Abstract
Human erythropoiesis is a complex multistep process that involves the differentiation of early erythroid progenitors to mature erythrocytes. Here we show that it is feasible to differentiate and mature human embryonic stem cells (hESCs) into functional oxygen-carrying erythrocytes on a large scale (1010-1011 cells/6-well plate hESCs). We also show for the first time that the oxygen equilibrium curves of the hESC-derived cells are comparable with normal red blood cells and respond to changes in pH and 2,3-diphosphoglyerate. Although these cells mainly expressed fetal and embryonic globins, they also possessed the capacity to express the adult β-globin chain on further maturation in vitro. Polymerase chain reaction and globin chain specific immunofluorescent analysis showed that the cells increased expression of β-globin (from 0% to > 16%) after in vitro culture. Importantly, the cells underwent multiple maturation events, including a progressive decrease in size, increase in glycophorin A expression, and chromatin and nuclear condensation. This process resulted in extrusion of the pycnotic nuclei in up to more than 60% of the cells generating red blood cells with a diameter of approximately 6 to 8 μm. The results show that it is feasible to differentiate and mature hESCs into functional oxygen-carrying erythrocytes on a large scale.
Introduction
Human embryonic stem cells (hESCs) can be propagated and expanded in vitro indefinitely, providing a potentially inexhaustible and donorless source of cells for human therapy. Hematopoietic differentiation of hESCs has been extensively investigated in vitro, and hematopoietic precursors as well as differentiated progeny representing erythroid, myeloid, macrophage, megakaryocytic, and lymphoid lineages have been identified in differentiating hESC cultures.1–8 Previous studies also generated primitive erythroid cells from hESCs by embryoid body formation and coculturing with stromal cells.8–10 However, the efficient and controlled differentiation of hESCs into homogeneous red blood cell (RBC) populations with oxygen-carrying capacity has not been previously achieved.
Mammalian erythropoiesis is a complex process that involves many steps, including the differentiation of early erythroid progenitors (burst-forming units-erythroid, BFU-E) via late erythroid progenitors (colony-forming units-erythroid, CFU-E), and finally morphologically recognizable erythroid precursors.11 Nuclear condensation is a key event in the late stages of erythropoiesis, and enucleation is the final step in the development of mature erythrocytes, although the molecular and cellular mechanisms involved in these processes are poorly understood.
Here we describe an efficient method to generate functional erythroid cells from hESCs under conditions suitable for scale-up. The cells possess oxygen-transporting capacity comparable with normal RBCs and respond to changes in pH and 2,3-diphosphoglycerate. We also show that they undergo a progressive decrease in size, chromatin condensation, and extrusion of the pycnotic nucleus to form enucleated erythrocytes with a diameter similar to normal RBCs. β-Globin chain specific antibody analysis showed that more than 16% of the cells after 28 days of culture express the adult β-globin chain.
Methods
Generation and expansion of erythroid cells from hESCs via hemangioblasts
Four human ESC lines were used in the current study: H1 (National Institutes of Health–registered as WA01), MA01 and MA99 (derived at Advanced Cell Technology), and HuES-3 (established by Cowan et al12 and obtained from the Harvard Stem Cell Institute). hESCs were grown on mitomycin C–treated mouse embryonic fibroblast (MEF) in complete hESC media until they reached 80% confluence. The detailed method for the generation of hemangioblasts (BCs) from hESCs has been described previously.13 A 4-step procedure was used for the generation and expansion of erythroid cells from hESCs.
Step 1. EB formation and hemangioblast precursor induction (day −3.5 to 0).
To induce hemangioblast precursor (mesoderm) formation, EBs were formed by plating 1 well of hESCs per EB culture well (ultra-low 6-well plates; Corning, Corning, NY) in 3 to 4 mL serum-free Stemline media (Sigma-Aldrich, St Louis, MO) with BMP-4, VEGF165 (50 ng/mL each; R&D Systems, Minneapolis, MN), and basic fibroblast growth factor (bFGF, 20 ng/mL; Invitrogen, Carlsbad, CA). Half of the media was refreshed 48 hours later with the addition of stem cell factor (SCF), thrombopoietin, and FLT3 ligand (20 ng/mL each; R&D Systems).
Step 2. Hemangioblast expansion (days 0-10).
After 3.5 days, EBs were collected and dissociated with trypsin. A single cell suspension was obtained by passing the cells through a G21 needle 3 times and filtering through a 40-μm filter. After resuspending in Stemline II medium, the cells were mixed with blast-colony growth media (BGM; 5 × 105 cells/mL) and plated in 100-mm ultra low dishes (10 mL/dish). The cultures were expanded for 9 to 10 days in BGM. The addition of 20 ng/mL bFGF and 2 μg/mL recombinant tPTD-HoxB4 fusion protein to BGM was found to significantly enhance hematopoietic cell proliferation. HoxB4 protein has been shown to promote hematopoietic development in both mouse and human ESC differentiation systems.14–19 The grape-like blast colonies were usually visible by microscopy after 4 to 6 days and expanded rapidly outward. Additional BGM was added to keep the density of blast cells at 1 to 2 × 106 cells/mL.
Step 3. Erythroid cell differentiation and expansion (days 11-20).
At the end of step 2, the cell density was often very high (≥ 2 × 106/mL). Equal volumes of BGM, containing 3 units/mL erythropoietin (Epo; total Epo is 6 units/mL) without HoxB4, were added to supplement the existing BGM. The blast cells were further expanded and differentiated into erythroid cells for an additional 5 days. For further expansion, the erythroid cells were transferred into 150-mm Petri dishes and Stemline II–based medium containing SCF (100 ng/mL), Epo (3 unit/mL), and 0.5% methylcellulose added every 2 to 3 days. (When the cells reached confluence, it was very important to split the cells at a ratio of 1:3 to allow maximum expansion for an additional 7 days [cell density, 2-4 × 106/mL].)
Step 4. Enrichment of erythroid cells (day 21).
Erythroid cells obtained from step 3 were diluted in 5 volumes of Iscove modified Dulbecco medium (IMDM) plus 0.5% bovine serum albumin (BSA) medium and collected by centrifugation at 1000g for 5 minutes. The cell pellets were washed twice with IMDM containing 0.5% BSA and plated in tissue culture flasks overnight to allow nonerythroid cells (usually the larger cells) to attach. The nonadherent cells were then collected by brief centrifugation.
