Abstract
A dynamic equilibrium between proliferation and programmed cell death (PCD) of auto reactive T-lymphocytes plays a pivotal role in the prevention of autoimmune diseases. We analyzed T lymphocytes myelin basic protein (MBP)-specific PCD and proliferation in demyelinating diseases. Results showed that MBP-specific PCD was significantly decreased in CD4+ and CD8+ T lymphocytes of progressive multifocal leukoencephalopathy (PML), not determined leukoencephalopathy (NDLE), and acute MS (AMS) patients compared to patients with stable MS (SMS) and healthy controls. MBP-specific proliferation/PCD rates were high in CD4+ T lymphocytes of PML, NDLE, and AMS patients, and in CD8+ T cells of PML and AMS individuals alone. Alterations of the balance between MBP-specific proliferation and PCD are present in demyelinating diseases and could play a major role in the pathogenesis of these diseases.
Keywords: PCD, Myelin Basic Protein, Demyelinating Diseases, Apoptosis, Multiple Sclerosis
INTRODUCTION
Demyelinating diseases are a heterogeneous group of diseases characterized by the destruction of myelin and extensive loss of myelin sheaths of the nerve fibers that can affect both the central and the peripheral nervous systems. Amongst these diseases both primarily autoimmune (e.g. multiple sclerosis) and primarily infectious (e.g. JC Virus-associated progressive multifocal leukoencephalopathy) conditions are included. The labile border between infection and autoimmunity in these diseases is nevertheless epitomized by progressive multifocal leukoencephalopathy (PML), a pathology that appears during the treatment of HIV infection. Thus, whereas highly active antiretroviral therapy (HAART) can lead to restoration of JC virus (JCV)-specific immune defenses, thus resulting in longer symptom-free survival of patients [1,2,3,4], HAART can in some cases induce new demyelinating lesions and worsen the prognosis of PML [5,6,7]. In this instance, it is suggested that HAART-associated immune reconstitution could activate immune competent cells that, probably through molecular mimicry with JCV epitopes, cross-react with self-component present in the Myelin Basic Protein (MBP).
Antigenic stimulation results in different possible outcomes including proliferation, tolerance, and programmed cell death (PCD). In the case of self antigens-specific cells, a physiologic dynamic equilibrium between these processes has a pivotal importance in preventing autoimmunity [8]. Previous results indicated that a deregulation of PCD could play a role in inducing or maintaining auto-reactive immune phenomena [9]. In particular, studies in multiple sclerosis (MS) clearly indicated that PCD of MBP-specific T lymphocytes is different when patients with acute (AMS) or stable (SMS) disease are compared. Thus, PCD is significantly augmented in SMS patients, suggesting that death of MBP-specific lymphocytes is associated with quiescent phases of the disease. [10]
In order to verify whether common pathogenic mechanisms could link demyelinating diseases with a different etiologies, we investigated by flow cytometry MBP-specific proliferation and PCD of T lymphocytes in three different demyelinating diseases: PML, MS, and not determined leuko encephalopathy (NDLE), a PML-like, JCV-negative disorder.
MATERIALS AND METHODS
Patients and controls
Twenty HIV+, HAART treated patients were enrolled in the study. Ten of these patients (7 males, 3 females; median age= 40 years; range = 21–60 years) were affected by PML as confirmed by the presence of white matter lesions on magnetic resonance imaging (MRI), and of JC Virus (JCV) DNA in the CSF; the CD4+ cells count mean was 170/μl (range: 96–651/μl). NDLE (white matter lesions in the absence of isolation of JCV from CSF) was diagnosed in the other 10 subjects (6 males and 3 females; median age= 47 years; range = 31–61 years); the CD4+ cells count mean was 275/μl (range: 153–445/μl).
All HIV+ patients were recruited from the Infectious Disease Departments of Policlinico S. Matteo in Pavia and from the Neurology Department of Mondino Hospital in Pavia and gave written consent for the enrollment in the study. Thirty-six patients with Multiple Sclerosis diagnosed by clinical and laboratory analyses were included in the study as well. These patients (33 females and 3 males; median age= 39 years; range = 25–53 years) were affected by Relapsing Remitting (RR) MS with or without sequelae and were followed by the Centro Sclerosi Multipla, Don Gnocchi Foundation, Milan, Italy. MS had been clinically stable for at least six months prior to the study period in 13 of these patients (12 females and 1 male), that were therefore classified as being affected by stable MS (SMS). The diagnosis of SMS was confirmed by brain and spinal cord MRI with gadolinium that showed no areas of enhancement at the time of enrollment. Twenty-three other RR MS (21 females and 2 males) patients were undergoing clinical relapses of the disease and were thus classified as patients with acute MS (AMS). The diagnosis was confirmed by MRI scans that showed the presence of enhancing lesions. None of the patients had received immunosuppressive drugs in the year prior to the study period. None of the MS patients was undergoing treatment with either interferon or statins at the time of the study. MS patients gave informed consent according to a protocol approved by the local ethics committee of Don Gnocchi Foundation. Finally, 20 age-and sex- matched healthy blood donors (HC)(6 males and 14 females; median age= 40 years; range = 32–54 years) were enrolled in the study as well.
