Abstract
We used controlled whisker deflections to examine the response properties of 208 primary afferent neurons in the trigeminal ganglion of adult mice. Proportions of rapidly adapting (RA, 47%) and slowly adapting (SA, 53%) neurons were equivalent, and most cells had low or no spontaneous activity. We quantified angular tuning and sensitivity to deflection amplitude and velocity. Both RA and SA units fired more frequently to larger deflections and faster deflections, but RA units were more sensitive to differences in velocity whereas SA units were more sensitive to deflection amplitudes. Almost all neurons were tuned for deflection angle, and the average response to the maximally effective direction was more than fourfold greater than the average response in the opposite direction; SA units were more tuned than RA units. Responses of primary afferent whisker-responsive neurons are qualitatively similar to those of the rat. However, average firing rates of both RA and SA neurons in the mouse are less sensitive to differences in deflection velocity, and RA units, unlike those in the rat, display amplitude sensitivity. Subtle observed differences between mice and rats may reflect greater mechanical compliance in mice of the whisker hairs and of the tissue in which they are embedded.
Keywords: Mechanoreceptor, primary afferent neuron, vibrissae, whiskers, stimulus coding
Introduction
In rodents, the mystacial vibrissae are components of a highly sensitive sensorimotor system that represents an important source of information about objects in the animal's immediate environment (Vincent 1912; Welker 1964). Behavioral studies have demonstrated that rats can use their vibrissae to detect and differentiate textures, forms, and vibrations (Huston and Masterton 1986; Guic-Robles et al. 1989; Carvell and Simons 1990, 1995; Brecht et al. 1997; Arabzadeh et al. 2004). During object exploration and texture discrimination, animals adjust whisking kinematics based on the tactile task (Carvell and Simons 1995; Harvey et al. 2001). Similarly, human subjects modify their hand and finger movements when discriminating objects of different shape, texture, and size (Lederman and Klatzky 1987). In rats, as in primates, threshold-level behavioral performance can be related to mechanoreceptive properties of primary afferent neurons, indicating a high degree of reliability and preservation of tactile information throughout the posterior column/medial lemniscus system and its trigeminal homolog (Mountcastle et al. 1972; LaMotte and Mountcastle 1975; Stuttgen et al. 2006).
Central somatosensory stations that process afferent information from the whiskers are characterized by anatomically well-defined structures, for example, cortical barrels which act as highly useful landmarks for studies of circuit function, neural coding, development, and plasticity (Woolsey and Van der Loos 1970; Van der Loos 1976; Ma 1991). Increasingly, mouse models are being employed for studying mammalian sensorimotor systems, including the whisker system (Berger et al. 2007; Ferezou et al. 2007). Additionally, comprehensive knowledge of the mouse genome has led to the generation of transgenic animals which are providing interesting insights into mammalian cortical function (Huber et al. 2008). However, differences exist between mice and rats and other rodent species (Krubitzer et al. 1986; Catania and Kaas 1995) in terms of the size and internal organization of whisker-related barrels (Welker and Woolsey 1974; Land and Erickson 2005) and in perhaps in other components of the system, such as the brainstem and thalamus. It is not clear, then, how findings in one species can be readily used to interpret related findings obtained in the other. Moreover, identifying similarities and differences in closely related systems may enable identification of key commonalities in neural organization as well as provide insights into subtle but important differences in local circuitry.
Here, we examine functional properties of trigeminal ganglion cells, the source of peripheral input to the whisker-barrel system. Vibrissal afferent neurons have been studied extensively in a variety of species (Zucker and Welker 1969; Dykes 1975; Lichtenstein et al. 1990; Swadlow and Gusev 2000; Jones et al. 2004a). Compared to investigations of rats, there are relatively few in vivo neurophysiological studies of the mouse vibrissa system and no basis for comparing the functional properties of the primary afferent neurons in the two species. Anatomically, the basic structure and innervation pattern of the follicle-sinus complex in mice and rats are nearly identical (Rice et al. 1986). Each whisker follicle is innervated by discrete bundles of large diameter, myelinated axons, and the follicle is populated in both species by similar types of mechanoreceptive endings located in similar positions within the sinus-hair complex. Reflecting the complex neural and mechanical properties of the sensory apparatus, primary afferent whisker-sensitive neurons in rats, cats, and seals are capable of encoding whisker deflection amplitude (position), velocity, duration, and direction in a highly reliable fashion (Dykes 1975; Jones et al. 2004a; Stuttgen et al. 2006).
Given the similarities between the anatomical structure of rat and mouse vibrissal follicles, we examined the extent to which functional properties of trigeminal ganglion cells are also similar. We employed single cell recordings, controlled whisker stimuli and data analyses highly similar to those used previously in studies of rat whisker-sensitive trigeminal ganglion cells (Lichtenstein et al. 1990; Shoykhet et al. 2000). We find that response properties of mouse primary afferent neurons are largely similar to those of the rat. There are however, subtle quantitative differences that may reflect mechanical properties of the whisker and facial tissues.
Methods
Surgical procedures
Fourteen adult C57 male mice weighing 20-25 g were used in this study. Under isoflurane anesthesia (1.5%), a venous catheter was inserted in the external jugular vein for subsequent administration of pentobarbital sodium, and a tracheotomy was performed in order to maintain a clear airway. Isoflurane was then discontinued, and for the remainder of the experiment mice were anesthetized with intravenously administered Nembutal. Depth of anesthesia was maintained by small injections at a level that abolished tail-pinch reflexes. Nembutal anesthesia was used in order to compare findings with previous studies in rats using similar recording and whisker stimuli (Lichtenstein et al. 1990).
Recordings were obtained from the left trigeminal ganglion. The dorsal surface of the skull overlying the ganglion was exposed, and a craniotomy was centered 1-2mm caudal to bregma and 1-2mm lateral to the midline. The trigeminal ganglion is located at the base of the skull approximately 5.7mm below the cortical surface. A steel post was fixed to the skull with dental acrylic for holding the animal's head without pressure points and for allowing unrestricted access to the left face. Throughout the experiment saline was applied to the craniotomy in order to prevent drying of the brain surface. Following the surgical procedures, the mice were moved to a vibration isolation table, and body temperature was maintained at 37°C with a servo-controlled heating blanket.
Unit recordings
Extracellular unit recordings were obtained with tungsten microelectrodes (2-5 MΩ at 1000 Hz; Frederick Haer). Electrodes were advanced perpendicular to the cortical surface using a hydraulic microdrive. Whiskers on the ipsilateral face were manually deflected during electrode advancement in order to detect units with low or no spontaneous activity. Especially in the case of slowly adapting cells, assessment of true spontaneous firing is made difficult by mechanical hysteresis of mystacial pad tissue, such that a whisker often fails to return to a neutral position even after manual stimulation (see Minnery and Simons 2003). Oftentimes, “spontaneous” firing can be eliminated by deflecting the whisker again so that it returns to a different “neutral” position, indicating that the “spontaneous” firing was not due to an injury discharge.