Plating in BGM after the 3.5-day EB dissociation step was denoted as day 0 of erythroid culture. The time period for the entire procedure was 19 to 21 days from the plating of EB cells in BGM, with a final culture volume of 3 to 4 L for 5 to 6 × 106 MA01 hESCs. We observed that the efficiency of RBC generation from MA99, H1, and HuES-3 was approximately 5 to 6 times less than from MA01 hESCs (with a correspondingly lower final culture volume). RBCs obtained from this procedure (before put into culture for further maturation and enucleation) were used for functional characterization, flow cytometry, and hemoglobin analyses. The large-scale culture experiments were carried out with hESC lines MA01 (n = 6), H1 (n = 2), HuES-3 (n = 2), and MA99 (n = 1).
For further maturation, cells collected at days 18 to 19 (step 3) were diluted with IMDM containing 0.5% BSA (1:5 dilution) and centrifuged at 450g for 10 minutes. To partially enrich the cells for RBCs, the top white portion of cell pellet was removed using a pipette with a long fine tip. The RBCs were then plated in StemPro-34 SCF (Invitrogen) medium containing SCF (100 ng/mL) and Epo (3 unit/mL) at a density of 2 × 106 cells/mL. The cells were cultured 6 days with media changes every 2 days and then switched to StemPro-34 containing Epo (3 unit/mL) for 4 to 5 more days. These cells were used for β-globin chain and benzidine stain analyses.
FACS analysis of erythroid cells
All of the conjugated antibodies and the corresponding isotype controls were purchased from BD Biosciences PharMingen (San Diego, CA), except for the RhD and HbF assay (ComDF), purchased from Chemicon International (Temecula, CA). The antibodies used were HLAabc, Duffy group, CD14, CD15, CD34, CD35, CD36, CD41, CD44, CD45, CD71, CD133, CD184 (CXCR4), GPA, RhD, and HbF. Erythroid cells were collected at 19 to 21 days and washed 2 times in phosphate-buffered saline (PBS) with 0.1% BSA and stained in accordance with the manufacturer's suggested concentration of conjugated antibody for 30 minutes at 4°C. The stained cells were then washed 2 times in PBS + 0.1% BSA and fixed with the wash buffer supplemented with 1% paraformaldehyde. The RhD and HbF assay was performed per manufacturer's protocol that included a 0.5% glutaraldehyde/0.1% BSA in PBS prefixing treatment and a 0.1% Triton-X/0.1% BSA in PBS permeabilization step before staining. After staining with the ComDF reagent for 15 minutes at room temperature, cells were washed 1 time in 0.1% BSA in PBS and fixed in wash buffer supplemented with 1% paraformaldehyde. The samples were then analyzed using a flow cytometer (FacScan; BD Biosciences, San Jose, CA). Cell populations were analyzed with the CellQuest program (BD Biosciences)
Functional analysis of hemoglobin
Cells collected at 19 to 21 days were washed 3 times in 0.9% NaCl, then suspended in 9 volumes of water, lysed with saponin, and clarified by centrifugation at 600g. Hemoglobins were then separated by cellulose acetate electrophoresis. Oxygen equilibrium curves were determined using a Hemox-Analyzer, Model B (TCS Scientific, New Hope, PA). The gas phase gradients were obtained using nitrogen and room air, and the curves were run in both directions Data were used only from runs showing negligible hysteresis as described previously.20,21
Globin mass spectra were obtained using a Voyager-DE Pro matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (Applied Biosystems, Foster City, CA) as described by Lee et al.22 In brief, ZipTips (Millipore, Billerica, MA), packed with C18 and C4 resin, were used to prepare the solution for MS analysis of peptide and protein, respectively. Cyano-4-hydroxycinnamic acid and sinapinic acid were used as the matrix for peptide and protein, respectively. Aliquots (1.3 mL) of the matrix solution (3-10 mg cyano-4-hydroxycinnamic acid or sinapinic acid in 1 mL aqueous solution of 50% acetonitrile containing 0.1% TFA) were used to elute the peptide/protein from ZipTips and spotted onto a matrix-assisted laser desorption/ionization time-of-flight target. A Voyager- DE PRO Mass Spectrometer (Applied Biosystems) equipped with a 337-nm pulsed nitrogen laser was used to analyze the samples. Protein mass was measured using the positive-ion linear mode. External mass calibration was performed using the peaks of a mixture of cytochrome c (equine) at m/z 12362, apomyoglobin (equine) at m/z 16952, and adolase (rabbit muscle) at m/z 39212.
RhD and ABO genotyping
RhD genotyping of hES cell lines by polymerase chain reaction (PCR) was reported by Arce et al23 and Simsek et al24 with minor modifications. Because all hES cells were maintained on MEF, we designed a pair of human DNA specific PCR primers that only amplified human DNA sequences. Genotyping of ABO blood group was developed based on the polymorphism of glycosyltransferase among ABO blood group individuals25 (see Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Characterization of hESC-derived erythroid cells
Cells collected at different time points were cytospun at low speed (< 1000g) on superfrost plus slides (VWR International, West Chester, PA). Slides were dried and stained with Wright-Giemsa dye for 5 minutes and washed 3 times with distilled water. For immunofluorescence staining, cytospun slides were fixed in 4% paraformaldehyde for 15 minutes, incubated in 1% BSA for 30 minutes, and incubated overnight at 4°C in 1:200 primary antibodies of CD235a/glycophorin A (Dako North America, Carpinteria, CA), CD71 (BD Biosciences), or human β-globin chain specific antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Cells were then incubated for 1 hour in 1:200 secondary antimouse IgG conjugated to rhodamine or fluorescein isothiocyanate (Jackson ImmunoResearch Laboratories, West Grove, PA). For total hemoglobin stain, cells at different stages of differentiation using the erythroid expansion maturation protocol outlined in “Generation and expansion of erythroid cells from hESCs via hemangioblasts” were collected and cytospun on slides. Air-dried cytospin samples were fixed in 100% methanol for 10 minutes. After washing with PBS for 10 minutes, cells were stained with 3′3-diaminobenzidine reagent (Sigma-Aldrich) according to the manufacturer's instructions. The cells (like all RBCs) containing hemoglobin stain brown and nuclei of cells stained blue with Wright-Giemsa.
For immunologic blood type characterization, erythroid cells were collected at 19 to 21 days, cytospun on glass slides, and stained with monoclonal antihuman blood group A and B antibodies (ViroGen, Watertown, MA) overnight at 4°C. Slides were then incubated with corresponding secondary antibodies labeled with rhodamine or fluorescein isothiocyanate (Jackson ImmunoResearch Laboratories) for 30 to 60 minutes. After a final wash, the cells were checked by fluorescence microscopy.
