Abstract
Graft-versus-tumor effects can be achieved after allogeneic bone marrow transplantation in patients with malignancies of the kidney or hematopoietic system but are often accompanied by severe graft-versus-host-disease (GVHD). We sought to maximize graft-versus-tumor while minimizing GVHD using tumor-specific allogeneic effector T cells rather than open-repertoire T cells. We transferred allogeneic CD8+ pmel-1 or CD4+ TRP-1 T cells specific for the melanoma-associated antigens, glycoprotein 100 (gp100) and tyrosinase-related protein-1 (TRP-1), respectively, into B16-melanoma–bearing mice. Mice receiving a preparative regimen of nonmyeloablating (5 Gy) total body irradiation experienced the rapid rejection of tumor-specific allogeneic lymphocytes with no impact on tumor growth. However, when mice were given more intense total body irradiation conditioning regimens combined with autologous bone marrow transplantation, adoptively transferred allogeneic tumor-specific T lymphocytes persisted at detectable levels for several weeks and mediated significant regression of large, vascularized tumors. We found that the risk of GVHD was low when tumor-specific T cells were transferred and significant toxicity was observed only when substantial numbers of open repertoire allogeneic naive T cells were mixed with the tumor-specific lymphocytes. Taken together, these data indicate that the use of tumor-specific allogeneic CD8+ T cells or CD4+ can result in significant antitumor effects in the absence of measurable GVHD.
Introduction
The ability of T lymphocytes to recognize antigens with a high degree of discrimination and mediate tissue destruction makes them excellent candidates for treating malignancies. After successes obtained in treating hematologic malignancies with allogeneic bone marrow (BM) transplantation (BMT), many investigators have tried to use the same approach for solid tumors, relying on the capacity of allogeneic lymphocytes to mediate a graft-versus-tumor (GVT) effect. Unfortunately, most solid tumors have proven to be resistant to this therapy, and beneficial clinical responses were observed only for renal cell carcinoma1,2 with anecdotal successes for other cancer types.3–8 Nevertheless, reports of regressions after the development of graft-versus-host disease (GVHD) in allogeneic BMT recipients constitute a proof-of-principle that solid tumors are susceptible to immune rejection.
A factor that might be important in limiting the successes of allogeneic BMT is the lack of tumor specificity. Antigenic differences between tumor cells and their nontransformed counterparts are poorly defined.9,10 Those differences might not be sufficient to reproducibly create a therapeutic window between GVT and GVHD. The toxicity of this therapy remains high, although it is diminished with reduced intensity conditioning regimens.1,11–14
Adoptive cell transfer (ACT) of autologous tumor-specific T cells represents a promising alternative to GVT using open-repertoire cells. ACT of tumor-specific cells derived from tumor infiltrating lymphocytes can lead to objective tumor regression in approximately 50% of treated patients who have metastatic melanoma with a favorable toxicity profile.15,16 This approach represents a good, though still experimental, treatment for patients with metastatic melanoma. Its use is limited by the fact that cells must be obtained through a surgical procedure, which is not always feasible, and the process of ex vivo expansion is labor intensive and requires a considerable amount of expertise and time.17
Allogeneic tumor-specific T lymphocytes could provide an alternative to bridging BMT and autologous T-cell therapy. There are clear precedents for the use of allogeneic T cell–based therapy in patients.18,19 In a recent publication by Haque et al, a bank of Epstein-Barr virus (EBV)–specific cytotoxic T-lymphocyte lines was established and used to treat patients affected by EBV-positive posttransplantation lymphoproliferative disorder in a multicenter phase 2 clinical trial.20 The results from this trial were very encouraging, with 64% of the patients achieving objective responses, including 43% complete responses at 6 months. The establishment of the cell bank in the latter clinical trial had the advantage of considerably shortening the time required to generate anti-EBV cytotoxic T lymphocyte and, more importantly, for providing cells for patients incapable of generating autologous EBV-specific cells. Another interesting study showed the use of allogeneic T cells to treat 4-day-old pulmonary metastases in the MC38 system.21 Additional preclinical results used a an experimental model of lymphoma treated with in vitro–derived allogeneic major histocompatibility complex (MHC)–mismatched T-cell precursors.22 However, the use of a similar strategy for vascularized solid tumors could be hampered by T-cell trafficking to tumors, low expression of MHC molecules, and the lack of highly immunogenic target antigens that are derived from foreign viral proteins.
One major concern is the survival of the effectors. The persistence of transferred cells might be crucial for a favorable clinical outcome.23–25 However, it still is not clear if persistence is a requirement for effective treatment or an epiphenomenon related to the other characteristics of therapeutically effective cells. Allogeneic cells with major MHC mismatches are rapidly rejected by the host immune system. In immunocompetent hosts, MHC-mismatched leukocytes present in nonirradiated blood products were shown to persist for not more than 5 to 6 days.26,27 Thus, although allogeneic cells specific for highly immunogenic viral antigens can be effective in the posttransplantation setting, it is not known whether allogeneic cells would be a viable option for adoptive immunotherapy of large, established solid tumors.
In the present paper, we sought to explore the feasibility of using tumor-specific allogeneic T cells in the treatment of the experimental mouse melanoma, B16, after a preparative regimen composed of total body irradiation (TBI) and autologous BMT. We use 2 different T-cell receptor (TCR) transgenic mouse models. The first is based on the CD8+, MHC class I–restricted, glycoprotein 100 (gp100)–specific pmel-1–transgenic model.28 The second is the recently described CD4+, MHC class II–restricted, tyrosinase-related protein-1 (TRP-1)–specific transgenic model.29 We also studied the ability of allogeneic cells to survive after transfer. As a final point, we explored the risk of inducing GVHD-like reactions when allogeneic T cells are used as a cell source.