Waveforms from well-isolated and stable units were parsed from the analog record using an analog-to-digital converter at a sampling rate of 32 kHz and saved to disk (National Instruments, Austin, TX, USA). Isolated units were sorted off-line using custom written software on a National Instruments LabView platform. The software allowed for visualization of selected unit waveforms during the sorting procedure. Single units were identified using analyses based on the first two principal components of the spike waveform and on interspike interval criterion.
Whisker stimulation
Individual trigeminal ganglion neurons responded to deflections of single vibrissae only. This was identified at the outset using hand-held probes to deflect individual whiskers. The relevant whisker was then attached to a computer-controlled piezoelectric stimulator that deflects the whisker in different directions (Simons and Land 1985). We positioned the stimulator on the whisker so that the “spontaneous” firing of the cell was unchanged. Similar to stimuli used previously (Lichtenstein et al. 1990), ramp-and-hold deflections having onset and offset velocities of 125 mm/s and intervening plateau durations of 200 ms, were applied with an interstimulus interval of 1.5 s. Individual whiskers were deflected in eight directions (in 45° increments relative to the horizontal alignment of the whisker row). The eight direction stimuli were delivered in pseudo-random fashion, and ten such blocks were used for a total of 80 stimuli using a deflection amplitude of 1.0 mm. The stimulator was attached to the whisker 10mm from the skin surface.
The velocity and the amplitude sensitivities of neurons were assessed by deflecting the whisker using 1.0 and 0.5 mm ramp-and-hold stimuli having average onset (and offset) velocities of 10, 50, 100, 150, and 200 mm/s. The average onset velocity was measured over the entire movement phase of the deflection ramp. To reduce mechanical ringing, the trapezoid ramp-and-hold waveforms were smoothed with a Bessel filter. The motion of the stimulator was calibrated with a photodiode device. For these stimuli, deflections were delivered in each cell's preferred direction determined first using the multi-angle stimuli described above. The ten stimuli (five velocities at 1.0mm and five velocities at 0.5mm) were delivered pseudo-randomly with an interstimulus interval of 1.5 s with ten repetitions for each amplitude-velocity combination.
Data analysis
Spike times were accumulated into peristimulus time histograms (PSTHs) having 0.1 or 1.0 ms bins. The short-duration bins were used for assessing amplitude and velocity sensitivities at the sub-millisecond scale; unless otherwise specified, all other analyses were based on spike times re-sampled to 1.0 ms. Firing rates were quantified by taking spike counts within selected time intervals corresponding to pre-stimulus (spontaneous) activity and responses to deflection onset (ON response), plateau, and offset (OFF response); time epochs for the various analyses are described in “Results”. Statistical analyses were performed in Microsoft Excel and Analyze-it, an Excel add-on, and in SPSS (Chicago). Between-group statistical comparisons were assessed with the non-parametric Mann-Whitney U-test. Within-group (individual neuron) comparisons were assessed with one-way analysis of variance (ANOVA) with repeated measures. We used paired t-tests to compare data for the two amplitude (1.0 and 0.5 mm) deflections. Categorized data were analyzed using a Chi-square test. For assessing two-factor interaction between amplitude and velocity, we used an ANOVA with repeated measures (SPSS).
Significant stimulus-evoked responses were defined as PSTH bins whose response magnitudes significantly exceeded pre-stimulus activity levels, computed from a 100 ms period preceding each stimulus. Hereafter, the terms pre-stimulus and spontaneous are used interchangeably with the caveat that measuring the true spontaneous activity of cells, especially in the case of slowly adapting units, can be problematic. The spontaneous spike activity of the cell was considered to be a Poisson process, and response onset latency was identified as the time of occurrence of the first post-stimulus bin displaying a significant response relative to preceding activity (95% confidence interval). Cells were classified as either slowly adapting or rapidly adapting following criteria described in Simons and Carvell (1989). Briefly, cells were considered slowly adapting if they had firing rates during the middle 100 ms stimulus plateau phase that were statistically greater than spontaneous firing rates (pπ.05, one-tailed). All other cells by default were classified as rapidly adapting.
The angular tuning properties of the cells were characterized by constructing polar plots from responses to stimulus onsets in the eight different stimulus directions. We quantified tuning using two methods. In the first, a tuning index was calculated as the ratio of the spike count at the maximally effective angle to the spike count averaged over all eight directions. A cell that responds similarly in all directions will have a tuning index of 1.0, whereas if the neuron responded only to deflection in one direction, the index will equal 8.0. A second method quantified the shape of the polar plots using a vector analysis. Response magnitude for each angle was converted to a vector of polar coordinates where
Vectors from the eight angles were then summed and the magnitude of the vector computed as: VM = (x2 + y2)1/2. Vector (or phase) angle, which can vary over 360 deg, reflects the preferred direction of movement. When polar plots are first normalized to the spike count at the maximally effective angle, which is assigned a value of 1.0, the vector magnitude reflects the degree of tuning (see Khatri and Simons 2007), such that large values indicate better tuning (see examples in Figure 1).
Figure 1.
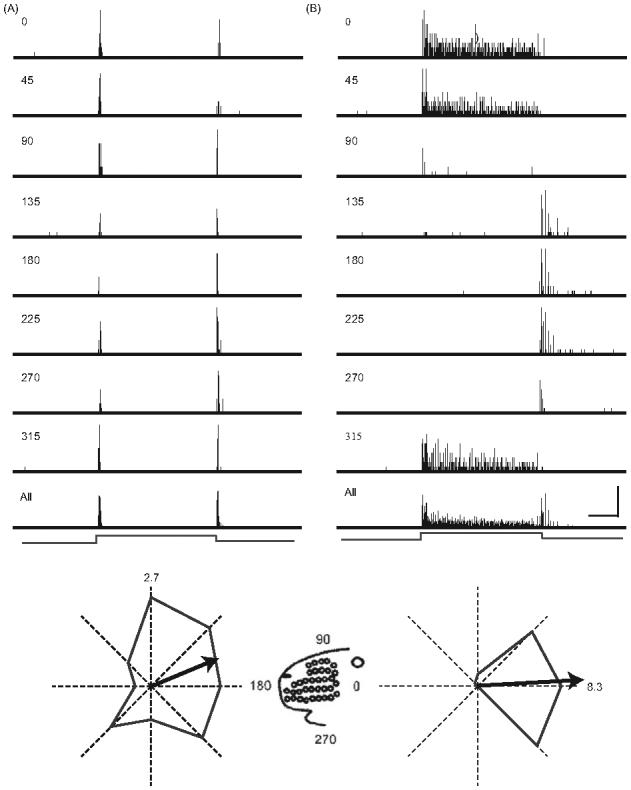
PSTHs depicting the response of a typical rapidly adapting neuron (A) and slowly adapting neuron (B) to whisker deflections in different directions. PSTHs having 1 ms bins were constructed from responses to ten deflections in each of the eight directions indicated (see figurine for orientation). The bottom-most PSTH is the response accumulated over all stimuli/angles. The duration of each trial was 500 ms with the ramp-and-hold stimulus centered in time (indicated at the bottom). Scale: horizontal = 50 ms; vertical = 8 spikes for the individual angle PSTHs, 35 spikes for the RA All-angle PSTH and 30 for the SA All-angle PSTH. Below each set of PSTHs is a polar plot constructed from responses evoked by stimulus onset; the maximal angle response is indicated in spikes/stimulus. Arrows indicate vector angle and magnitude.