RT-PCR analysis
Erythroid cells differentiated at different stages using the erythroid expansion protocol outlined in “Generation and expansion of erythroid cells from hESCs via hemangioblasts” were collected, and the expression of β-, γ-, and ϵ-globin genes was analyzed by RT-PCR. In brief, total RNA was isolated using an RNAeasy Micro Kit (QIAGEN, Valencia, CA), cDNA pools were constructed using the SMART cDNA synthesis kit (Clontech, Mountain View, CA) as previously reported.2 Primers specific for β-, γ-, and ϵ-globin genes, as reported previously,10 were used to amplify corresponding messages. PCR products were separated on a 2.5% agarose gel and visualized by ethidium bromide fluorescence.
Enucleation of hESC-derived erythroid cells in vitro
Blast cells were cultured as described above up until day 7.
Step 1.
Day 7 blast cells in BGM were filtered and plated in Stemline II (Sigma-Aldrich) with supplements based on Giarratana et al.26 These included 40 μg/mL of inositol, 10 μg/mL of folic acid, 160 μM of monothioglycerol, 120 μg/mL of transferrin, 10 μg/mL of insulin, 90 ng/mL of ferrous nitrate, 900 ng/mL of ferrous sulfate, 10 mg/mL of BSA (StemCell Technologies, Vancouver, BC), 4 mM of l-glutamine (Invitrogen), and 1% penicillin-streptomycin (Invitrogen). All reagents were from Sigma-Aldrich unless otherwise noted.
Step 2.
For the first 7 days in this media (days 7-14), cells were cultured in 1 μM of hydrocortisone, 100 ng/mL of SCF (Invitrogen), 5 ng/mL of interleukin-3 (Invitrogen), and 3 IU/mL of Epo (Cell Sciences, Canton, MA) and maintained at 106 cells/mL.
Step 3.
From day 14 onward, SCF and interleukin-3 were discontinued and Epo was continued. Cells were maintained at a density of 2 × 106 cells/mL. Medium was changed every few days.
Step 4.
Cells were cocultured with human mesenchymal stem cells (MSCs; Lonza Walkersville, Walkersville, MD) or OP9 mouse stromal cells at various time points (days 19-36) in Stemline II with supplements described in steps 1 to 3 of this section and Epo. Before coculture, MSCs were expanded in MSC Growth Medium (MSCGM; Lonza Walkersville) and OP9 cells were expanded in 20% FBS (Atlas Biologicals, Fort Collins, CO) in α-MEM (Invitrogen) with 4 mM of l-glutamine and 1% penicillin-streptomycin (Invitrogen).
Statistical analysis of cell dimensions
The area of cells and nuclei on cytospun Wright-Giemsa–stained slides was measured during the enucleation protocol using Scion Image. The area of the cytoplasm was calculated as the difference between the total cell area and nuclear area and nuclear-to-cytoplasmic ratio (N/C). Diameter was calculated from the area of the nucleus. Differences between diamater and N/C at each time point were measured by an analysis of variance, followed by the Holm test. Data are presented as mean plus or minus SD with significance of at least P less than .05.
Image acquisition
Slides were viewed with an Olympus BX51 research microscope (Olympus America, Center Valley, PA) using lenses of UPlan FLN 20×/0.50, UPlan FI 40×/0.75, and PlanApo 100×/1.40 oil. Wright-Giemsa– and benzidine-stained images were acquired using a PAXcam EDU camera (Midwest Information Systems, Franklin Park, IL) and were processed with PAX-it (Midwest Information Systems) and Adobe Photoshop element (Adobe Systems, San Jose, CA) software. Fluorescent images were acquired using a QImaging QIC Fast 1394 Mono Cooled camera (Surry, BC) and were processed with Qcapture Pro version 5.1 (QImaging) and Adobe Photoshop element software (Adobe Systems).
Results
Differentiation of hESCs into RBCs
BCs were generated from hESCs as previously described.13 A 4-step protocol was used to differentiate the BCs toward the erythroid lineage, which included: (1) EB formation from undifferentiated hESCs, (2) BC formation and expansion, (3) erythroid differentiation and amplification into a mass population of RBCs, and (4) enrichment of RBCs (“Generation and expansion of erythroid cells from hECSs via hemangioblasts”). Early-stage EBs were generated from hESCs cultured in serum-free media supplemented with a combination of morphogens and early hematopoietic cytokines. The EBs were then dissociated, and individual cells were plated in serum-free semisolid BGM for the growth and expansion of BCs. Grape-like blast colonies appeared at the beginning of 3 days and rapidly expanded from 4 days. The BCs were then induced to proliferate and differentiate into erythrocytes by adding BGM and Epo for several days. To further expand the erythroid cells, Stemline II-based media containing SCF, Epo, and methylcellulose was added every 2 or 3 days for 1 week. Cells were then diluted in IMDM with added BSA, collected by brief centrifugation and plated in tissue culture flasks overnight to allow the nonerythroid cells to attach. The remaining nonadherent cells were collected (representing > 95% erythroid cells; Figure 1A-D). Using this optimized (19-21 days) protocol of expansion and differentiation with the addition of bFGF (20 ng/mL) and HoxB4 protein (2 μg/mL) in BGM, 3.86 (± 1.19 × 1010, mean ± SD, n = 6) RBCs were generated from one 6-well plate of MA01 hESCs (∼ 1.2 × 107 cells). RBCs were also generated with high efficiency from H1 (n = 2), HuES-3 (n = 2), and MA99 (n = 1) hESCs, but the yield was 5 to 6 times less that obtained from MA01 hESCs. We found that the quality of hESCs is one of the most important factors for high-efficient generation of RBCs; high-quality hESCs (ie, hESC culture should be composed of colonies with tight borders with minimal signs of differentiation as seen under microscope at approximately 80% confluent but not touching each other; grown at moderated rate: 1:3 split getting confluent in 3-5 days; stained positive with markers of pluripotency for almost every cells; and formed uniform EBs 24 hours after replating) usually generate a high number of EB cells (eg, 2 × 106 high-quality hESCs will generate ∼ 2-3 × 106 EB cells after 3.5 days). We also noted that the presence of 0.2% to 0.5% methylcellulose in the differentiation and expansion medium prevents cells from aggregating, resulting in enhanced expansion.
Figure 1.