Methods
Mice and tumor lines
All mice used in these experiments were bred and housed at National Institutes of Health facilities. Female pmel-1 TCR-transgenic (Tg) mice were crossed with C57BL/6-Thy1.1+-Tg (The Jackson Laboratory, Bar Harbor, ME) to derive pmel-1-Thy1.1+ double-Tg mice. Rag1−/− BW TRP-1 mice were bred at the National Institutes of Health and have been previously described.29 Male Rag1−/− BW TRP-1 mice were crossed with Rag1−/− BALB/c mice to derive TRP-1 F1 mice expressing an H-2b/d phenotype. Female pmel-1 TCR-Tg (H-2b/b) mice were crossed with BALB/c mice (H-2d/d), C3H mice (H-2k/k), and DBA mice (H-2d/d) (The Jackson Laboratory) to derive pmel-1-F1 expressing different MHC haplotypes (H-2b/d, H-2b/k, and H-2b/d, respectively). Female C57BL/6 wild-type and C57BL/6 Rag1−/− (both H-2b/b) were used as recipients in ACT experiments. BALB/c mice (H-2d/d), C3H mice (H-2k/k), DBA/2J mice (H-2d/d), B6-A F1 mice (H-2b/a), and SJL/J mice (H-2b/s) were purchased from The Jackson Laboratory. Rag1−/− BW TRP-1 TCR-Tg mice (H-2b/b) were previously described.29 Rag1−/− BW TRP-1 TCR-Tg mice were crossed with Rag1−/− BALB/c mice to obtain Rag1−/− TRP-1-BALB/c F1 mice (H-2b/d). Experiments were conducted with the approval of the National Cancer Institute Animal Use and Care Committee. B16-F10 (H-2b/b), a transplantable gp100+ murine melanoma, was maintained in culture medium (CM).28
In vitro activation of pmel-1 CD8+ T cells, TRP-1 CD4+ T cells, and cytokine release assays
pmel-1 splenocytes were isolated from spleens of donor mice and cultured in the presence of 1 μM human (h) gp10025-33 and CM containing 30 IU/mL recombinant human (rh) IL-2 (Chiron, Emeryville, CA). For the TRP-1 CD4+ cell cultures, splenocytes from Rag1−/− BW TRP-1 TCR-Tg mice were plated at 106 cells/mL in CM containing 30 IU/mL rhIL-2 (Chiron). One million C57BL/6 3000-cGy–irradiated splenocytes pulsed with 1 μM TRP-1106-130 (SGHNCGTCRPGWRGAACNQKILTVR) peptide were added as stimulators. Cells were used for adoptive transfer 6 to 7 days after the start of the culture. The same procedure was followed for all different pmel-1 backgrounds used in the present manuscript. We used the R&D Systems (Minneapolis, MN) antibody pair according to the manufacturer's protocol for testing the release of interferon-γ (IFN-γ).
Adoptive cell transfer
Mice 6 to 12 weeks of age (n = 5-6 for all groups) were injected subcutaneously with 2 × 105 to 5 × 105 B16-F10 melanoma cells and treated 10 to 14 days later with intravenous adoptive transfer of in vitro–activated pmel-1 CD8+ T splenocytes or Rag1−/− BW TRP-1 CD4+ cells derived from different F1 donors as indicated. Recombinant vaccinia virus expressing human gp100 (2 × 107 plaque-forming units) was administered intravenously, and interleukin-2 (IL-2) was given as 600 000 international units (IU) intraperitoneally twice daily for 6 doses. Lymphopenia was induced by nonmyeloablative (5 Gy) or myeloablative (9 Gy) TBI on the day before the cell transfer, and hematopoietic stem cells derived from bone marrow of donor animals were transferred on day 1 after treatment; 106 unsorted bone marrow cells were administered. Tumors were measured using calipers, and the tumor area was calculated as the products of the perpendicular diameters. All experiments were performed in a blinded, randomized fashion and performed independently at least twice, with similar results.
Enumeration of cells in vivo
At indicated time points, spleens were resected, and total cell numbers were enumerated by trypan blue exclusion. Samples were analyzed by flow cytometry for Thy1.1, CD8a, Vβ14, H-2 Dd, and Vβ13 (BD Biosciences, San Jose, CA). Cytofluorimetric analysis was done on a “lymphocytes gate” identified by physical parameters and staining with propidium iodide. Numbers of adoptively transferred cells were calculated by multiplying the total number of live splenocytes by the percentage of CD8+ Vβ13+ cells.
Histology
Eyes were enucleated 14 days after adoptive transfer, fixed in 10% formalin, embedded in methylacrylate, sectioned via papillary-optic nerve axis, and stained with hematoxylin and eosin. Images were obtained using a Nikon Eclipse E400 microscope (Tokyo, Japan) equipped with Nuance Multispectral Imaging System VIS and related software (CRI, Woburn, MA). Original magnification was ×100. Images were processed using Adobe Photoshop, version 7 (Adobe Systems, San Jose, CA).
Statistics
Tumor graphs were compared using analysis of variance. P values less than .05 were considered significant.
Results
The intensity of the conditioning regimen directly correlates with cell persistence after transfer
To address whether allogeneic tumor-specific T cells could be effectively used to treat established tumors, we created an allogeneic adoptive immunotherapy model based on the B16/pmel-1 system that we previously described.28 pmel-1 mice are Tg for a TCR that recognize the H-2 Db-restricted epitope of the melanocyte differentiation antigen gp100 corresponding to amino acids 25 to 33 that is naturally expressed on the B16 melanoma tumor. To create a source of tumor-specific allogeneic effector cells, we crossed pmel-1 mice (H-2b/b) with wild-type BALB/c mice (H-2d/d) to generate F1-pmel-1 mice with a MHC H-2b/d genotype (pmel-1b/d).
To asses the ability of allogeneic effector cells to persist in MHC-mismatched hosts, we transferred pmel-1b/d cells into either unmanipulated C57BL/6 mice or C57BL/6 mice that received 5 Gy TBI as lymphodepleting regimen before the transfer. The administration of 5 Gy TBI is a well-established conditioning regimen that induces a profound but transient lymphodepletion lasting for 7 to 10 days.30,31 This dose has previously been shown to augment the effectiveness of adoptive cell transfer therapy in the pmel-1 model through several mechanisms, including the removal of immunoregulatory elements, “cytokine sinks,” and activation of antigen-presenting cells.30,32 We wanted to investigate whether this conditioning regimen induced a sufficient degree of immunosuppression that would enable the transient engraftment of allogeneic cells. As a control, we transferred allogeneic pmel-1 cells into Rag1−/− hosts, which genetically lack T and B lymphocytes. In both nonirradiated and 5 Gy TBI preconditioned mice, allogeneic cells were rapidly rejected with very little persistence observed at day 5 and no detectable cells at day 10 after transfer. In contrast, allogeneic cells that were transferred into immunodeficient Rag1−/− recipients not only persisted but expanded over time (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
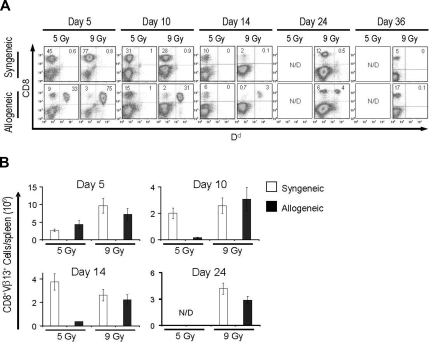
Because 5 Gy TBI did not confer any significant survival advantage to adoptively transferred allogeneic T cells, even at shorter time points, we sought to test whether a stronger lymphodepleting/immunosuppressive regimen would lead to a better engraftment. We treated tumor-bearing C57BL/6 mice preconditioned with 5 or 9 Gy TBI with pmel-1b/d cells and added a specific vaccination and exogenous IL-2. We analyzed the animals at different time points for the presence of allogeneic cells expressing the MHC class I H-2 Dd molecule. Because 9 Gy is a myeloablative dose of TBI, all groups received an autologous BMT on the day after the transfer of allogeneic pmel-1 T cells. In mice that received 5 Gy TBI, allogeneic cells briefly persisted and were detectable in secondary lymphoid organs at day 5 but completely disappeared by day 10 (Figure 1A). In contrast, allogeneic donor cells were clearly measurable in 9 Gy irradiated hosts up to 24 days after transfer and were rejected only by day 36 (Figure 1A). Allogeneic CD8+ lymphocytes were also present at later time points in the draining lymph nodes and at the tumor site, indicating that they not only persisted but were capable of trafficking to peripheral sites (Figure S2).