Quantitative Real-Time in CSF sample
Cerebrospinal Fluid samples were collected from HIV+ patients to verify the presence of JCV DNA and thus allow the differential diagnosis between PML and NDLE. JCV DNA viral load was determined using the TaqMan Q-PCR methodology. Amplification and detection were performed using an ABI PRISM 7000 Sequence Detection System. The real-time PCR assay used forward (5′-GAG TGT TGG GAT CCT GTG TTT TC-3′) and reverse (5′-GAG AAG TGG GAT GAA GAC CTG TTT-3′) primers as well as the fluorescent TaqMan probe (5′-6-FAM- TCA TCA CTG GCA AAC ATT TCT TCA TGG C-TAMRA-3′) (PE Applied Biosystems, Cheshire, United Kingdom) to amplify and detect a 54 bp amplicon in the JCV Large T antigen region. A standard curve of the Ct values was generated using serial ten-fold dilutions of an external JCV standard. Each standard, each sample, and one negative control were analyzed in triplicate; 10 μl of the sample were added to 40 μl of the reaction mixture consisting of 25 μl of Universal Mastermix (PE Applied Biosystems) and of primers and probe, at a concentration of 900 nM and 200 nM, respectively. The cycling parameters were: 50°C for 2′, 95°C for 10′, 40 cycles at 95°C for 15″ and 60°C for 1′ [11].
Blood sample collection and cell separation
Whole blood was collected by venopuncture in vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA) (Becton Dickinson & Co., Rutherford, NJ, USA). Peripheral blood mononuclear cells (PBMC) were separated on lymphocyte separation medium (Organon Teknika Corp., Durham, NC), washed twice in PBS, and the number of viable leukocytes was determined by trypan blue exclusion.
Peptides Synthesis
Thirty-one HLA class I-restricted and 7 HLA class II-restricted promiscuous peptides partially overlapping and spanning the whole Myelin Basic Protein (MBP), as well as 14 HLA class I-restricted and 11 HLA class II-restricted promiscuous peptides of major capside of JCV (VP-1) were synthesized, using Fmoc chemistry. Peptides purity, as assayed by HPLC, was > 70%, the composition of each peptide was verified by mass spectrometry [12]. Peptides with no immunological properties (peptide control pool) to be used as a negative control, were synthesized and purified under same conditions as MBP or VP-1 peptides.
To selected amino acid sequences for non immunogenic peptides we utilized on-line resource RANKPEP (http://immunax.dfci.harvard.edu/Tools/rankpep_help.html#pssm). This server predicts peptide binders to MHCI and MHCII molecules from protein sequence/s or sequence alignments using Position Specific Scoring Matrices (PSSMs). In addition, it predicts those MHCI ligands whose C-terminal end is likely to be the result of proteasomal cleavage. Currently, 88 and 50 different MHC I and MHC II molecules, respectively, can be target for peptide binding predictions. The peptides predicted to bind with maximal score (> 80% ), HLA I and II alleles included in RANKPEP were considered promiscuous for binding. Peptides with lowest binding potential (score) to the selected MHC molecule were considered as negative control peptide. In particular two 9 mer MBP peptides and one 15 mer MBP peptide with lowest score were respectively chosen as peptide control for HLA I and HLA II MBP restricted peptides.(Tab. 1)
Lyophilized peptides were dissolved at 25mg/ml in DMSO or sterile water to prepare peptide pool at 10ug/ml final concentration and storing at −20°C until use.
Cell cultures
PBMC resuspended (3×106/ml) in RPMI 1640 (Life Tecnologies, Grand Island, NY) supplemented with 10% heat-inactivated human AB (Sigma, St. Louis, MO), 2mM L-glutamine (Sigma) and 1% penicillin (Sigma), were either unstimulated or were stimulated with the MBP peptide pools or the peptide control pool (10μg/ml in both cases). Anti human CD-28 (1μg/ml) was added to the cell cultures; cultures were kept at 37° C in a humidified 5% CO2 atmosphere for 4 days.
For intracellular cytokine detection PBMC were stimulated with VP-1; MBP; or peptide control pools (10μg/ml in all cases) in the presence of anti human CD-28. Cultures were kept at 37° C in a humidified 5% CO2 atmosphere for 18 hours; 10 μg/ml of Brefeldin A (Sigma), were added to the cell cultures in the last 5 hours of incubation.