Large-scale production of erythroid cells from hESCs. (A) Erythroid cells (pellet) derived from 2 × 106 human ESCs. (B) Erythroid cells from panel A were resuspended in equivalent hematocrit of human whole blood. (C,D) Morphology of erythroid cells derived from human ESCs (C: original magnification ×200; D: original magnification ×1000). (E) Electrospray ionization mass spectra of globin chains in hemoglobins from hESC-derived erythroid cells, confirming the presence of α, ζ, ϵ, and γ globins. The observed molecular weight for each of the globins is shown. (F) Flow cytometric analysis of hESC-derived erythroid cells. Erythroid cells derived from hESCs were labeled with specific antibodies conjugated with phycoerythrin and analyzed on a FacScan flow cytometer (BD Biosciences) with the CellQuest program. Corresponding unspecific isotype antibodies conjugated with the same dyes were used as negative controls.
Characterization of hESC-derived RBCs
Morphologically, the RBCs obtained using the (19-21 days) protocol as described in “Generation and expansion of erythroid cells from hESCs via hemangioblasts” were nucleated (> 95%) and substantially larger than definitive erythocytes with an average diameter of approximately 10 μm. Giemsa-Wright staining showed an abundance of hemoglobin in the cytoplasm (Figure 1C,D). The identity of the cells was confirmed by immunologic characterization (Table 1; Figure 1F). More than 65% of the cells expressed fetal hemoglobin (HbF), more than 75% were CD71 positive, and 30% of the cells expressed CD235a, whereas the majority of the cells did not express myelomonocytic or megakaryocytic antigens (all cells were negative for CD14, whereas 0.4% of cells expressed CD15; 8.6% of cells expressed CD41) and progenitor antigens (0.3% cells were positive for CD34; 10% cells expressed CD35, and 5% cells were positive for CD36; Table 1). We have previously shown that BCs express the chemokine receptor CXCR4.13 However, we did not detect the expression of CXCR4 or CD133 on the surface of the hESC-derived RBCs, which is consistent with the findings from erythroid cells expanded from cord blood progenitors in vitro.26,27 Interestingly, few or none of the cells expressed HLA (< 5%) or Duffy (0%) group antigens, a finding that has also been observed for CD34+CD38− hematopoietic precursors derived from hESCs.2
Table 1.
Characterization of hESC-derived erythroid cells by FACS analysis
| Antibody | Positive range, % (n = 5) | Mean ± SE |
|---|---|---|
| HbF | 40.03-96.60 | 66.79 ± 9.88 |
| CD47 | 95.00-99.21 | 97.51 ± 0.85 |
| GPA | 21.31-41.93 | 30.10 ± 3.79 |
| CD71 | 59.40-83.39 | 76.07 ± 4.33 |
| CD44 | 18.61-44.56 | 30.72 ± 4.55 |
| CD45 | 10.06-40.21 | 22.23 ± 5.45 |
| CD41 | 4.44-20.16 | 8.61 ± 2.98 |
| CD14 | 0 | 0 |
| CD15 | 0.20-0.60 | 0.38 ± 0.08 |
| CD34 | 0-1.62 | 0.34 ± 0.32 |
| CD35 | 5.82-17.46 | 9.79 ± 2.00 |
| CD36 | 1.08-13.30 | 4.99 ± 2.14 |
| CD133 | 0 | 0 |
| CD184 (CXCR-4) | 0 | 0 |
| Duffy | 0 | 0 |
| HLAabc | 0.75-6.25 | 4.15 ± 1.14 |
Mass spectral analysis showed that the main globin types found in the RBCs obtained at days 19 to 21 from MA01 and H1 hESCs included the embryonic ζ- and ϵ-chains, and the fetal Gγ-chain (Figure 1E). Substantial quantities of α-chains were also present, but neither Aγ- nor adult β-globin chains could be detected. Nevertheless, these results demonstrate that hemoglobin synthesis in these cells corresponds to the embryonic and early fetal developmental stage and are consistent with recent reports showing that, even definitive-appearing erythroid cells derived from hESCs coexpress high levels of embryonic and fetal globins with little or no adult globin.2,8,10,19
Functional analysis
In 6 separate experiments, the oxygen equilibrium curves of the hESC-derived erythroid cells (day 19-21 cultures) were either very similar to (Figure 2A) or somewhat rightward-shifted (data not shown), relative to that of normal adult RBCs. The oxygen equilibrium curve illustrated in Figure 2A has a biphasic appearance. At the low end of the oxygen saturation, its curve is to the left of the normal, and it is hyperbolic in shape (arrow). At their midpoint, the 2 curves are virtually identical, and at higher saturation levels, the curve of ESC-derived erythroid cells is again displaced slightly to the left of the normal (arrowhead). The Hill n coefficient was also similar to that of the normal control (Figure 2C). The ESC-derived erythroid cells showed a comparable Bohr effect at physiologic and higher pH values but a lesser shift at lower pH (Figure 2B). The response to 2,3-diphosphoglycerate (2,3-DPG) depletion of these cells was significantly less than in the normal control (Figure 2C), consistent with the known lack of interaction between Hb F and 2,3-DPG.28 These findings demonstrate that the hESC-derived RBCs have oxygen-carrying properties that are comparable with those of normal adult erythrocytes.
Figure 2.
Functional characterization of hESC-derived erythroid cells. (A) Oxygen equilibrium curves of normal human RBCs and human ESC-derived erythroid cells. The 2 curves are virtually indistinguishable at their midpoints, whereas the curve of human ESC-derived erythroid cells is leftward shifted at low (→) and high (▾) oxygen saturation percentages. (B) The Bohr effect. (C) Effects of 2,3-DPG depletion. The solid lines represent the normal RBC control; dashed lines, the human ESC-derived erythroid cells. For each pair, the line on the right represents the fresh cells and the one to the left is the curve from cells depleted of 2,3-DPG.