Figure 1.
Allogeneic antitumor T lymphocyte persistence in vivo. C57BL/6 mice bearing B16 tumors were irradiated with 5 or 9 Gy TBI and injected on day 0 with 106 allogeneic pmel-1b/d cells, vaccinia virus encoding hgp10025-33, and exogenous rhIL-2 (alloPVI) or with 106 syngeneic pmel-1b/b cells, vaccinia virus encoding hgp10025-33, and exogenous rhIL-2 (synPVI). All groups received syngeneic BMT with 106 unsorted bone marrow cells the day after the transfer of the effector cells. At the indicated time points, mice were killed, and the spleens were analyzed by flow cytometry for the presence of the transferred cells. (A) The dot plots show the percentage of CD8+ H-2 Dd+ cells. Allogeneic cells were detectable up to day 24 after transfer. (B) Absolute numbers of CD8+Vβ13+ cells present in the spleens of the animals. Each bar represents 3 mice plus or minus SE. Data are representative of 3 independent experiments.
We sought to have a more reliable quantitative comparison of adoptively transferred syngeneic and allogeneic T cells. Because pmel-1 T cells are CD8+ and express the Vβ13 rearrangement of the TCR, we analyzed the absolute numbers of CD8+Vβ13+ cells present in the spleens. As shown in Figure 1B, pmel-1 cells reached a peak of expansion around day 5 in both the allogeneic and syngeneic groups and subsequently declined. However, in the 5-Gy setting, allogeneic cells did not persist after day 10. CD8+Vβ13+ cells observed in the allogeneic group in Figure 1B past day 5 represented endogenous Vβ13+ cells because they did not express H-2 Dd (Figure 1A). In contrast, in the 9-Gy setting, allogeneic cells persisted for at least 24 days. These data indicated that, despite the MHC barrier, allogeneic effector cells can transiently engraft for relatively long periods after an increased intensity conditioning regimen.
Effective tumor treatment with allogeneic effector cells is observed after administration of a highly immunosuppressive conditioning regimen
Next, we wanted to investigate the ability of allogeneic effector cells to treat an established tumor. We used pmel-1b/d cells to treat B16 melanoma-bearing C57BL/6 mice. This model eliminates the possibility of a GVHD-like reaction because F1 lymphocytes are tolerant to all antigens in the C57BL/6 background, but rejection of the donor cells remained efficient (Figure S1, Figure 1). This is because the endogenous lymphocytes present in the recipient recognize allogeneic MHC molecules expressed by the tumor-specific lymphocytes. Tumors were implanted subcutaneously and allowed to grow for 8 to 10 days before treatment. On the day of the transfer, recipient mice were preconditioned with 9 Gy TBI and received vaccination and adjuvant administration of exogenous recombinant IL-2. One day after treatment, all groups were injected with syngeneic (C57BL/6 H-2b/b) bone marrow cells. This tripartite regimen was previously published by our group and proven highly effective in the eradication of well-established, large vascularized tumors.28,30,33 We chose a 9 Gy TBI dose as this was previously established to be effective in our model.31
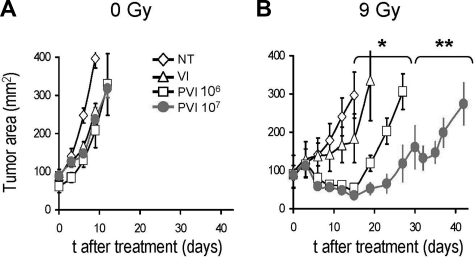
Allogeneic haploidentical effector cells did not cause significant tumor regression in nonirradiated (0 Gy) mice (Figure 2A). In contrast, when mice were subjected to an intense conditioning regimen of 9 Gy TBI, we observed significant tumor regression (Figure 2B; no treatment vs pmel-1 T cells [106] with vaccine and IL-2 [PVI]; P < .001). Increasing the dose of allogeneic effector cells by 10-fold (ie, 107 cells instead of 106) led to a better tumor treatment (106 vs 107 pmel-1 T cells, P < .05) in the 9-Gy group, but it did not impact the nonirradiated animals (Figure 2A,B).
Figure 2.
Allogeneic tumor-specific lymphocytes can mediate tumor regression after intensive lymphodepleting preparatory regimen. C57BL/6 mice bearing B16 tumors were left untreated (A) or irradiated with 9 Gy TBI (B). Mice were left untreated as a control (NT) or injected on day 0 with vaccinia virus encoding hgp100 and exogenous IL-2 (VI) or injected with 106 or 107 (as indicated) allogeneic pmel-1b/d cells, vaccine, and IL-2 (PVI). *P < .001. **P < .05. All groups received syngeneic BMT with 106 unsorted bone marrow cells the day after the transfer of the effector cells (day 1). Results of tumor area are the mean of measurements of at least 5 mice per group (± SEM). Data are representative of 4 independent experiments.
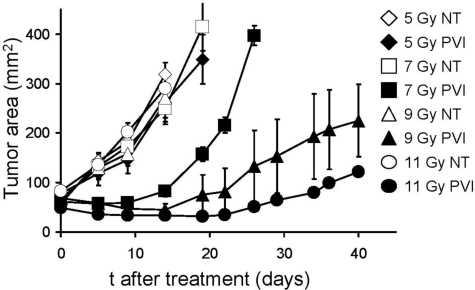
To further test the association between the intensity of conditioning TBI and therapeutic efficacy, we exposed tumor-bearing mice to increasing doses of irradiation, starting at 5 Gy and escalating up to 11 Gy (Figure 3). Consistent with the previous findings, treatment outcome directly correlated with the dose of TBI. In particular, significant tumor treatment was evident when mice received at least 7 Gy TBI, whereas transfer of allogeneic antitumor cells did not show any effect in 5 Gy-irradiated hosts (5 Gy PVI vs 7 Gy PVI, P < .04). Similar data were also obtained using a different pmel-1-F1 (B6-C3H F1 H-2b/k) as a source of donor cells (data not shown). Taken together, these data suggest that an intense lymphodepleting/immunosuppressive regimen is critical for the therapeutic effectiveness of allogeneic tumor-specific lymphocytes, with obvious implications for the design of future clinical trials.
