potential for lipid peroxidation as an important mechanism in endothelial cell injury caused by R. rickettsii is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. S., Walker D. H. The liver in Rocky Mountain spotted fever. Am J Clin Pathol. 1981 Feb;75(2):156–161. doi: 10.1093/ajcp/75.2.156. [DOI] [PubMed] [Google Scholar]
- Arstila A. U., Smith M. A., Trump B. F. Microsomal lipid peroxidation: morphological characterization. Science. 1972 Feb 4;175(4021):530–533. doi: 10.1126/science.175.4021.530. [DOI] [PubMed] [Google Scholar]
- Black M. J., Brandt R. B. Spectrofluorometric analysis of hydrogen peroxide. Anal Biochem. 1974 Mar;58(1):246–254. doi: 10.1016/0003-2697(74)90464-3. [DOI] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Ames B. N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983 Oct 1;134(1):111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986 May 15;247(1):1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Green W. R., Walker D. H., Cain B. G. Fatal viscerotropic Rocky Mountain spotted fever. Report of a case diagnosed by immunofluorescence. Am J Med. 1978 Mar;64(3):523–528. doi: 10.1016/0002-9343(78)90247-4. [DOI] [PubMed] [Google Scholar]
- HARRELL G. T. Rocky Mountain spotted fever. Medicine (Baltimore) 1949 Dec;28(4):333–370. doi: 10.1097/00005792-194912000-00001. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Grootveld M. The measurement of free radical reactions in humans. Some thoughts for future experimentation. FEBS Lett. 1987 Mar 9;213(1):9–14. doi: 10.1016/0014-5793(87)81455-2. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8(2):89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Hand W. L., Miller J. B., Reinarz J. A., Sanford J. P. Rocky Mountain spotted fever. A vascular disease. Arch Intern Med. 1970 May;125(5):879–882. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Randall M. B., Walker D. H. Rocky Mountain spotted fever. Gastrointestinal and pancreatic lesions and rickettsial infection. Arch Pathol Lab Med. 1984 Dec;108(12):963–967. [PubMed] [Google Scholar]
- Silverman D. J. Adherence of platelets to human endothelial cells infected by Rickettsia rickettsii. J Infect Dis. 1986 Apr;153(4):694–700. doi: 10.1093/infdis/153.4.694. [DOI] [PubMed] [Google Scholar]
- Silverman D. J., Bond S. B. Infection of human vascular endothelial cells by Rickettsia rickettsii. J Infect Dis. 1984 Feb;149(2):201–206. doi: 10.1093/infdis/149.2.201. [DOI] [PubMed] [Google Scholar]
- Silverman D. J. Rickettsia rickettsii-induced cellular injury of human vascular endothelium in vitro. Infect Immun. 1984 Jun;44(3):545–553. doi: 10.1128/iai.44.3.545-553.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr In vitro studies of rickettsia-host cell interactions: ultrastructural changes induced by Rickettsia rickettsii infection of chicken embryo fibroblasts. Infect Immun. 1979 Nov;26(2):714–727. doi: 10.1128/iai.26.2.714-727.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr, Waddell A. In vitro studies of Rickettsia-host cell interactions: ultrastructural study of Rickettsia prowazekii-infected chicken embryo fibroblasts. Infect Immun. 1980 Aug;29(2):778–790. doi: 10.1128/iai.29.2.778-790.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuckler E. A. Alterations produced in the endoplasmic reticulum by carbon tetrachloride. Panminerva Med. 1976 Sep-Oct;18(9-10):292–309. [PubMed] [Google Scholar]
- Walker D. H., Cain B. G. The rickettsial plaque. Evidence for direct cytopathic effect of Rickettsia rickettsii. Lab Invest. 1980 Oct;43(4):388–396. [PubMed] [Google Scholar]
- Walker D. H., Crawford C. G., Cain B. G. Rickettsial infection of the pulmonary microcirculation: the basis for interstitial pneumonitis in Rocky Mountain spotted fever. Hum Pathol. 1980 May;11(3):263–272. doi: 10.1016/s0046-8177(80)80008-6. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Firth W. T., Ballard J. G., Hegarty B. C. Role of phospholipase-associated penetration mechanism in cell injury by Rickettsia rickettsii. Infect Immun. 1983 May;40(2):840–842. doi: 10.1128/iai.40.2.840-842.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Paletta C. E., Cain B. G. Pathogenesis of myocarditis in Rocky Mountain spotted fever. Arch Pathol Lab Med. 1980 Apr;104(4):171–174. [PubMed] [Google Scholar]
- Walker D. H., Tidwell R. R., Rector T. M., Geratz J. D. Effect of synthetic protease inhibitors of the amidine type on cell injury by Rickettsia rickettsii. Antimicrob Agents Chemother. 1984 May;25(5):582–585. doi: 10.1128/aac.25.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Burgdorfer W. Plaque formation in tissue cultures by Rickettsia rickettsi isolated directly from whole blood and tick hemolymph. Infect Immun. 1972 Nov;6(5):736–738. doi: 10.1128/iai.6.5.736-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Edlinger E. A., Waddell A. D., Jones M. R. Infection cycle of Rickettsia rickettsii in chicken embryo and L-929 cells in culture. Infect Immun. 1976 Oct;14(4):1052–1064. doi: 10.1128/iai.14.4.1052-1064.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]