Results
We studied 208 well-isolated single units from 14 C57 male mice. Ninety-eight cells (47%) were classified as rapidly adapting (RA) and 110 (53%) as slowly adapting (SA). The proportion of RA units is underestimated in our studied sample, because high velocity threshold neurons (~10% of all cells) could not be examined using our stimulators. High velocity deflections, achieved only with hand-held probes, evoked a brief transient response in these neurons. Figures 1A and B show PSTHs from representative RA and SA units, respectively. Both cells fired to deflection onset and offset in a directionally dependent fashion; the slowly adapting cell additionally fired during the stimulus plateau. Figure 1 also illustrates the general finding that SA units respond more robustly to stimulus onset than RA units (see below). In addition, for SA units the preferred direction (or maximally effective deflection angle) was similar for responses to stimulus onset and sustained deflection; OFF responses were typically maximal in the opposite direction (see below). Figures 2A and B are population PSTHs constructed from the 98 RA units and 110 SA units. Qualitatively, response characteristics are highly similar to those previously described for the rat (see Figures 1 and 2 in Lichtenstein et al. 1990).
Figure 2.
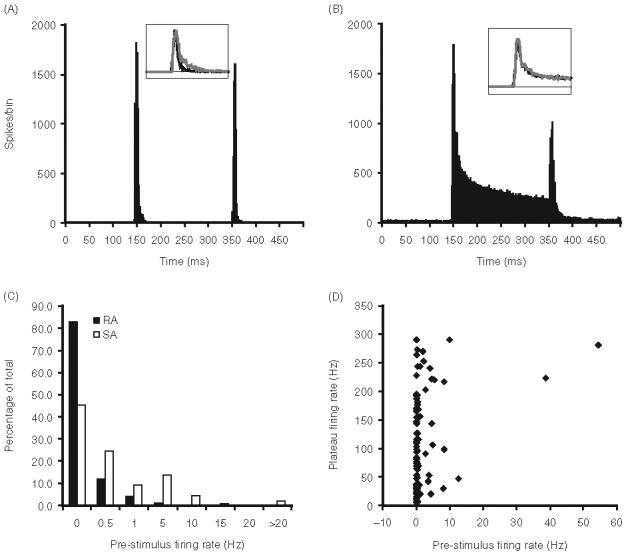
(A) Population PSTHs constructed from the responses of 98 RA neurons accumulated overall deflection angles. Inset shows population 70 ms long PSTHs constructed from the ON response of RA units in the mouse (black) and rat (gray); PSTHs were scaled to probability of spike per 1 ms bin. Stimulus onsets and offset occur at the 146 and 350 ms bins. (B) Population PSTHs from 110 SAs; conventions as in panel A. (C) Bar graphs illustrating the proportion of trigeminal ganglion neurons that display different levels of spontaneous firing. (D) Scatter plots of the relationship between spontaneous activity and maximum-angle plateau firing rates of SA neurons.
Most trigeminal ganglion cells lacked spontaneous activity (Figure 2C). Of the 98 rapidly adapting neurons, 17 (17.3%) had spontaneous activity, whereas 60 (54.5%) of the 110 slowly adapting neurons fired spontaneously. Overall, the average spontaneous firing rate for spontaneously active RA and SA neurons was 2.73±7.74 Hz. The mean firing rate of spontaneously active SA neurons was greater than that of RA neurons (Mann-Whitney U; p = 0.01: 3.38±8.67 Hz vs 0.41±0.52 Hz, respectively); the higher pre-stimulus firing of SA units may reflect, in part, mechanical hysteresis of the whisker follicle (see “Discussion”). Especially for cells having no spontaneous activity, even low levels of firing evoked during the stimulus plateau could lead to a cell being classified as slowly adapting. Figure 2D plots the plateau firing rates of the 110 cells classified as SA as a function of their spontaneous activities. Mean plateau firing was 98.43±82.90 Hz and 88% fired at>10 Hz. Thus, as a population, SA units fired robustly during sustained whisker deflections.
Response magnitudes
All neurons fired at short latency to whisker movement. Response latency to stimulus onset (initial movement of the whisker from its neutral or resting position) was defined as the time of occurrence of the first post-stimulus 1 ms bin displaying a statistically significant response (see “Methods”); latency was computed for the response in the maximally effective deflection angle. Mean RA latency, 2.97±1.58 ms, is comparable to that of SA units (3.33±1.97 ms; two-tailed test, Mann-Whitney U; p=0.16). These values are smaller than those in the rat (see below), likely reflecting the smaller face and hence the shorter conduction distance.
ON and OFF responses to whisker movement were quantified by spike counts during the 20 ms period following the beginning of the response to stimulus onsets and offsets, respectively. Examination of population PSTHs indicated that this 20 ms epoch captures the transient response to whisker movement. Spike counts were calculated as an average over all eight deflection angles and also for the angle evoking the strongest response. Figure 3 shows data for stimulus onsets and offsets. RA neurons fire less robustly to stimulus onsets than SA neurons (Mann-Whitney U; p0.0001); similarly in rats, SA responses are more robust than RA responses (Lichtenstein et al. 1990). On average, best-angle whisker deflection evoked 2.36±1.48 spikes/stimulus in RA neurons and 5.15±2.70 spikes/stimulus in SA neurons. The average whisker-evoked firing rate over all eight angles was 1.21±0.93 spikes/stimulus for rapidly adapting neurons and 2.05±1.22 spikes/stimulus for slowly adapting neurons. The larger values for SA units reflect their more sustained firing and longer duration ON responses; spike counts computed during the first 2 ms of the response are similar for SA and RA neurons (0.98±0.53 vs 1.03±0.59 spikes/stimulus, respectively; Mann-Whitney U; p=0.85). Proportional differences between best-angle and all-angle mean firing rates were somewhat greater in the case of SA units, indicating that they are more tuned for deflection angle (see below). OFF responses of RA and SA units were similar (Figure 3B).
Figure 3.