Figure 3.
Therapeutic efficacy of allogeneic cells correlates with the intensity of the lymphodepleting preparatory regimen. C57BL/6 mice bearing B16 tumors were irradiated with 5, 7, 9, or 11 Gy TBI. For each irradiation dose, mice were left untreated as a control (NT) or injected on day 0 with 106 allogeneic pmel-1b/d cells, vaccinia virus encoding hgp100, and exogenous rhIL-2 (PVI). All groups received syngeneic BMT with 106 unsorted bone marrow cells the day after the transfer of the effector cells. Statistical results are as follows: 5 Gy NT versus 5 Gy PVI, not significant; 11 Gy NT versus 11 Gy PVI, P < .02; 5 Gy PVI versus 7 Gy PVI, P < .04; 7 Gy PVI versus 9 Gy PVI, P < .03. There was no statistical difference between mice receiving 9 Gy versus 11 Gy plus PVI. Results of tumor area are the mean of measurements of at least 5 mice per group (± SEM). Data are representative of 2 independent experiments.
Adoptive transfer of allogeneic CD4+ T cells mediates effective tumor regression and ocular autoimmunity
We wanted to test whether our findings could be extended to adoptive immunotherapy based on allogeneic antitumor CD4+ T helper cells. We used CD4+ T lymphocytes expressing a Tg TCR-specific melanocyte differentiation antigen, TRP-1.29 These cells were recently described to be able to eradicate large tumors in an autologous setting.29 TRP-1–Tg mice were bred on a C57BL/6 Rag1−/− H-2b/b background, and allogeneic effectors were generated by crossing them with BALB/c Rag1−/− H-2d/d mice to obtain TRP-1 H-2b/d Tg cells.
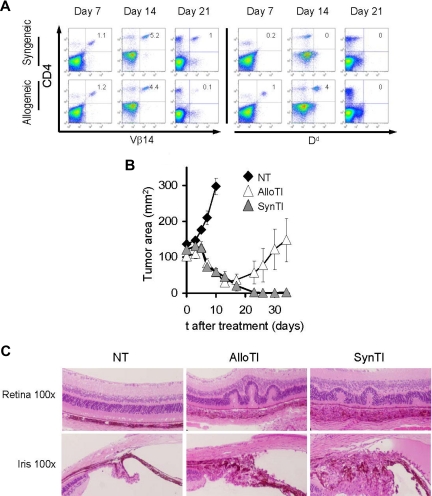
When given after a preparative regimen of 9 Gy TBI and autologous BMT, TRP-1d/b cells were detectable for 2 weeks after transfer, as measured by Vβ14 (the TCR variable chain used by TRP-1) and Dd (Figure 4A). As was observed with the pmel-1 model, allogeneic TRP-1 cells induced tumor regression (P < .02 NT vs AlloTI) and complete tumor cure was observed in 3 of 8 mice. Tumor regression using syngeneic T cells seemed more efficient than allogeneic T cells, but the difference did not achieve statistical significance (AlloTI vs SynTI, P = .062; Figure 4B). Note that, in the CD8+ pmel-1 model, syngeneic treatment was consistently statistically significantly better than allogeneic treatment, but the relative efficacies of allogeneic and syngeneic cells were not compared between the 2 systems. Furthermore, as was previously observed using syngeneic CD4+ cells,29 mice that received allogeneic CD4+ T cells developed ocular autoimmunity with edema, retinal folding, disruption of pigmented epithelium, and inflammatory infiltration of the choroid (Figure 4C). Thus, ACT using allogeneic MHC class II–restricted effector cells that are specific for the self/tumor-associated antigen, TRP-1, triggered both tumor immunity and autoimmunity. This finding could be of particular importance given the ability of CD4+ of recruiting other endogenous effector cells that might persist after the allogeneic tumor-specific CD4+ have been rejected.34–36
Figure 4.
Allogeneic tumor-specific CD4+ cells cause tumor regression and ocular autoimmunity in lethally irradiated hosts. C57BL/6 mice bearing B16 tumors established for 12 days were irradiated with 9 Gy TBI. Mice were left untreated as a control (NT) or injected on day 0 with 106 allogeneic TRP-1b/d cells and exogenous IL-2 (AlloTI) or 106 syngeneic TRP-1b/b cells and exogenous IL-2 (SynTI). All groups received syngeneic BMT with 106 unsorted bone marrow cells the day after the transfer of the effector cells. (A) Spleens analyzed for the presence of transferred T cells at the indicated time points. The experiment was independently repeated with similar results. (B) B16 tumor growth in the different treatment groups. Results of tumor area are the mean of measurements of at least 5 mice per group (± SEM) (n = 5, 9, and 10 mice for NT, AlloTI, and SynTI, respectively, in the experiment shown). Data are representative of 3 independent experiments. (C) Hematoxylin and eosin staining of ocular tissue of mice killed at day 14. Images were obtained using a Nikon Eclipse E400 microscope equipped with Nuance Multispectral Imaging System VIS (original magnification ×100). Images were processed using Adobe Photoshop, version 7, as described in “Histology.”
Rejection of adoptively transferred lymphocytes is mediated by endogenous T cells rather than those derived from transplanted bone marrow
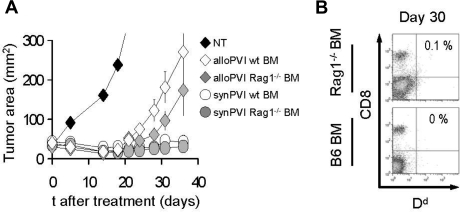
We sought to understand whether allogeneic effectors transferred after 9 Gy TBI were rejected by host lymphocytes that differentiate from the bone marrow stem cells or by surviving lymphocytes that repopulate the host by homeostatic expansion. We treated tumor-bearing C57BL/6 (H-2b/b) animals with a myeloablative dose of 9 Gy TBI and adoptive transfer of pmel-1b/d cells along with vaccination and exogenous rhIL-2 administration. One day after ACT, we injected the animals with bone marrow derived either from wild-type C57BL/6 or Rag1−/− mice. In animals transplanted with Rag1−/− bone marrow, the host lymphocyte populations can only be replaced by surviving cells that homeostatically proliferate after TBI. Interestingly, we did not observe any statistically significant differences between the groups that received normal or Rag1−/− bone marrow in terms of tumor treatment (Figure 5A). Furthermore, allogeneic cells were not detectable at day 30 in both groups (Figure 5B). These results indicate that allogeneic donor cells are likely rejected by the host lymphocytes that survived the irradiation. Surviving host lymphocytes have been shown in previous studies to play a critical role in early immune-reconstitution of irradiated animals.37
Figure 5.