Generation of RhD(−) RBCs from hESCs
The manufacture of O/RhD(−) RBCs would substantially aid in the prevention of alloimmunization when transfused into RhD(−)-mismatched patients. The anticipated need for universal donor RBCs (O−) in Western countries is greater than in Asian countries, such as Korea, Japan, and China, where the RhD(−) type is less prevalent (< 0.5% vs 15%, respectively). Genotype analysis by PCR showed that only 2 of 20 hESC lines studied, MA99 and MA133, were RhD(−) (Figure 3A). Erythroid cells from 19- to 21-day cultures were used for FACS and immunologic analyses. FACS analyses demonstrated that RBCs generated from MA01 expressed RhD antigen on their surfaces, whereas cells derived from MA99 lacked the expression of RhD antigen (Figure 3D), confirming the results of genomic DNA PCR analysis (Figure 3A). Immunocytochemical analysis using monoclonal antibodies against the A and B antigens showed that approximately 5% of RBCs generated from MA01 cells expressed the A, but not the B antigen (Figure 3E), demonstrating that MA01 cells have a phenotype of A(+); approximately 5% of RBCs derived from MA99 cells expressed the B, but not the A antigen (Figure 3E), suggesting that MA99 cells have a B(−) phenotype, whereas RBCs derived from WA01 cells expressed neither A nor B antigens, confirming WA01 cells as O-type, consistent with the results of genomic PCR analysis (Figure 3B,C). However, it is worth noting that not all erythroid cells expressed the A or B antigen, which may reflect the early developmental stage of the cells.29,30
Figure 3.
Characterization of Rh(D) and ABO genotype of hESC lines by PCR. (A) Genotyping of RhD locus: Specific primers were designed for the Rh locus that when Rh(D) positive DNA was used, 1200-bp (weak) and 600-bp PCR products were amplified; whereas DNA from RhD-negative cells generated only the 1200-bp fragment. (B,C) Genotyping of the ABO locus: 2 pairs of primers were designed to amplify 2 regions of the ABO locus. The PCR products were digested with restriction enzymes to distinguish ABO types. Vertical white lines have been inserted in panels B and C to indicate repositioned gel lanes. ABO and Rh(D) genotypes are as follows: WA01, O( + ); MA99, B(−); MA133, A(−); WA07 and MA09, B( + ); and WA09 and MA01, A( + ). (D) RhD antigen expression analysis on erythroid cells derived from MA01 and MA99 hESCs by FACS. Erythroid cells generated from MA01 and MA99 hESCs were stained with phycoerythrin-labeled monoclonal anti-RhD antibody and analyzed by FACS. (E) ABO type characterization of hESC-derived erythroid cells. (i) Cells stained with monoclonal antibody against A-antigen (original magnification ×400). (ii) Cells stained with monoclonal antibody against B-antigen (original magnification ×400).
Enucleation and maturation of hESC-derived erythroid cells in vitro
A critical scientific and clinical issue is whether hESC-derived erythroid cells can be matured in vitro to generate enucleated erythrocytes. To investigate this, we studied several different strategies and culture conditions. We found that hematopoietic stem cell expansion medium Stemline II plus supplements and cytokines reported by Giarratana et al26 supported the growth, expansion, maturation, and enucleation of hESC-derived erythroid cells with significantly higher efficiency than other tested conditions. Blast cells cultured in this condition without stromal layers resulted in 10% to 30% enucleation, whereas culturing on MSC stromal cells resulted in approximately 30% enucleation and OP9 stromal cell layers further enhanced the enucleation process. Approximately 30% to 65% of erythroid cells (40% ± 17% [mean ± SD, n = 4]) were enucleated when these cells were transferred to OP9 stromal layers from nonstromal 5-week cultures and cocultured from days 36 to 42 (Figure 4C,E). The enucleated erythrocytes (Figure 4C,E) show similar staining pattern and size as mature RBCs from normal human blood (Figure 4D,F). These erythroblasts were derived from hESCs grown without MEFs using the BD Matrigel system. The fact that erythroblasts kept in nonstromal conditions (without transfer to MSC or OP9) could enucleate 10% to 30% suggests that enucleation could be achieved completely feeder-free.
Figure 4.
Enucleation of hESC-derived erythroid cells in vitro. (A) Diameter decreases with time in culture. Data for each day represent diameters of nucleated cells, except “27e” represents diameters of enucleated cells at 27 days. Enucleated cells decrease to less than half the original diameter on day 8. (B) Nuclear-to-cytoplasm ratio decreases with time in culture. Samples significantly different from day 8: *P < .05, **P < .001, #P < .002. (C,E) Erythroid cells derived from human ESCs were cultured in vitro for 4 weeks in Stemline II media with supplements and cocultured with OP9 stromal cells on day 36. On day 42, cells were cytospun and stained with Wright-Giemsa dye (C: original magnification ×200; E: original magnification ×1000). (D,F) Red blood cells from human blood were also cytospun and stained with Wright-Giemsa and compared with hESC-derived erythroid cells (D: original magnification ×200; F: original magnification ×1000). Scale bar represents 10 μm.
A total of 6 experiments were performed with hESC lines H1 (n = 3), MA01 (n = 2), and huES-3 (n = 1), all exhibiting various levels of enucleation and expansion of 30- to 50-fold. Stromal cells, especially OP9, were able to enhance survival of the cells after long-term culture compared with nonstromal conditions.
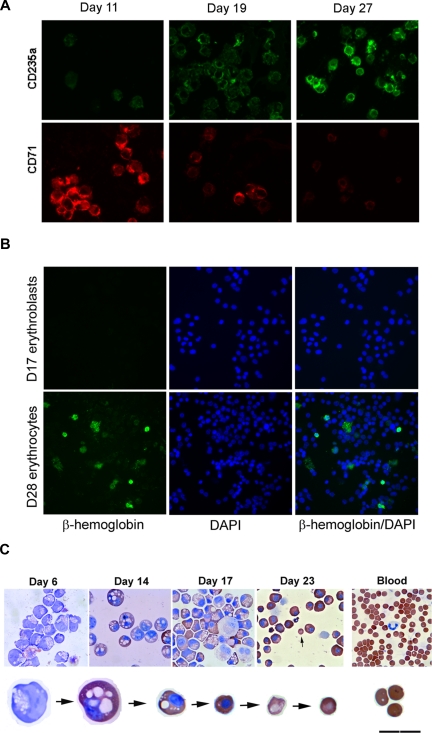
To further investigate the events associated with enucleation, we examined multiple characteristics related to the process of erythrocyte maturation. We observed a progressive decrease in cell size and N/C ratio before enucleation occurred. Before transfer to the OP9 stomal layer, the size and N/C of these cells decreased significantly from 18.3 μm in diameter on day 8 to 12.9 μm for nucleated cells (P < .001) and to 7.5 μm for enucleated cells on day 27 (P < .001), and N/C ratios from 0.82 on day 8 to 0.30 by day 27 (P < .001, Figure 4A,B), indicating substantial nuclear condensation during the process. Wright-Giemsa stains demonstrated a gradual progression from blue to purple to pink stain, indicative of pronormoblast to polychromatic erythroblast to orthochromatic normoblast transition (data not shown). These cells expressed a high level of CD71, an early erythroblast marker, on day 8 and decreased their expression over time, although they showed low to negligible level of CD235a (glycophorin A) protein, a mature erythrocyte marker, in the beginning, but increased their expression dramatically with their maturation (Figures 5A, S1). Benzidine stains also showed a progressive accumulation of hemoglobins in these cells and a decrease in cell size over time (Figure 5C).