Monoclonal antibodies (mAbs)
The antibody used in the cell-stimulation assays was a monoclonal anti-human CD28 (CD28.2)(mouse IgG1). Cell-surface antigens staining monoclonal antibodies (mAb) were anti-CD3(UCHT 1)(mouse IgG1), anti-CD4(SFCI12T4D11)(mouse IgG1) and anti-CD8 (SFC121Thy2D3), directly coupled to phycoerythrin (R-PE), Phycoerythrin-Texas Red (ECD) and Phycoerythrin-Cyanin-7 (PC7). The mAb used for intracellular cytokine detection was an anti-IFNγ (45.15)(mouse IgG1) fluorescein isothiocyanate (FITC)- conjugated reagent (Beckman-Coulter, CA). Cells were prepared, stained and analyzed using Cytomics FC-500 flow cytometer (Beckman-Coulter)
Intracellular cytokine assay
VP-1-, MBP-, or control peptide pool- stimulated PBMC were washed in PBS and incubated with mAb directed to surface antigens (CD3+, CD4+ and CD8+) for 30 minutes at 4°C in the dark. After staining PBMC were washed and fixed (FIX & PERM kits; Invitrogen-Caltag Lab Carlsbad, CA, USA) for 10 minutes at room temperature in the dark. The cells were washed in PBS, permeabilized and stained with mAb specific for IFNγ. After 30 minutes at 4°C cells were washed and 1% paraformaldeid in PBS was added.
Apoptosis staining assay
Apoptotic cells were analyzed using two-color analysis for the simultaneous detection of surface (CD4 and CD8) and 7-AAD. During the cell death process, the plasma membrane is progressively altered and becomes permeable to the dye. 7-AAD staining discriminates between early (7-AADLo) and late (7-AADHi) apoptotic cells, and the increase in 7-AAD fluorescence is due to an alteration of their permeability. To exclude the possibility that 7AADHi CD4+ or CD8+ subsets included necrotic cells, a morphological gate capable to distinguish SSCHi FSCHi (necrotic cells) from SSCHi FSCLo (late apoptotic cells) was selected [see ref. 13]. Briefly, cultured PBMC were incubated with mAb directed to surface antigens (CD3+, CD4+ and CD8+) and with 20μg/ml of 7-AAD for 20 minutes at 4°C in the dark. Stained cells were further fixed with 1% paraformaldehyde (PFA) in PBS in the presence of 20μg/ml of non-fluorescent amino-actinomycin D (AAD) (Sigma-Aldrich) to block 7-AAD staining within apoptotic cells and to avoid non-specific labeling of viable cells. After 2 washes the PBMC were fixed in PFA-AAD buffer and immediately analyzed [14].
CFDA-SE labeling
PBMC resuspended at 107/ml in PBS were added to an equal volume of 5 μM of 5 and 6 carboxyfluorescein diacetate succimidyl ester (CFSE) (Molecular Probe, Eugene, OR) in PBS and mixed gently for 3 minutes at room temperature. This procedure has a labeling efficiency exceeding 99%, and the cells remain labeled for at least 10 days during tissue culture [15]. CFSE, reacts with secondary amino of intracellular proteins providing a uniform fluorescent label to cells. Upon cell division a CFSE high cell will lose half of its CFSE label resulting in populations of CFSE low daughter cells (proliferating cells) which can be visualized by flow cytometry.
Proliferation Assay
CFSE-labeled cells were washed twice and resuspended at 1×106 cells/ml in polystyrene tissue culture tubes containing 1 ml RPMI-1640 medium supplemented with 10% AB serum alone and anti-CD28 (2μg/ml)( Beckman-Coulter). Cells were then stimulated with either the MBP or the control peptide pool (10μg/ml in both cases). Culture tubes were incubated at 37° C in a humidified 5% CO2 atmosphere for 5 days. Cells were subsequently harvested and washed twice in PBS. Surface staining for CD3+, CD4+ and CD8+ was performed for 30 minutes at 4°C, finally the cells were washed in PBS and fixed in 1% of paraformaldehyde. Flow-cytometric data (100.000 non-gated events) were acquired on a Beckman-Coulter Cytomics FC-500 flow cytometer. For analysis the CXP Analysis software (Beckman-Coulter was used to gate on CD4+/CD8− or CD8+/CD4−CD3+ T-cell populations. Proliferation observed in unstimulated sample was considered to be the background value. The Δ Proliferating Fraction (PF) was calculated by subtracting background proliferation from the antigen-specific proliferation. The stimulation index (SI) was calculated by dividing antigen-induced proliferation by background proliferation. Both a Δ PF > 1% and an SI > 2.0 were required to classify a response as positive [16].
Statistical analyses
Data were summarized according to standard statistical tests; non-parametric descriptive statistics were preferred in order to avoid assuming a definite theoretical distribution for each data set. Non-parametric tests were performed to evaluate differences between PML, NDLE, and MS patients, divided according to their clinical status (i.e., SMS and AMS), and HC. For each variable, a Kruskal-Wallis one-way analysis of variance was performed. If this analysis showed significant differences between the groups of individuals, non-parametric subsets (Mann-Whitney U tests) were then used to examine differences between the MS patients classified according to clinical status. All p values were two-sided.