Bone marrow–derived lymphocytes are not necessary for allogeneic effector cell rejection. (A) C57BL/6 mice bearing B16 tumors were irradiated with 9 Gy TBI and injected on day 0 with 106 allogeneic pmel-1b/d cells, vaccinia virus encoding hgp100, and exogenous rhIL-2 (alloPVI) or with 106 syngeneic pmel-1b/b cells, vaccinia virus encoding hgp100, and exogenous rhIL-2 (synPVI). On the day after cell transfer, the mice received syngeneic BMT with 106 unsorted bone marrow cells derived either from wild-type animals (wt BM) or Rag1−/− (Rag1−/− BM) animals. Results of tumor area are the mean of measurements of at least 5 mice per group (± SEM). Data are representative of 2 independent experiments. (B) After 30 days, mice that received allogeneic effector cells were killed, and the spleens were analyzed by flow cytometry for the presence of the transferred cells. Allogeneic CD8+ H-2d+ cells were undetectable at day 30 in both allogeneic PVI groups independently of the type of bone marrow cells they received.
In the same experiment, we compared allogeneic cell treatment with syngeneic effector cells (Figure 5A). We injected syngeneic pmel-1b/b into C57BL/6 mice transplanted either with wild-type or Rag1−/− bone marrow. We did not observe any difference between the 2 groups that received syngeneic cells but different bone marrow. However, syngeneic cell treatment was superior to allogeneic adoptive transfer (alloPVI wild type [wt] BM vs synPVI wt BM, P < .008; alloPVI Rag1−/− BM vs synPVI Rag1−/− BM, P < .03). Similar differences between allogeneic and syngeneic therapy were observed in other experiments (data not shown). These data suggest that transient engraftment is sufficient to mediate antitumor activity, but the potency of the treatment is reduced compared with syngeneic treatment.
The risk of inducing a GVHD-like reaction is minimal when the TCR repertoire is confined
Allogeneic cell lines can induce GVHD-like reactions, especially considering the important requirement of a lymphodepleting regimen before cell transfer.15,30 For example, transfusion-associated GVHD could be a serious, even lethal, complication, and it might occur in immunosuppressed persons that receive nonirradiated blood components.38 We wanted to explore whether treatment with allogeneic CD8+ T lymphocytes bearing a restricted/monoclonal TCR repertoire had a potential for inducing a GVHD-like reaction. Theoretically, because the pmel-1 TCR is selected on the H-2 Db MHC class I molecule, it could cross-react with self-antigens expressed on a different haplotype or even to react with a different MHC class I antigens in a peptide-independent manner.39 We first assessed the ability of pmel-1 cells to react against several different allogeneic MHC. C57BL/6–pmel-1 (H-2b/b) cells cocultured with irradiated splenocytes derived from different inbred mouse strains and F1 mice displaying several allogeneic haplotypes in the presence or the absence of the relevant gp10025-33 peptide (Figure 6A,B). IFN-γ release was observed only in the presence of the relevant peptide and the proper restriction element (H-2 Db), suggesting that the pmel-1 TCR cross-reactivity with allogeneic antigens is minimal.
Figure 6.
GVHD-like reactions are doubtful when the TCR repertoire is limited. (A,B). C57BL/6–pmel-1 (H-2b/b) cells were stimulated with the relevant peptide and cultured for 1 week in IL-2 and subsequently challenged in overnight cocultures against irradiated splenocytes derived from different inbred mouse strains (SJL, H-2s/s and DBA, H-2d/d) and F1 mice (B6-A F1, H-2b/a; B6-C3H F1, H-2b/k; and B6-BALB/c F1, H-2b/d) displaying different allogeneic MHC haplotypes in the presence or the absence of the relevant peptide gp10025-33 (+ pep). Syngeneic C57BL/6 H-2b/b irradiated splenocytes in the presence or the absence of the relevant gp10025-33 peptide were used as positive and negative controls, respectively. Panels A and B are representative of 2 independent experiments. (C) pmel-1 cells were generated on a B6-C3H F1 background (H-2b/k) and used in combination with vaccinia virus encoding hgp100 and exogenous rhIL-2 to treat B16 tumors established for 10 days in either B6-C3H F1 (SynPVI) or in B6-DBA F1 mice (H-2d/b) (AlloPVI). All groups were irradiated with 9 Gy TBI and given autologous BMT. Some groups also received different doses (104 or 105) of open repertoire B6-C3H F1 CD8+ naive T cells in conjunction with pmel-1 cells, vaccine, and rhIL-2 (AlloPVI + 105 CD8 and AlloPVI + 104 CD8). Results of tumor area are the mean of measurements of at least 5 mice per group (± SEM). Data are representative of 2 independent experiments. (D) Percentage of initial weight of mouse groups is shown.
However, many more self-antigens are presented in vivo; thus, this kind of in vitro mixed leukocyte reaction might not be an accurate predictor of what would happen after transfer in a living host. A small number of non–tumor-specific T cells might contaminate cell cultures, even after several rounds of in vitro expansions and selection. To address this point, we transferred pmel-1b/k cells into 9 Gy-irradiated allogeneic haploidentical B6-DBA F1 (H-2b/d) or in 9 Gy-irradiated syngeneic B6C3H F1 (H-2b/k) mice as a control. In some groups, we cotransferred increasing amounts of purified B6-C3H F1 naive open repertoire CD8+ T cells. B16 tumor growth was comparable on different F1 mice (Figure S3). Transfer of pmel-1b/k cells alone or cotransfer of transgenic cells and small numbers of open repertoire CD8+ T cells (ie, 2 × 104) did not result in any measurable toxicity. In contrast, cotransfer of greater quantities (ie, 2 × 105) of open repertoire CD8+ T cells caused a significant loss of weight and cachexia, and all mice eventually died of a lethal GVHD reaction (Figure 6C,D). We did not detect differences between mice treated with pmel-1 cells alone or with pmel-1 cells and the lower dose of open repertoire CD8+ T cells (Figure 6C) (AlloPVI + 104 CD8 vs AlloPVI, P = .22; AlloPVI vs NT, P < .015). Similar to what was observed in other experiments, syngeneic cells were consistently more effective than allogeneic cells (AlloPVI vs SynPVI, P < .05). We observed a trend toward a better tumor treatment in mice that received the higher dose of allogeneic CD8+ T cells (AlloPVI vs AlloPVI + 105 CD8+, P < .02; AlloPVI + 104 CD8+ vs AlloPVI + 105 CD8+, P < .02; SynPVI vs AlloPVI + 105 CD8+, P = .602), but it is possible that tumor growth was impaired because of the severely compromised general health in this group. These latter experiments show that the risk of GVHD is small when the TCR repertoire of the allogeneic effector cells is restricted or monoclonal, but significant toxicity might result after transfer of nonselected T lymphocytes.