Figure 5.
Maturation of hESC-derived erythroid cells mimic erythroid development. (A) Expression of CD235a, a mature erythrocyte marker, increases with time; and CD71, an immature RBC marker, shows a decrease in expression over time. (B) Expression of β-globin chain in hESC-derived erythroid cells. Cytospin samples of hESC-derived erythroid cells collected from day17 and day 28 differentiation and maturation cultures were stained with human β-globin chain specific antibody. (C) Progressive maturation of hESC-derived erythroid cells in vitro. Progressive morphologic changes from blast cells to erythroblasts, and eventually matured erythrocytes are accompanied by significant increase of hemoglobin and decrease in size during their in vitro differentiation and maturation. Cells were stained with both Wright-Giemsa and benzidine (A,B: original magnification ×200).
Preliminary experiments confirmed that the immature enucleated erythroid cells mainly expressed the embryonic ζ- and ϵ-globin chains, and the fetal γ-globin chain (Figure 1E). Although substantial quantities of α-chains were present in these cells, adult β-globin chains were not detected. Subsequent studies were carried out to determine whether the erythroid cells possess the capacity to express the adult definitive β-globin chain on further differentiation and maturation in vitro. Globin chain specific immunofluorescent analysis showed that the cells increased expression of the adult β-globin chain (0% at day 17, Figure 5B) to approximately 16.37% after 28 days of in vitro culture (some cells expressed the β-globin chain at very high levels, Figures 5B, S2). The expression of β-globin chain gene in these cells was confirmed by globin chain specific RT-PCR analysis10 (Figure S3). Consistent with a recent report,31 we also observed that all the cells expressed the fetal γ- globin chain irrespective of the β-globin chain expression status (data not shown).
Discussion
We describe a system that efficiently supports the development of RBCs from hESC-derived hemangioblasts. This simplified system can be used to reproducibly generate large numbers (1010-1011 cells cells/6-well plate of hESCs) of erythroid cells under serum-free conditions. The use of serum, which contains a variable mixture of cytokines, inducers, and inhibitors, contributes to wide variations in efficiency and reproducibility of hESC differentiation. Olivier et al9 recently reported the production of early-stage (nucleated) RBCs from hESCs, although the system required the use of serum, and no studies were carried to determine if the cells were functional. In addition, the efficiency was less than 0.1% of that reported here.
The expression of both α- and β-globin cluster genes is regulated by upstream locus control regions. Although the mechanisms that control the timing of globin switching are poorly understood,32 normal expression of the various globin genes, especially the non–α-cluster genes, with their distinct expression patterns corresponding to the embryonic, fetal, and adult developmental stages, provides a particularly useful measure of the maturation stage in primates and humans.33 The erythroid cells produced using this methodology express mainly the embryonic ϵ- and ζ- and the fetal Gγ-and α-globin genes, but neither Aγ- nor adult β-globin chains could be detected; whereas one group reported the presence of both Gγ- and Aγ-globin chains in similar cells obtained from hESCs.9,10 It is possible that the cells obtained in the current study are at a different developmental stage and that early developmental stage of human embryos does not express the Aγ-globin chain.34 Another possibility is the methods used by us, Olivier et al,9 and Qiu et al10 are different. The cells in the present study were derived via hemangioblasts (blast cells), and they generated erythroid cells via CD34+ cells isolated from hESCs cocultured on irradiated FH-B-hTERT feeder cells.9,10 Or this may be the result of a genetic polymorphism that the 2 cell lines (MA01 and H1) have 2 Aγ- but no Gγ-globin gene. These observations are consistent with previous studies showing that hematopoietic cells derived from both human and rhesus ESC differentiation dominantly express embryonic ϵ and fetal γ, but low to negligible levels of the adult β-globin gene.2,8,10,19,35,36 However, we also observed that more than 15% of these cells expressed the adult β-globin chain after further in vitro culturing and maturation, suggesting that these cells are able to switch to definitive adult globins.
We demonstrate here for the first time that erythroid cells derived from hESCs possess oxygen equilibrium curves comparable with normal transfusable RBCs and also respond to pH changes (Bohr effect) and depletion of 2,3-diphosphoglyerate. Another critical issue for clinical utilization of hESC-derived RBCs is whether they can be enucleated in vitro. We show that 30% to 65% of the RBCs underwent multiple differentiation events, including a progressive decrease in size and increase in glycophorin A expression (a mature RBC marker) and chromatin/nuclear condensation, which resulted in the extrusion of the pycnotic nucleus to form enucleated erythrocytes with a diameter of 6 to 8 μm, which is similar to normal RBCs. Although efficiency was not as high, hESC-derived RBCs could enucleate under MEF-free and OP9-free conditions, indicating that a mouse feeder-free system is attainable for enucleation. Human MSC coculture improved efficiency of enucleation, but even without these cells, enucleation could be achieved for a completely feeder-free system. Although generation of enucleated erythrocytes has been achieved from the mouse ESC system,37 only background enucleation rates (< 1%-7%) have been reported in the human ESC system.10 However, further study will be needed to investigate their in vivo function.
Erythropoiesis in mammals consists of 2 waves: (1) primitive erythropoiesis initiated in the yolk sac with the generation of large nucleated erythroblasts and (2) definitive erythropoiesis arising from the fetal liver with the development of smaller enucleated erythrocytes. Thus, the presence or absence of a nucleus has long been accepted as a key distinguishing feature for primitive and definitive erythroid cells. Several reports have recently demonstrated that primitive erythroblasts undergo differentiation events with the generation of enucleated erythrocytes in the mouse embryo.38–40 Our data clearly demonstrate that hESC-derived primitive erythroblasts are also capable of enucleation in vitro.