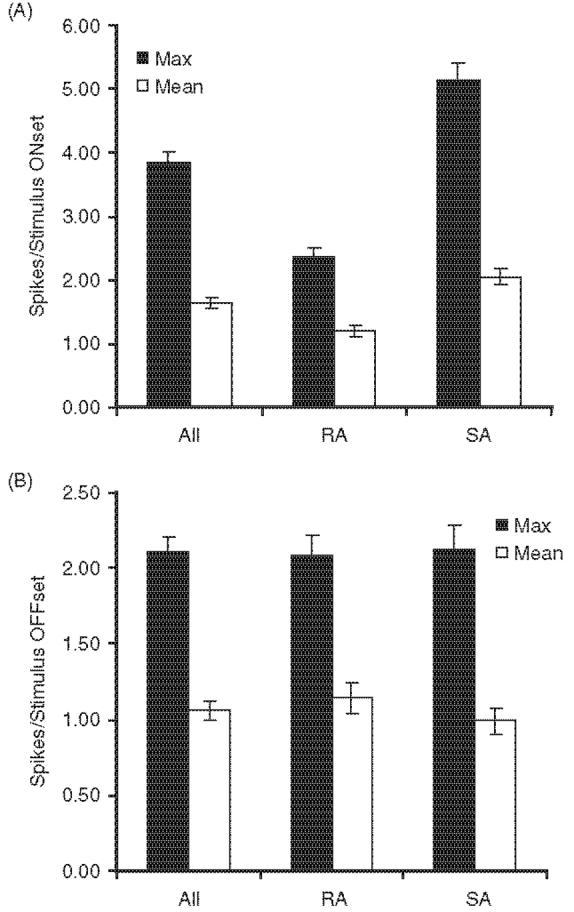
Average ON and OFF response magnitudes evoked by each cell's maximally effective (Max) angle and averaged over all eight angles (Mean) for All neurons, 98 RA neurons and 110 SA neurons. Error bars=standard error of the mean.
Angular tuning
A prominent feature of vibrissa-sensitive neurons in both the peripheral and central nervous systems is directional, or angular, tuning. Figure 4A shows population polar plots for ON responses of RA and SA neurons. To construct these, the spike count at each direction was normalized to the spike count at the maximally effective angle. Individual polar plots were rotated to the same deflection angle (arbitrarily chosen as 270 deg or downward), and then averaged across units. As a population, neurons fired on average approximately fivefold more spikes at the preferred angle than at the opposite one. Proportional differences are greater in the case of SA units (Figure 4A). Figure 4B shows the average vector angle and magnitude computed from polar plots obtained from all RA and SA units. Consistent with the rotated, average polar plots of panel A, SA units are more selective, as indicated by the longer vector (Mann-Whitney U; p0.0001). Also, SAs, as a population, had a slightly more ventral orientation than RAs.
Figure 4.
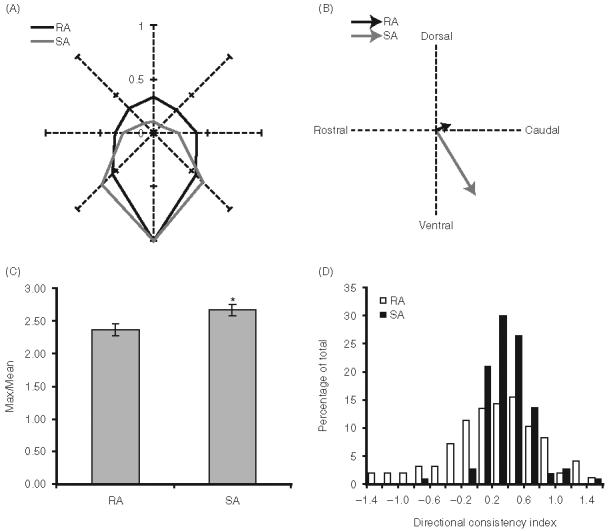
Angular tuning and directional consistency of RA and SA neurons. (A) Population polar plots of RA (black; n98) and SA (gray; n110) units. For each cell ON response magnitudes were normalized to the response evoked by the maximally effective angle. Individual polar plots were then rotated downwards and averaged across the population. (B) Vector angles and magnitudes calculated population polar plots in panel A. (C) Bar graphs depicting average angular tuning indices, calculated as the ratio of the maximal angle response (in spikes/stimulus onset) to the mean over all eight angles. Error bars=standard error of the mean. Asterisk denotes a statistically significant difference (p<0.05) between the two populations. (D) Directional consistency indices. SA neurons are more likely to respond to a given deflection direction regardless of the starting position of the deflection (see text).
We also quantified the tuning of individual neurons as the ratio of the maximal angle response to the response averaged over all eight directions. On average, maximal angle responses are 2.5-fold larger than responses averaged over all eight angles (mean=2.53±0.78); as in the rat (see “Discussion”) SA units are slightly more tuned than RA units (2.67±0.62 vs 2.37±0.90, respectively; Mann-Whitney U: p<0.0001; Figure 4C).
Many vibrissa-sensitive cells are directionally consistent in that they fire to whisker movement in a particular direction regardless of the starting point of the deflection (Lichtenstein et al. 1990). This is illustrated by the unit in Figure 1B. For example, the SA unit responds to stimulus onsets (and sustained deflections) in caudal (0 deg) directions and to stimulus offsets following an initial rostral deflection. In the latter case, the movement of the whisker is caudal. By contrast, the RA unit (Figure 1A), which was also well-tuned, responded at all deflection angles, albeit more vigorously at 90 deg. For a directionally consistent cell such as the one depicted in Figure 1B, the correlation coefficient (r) between the ON and OFF responses at the eight different directions approaches -1.0. After Simons and Carvell (1989) we quantified directional consistency using an index that compares ON and OFF response magnitudes at each deflection angle:
Directional consistency index
= MaxOFF/MaxON × r × (-1.0).
More directionally consistent cells will have more positive indices. Figure 4D shows the distribution of directional consistency indices for slowly adapting and rapidly adapting neurons. The mean directional consistency index for RA units was 0.04±0.64 whereas that for SA units was 0.20±0.28 (p=0.02). Similarly in rats, SA units are more directionally consistent (Lichtenstein et al. 1990).
Velocity and amplitude sensitivity
Previous studies have shown that, as a population, barrel cortex neurons are strongly sensitive to whisker deflection velocity, a feature that appears to reflect the timing and number of spikes evoked in trigeminal ganglion cells (see “Discussion”). For 44 trigeminal ganglion neurons (21 RA and 23 SA neurons), we examined responses to whisker deflections at five different velocities (10, 50, 100, 150, and 200 mm/s) and two amplitudes (1.0 and 0.5 mm). For each velocity, we defined the onset latency of the population PSTH as the first 0.1 ms bin that was statistically larger than expected on the basis of the spontaneous firing rate. We then computed response magnitude (in spikes/stimulus) over five time windows (1.2, 2.0, 5.0, 8.0, and 25.0 ms) from this onset time. These time epochs, chosen to match those used previously in the rat (Shoykhet et al. 2000), are skewed towards the early part of the response where firing rates change rapidly.
Figure 5 shows population PSTHs constructed from responses of 23 SA and 21 RA neurons to two whisker deflection amplitudes and velocities; the lower velocity stimulus (10 mm/s) is close to threshold for RA units whereas the higher velocity stimulus (100 mm/s) is supra-threshold for all units in our sample. The sensitivity of slowly adapting neurons to deflection velocity is reflected in the rise time of the population PSTHs. At low velocities (10 mm/s) the SA PSTH (Figure 5A) displays a slow rise time, whereas at higher velocities (100 mm/s) the PSTH has a fast rise time. The smaller height of the PSTH for 0.5 mm deflections at both velocities reflects the amplitude sensitivity of SA neurons. Further, close examination of the 10 mm/s PSTHs shows that for 1.0 mm deflections, the time to peak is longer than that of responses to 0.5 mm deflections. This is due to the longer time it takes to reach maximum deflection for the 1.0 mm deflection.