Discussion
In the present manuscript, we sought to explore the use of allogeneic MHC partially matched effector cells for adoptive immunotherapy of cancer. Our results indicate that this approach is a safe, feasible, and effective alternative to allogeneic BMT or autologous antitumor T cells in 2 TCR-Tg mouse models. We observed tumor regression in the absence of severe GVHD when a narrow repertoire of T cells was transferred. In vivo survival and persistence of allogeneic haploidentical cells were critically dependent on the administration of an immunoablating/immunosuppressive treatment before the transfer. With a strong lymphodepleting preparative regimen of 9 GyTBI, allogeneic lymphocytes could survive in vivo for several weeks before being eventually rejected. In addition, allogeneic T cells were able to efficiently migrate to secondary lymphoid organs as well as to the tumor site, where they were detectable in significant numbers.
Transient engraftment was necessary and sufficient to induce significant tumor regression. The persistence of adoptively transferred T cells has been previously linked to favorable outcomes in clinical trials of adoptive cell therapy, but it is uncertain whether this is a prerequisite for effective treatment or only an epiphenomenon correlated with successful immunotherapy but not causal.25 After the initial peak of activation and proliferation, T lymphocytes might become functionally exhausted or anergic.40,41 In the pmel-1 model, antitumor T lymphocytes recovered 2 weeks after transfer are no longer capable of readily secreting cytokines and proliferating on antigen reencounter in vitro (data not shown). Moreover, our group has recently shown that elimination of effector cells using anti-CD8 antibody after adoptive cell transfer has no impact on tumor treatment as well as on the development of ocular autoimmunity when done 9 days after infusion.42 Long-term persistence might not be a crucial requirement for ACT. On the other hand, we observed diminished effectiveness of allogeneic compared with syngeneic effectors that is probable because of immune-mediated rejection. Interestingly, allogeneic CD4+ TRP-1–specific cells mediated a surprisingly strong antitumor effect in the absence of specific vaccination, even though they had a shorter survival time compared with allogeneic CD8+ pmel-1 cells. Furthermore, the difference between allogeneic and syngeneic CD4+ TRP-1 cells was much less pronounced than in the pmel-1 setting. In contrast to the CD8+ cell-based model, the differences between the 2 groups in the CD4+ cell-based therapy never reached statistical significance, although we did observe a trend toward a better treatment with the use of syngeneic TRP-1 cells. Although definitive conclusions are not easy to draw with the currently available data, these findings might be explained by the ability of T helper cells to recruit and activate endogenous effector cells from both the innate and adaptative branch of the immune system to the tumor site.34,43,44 In addition, CD4+ T cells facilitate the priming of specific antitumor CD8+ T cells in the tumor-draining lymph nodes.45–47 Thus, the requirement for long-term engraftment might be of lesser importance for antitumor CD4+ T cell–based therapies, making allogeneic T helper cells a particularly viable approach deserving a more detailed investigation.
In the trial published by Haque et al, patients received immunosuppression before ACT to prevent graft rejection.20 In that setting, immunosuppression was a mandatory component of a solid organ transplantation, which triggered posttransplantation lymphoproliferative disease, but it also enabled the allogeneic EBV-specific T cells to survive and function. Immunosuppression could be achieved in several ways, but our data indicate that intense lymphodepletion will be required for successful ACT.15,48 Lymphodepleting regimens offer allogeneic effector cells both the possibility to engraft and the chance to expand under the drive of homeostatic cytokines and the elimination of regulatory elements.30,31,49 In addition, TBI and chemotherapy can promote the generation of a proinflammatory environment that supports lymphocyte activation and function.32,50 It might be possible to take advantage of the myelotoxic/immunosuppressive properties of many chemotherapeutic agents by administering antitumor allogeneic cells as an adjunct to already existing regimens. Antimetabolite drugs such as fludarabine, which have already been used as preparatory regimen for adoptive cell transfer therapy,15 profoundly deplete host lymphocyte populations. Fludarabine has been linked to the development of transfusion associated GVHD several months after its administration.51
Based on previous findings, hematopoietic stem cell transplantations might also be useful in promoting cell expansion.31 Immunosuppressive strategies aimed at decreasing host lymphocyte activation and function might be used to prolong in vivo survival and prevent the development of immunity against alloantigens. Steroids might delay the rejection of allogeneic cells without having a negative impact on the tumor therapy.52 Selective in vivo depletions of host lymphocyte subpopulations with specific antibodies could also be used.53,54
Our data indicate that the risk of adverse reaction because of “off target” recognition may be low if a restricted or monoclonal TCR repertoire is used. We did not detect the induction of GVHD unless significant numbers of allogeneic open repertoire cells were admixed with tumor-specific cells. We observed a trend toward an improved tumor treatment in animals that died of GVHD, but this finding is difficult to interpret in the absence of further data. Many publications have suggested that a GVT reaction might effectively destroy solid tumors in the setting of allogeneic BMT,55,56 but we cannot exclude that slower tumor growth was not because of the severely compromised general health of the hosts in our experiments represented by Figure 6. Interestingly, even though allogeneic open repertoire cells were subjected to rejection, they were capable of causing fatal GVHD. A similar observation was made in a phase 1/2 clinical trial where patients were treated with autologous hematopoietic stem cell transplantation followed by the administration of low or high doses of allogeneic haploidentical donor lymphocytes.57 In this trial, patients treated with high-dose donor lymphocyte infusion developed severe adverse reactions and died of GVHD and bone marrow failure. Thus, it might be useful to screen allogeneic T-cell effectors and remove those cells that are alloreactive.58–60 However, because antitumor T cells generated from tumor-infiltrating lymphocytes have an effector/effector memory phenotype and possess a limited TCR repertoire, the risk of GVHD in this setting should be considerably lower. Indeed, several reports have shown that naive T cells are much more efficient in initiating graft-versus-host reaction compared with memory T cells.61–64
Further applications might include the generation of genetically engineered cell lines, which might be modified to improve survival, proliferation, and effector functions or to deliver proinflammatory molecules to the tumor microenvironment.65–69 In this scenario, short-term engraftment might be an advantage, possibly preventing cells to cause excessive normal tissue damage42 or to undergo malignant transformation.70–74
Taken together, our findings show that allogeneic CD4+ and CD8+ T cells might have a future role in the cell-based immunotherapy of cancer. There are several reasons that make this strategy appealing. “Off-the shelf” cell products would ensure improved selection, standardization, and quality and safety controls. The establishment of a bank of highly reactive tumor-specific T cells would allow the treatment of more patients and to spread this therapy where extensive laboratory expertise and equipment are not available. Multiple administrations, perhaps targeting different antigens, might be more feasible, but development of host immunity against allogeneic targets might thwart the effectiveness of repeat treatments.