Although enucleation of erythroblasts was structurally studied by electron microscopy almost half a century ago,41 little is known about the underlying mechanism(s). It has been suggested that enucleation is the result of asymmetric cell division involving extrusion of a pycnotic nucleus enveloped by the plasma membrane,42 and that Rac GTPases and their effector mDia2 play important roles in the process.43 Studies also suggest that direct contact of erythroblasts with macrophages promotes nuclear extrusion and that knockout of erythroblast-macrophage-protein (Emp) results in the failure of enucleation.44–46 However, 2 groups recently demonstrated that, although macrophages play a role in the maturation of erythroblasts, they are neither sufficient nor required for red cell enucleation in the mouse system.47,48 The current system provides an excellent model to investigate these and various other molecular and cellular mechanisms involved in the enucleation of human RBCs, and could help further optimize conditions to induce synchronous terminal differentiation of these cells.
Limitations in the supply of RBCs can have potentially life-threatening consequences for patients with massive blood loss resulting from trauma or surgery or who have diseases that cause severe anemia. Although alternative sources of progenitors for the generation of large-scale transfusable RBCs have been investigated, including cord blood, bone marrow, and peripheral blood,26,27,49–51 it is clear that, even after expansion and differentiation, these progenitors represent donor-limited sources of RBCs. Moreover, the low prevalence of O(−) type blood in the general population (< 8% in Western countries and < 0.3% in Asia) further intensifies the consequences of blood shortages for emergency situations where blood typing may not be possible. In the present study, we have developed a reproducible system to generate erythroid cells with oxygen-carrying ability from hESCs that is suitable for scale-up. The successful enucleation of these cells suggests a potential future role for hESCs as a donorless source of RBC for transfusion. The identification of a hESC line with a O(−) genotype would permit the production of ABO and RhD compatible (and pathogen-free) “universal donor” RBCs. Work is also currently under way to generate multiple induced pluripotent stem cell lines52,53 from fibroblasts obtained from individuals with type O(−) blood.
Supplementary Material
Acknowledgments
The authors thank Chenmei Luo, Katherine Holton, Yordanka Ivanova, and Feng Li for technical assistance.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.-J.L. designed research, collected and analyzed data, and wrote the paper; Q.F. and J.S.P. performed research, collected and analyzed data, and contributed toward writing the paper; L.V., B.-S.L., M.S., P.J.W., and G.R.H. performed research and collected and analyzed data; and R.L. conceptualized the study and design and wrote the paper.
Conflict-of-interest disclosure: S.-J.L., Q.F., J.S.P., and R.L. are employees of Advanced Cell Technology, a stem cell company in the field of regenerative medicine. L.V., B.-S.L., M.S., P.J.W., and G.R.H. declare no competing financial interests.
Correspondence: George R. Honig, Department of Pediatrics, University of Illinois at Chicago, Chicago, IL 60612; e-mail: ghonig@uic.edu; or Robert Lanza, Advanced Cell Technology, 381 Plantation Street, Worcester, MA 01605; e-mail: rlanza@advancedcell.com.
References
- 1.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu S-J, Li F, Vida L, Honig GR. CD34+CD38- hematopoietic precursors derived from human embryonic stem cells exhibit an embryonic gene expression pattern. Blood. 2004;103:4134–4141. doi: 10.1182/blood-2003-10-3575. [DOI] [PubMed] [Google Scholar]
- 3.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Menendez P, Shojaei F, et al. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med. 2005;201:1603–1614. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 6.Woll PS, Martin CH, Miller JS, Kaufman DS. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol. 2005;175:5095–5103. doi: 10.4049/jimmunol.175.8.5095. [DOI] [PubMed] [Google Scholar]
- 7.Zambidis ET, Peault B, Park TS, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang KH, Nelson AM, Cao H, et al. Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood. 2006;108:1515–1523. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivier EN, Qiu C, Velho M, Hirsch RE, Bouhassira EE. Large-scale production of embryonic red blood cells from human embryonic stem cells. Exp Hematol. 2006;34:1635–1642. doi: 10.1016/j.exphem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Qiu C, Olivier EN, Velho M, Bouhassira EE. Globin switches in yolk-sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood. 2008;111:2400–2408. doi: 10.1182/blood-2007-07-102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ney PA. Gene expression during terminal erythroid differentiation. Curr Opin Hematol. 2006;13:203–208. doi: 10.1097/01.moh.0000231415.18333.2c. [DOI] [PubMed] [Google Scholar]
- 12.Cowan CA, Klimanskaya I, McMahon J, et al. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med. 2004;350:1353–1356. doi: 10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- 13.Lu SJ, Feng Q, Caballero S, et al. Generation of functional hemangioblasts from human embryonic stem cells. Nat Methods. 2007;4:501–509. doi: 10.1038/nmeth1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helgason CD, Sauvageau G, Lawrence HJ, Largman C, Humphries RK. Overexpression of HOXB4 enhances the hematopoietic potential of embryonic stem cells differentiated in vitro. Blood. 1996;87:2740–2749. [PubMed] [Google Scholar]
- 15.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Yates F, Naveiras O, Ernst P, Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci U S A. 2005;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowles KM, Vallier L, Smith JR, Alexander MR, Pedersen RA. HOXB4 overexpression promotes hematopoietic development by human embryonic stem cells. Stem Cells. 2006;24:1359–1369. doi: 10.1634/stemcells.2005-0210. [DOI] [PubMed] [Google Scholar]
- 18.Pilat S, Carotta S, Schiedlmeier B, et al. HOXB4 enforces equivalent fates of ES-cell-derived and adult hematopoietic cells. Proc Natl Acad Sci U S A. 2005;102:12101–12106. doi: 10.1073/pnas.0505624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu SJ, Feng Q, Ivanova Y, et al. Recombinant HoxB4 fusion proteins enhance hematopoietic differentiation of human embryonic stem cells. Stem Cells Dev. 2007;16:547–560. doi: 10.1089/scd.2007.0002. [DOI] [PubMed] [Google Scholar]