RESULTS
Intracellular IFNγ-producing CD4+ and CD8+ T lymphocytes upon stimulation with VP-1 and MBP peptide pools
The percentage of VP1-stimulated IFNγ-producing CD4+ T lymphocytes was significantly increased in PML patients compared to all other groups (PML vs. HC p<0.05)(Figure 1, Panel A); similar results were obtained when VP1-stimulated IFNγ-producing CD8+ T lymphocytes were analyzed. Thus, these cells were increased in PML compared to all of the groups (PML vs. AMS, vs. SMS, vs. HC p<0.05)(Figure 1, Panel B).Finally, whereas no significant differences were seen in CD4+ T lymphocytes (data not shown), MBP stimulated, IFNγ-producing CD8+ T cells were increased in PML, NDLE, and AMS patients in comparison to HC (p<0.05 in all cases)(Figure 1, Panel C).
Figure 1.
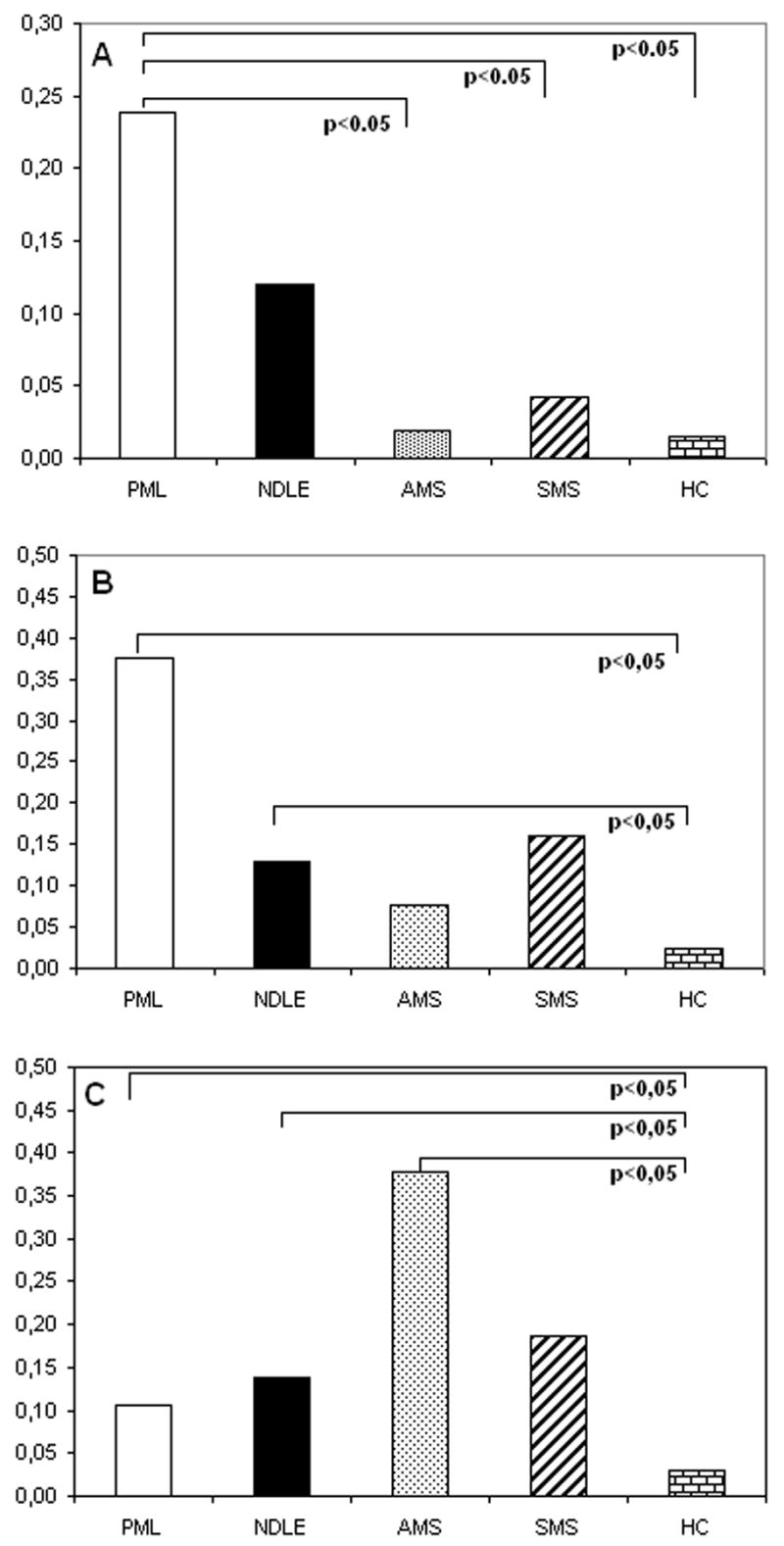
VP-1-stimulated, IFNγ-producing CD4+ (panel A) and CD8+ (panel B) T lymphocytes of patients with PML, NDLE, acute (AMS), or stable (SMS) multiple sclerosis, and healthy controls (HC). Panel C shows MBP-stimulated, IFNγ-producing CD8-T cells from the same patients and controls. Means values and statistically significant differences are shown.