In conclusion, a quickly available and reliable “off-the-shelf” cell source would reduce the time and labor required, speeding delivery of the treatment to the patient and reducing the associated costs.
Supplementary Material
Acknowledgment
This study was supported by the intramural program of the National Cancer Institute, National Institutes of Health (Bethesda, MD).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.M. and A.B. wrote the paper and designed and performed experiments; L.C., C.W., C.M.P., D.C.P., L.G., C.S.H., and C.-C.C. helped with some of the experiments and wrote the paper; and S.A.R. and N.P.R. supervised the research and design and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicholas P. Restifo, National Cancer Institute, National Institutes of Health, 10 Center Dr, Bldg CRC, Room 3-5762, Bethesda, MD 20892; e-mail: restifo@nih.gov.
References
- 1.Childs R, Chernoff A, Contentin N, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI, Zimmerman T, Stadler WM, Gajewski TF, Vogelzang NJ. Allogeneic stem-cell transplantation of renal cell cancer after nonmyeloablative chemotherapy: feasibility, engraftment, and clinical results. J Clin Oncol. 2002;20:2017–2024. doi: 10.1200/JCO.2002.08.068. [DOI] [PubMed] [Google Scholar]
- 3.Hentschke P, Barkholt L, Uzunel M, et al. Low-intensity conditioning and hematopoietic stem cell transplantation in patients with renal and colon carcinoma. Bone Marrow Transplant. 2003;31:253–261. doi: 10.1038/sj.bmt.1703811. [DOI] [PubMed] [Google Scholar]
- 4.Bishop MR, Fowler DH, Marchigiani D, et al. Allogeneic lymphocytes induce tumor regression of advanced metastatic breast cancer. J Clin Oncol. 2004;22:3886–3892. doi: 10.1200/JCO.2004.01.127. [DOI] [PubMed] [Google Scholar]
- 5.Carella AM, Beltrami G, Corsetti MT, et al. Reduced intensity conditioning for allograft after cytoreductive autograft in metastatic breast cancer. Lancet. 2005;366:318–320. doi: 10.1016/S0140-6736(05)66989-9. [DOI] [PubMed] [Google Scholar]
- 6.Omuro Y, Matsumoto G, Sasaki T, et al. Regression of an unresectable pancreatic tumor following nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. Bone Marrow Transplant. 2003;31:943–945. doi: 10.1038/sj.bmt.1703932. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Omuro Y, Matsumoto G, et al. Nonmyeloablative allogeneic stem cell transplantation for patients with unresectable pancreatic cancer. Pancreas. 2004;28:e65–e69. doi: 10.1097/00006676-200404000-00027. [DOI] [PubMed] [Google Scholar]
- 8.Bay JO, Fleury J, Choufi B, et al. Allogeneic hematopoietic stem cell transplantation in ovarian carcinoma: results of five patients. Bone Marrow Transplant. 2002;30:95–102. doi: 10.1038/sj.bmt.1703609. [DOI] [PubMed] [Google Scholar]
- 9.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renkvist N, Castelli C, Robbins PF, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2001;50:3–15. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Childs RW, Barrett J. Nonmyeloablative allogeneic immunotherapy for solid tumors. Annu Rev Med. 2004;55:459–475. doi: 10.1146/annurev.med.55.091902.104511. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons C, Sykes M. Manipulating the immune system for anti-tumor responses and transplant tolerance via mixed hematopoietic chimerism. Immunol Rev. 2008;223:334–360. doi: 10.1111/j.1600-065X.2008.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehr T, Haspot F, Mollov J, Chittenden M, Hogan T, Sykes M. Alloreactive CD8 T cell tolerance requires recipient B cells, dendritic cells, and MHC class II. J Immunol. 2008;181:165–173. doi: 10.4049/jimmunol.181.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sykes M. Mechanisms of tolerance induced via mixed chimerism. Front Biosci. 2007;12:2922–2934. doi: 10.2741/2282. [DOI] [PubMed] [Google Scholar]
- 15.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lympho-depleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wynn RF, Arkwright PD, Haque T, et al. Treatment of Epstein-Barr-virus-associated primary CNS B cell lymphoma with allogeneic T-cell immunotherapy and stem-cell transplantation. Lancet Oncol. 2005;6:344–346. doi: 10.1016/S1470-2045(05)70171-6. [DOI] [PubMed] [Google Scholar]
- 19.Haque T, Wilkie GM, Taylor C, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002;360:436–442. doi: 10.1016/S0140-6736(02)09672-1. [DOI] [PubMed] [Google Scholar]
- 20.Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 21.Bartels CJ, Rosenberg SA, Yang JC. Adoptive cellular immunotherapy of cancer in mice using allogeneic T-cells. Ann Surg Oncol. 1996;3:67–73. doi: 10.1007/BF02409054. [DOI] [PubMed] [Google Scholar]
- 22.Zakrzewski JL, Suh D, Markley JC, et al. Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nat Biotechnol. 2008;26:453–461. doi: 10.1038/nbt1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Khong HT, Dudley ME, et al. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28:258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee TH, Donegan E, Slichter S, Busch MP. Transient increase in circulating donor leukocytes after allogeneic transfusions in immunocompetent recipients compatible with donor cell proliferation. Blood. 1995;85:1207–1214. [PubMed] [Google Scholar]
- 27.Adams PT, Davenport RD, Reardon DA, Roth MS. Detection of circulating donor white blood cells in patients receiving multiple transfusions. Blood. 1992;80:551–555. [PubMed] [Google Scholar]
- 28.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wrzesinski C, Paulos CM, Gattinoni L, et al. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulos CM, Wrzesinski C, Kaiser A, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong SB, Bos R, Sherman LA. Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J Immunol. 2008;180:3122–3131. doi: 10.4049/jimmunol.180.5.3122. [DOI] [PubMed] [Google Scholar]
- 35.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19:318–330. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leitman SF, Tisdale JF, Bolan CD, et al. Transfusion-associated GVHD after fludarabine therapy in a patient with systemic lupus erythematosus. Transfusion. 2003;43:1667–1671. doi: 10.1046/j.0041-1132.2003.00579.x. [DOI] [PubMed] [Google Scholar]