Figure 5.
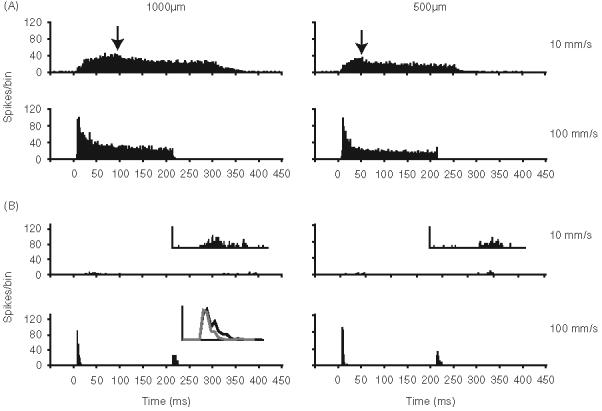
Amplitude and velocity sensitivity of 23 SA (panel A) and 21 RA (panel B) neurons. Population PSTHs having 1 ms bins depicting responses evoked at two deflection amplitudes and velocities. PSTHs (time 0) are aligned to stimulus onsets; ramp durations are considerably longer for the 1.0mm amplitude, 10 mm/s stimuli. Arrows indicate peak of the PSTHs for 10 mm/s deflections. Insets for the 10 mm/s PSTHs in B show the small RA ON responses at higher resolution. The top panel (12-fold magnification of y-axis). Inset in the left lower panel (1000 mm deflection at 100 mm/s) shows overlaid population PSTHs ON responses to 1000 mm (black) and 500 mm (gray) deflections. For each neuron, the whisker was deflected ten times in its maximally effective direction.
Figure 5B illustrates the amplitude and velocity sensitivity of RA neurons. At low velocities (10 mm/s), RA neurons barely respond to whisker deflection (inset is a 12-fold magnification of the PSTH). At higher velocities (100 mm/s), RA neurons fire robustly to whisker deflection; these responses also vary with deflection amplitude. As evident in the inset PSTHs, smaller amplitude deflections elicit shorter duration responses.
Figure 6A shows the relationship between firing rate and deflection velocity in the five different time windows. For the RA population (Figure 6A), firing rates within all time windows examined showed a strong correlation with changes in deflection velocity (one-way ANOVA with repeated measures; p<0.0001). The relationship between firing rates and deflection velocity was non-linear. The earliest firing, for instance, spikes occurring within the 1.2 and 2.0 ms windows, was most strongly correlated with velocity in that a 20-fold increase in velocity resulted in 150-fold (0.005-0.71) and ∼230-fold (0.005-1.10) increases in firing rates, respectively. A fourfold increase in deflection velocity (from 50 to 200 mm/s) resulted in a smaller proportional increase in firing rates (4.1-fold and 3.7-fold increases in firing rates for the 1.2 and 2.0 ms windows, respectively). Firing rates within the 5.0, 8.0, and 25.0 ms time epochs also followed such non-linear trends and were similarly correlated with increases in whisker deflection velocity.
Figure 6.
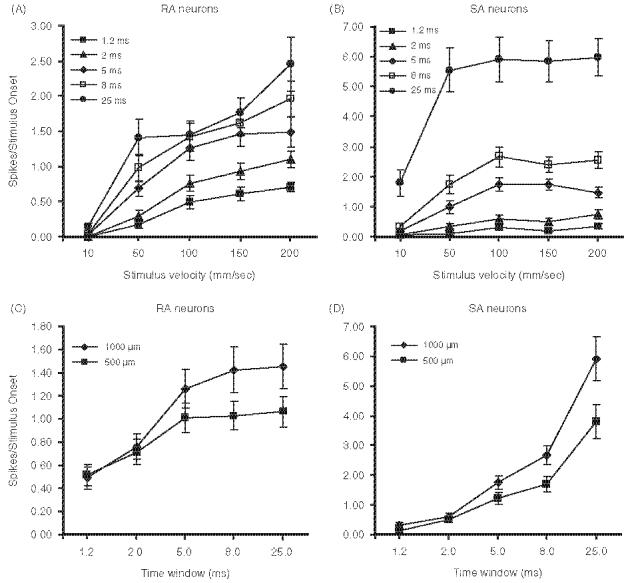
Stimulus-response functions of 23 SA and 21 RA calculated over different response windows (see legend in figure panels). (A, B) Whisker was deflected with different velocities at an amplitude of 1.0 mm. (C, D) Whisker was deflected 0.5 or 1.0 mm at 100 mm/s. Error bars=standard error of mean.
Non-linear effects are observed for the SA units, too (Figure 6B). However, SA units are less sensitive to deflection velocity than RA units. In the 1.2 and 2.0 ms window epochs, the 20-fold increase in deflection velocity (10-200 mm/s) resulted in 6.7-fold and 10.6-fold increases in firing rates, respectively. The fourfold increase in velocity from 50 to 200 mm/s resulted in a 3.6-fold increase in firing rates for the 1.2 ms window and a 2.2-fold increase for the 2.0 ms window. Firing rates within the 5.0, 8.0, and 25.0 ms window epochs showed smaller increases in firing rates with increase in deflection velocity. Particularly for the 25 ms window, the effect of deflection velocity on firing rates asymptotes above 50 mm/s (Figure 6B). A fourfold increase in deflection velocity (from 50 to 200 mm/s) for the 25 ms window epoch resulted in no change in firing rates (one-way ANOVA with repeated measures; p=0.14). This shallower slope with the longer response windows reflects the fact that SA firing becomes increasingly determined by amplitude (displacement) as the response evolves in time (see below).
The analyses for velocity sensitivity were based on data obtained with 1.0 mm deflection amplitudes. In an attempt to dissociate the effect of amplitude from velocity, we also deflected the whiskers using 0.5 mm amplitude deflections at the different velocities. We assessed amplitude sensitivity at a medium velocity of 100 mm/s, thereby avoiding the non-linear effects of velocity on firing rate at the two ends of the velocity spectrum tested. Figures 6C and D show the amplitude sensitivity of RA and SA units. At the earliest time windows (1.2 and 2.0 ms), where both RA and SA units are most sensitive to whisker velocity, there is no dependence of either SA or RA firing rates on deflection amplitude (Figures 6C and D). Amplitude effects are observed first in SA units at the 5.0 ms response window, with a 2-fold increase in deflection amplitude resulting in a 43.6% increase in firing rate (two-tailed paired t-test; p<0.0001). With the 8.0 and 25.0 ms window epochs, firing rate increases were larger (57.6 and 55.5%, respectively; two-tailed paired t-test; p<0.0001), again reflecting the strong amplitude sensitivity of SA units. Additionally, for slowly adapting cells, amplitude effects were readily apparent during the sustained firing rates in response to stimulus plateau. The mean plateau response for 1.0 mm amplitude deflections was 126.0% greater than the mean plateau response to 0.5 mm amplitude deflection (152.2±108.9 Hz vs 67.3±70.6 Hz, respectively; two-tailed paired t-test; p<0.0001).