- 20.Honig GR, Vida LN, Latorraca R, Divgi AB. Hb south Milwaukee [beta 105 (G7) Leu-Phe]: a newly-identified hemoglobin variant with high oxygen affinity. Am J Hematol. 1990;34:199–203. doi: 10.1002/ajh.2830340308. [DOI] [PubMed] [Google Scholar]
- 21.Honig GR, Vida LN, Rosenblum BB, Perutz MF, Fermi G. Hemoglobin Warsaw (Phe beta 42(CD1)-Val), an unstable variant with decreased oxygen affinity: characterization of its synthesis, functional properties, and structure. J Biol Chem. 1990;265:126–132. [PubMed] [Google Scholar]
- 22.Lee BS, Krishnanchettiar S, Lateef SS, Lateef NS, Gupta S. Characterization of oligosaccharide moieties of intact glycoproteins by microwave-assisted partial acid hydrolysis and mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:2629–2635. doi: 10.1002/rcm.2096. [DOI] [PubMed] [Google Scholar]
- 23.Arce MA, Thompson ES, Wagner S, et al. Molecular cloning of RhD cDNA derived from a gene present in RhD-positive, but not RhD-negative individuals. Blood. 1993;82:651–655. [PubMed] [Google Scholar]
- 24.Simsek S, Faas BH, Bleeker PM, et al. Rapid Rh D genotyping by polymerase chain reaction-based amplification of DNA. Blood. 1995;85:2975–2980. [PubMed] [Google Scholar]
- 25.Yamamoto F, Clausen H, White T, Marken J, Hakomori S. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990;345:229–233. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]
- 26.Giarratana MC, Kobari L, Lapillonne H, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 27.Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006;24:1255–1256. doi: 10.1038/nbt1245. [DOI] [PubMed] [Google Scholar]
- 28.Maurer HS, Behrman RE, Honig GR. Dependence of the oxygen affinity of blood on the presence of foetal or adult haemoglobin. Nature. 1970;227:388–390. doi: 10.1038/227388a0. [DOI] [PubMed] [Google Scholar]
- 29.Wada H, Suda T, Miura Y, et al. Expression of major blood group antigens on human erythroid cells in a two phase liquid culture system. Blood. 1990;75:505–511. [PubMed] [Google Scholar]
- 30.Hosoi E, Hirose M, Hamano S. Expression levels of H-type alpha(1,2)-fucosyltransferase gene and histo-blood group ABO gene corresponding to hematopoietic cell differentiation. Transfusion. 2003;43:65–71. doi: 10.1046/j.1537-2995.2003.00277.x. [DOI] [PubMed] [Google Scholar]
- 31.Zambidis ET, Park TS, Yu W, et al. Expression of angiotensin-converting enzyme identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells [abstract].. 6th ISSCR Annual Meeting; Philadelphia, PA. 2008. p. 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Exp Hematol. 2005;33:259–271. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peschle C, Migliaccio AR, Migliaccio G, et al. Embryonic-fetal Hb switch in humans: studies on erythroid bursts generated by embryonic progenitors from yolk sac and liver. Proc Natl Acad Sci U S A. 1984;81:2416–2420. doi: 10.1073/pnas.81.8.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Migliaccio A R, Papayannopoulou T. Erythroporesis. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge, United Kingdom: Cambridge University Press; 2001. pp. 52–71. [Google Scholar]
- 35.Honig GR, Li F, Lu S-J, Vida L. Hematopoietic differentiation of Rhesus monkey embryonic stem cells. Blood Cells Mol Dis. 2004;32:5–10. doi: 10.1016/j.bcmd.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Qiu C, Hanson E, Olivier E, et al. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp Hematol. 2005;33:1450–1458. doi: 10.1016/j.exphem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Carotta S, Pilat S, Mairhofer A, et al. Directed differentiation and mass cultivation of pure erythroid progenitors from mouse embryonic stem cells. Blood. 2004;104:1873–1880. doi: 10.1182/blood-2004-02-0570. [DOI] [PubMed] [Google Scholar]
- 38.Fraser ST, Isern J, Baron MH. Maturation and enucleation of primitive erythroblasts during mouse embryogenesis is accompanied by changes in cell-surface antigen expression. Blood. 2007;109:343–352. doi: 10.1182/blood-2006-03-006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGrath KE, Kingsley PD, Koniski AD, et al. Enucleation of primitive erythroid cells generates a transient population of “pyrenocytes” in the mammalian fetus. Blood. 2008;111:2409–2417. doi: 10.1182/blood-2007-08-107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kingsley PD, Malik J, Fantauzzo KA, Palis J. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood. 2004;104:19–25. doi: 10.1182/blood-2003-12-4162. [DOI] [PubMed] [Google Scholar]
- 41.Skutelsky E, Danon D. An electron microscopic study of nuclear elimination from the late erythroblast. J Cell Biol. 1967;33:625–635. doi: 10.1083/jcb.33.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chasis JA, Prenant M, Leung A, Mohandas N. Membrane assembly and remodeling during reticulocyte maturation. Blood. 1989;74:1112–1120. [PubMed] [Google Scholar]
- 43.Ji P, Jayapal SR, Lodish HF. Enucleation of cultured mouse fetal erythroblasts requires Rac GTPases and mDia2. Nat Cell Biol. 2008;10:314–321. doi: 10.1038/ncb1693. [DOI] [PubMed] [Google Scholar]
- 44.Hanspal M, Hanspal JS. The association of erythroblasts with macrophages promotes erythroid proliferation and maturation: a 30-kD heparin-binding protein is involved in this contact. Blood. 1994;84:3494–3504. [PubMed] [Google Scholar]
- 45.Soni S, Bala S, Gwynn B, et al. Absence of erythroblast macrophage protein (Emp) leads to failure of erythroblast nuclear extrusion. J Biol Chem. 2006;281:20181–20189. doi: 10.1074/jbc.M603226200. [DOI] [PubMed] [Google Scholar]
- 46.Hanspal M, Smockova Y, Uong Q. Molecular identification and functional characterization of a novel protein that mediates the attachment of erythroblasts to macrophages. Blood. 1998;92:2940–2950. [PubMed] [Google Scholar]
- 47.Spike BT, Dibling BC, Macleod KF. Hypoxic stress underlies defects in erythroblast islands in the Rb-null mouse. Blood. 2007;110:2173–2181. doi: 10.1182/blood-2007-01-069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isern J, Fraser ST, He Z, Baron MH. The fetal liver is a niche for maturation of primitive erythroid cells. Proc Natl Acad Sci U S A. 2008;105:6662–6667. doi: 10.1073/pnas.0802032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leberbauer C, Boulme F, Unfried G, et al. Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood. 2005;105:85–94. doi: 10.1182/blood-2004-03-1002. [DOI] [PubMed] [Google Scholar]
- 50.Malik P, Fisher TC, Barsky LL, et al. An in vitro model of human red blood cell production from hematopoietic progenitor cells. Blood. 1998;91:2664–2671. [PubMed] [Google Scholar]
- 51.Neildez-Nguyen TM, Wajcman H, Marden MC, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002;20:467–472. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- 52.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.