- 39.Felix NJ, Allen PM. Specificity of T-cell alloreactivity. Nat Rev Immunol. 2007;7:942–953. doi: 10.1038/nri2200. [DOI] [PubMed] [Google Scholar]
- 40.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer DC, Chan CC, Gattinoni L, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci U S A. 2008;105:8061–8066. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corthay A, Skovseth DK, Lundin KU, et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 46.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 47.Smith CM, Wilson NS, Waithman J, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 48.Muranski P, Boni A, Wrzesinski C, et al. Increased intensity lymphodepletion and adoptive immunotherapy: how far can we go? Nat Clin Pract Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sprent J, Cho JH, Boyman O, Surh CD. T cell homeostasis. Immunol Cell Biol. 2008;86:312–319. doi: 10.1038/icb.2008.12. [DOI] [PubMed] [Google Scholar]
- 50.Bellone M, Mondino A, Corti A. Vascular targeting, chemotherapy and active immunotherapy: teaming up to attack cancer. Trends Immunol. 2008;29:235–241. doi: 10.1016/j.it.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Williamson LM, Wimperis JZ, Wood ME, Woodcock B. Fludarabine treatment and transfusion-associated graft-versus-host disease. Lancet. 1996;348:472–473. doi: 10.1016/S0140-6736(05)64563-1. [DOI] [PubMed] [Google Scholar]
- 52.Hinrichs CS, Palmer DC, Rosenberg SA, Restifo NP. Glucocorticoids do not inhibit antitumor activity of activated CD8+ T cells. J Immunother. 2005;28:517–524. doi: 10.1097/01.cji.0000177999.95831.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Popat U, Carrum G, May R, et al. CD52 and CD45 monoclonal antibodies for reduced intensity hemopoietic stem cell transplantation from HLA matched and one antigen mismatched unrelated donors. Bone Marrow Transplant. 2005;35:1127–1132. doi: 10.1038/sj.bmt.1704975. [DOI] [PubMed] [Google Scholar]
- 54.Ge X, Brown J, Sykes M, Boussiotis VA. CD134-allodepletion allows selective elimination of alloreactive human T cells without loss of virus-specific and leukemia-specific effectors. Biol Blood Marrow Transplant. 2008;14:518–530. doi: 10.1016/j.bbmt.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Demirer T, Barkholt L, Blaise D, et al. Transplantation of allogeneic hematopoietic stem cells: an emerging treatment modality for solid tumors. Nat Clin Pract Oncol. 2008;5:256–267. doi: 10.1038/ncponc1104. [DOI] [PubMed] [Google Scholar]
- 56.Lundqvist A, Childs R. Allogeneic hematopoietic cell transplantation as immunotherapy for solid tumors: current status and future directions. J Immunother. 2005;28:281–288. doi: 10.1097/01.cji.0000165354.19171.8f. [DOI] [PubMed] [Google Scholar]
- 57.Nagler A, Ackerstein A, Or R, Naparstek E, Slavin S. Adoptive immunotherapy with haploidentical allogeneic peripheral blood lymphocytes following autologous bone marrow transplantation. Exp Hematol. 2000;28:1225–1231. doi: 10.1016/s0301-472x(00)00533-6. [DOI] [PubMed] [Google Scholar]
- 58.Kennedy-Nasser AA, Brenner MK. T-cell therapy after hematopoietic stem cell transplantation. Curr Opin Hematol. 2007;14:616–624. doi: 10.1097/MOH.0b013e3282ef615a. [DOI] [PubMed] [Google Scholar]
- 59.Amrolia PJ, Muccioli-Casadei G, Huls H, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108:1797–1808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mielke S, Nunes R, Rezvani K, et al. A clinical-scale selective allodepletion approach for the treatment of HLA-mismatched and matched donor-recipient pairs using expanded T lymphocytes as antigen-presenting cells and a TH9402-based photodepletion technique. Blood. 2008;111:4392–4402. doi: 10.1182/blood-2007-08-104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L- memory T cells without graft-versus-host disease. Blood. 2004;103:1534–1541. doi: 10.1182/blood-2003-08-2987. [DOI] [PubMed] [Google Scholar]
- 62.Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112:101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson BE, Taylor PA, McNiff JM, et al. Effects of donor T-cell trafficking and priming site on graft-versus-host disease induction by naive and memory phenotype CD4 T cells. Blood. 2008;111:5242–5251. doi: 10.1182/blood-2007-09-107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fowler DH, Breglio J, Nagel G, Eckhaus MA, Gress RE. Allospecific CD8+ Tc1 and Tc2 populations in graft-versus-leukemia effect and graft-versus-host disease. J Immunol. 1996;157:4811–4821. [PubMed] [Google Scholar]
- 65.Labbe A, Nelles M, Walia J, et al. IL-12 Immunotherapy of murine leukemia: comparison of systemic versus gene modified cell therapy. J Cell Mol Med. 2008 Jun 28; doi: 10.1111/j.1582-4934.2008.00412.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heinzerling L, Burg G, Dummer R, et al. Intratumoral injection of DNA encoding human interleukin 12 into patients with metastatic melanoma: clinical efficacy. Hum Gene Ther. 2005;16:35–48. doi: 10.1089/hum.2005.16.35. [DOI] [PubMed] [Google Scholar]
- 67.Gao JQ, Kanagawa N, Motomura Y, et al. Cotransduction of CCL27 gene can improve the efficacy and safety of IL-12 gene therapy for cancer. Gene Ther. 2007;14:491–502. doi: 10.1038/sj.gt.3302892. [DOI] [PubMed] [Google Scholar]
- 68.Gao JQ, Eto Y, Yoshioka Y, et al. Effective tumor targeted gene transfer using PEGylated adenovirus vector via systemic administration. J Control Release. 2007;122:102–110. doi: 10.1016/j.jconrel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 69.Quintarelli C, Vera JF, Savoldo B, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunbar CE. The yin and yang of stem cell gene therapy: insights into hematopoiesis, leukemogenesis, and gene therapy safety. Hematology Am Soc Hematol Educ Program. 2007;2007:460–465. doi: 10.1182/asheducation-2007.1.460. [DOI] [PubMed] [Google Scholar]
- 71.Hsu C, Jones SA, Cohen CJ, et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood. 2007;109:5168–5177. doi: 10.1182/blood-2006-06-029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 73.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 74.Woods NB, Bottero V, Schmidt M, von Kalle C, Verma IM. Gene therapy: therapeutic gene causing lymphoma. Nature. 2006;440:1123. doi: 10.1038/4401123a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.