Similar to SA neurons, the first indication of amplitude-dependent firing in RA units is observed in the 5.0 ms window. However, a 2-fold increase in deflection amplitude produced only a 25% increase in firing (two-tailed paired t-test; pM=0.02). The 8.0 and 25.0 ms window epochs also showed greater increases in firing rates (38.4 and 36.8%, respectively, two-tailed paired t-test; p<0.006 and 0.005). The proportional increases in RA firing were smaller than those observed from comparable response windows in SA units. Indeed, the shapes of the stimulus-response functions are nearly the converse in RA and SA units (compare Figures 6C and D).
For both SA and RA neurons, we considered the possibility of an interaction between the effect of deflection amplitude and velocity on evoked firing rates. Such an interaction may obscure the effect of velocity especially in larger integration time windows (for example, the 8.0 and 25.0 ms windows). This is because with larger integration windows, the full effect of the total displacement of the whisker becomes evident in the firing rates. For the 0.5 mm amplitude deflections, as with the 1.0 mm stimulus, both RA and SA units exhibited velocity modulated firing rates within all time windows, with the greatest effects occurring during the earlier time windows when the whisker is first displaced from rest (i.e., high acceleration). Similar to 1.0 mm deflections, RA units were more sensitive to deflection velocity than SA units. To assess the possible interaction between velocity and amplitude, we analyzed the data within the time windows where response magnitude is sensitive to deflection amplitude. For RA neurons there was a trend-level interaction between velocity and amplitude in the 8.0 ms epoch window (ANOVA, p=0.05). For the 5.0 and 25.0 ms windows there was no significant interaction between velocity and amplitude (p=0.10 and 0.20, respectively). Note that for the 8.0ms window, the time epoch having the strongest interaction, the ON-ramp of the 0.5mm movement has ended (the whisker is fully deflected) while that of the 1.0mm movement continues. For SA units, on the other hand, significant interactions between deflection amplitude and velocity were observed for all time window (5.0, 8.0, and 25.0 ms) epochs tested (GLM with repeated measures; p<0.0001).
Discussion
In this study we describe the response properties of mouse trigeminal ganglion neurons. We find that mouse primary afferent neurons respond with short latency and are highly time-locked to the transient phase of the stimulus. The response properties of mouse primary afferents are largely similar to those previously described for rats, cats, and seals (Zucker and Welker 1969; Dykes 1975; Lichtenstein et al. 1990; Shoykhet et al. 2000; Jones et al. 2004b). Similarities include substantial proportions of slowly adapting type responses, low spontaneous activity, and sensitivity of evoked firing rates to whisker deflection velocity and/or amplitude. Also, both RA and SA neurons display strong preferences for the direction of whisker deflection. Taken together, findings across species are consistent with the view that mystacial vibrissae provide highly reliable and detailed information about the tactile environment around the animal's face.
We found in mice that the proportions of slowly adapting and rapidly adapting neurons are equivalent. Percentages of slowly adapting cells differ, often substantially, in different studies even in the same species (see Lichtenstein et al. 1990). In rats the proportion of SA units has been reported to be as low as 25% and as high as 75% (Zucker and Welker 1969; Lichtenstein et al. 1990; Shoykhet et al. 2000; Jones et al. 2004b; Stuttgen et al. 2006). Reported differences likely reflect methodological and sampling factors and the criterion used to distinguish rapidly adapting from slowly adapting responses. In this regard, our data probably underestimate the proportion of rapidly adapting neurons, because our stimulators do not produce high amplitude/velocity movements necessary to activate higher threshold neurons.
Most trigeminal ganglion neurons in the mouse have no spontaneous activity and of those that do, the spontaneous activity is very low. Similar to cats, seals (Dykes 1975), and rats (Zucker and Welker 1969; Lichtenstein et al. 1990), the spontaneous activities of SA neurons in mice are higher than that of RA neurons. Also, spontaneous activities of SA neurons in the mouse are larger than those reported in a similar study in the rat (Lichtenstein et al. 1990). Observed spontaneous activity, particularly of SA units, may in part reflect failure of the whisker hair to return to a neutral position following a displacement with hand-held probes (see Minnery and Simons 2003). When the whisker is firmly attached to a stimulator, as was the case here, the hysteresis may reflect failure of the follicle to return to its neutral position within the supporting skin and muscle (Minnery and Simons 2003; Fraser et al. 2006). Such mechanical hysteresis effects may be more pronounced in mice relative to rats because the mystacial tissue may be more pliable (see below).
We also find that the response latency of both SA and RA units is much shorter in the mouse compared to the rat. This short latency can be attributed to the shorter distance between the whisker follicle and the trigeminal ganglion in the mouse. In addition, ON response durations of RA neurons are shorter in the mouse (see Figure 2 inset). This difference may reflect the viscoelastic properties of the face in the two species (see below).
Angular tuning
Angular tuning is thought to be due to a non-uniform circumferential distribution within the sinus hair follicle of mechanoreceptor endings associated with individual primary afferent axons (Gottschaldt et al. 1973; Gottschaldt and Vahle-Hinz 1981). For a given unit, the specific location(s) of the mechanoreceptor(s) from which they derive their input determines its preferred deflection angle. Slowly adapting neurons are more angularly tuned and more directionally consistent than rapidly adapting neurons (see Figure 4). This property is similar to that observed in rats (Lichtenstein et al. 1990). Using the same whisker stimuli and analyses as in Lichtenstein et al. (1990), we found that mouse trigeminal ganglion neurons are slightly more tuned than their counterparts in the rat. We attribute this to differences in the viscoelastic properties of the mouse face (see below) rather than differences in patterns of follicle innervation by single fibers.
Amplitude and velocity sensitivities
Responses of trigeminal ganglion cells are robust and velocity-dependent, at least over the range of 10-200 mm/s. Data, including those from 44 other neurons examined during pilot experiments, indicate that response magnitudes are nearly asymptotic at 200 mm/s, corresponding to a velocity of 1150 deg/s. The highest peak velocity (the fastest portion of the filtered ramp) is 330 mm/s (1900 deg/s). The sensitivity of RA neurons appears to extend to higher velocities in rats (Shoykhet et al. 2000; Stuttgen et al. 2006). Even in mice, however, a saturating limit of nearly 2000 deg/s is within the range of the majority of rapid grip-slip movements observed in rats palpating textured surfaces (Ritt et al. 2008).
The most prominent effect of velocity on firing rates was evident in the first few milliseconds of the response for both RA and SA units. As the response evolves over time, the effect of velocity on response magnitude diminishes, especially for SA units. At the time epochs wherein the effect of velocity is evident, stimulus-response relationships are non-linear. This is especially the case for RA units, many of which have velocity thresholds (see Stuttgen et al. 2006). Though we did not assess velocity thresholds, we found a substantial increase in firing rates when deflection velocity increased from 10 to 50 mm/s. The velocity sensitivity of slowly adapting neurons is also non-linear, though the slope of the stimulus-response function is shallower than that of RA units. This is because SA units, which derive their inputs from pressure-sensitive Merkel cell endings (Gottschaldt and Vahle-Hinz 1981), are more sensitive to static whisker position than movement per se and because SA units are less likely to have velocity thresholds (see Stuttgen et al. 2006). In the rat trigeminal ganglion, angular tuning also exhibits a non-linear relationship with deflection velocity (Lee and Simons 2004).