PCD of MBP-specific T lymphocytes in patients with demyelinating diseases
Programmed cell death was evaluated in patients and controls after stimulation of lymphocytes with overlapping MBP peptides; cells undergoing PCD were quantified by analysis of 7AAD permeability. Representative results obtained in the different groups of individuals enrolled in the study are shown in Figures 2 and 3.
Figure 2.
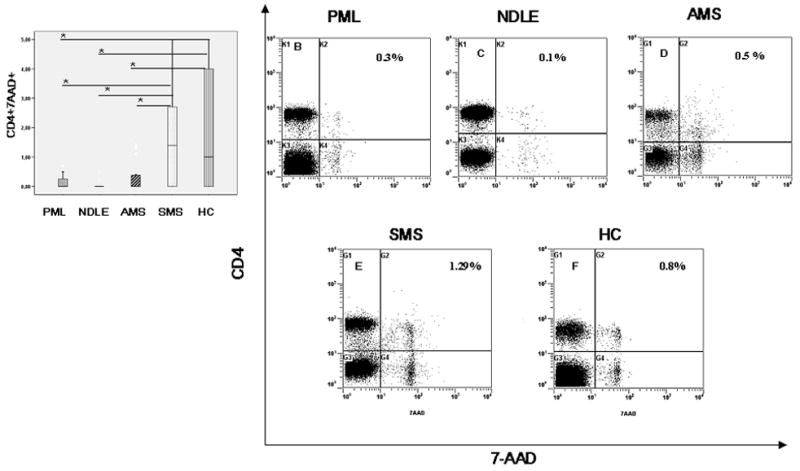
Median percentages of MBP-stimulated CD4+ T lymphocytes that express 7-AAD (apoptotic lymphocytes) are shown. Data obtained in peripheral blood of patients affected by PML, NDLE, acute (AMS) or stable (SMS) multiple sclerosis and healthy control (HC) are presented. In panel A the boxes stretch indicate from the 25th to the 75th percentile; the lines across the boxes indicate the median values; the lines stretching from the boxes indicate interquartile ranges. Statistical significance is shown:*indicates p<0.05; **indicates p<0.01. Representative results obtained in a PML (Panel B), a NDLE (panel C), an AMS (Panel D) SMS (panels E) patients and a HC (panels F) are shown. The percentage of apoptotic cells appears in the upper right corner.
Figure 3.
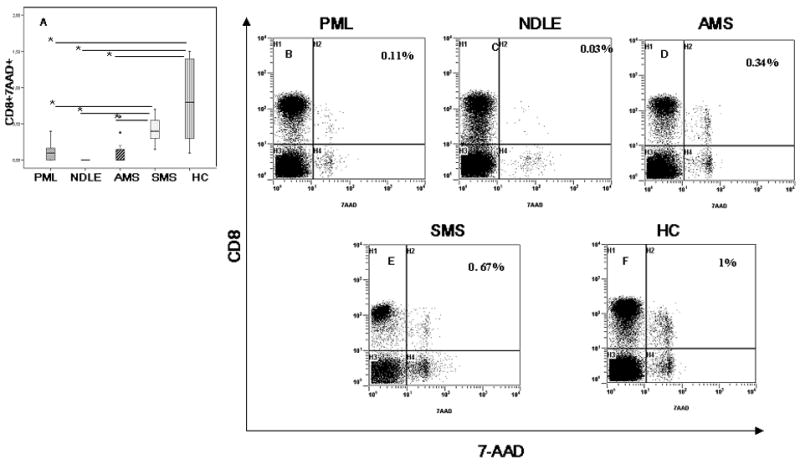
Median percentages of MBP-stimulated CD8+ T lymphocytes that express 7-AAD (apoptotic lymphocytes) are shown. Data obtained in peripheral blood of patients affected by PML, NDLE, acute (AMS) or stable (SMS) multiple sclerosis and healthy control (HC) are shown. In panel A the boxes stretch indicate from the 25th to the 75th percentile; the lines across the boxes indicate the median values; the lines stretching from the boxes indicate interquartile ranges. Statistical significance is shown:*indicates p<0.05; **indicates p<0.01. Representative results obtained in a PML (Panel B), a NDLE (panel C), an AMS (Panel D), a SMS (panels E) patients and a HC (panels F) are shown. The percentage of apoptotic cells appears in the upper right corner.