We also examined amplitude sensitivity of RA and SA neurons over the time-course of their responses. The earliest response windows, which displayed the most prominent relationship with velocity, show no effect of amplitude on firing rates. This is because our stimuli were delivered from the resting position of the whisker so that in the earliest time windows, movement velocity (acceleration) is high even though the displacement is small. Integration over longer time windows takes into account the response of the cell to the total displacement of the whisker. Our data indicate that SA units are more sensitive to deflection amplitude than RA units. On the other hand, Jones et al. (2004a), using white noise stimuli, report that RA and SA units in rats are equivalent with respect to their ability to extract instantaneous position information from a rapidly and continually varying, complex stimulus. We find that amplitude sensitivity, observed most strongly in SA units, is only evident with relatively long-lasting deflections and long analysis windows.
Mechanical properties of the face/whisker
Detailed anatomical studies indicate that whisker follicles of mice and rats are similar with respect to their structure, their neural innervation, and the types of mechanoreceptors located within them (Rice et al. 1986). Mouse whiskers are thinner than those in rats, and the stiffness of the hair is known to affect its mechanical properties (Hartmann et al. 2003; Neimark et al. 2003). We found that RA units in mice are amplitude sensitive, a finding at variance with two studies in rats that also used ramp-and-hold stimuli and reported that RA firing is unrelated to deflection amplitude (Shoykhet et al. 2000; Stuttgen et al. 2006). In our study and in that of Shoykhet et al. (2000), both RAs and SAs were equivalently velocity sensitive during the first 1-2 ms of the response, suggesting that initial changes in force were being transmitted similarly in the two species. For a similar point of stimulator attachment, mouse whiskers may be more likely than rat whiskers to bend during a large deflection. The bending could enhance the bowing of the whisker within the follicle (see Gottschaldt et al. 1973), adding an additional force component to the stimulus that would be evident in firing rates using large amplitude deflections and long integration windows.
Qualitative observation suggests that the mouse mystacial pad is considerably more pliable than that of the rat, perhaps because of less muscle mass in the smaller mystacial pad. Fraser et al. (2006) have provided indirect evidence that muscle tone affects responses of trigeminal ganglion cells to transient, periodic whisker deflections by determining the extent to which the whisker follicle tracks the motion of the deflected whisker. Concurrent movement of the follicle in the same direction as the whisker will result in less relative displacement of the whisker hair, less receptor deformation in the whisker follicle, and hence a smaller neural response. Due to inertia, the initiation of whisker follicle movement may lag that of the whisker tip, so that later in the stimulus the relative velocity would be reduced. This could explain the lower velocity sensitivity of mouse RA and SA units, relative to those in the rat, when the full-duration response is examined. In the earliest time windows, the responses of SA and RA units in mice and rats are comparable, but with longer time windows responses are less robust in the mouse and the slopes of the stimulus-response functions are shallower (compare the present Figure 6 with Figure 2 in Shoykhet et al. 2000).
Rapid, early onset rotation of the follicle in the direction of an applied whisker stimulus would truncate the neural response by reducing some of the force transmitted onto mechanoreceptor endings near the base of the hair. This may explain why RA responses in mice are shorter in duration than those in the rat. Such effects would be less pronounced, and possibly undetectable in SA neurons, because they are more sensitive to displacement than to movement per se. SA mechanoreceptors are sensitive to sustained pressure, and thus SA neurons will continue to fire, and do so at increasingly high rates, as the whisker attains a progressively more displaced position during the ramp stimulus. Less effective transmission of force to the follicle could also yield somewhat more angularly tuned responses in mice due to spike threshold non-linearities.
The whisker sensorium, including the hair, follicle, and surrounding tissue, is moved by a complex system of muscles (Hill et al. 2008) and sustained by a blood supply that may affect the transmission of force with the whisker/sinus hair follicle. Response properties of trigeminal neurons could vary subtly with behavioral conditions, as it is unlikely that during arousal and active touch the mechanical properties of the peripheral system remain static (Szwed et al. 2003). The present study in mice, like other quantitative studies of primary afferent neurons innervating whiskers, was conducted in anesthetized animals. Available evidence from these and other related investigations of whisker mechanics (Hartmann et al. 2003; Neimark et al. 2003) indicates that, as a population, trigeminal ganglion neurons are capable of providing highly detailed and accurate information about even minute perturbations of the whiskers in whatever physiological context they might occur.
Acknowledgements
We are grateful to Dr G. Carvell for insightful comments and discussions. This work was supported by NIH grant NS-19950.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Arabzadeh E, Petersen RS, Diamond ME. Encoding of whisker vibration by rat barrel cortex neurons: Implications for texture discrimination. J Neurosci. 2004;23:9146–9154. doi: 10.1523/JNEUROSCI.23-27-09146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Borgdorff A, Crochet S, Neubauer FB, Lefort S, Fauvet B, Ferezou I, Carleton A, Luscher HR, Petersen CC. Combined voltage and calcium epifluorescence imaging in vitro and in vivo reveals subthreshold and suprathreshold dynamics of mouse barrel cortex. J Neurophysiol. 2007;97:3751–3762. doi: 10.1152/jn.01178.2006. [DOI] [PubMed] [Google Scholar]
- Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res. 1997;84:81–97. doi: 10.1016/s0166-4328(97)83328-1. [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci. 1990;10:2638–2648. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Task- and subject-related differences in sensorimotor behavior during active touch. Somatosens Mot Res. 1995;12:1–9. doi: 10.3109/08990229509063138. [DOI] [PubMed] [Google Scholar]
- Catania KC, Kaas JH. Organization of the somatosensory cortex of the star-nosed mole. J Comp Neurol. 1995;351:549–567. doi: 10.1002/cne.903510406. [DOI] [PubMed] [Google Scholar]
- Dykes RW. Afferent fibers from mystacial vibrissae of cats and seals. J Neurophysiol. 1975;38:650–662. doi: 10.1152/jn.1975.38.3.650. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CC. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Fraser G, Hartings JA, Simons DJ. Adaptation of trigeminal ganglion cells to periodic whisker deflections. Somatosens Mot Res. 2006;23:111–118. doi: 10.1080/08990220600906589. [DOI] [PubMed] [Google Scholar]