Results showed that the percentage of MBP-specific CD4+ T cells undergoing PCD was significantly reduced in PML, NDLE, and AMS subjects compared to SMS patients (p<0.05 in all cases) and healthy controls (p<0.05 in all cases)(Figure 2).
Similar results were observed when PCD was analyzed in MBP-specific CD8+ T cells. Thus, the percentage of MBP-specific CD8+ T cells undergoing PCD was comparable in SMS individuals and HC, but was greatly reduced in PML (vs. SMS and HC p<0.05); NDLE (vs. SMS and HC p<0.05) and AMS (vs. HC and p<0.05) patients. These data are shown in Figure 3.
MBP peptides-stimulated proliferation in patients with demyelinating diseases
Representative MBP-stimulated proliferative responses of CD4+ and CD8+ of representative PML, NDLE, AMS, SMS patients and HC are shown in Figure 4. MBP-stimulated proliferation was measured in all patients and controls by evaluating cell division using CFSE staining. Results indicated that the stimulation index relative to MBP peptides-stimulated proliferation of CD4+ lymphocytes was significantly augmented in NDLE and AMS compared to PML (p<0.05 in both cases) and SMS (p<0.05 in both cases) patients. Interestingly, the highest stimulation indexes were detected in healthy controls.
Figure 4.
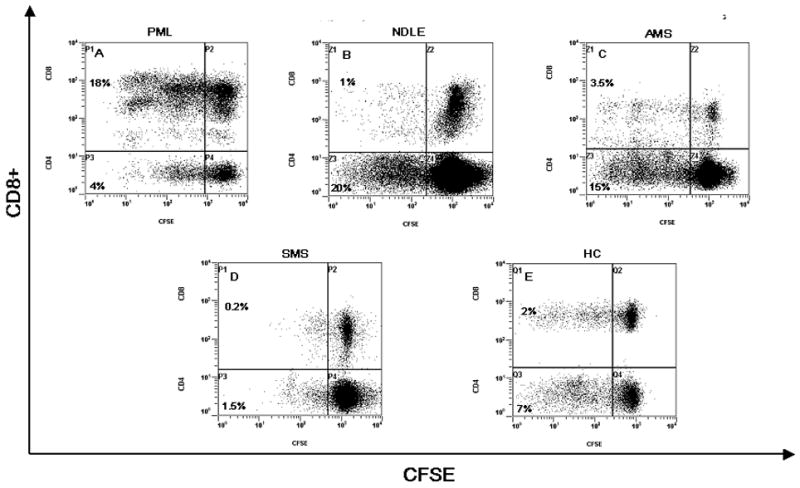
MBP peptide pool-stimulated proliferation (CFSE –staining). Representative results obtained in a PML (Panel A), a NDLE (panel B), an AMS (panel C), a SMS (panels D) patients and a HC (panels E) are shown. These data represent gated CD3+ T cells that are further gated for CD4+/CD8− or CD4−/CD8+ T cells. CFSE staining is shown on x-axis and CD8 staining on the y-axis. The CD8− populations in the dot plots represent gated CD4+ T cells. The numbers next to the populations represent the proliferating fraction of CD4+ T cells and CD8+ T cells. Non-divided CFSEhigh labeled cells are shown in the upper(CD8+) and lower(CD4+) right quadrants, whereas daughter cell populations (CFSElow) are shown in upper (CD8+) and lower (CD4+) left quadrants because have subsequently lost half of their CFSE signal with each division round.
Different results were seen when stimulation indexes were measured in MBP peptides-stimulated CD8+ lymphocytes. In this case, SI of PML and AMS patients were significantly higher compared to results obtained in NDLE (p<0.05 in both cases) and SMS (p<0.05 in both cases) individuals. Opposite to what was observed for CD4+ T cells, the lowest values for MBP-stimulated CD8+ T cells was detected in healthy controls. These results are presented in Figure 5.
Figure 5.
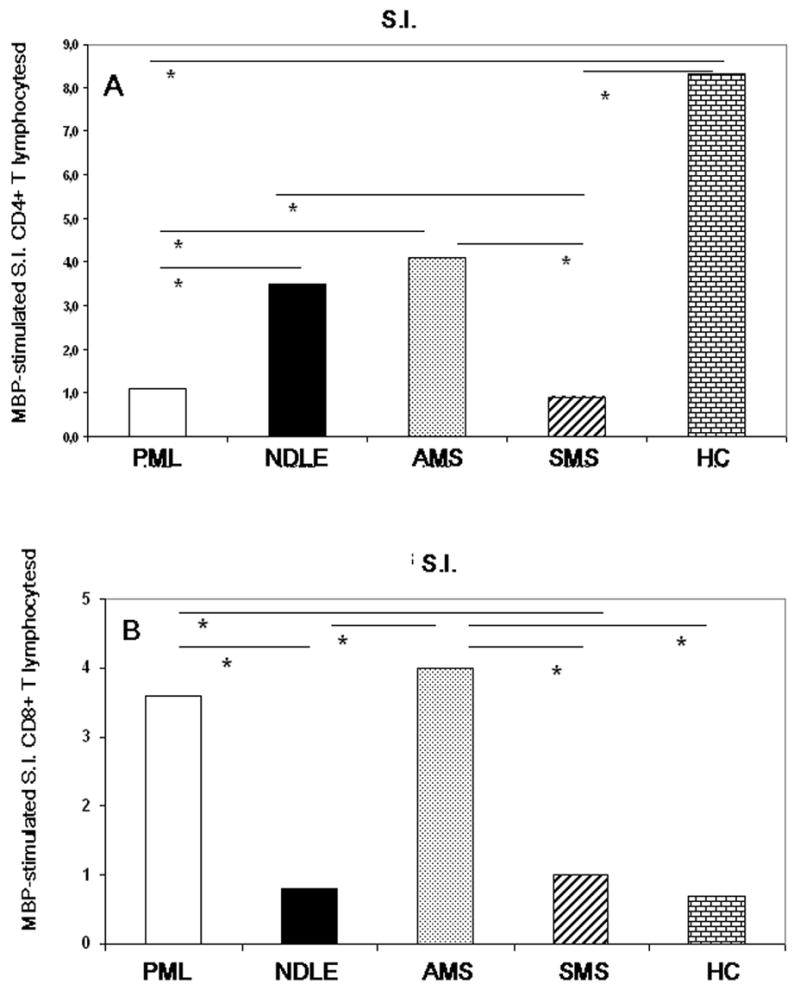
Stimulation index (S.I.) of CD4+ (panel A) and CD8+ (panel B) T lymphocytes stimulated with MBP peptide pools in patients affected by PML, NDLE, acute (AMS) or stable (SMS) multiple sclerosis and healthy control (HC). Median values and statistically significant differences are shown: *indicates p<0.05.
MBP-stimulated proliferation/PCD rates in patients with demyelinating diseases
Antigen stimulation of T lymphocytes can result in proliferation or in the initiation of the apoptotic cascade; an imbalance between these two processes could be involved in the development of autoimmune conditions. Hence we measured MBP-specific proliferation/PCD rates in CD4+ and CD8+ T cells of all individuals enrolled in the study. Calculation of this ratio for MBP-specific CD4+ T lymphocytes showed higher values in PML (6.5), NDLE (12), and AMS (16) patients; these ratios were much lower in SMS (<1) individuals and HC (2). The proliferation/PCD MBP-specific rates for CD8+ T cells showed the presence of high values in PML (8) and AMS (14,5) individuals; this rate was <0.5 in NDLE and SMS patients as well as in healthy controls. These results are presented in Figure 6.
Figure 6.
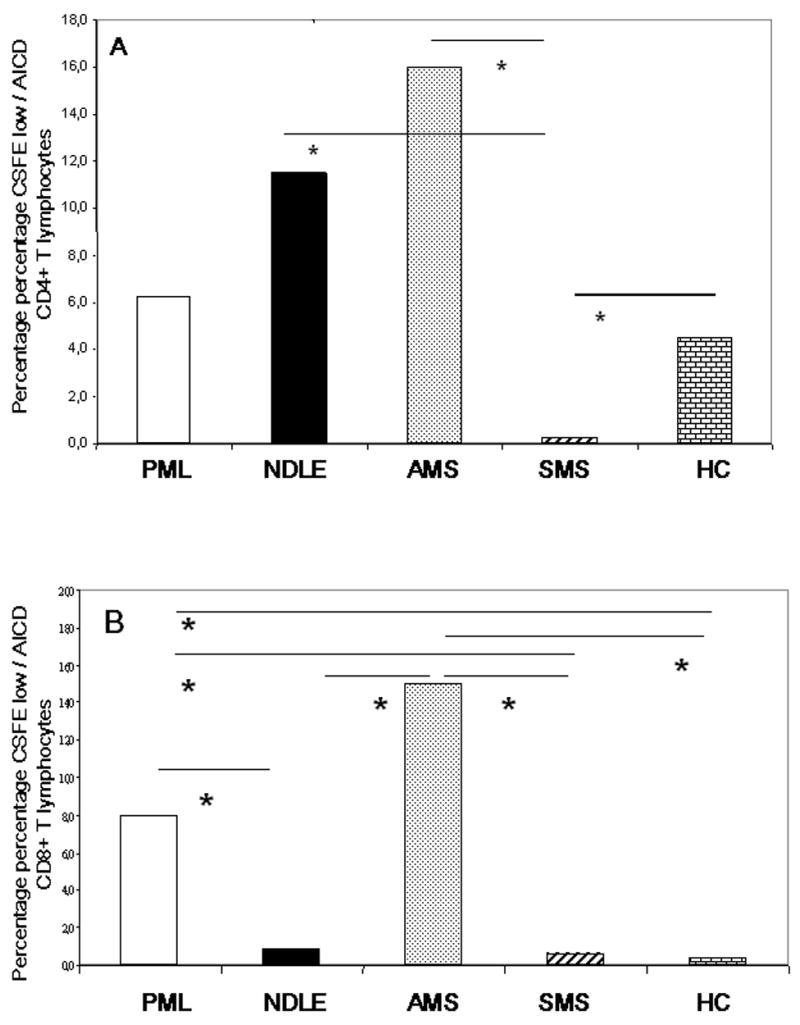
Proliferation/PCD indices in MBP peptide pools-stimulated CD4+ (panel A) or CD8+ (panel B) T lymphocytes of patients affected by PML, NDLE, either acute (AMS) or stable (SMS) multiple sclerosis and healthy control (HC). Median values and statistically significant differences are shown: :*indicates p<0.05.