- Gottschaldt K-M, Iggo A, Young DW. Functional characteristics of mechanoreceptors in sinus hair follicles of the cat. J Physiol (Lond) 1973;235:287–315. doi: 10.1113/jphysiol.1973.sp010388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschaldt K-M, Vahle-Hinz C. Merkel cell receptors: Structure and transducer function. Science. 1981;214:183–186. doi: 10.1126/science.7280690. [DOI] [PubMed] [Google Scholar]
- Guic-Robles E, Valdivieso C, Guajardo G. Rats can learn a roughness discrimination using only their vibrissal system. Behav Brain Res. 1989;31:285–289. doi: 10.1016/0166-4328(89)90011-9. [DOI] [PubMed] [Google Scholar]
- Hartmann MJ, Johnson NJ, Towal RB, Assad C. Mechanical characteristics of rat vibrissae: Resonant frequencies and damping in isolated whiskers and in the awake behaving animal. J Neurosci. 2003;23:6510–6519. doi: 10.1523/JNEUROSCI.23-16-06510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Bermejo R, Zeigler HP. Discriminative whisking in the head-fixed rat: Optoelectronic monitoring during tactile detection and discrimination tasks. Somatosens Mot Res. 2001;18:211–222. doi: 10.1080/01421590120072204. [DOI] [PubMed] [Google Scholar]
- Hill DN, Bermejo R, Zeigler HP, Kleinfeld D. Biomechanics of the vibrissa motor plant in rat: Rhythmic whisking consists of triphasic neuromuscular activity. J Neurosci. 2008;28:3438–3455. doi: 10.1523/JNEUROSCI.5008-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston KA, Masterton RB. The sensory contribution of a single vibrissa's cortical barrel. J Neurophysiol. 1986;56:1196–1223. doi: 10.1152/jn.1986.56.4.1196. [DOI] [PubMed] [Google Scholar]
- Jones LM, Depireux DA, Simons DJ, Keller A. Robust temporal coding in the trigeminal system. Science. 2004a;304:1986–1989. doi: 10.1126/science.1097779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LM, Lee S, Trageser JC, Simons DJ, Keller A. Precise temporal responses in whisker trigeminal neurons. J Neurophysiol. 2004b;92:665–668. doi: 10.1152/jn.00031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri V, Simons DJ. Angularly nonspecific response suppression in rat barrel cortex. Cereb Cortex. 2007;17:599–609. doi: 10.1093/cercor/bhk006. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Sesma MA, Kaas JH. Microelectrode maps, myeloarchitecture, and cortical connections of three somatotopically organized representations of the body surface in the parietal cortex of squirrels. J Comp Neurol. 1986;250:403–430. doi: 10.1002/cne.902500402. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Mountcastle VB. Capacities of humans and monkeys to discriminate vibratory stimuli of different frequency and amplitude: A correlation between neural events and psychological measurements. J Neurophysiol. 1975;38:539–559. doi: 10.1152/jn.1975.38.3.539. [DOI] [PubMed] [Google Scholar]
- Land PW, Erickson SL. Subbarrel domains in rat somatosensory (S1) cortex. J Comp Neurol. 2005;490:414–426. doi: 10.1002/cne.20677. [DOI] [PubMed] [Google Scholar]
- Lederman SJ, Klatzky RL. Hand movements: A window into haptic object recognition. Cognit Psychol. 1987;19:342–368. doi: 10.1016/0010-0285(87)90008-9. [DOI] [PubMed] [Google Scholar]
- Lee SH, Simons DJ. Angular tuning and velocity sensitivity in different neuron classes within layer 4 of rat barrel cortex. J Neurophysiol. 2004;91:223–229. doi: 10.1152/jn.00541.2003. [DOI] [PubMed] [Google Scholar]
- Lichtenstein SH, Carvell GE, Simons DJ. Responses of rat trigeminal ganglion neurons to movements of vibrissae in different directions. Somatosens Mot Res. 1990;7:47–65. doi: 10.3109/08990229009144697. [DOI] [PubMed] [Google Scholar]
- Ma PM. The barrelettes—Architectonic vibrissal representations in the brainstem trigeminal complex of the mouse. I. Normal structural organization. J Comp Neurol. 1991;309:161–199. doi: 10.1002/cne.903090202. [DOI] [PubMed] [Google Scholar]
- Minnery BS, Simons DJ. Response properties of whisker-associated trigeminothalamic neurons in rat nucleus principalis. J Neurophysiol. 2003;89:40–56. doi: 10.1152/jn.00272.2002. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, LaMotte RH, Carli G. Detection thresholds for stimuli in humans and monkeys: Comparison with threshold events in mechanoreceptive afferent nerve fibers innervating the monkey hand. J Neurophysiol. 1972;35:122–136. doi: 10.1152/jn.1972.35.1.122. [DOI] [PubMed] [Google Scholar]
- Neimark MA, Andermann ML, Hopfield JJ, Moore CI. Vibrissa resonance as a transduction mechanism for tactile encoding. J Neurosci. 2003;23:6499–6509. doi: 10.1523/JNEUROSCI.23-16-06499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice FL, Mance A, Munger BL. A comparative light microscopic analysis of the sensory innervation of the mystacial pad. I. Innervation of the vibrissal follicle-sinus complexes. J Comp Neurol. 1986;252:154–174. doi: 10.1002/cne.902520203. [DOI] [PubMed] [Google Scholar]
- Ritt JT, Andermann ML, Moore CI. Embodied information processing: Vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron. 2008;57:599–613. doi: 10.1016/j.neuron.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoykhet M, Doherty D, Simons DJ. Coding of deflection velocity and amplitude by whisker primary afferent neurons: Implications for higher level processing. Somatosens Mot Res. 2000;17:171–180. doi: 10.1080/08990220050020580. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol. 1989;61:311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Land PW. Angular sensitivities of SmI barrel neurons and the effects of fentanyl anesthesia. Neurosci Abstr. 1985:11751. [Google Scholar]
- Stuttgen MC, Ruter J, Schwarz C. Two psychophysical channels of whisker deflection in rats align with two neuronal classes of primary afferents. J Neurosci. 2006;26:7933–7941. doi: 10.1523/JNEUROSCI.1864-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG. The influence of single VB thalamocortical impulses on barrel columns of rabbit somato-sensory cortex. J Neurophysiol. 2000;83:2802–2813. doi: 10.1152/jn.2000.83.5.2802. [DOI] [PubMed] [Google Scholar]
- Szwed M, Bagdasarian K, Ahissar E. Encoding of vibrissal active touch. Neuron. 2003;40:621–630. doi: 10.1016/s0896-6273(03)00671-8. [DOI] [PubMed] [Google Scholar]
- Van der Loos H. Barreloids in mouse somatosensory thalamus. Neurosci Lett. 1976;2:1–6. doi: 10.1016/0304-3940(76)90036-7. [DOI] [PubMed] [Google Scholar]
- Vincent SB. The function of vibrissae in the behavior of the white rat. Behav Monographs. 1912;1:7–85. [Google Scholar]
- Welker C, Woolsey TA. Structure of layer IV in the somatosensory neocortex of the rat: Description and comparison with the mouse. J Comp Neurol. 1974;158:437–453. doi: 10.1002/cne.901580405. [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat. Behaviour. 1964;22:223–244. [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Zucker E, Welker WI. Coding of somatic sensory input by vibrissal neurons in the rat's trigeminal ganglion. Brain Res. 1969;12:138–156. doi: 10.1016/0006-8993(69)90061-4. [DOI] [PubMed] [Google Scholar]