DISCUSSION
Antigen-stimulated lymphocytes can undergo proliferation, tolerance, or death; the rupture of these finely regulated processes favors the emergence of autoimmunity[17]. To evaluate possible imbalances between proliferation and PCD in demyelinating diseases with different etiologies we verified MBP-stimulated proliferation and PCD in patients affected by either PML, NDLE, or MS. Results indicate that an imbalance in the proliferation/PCD equilibrium, and in particular the prevalence of MBP-specific proliferation over PCD, is indeed associated with the presence of demyelinating diseases.
MBP-stimulated PCD was significantly reduced in both CD4+ and CD8+ T lymphocytes of patients with all the demyelinating disease we considered, with the predictable exception of multiple sclerosis patients in phases of quiescent disease. Interestingly, different results were obtained when MBP-stimulated proliferation was compared in CD4+ and CD8+ T cells. Thus, CD4+ MBP-stimulated proliferation was reduced in PML but not in NDLE individuals. This finding is probably justified by the fact that PML appears in severely immunocompromised hosts. In particular, in our study we enrolled patients in whom PML developed concomitantly with HIV infection. Defective antigen-stimulated proliferation and cytokine production have been described in HIV-infected patients and are a hallmark of this infection. It is therefore likely that the immune impairment characteristic of HIV infection would result in the impossibility of MBP-specific CD4+ T cells to proliferate. MBP-specific proliferation was strong in CD4+ T cells of NDLE patients. Although clinically very similar, PML and NDLE could be etiologically different. Thus, the human polyomavirus JC (JCV), a virus that infects and destroys oligodendrocites, is the causative agent of PML [18]; JCV is not observed in the CSF of patients with NDLE. This raises two possibilities: 1) NDLE is simply a CSF- JCV-negative form of PML [19,20,21]; or 2) NDLE [22] and PML are distinct diseases. These possibilities notwithstanding, NDLE is clinically very similar to PML, but it is characterized by milder symptoms and slower progression. The observation that MBP-specific proliferation was indeed preserved in NDLE individuals could be explained by a minor degree of functional impairment in these patients. This suggestion is supported by the clinical evidence that NDLE appears in hosts that are not as immunocompromised as those in whom PML is seen. The prognosis of NDLE is also better, and the signs of demyelinization are scarce in NDLE compared to PML patients. A final possibility is that JCV could have role in altering CD4+ T lymphocytes-mediated immune functions secondary to either a direct or an indirect effect, possibly mediated by putative soluble suppressive factors.
The highest values of MBP-specific proliferation of CD8+ T cells were observed in PML and AMS patients; very low stimulation indexes were detected in NDLE and SMS individuals. This result could explain the lesser clinical severity of NDLE compared to PML. Alternatively, this low proliferation might be the result of a compartmentalization of the immune response. Thus, in NDLE low frequency of MBP-specific CD8+ T cells might be segregated within the CSF. Finally, the suggested presence or regions of mimicry between JCV and MBP could justify the high CD8+ T cells proliferation rates observed in PML [23], but not in NDLE patients.
MBP-stimulated proliferation was very elevated in CD4+ T lymphocytes of HC in whom, by definition, no signs of demyelinization were present. This interesting observation indicates that antigen-specific proliferation directed toward components of self proteins might not be pathologic per se; self proteins-specific proliferation could become pathologic when it is not balanced by an adequate degree of cells death.
A rough way to measure possible imbalances between MBP-stimulated proliferation and PCD was to calculate proliferation/PCD rates in all the individuals included in the study. The results indicated that health, lack of disease progression (SMS), or moderate disease activity (NDLE) are associated with CD8+ T lymphocytes indexes <1. These findings seem to support the idea that autoimmune diseases, and in this particular instance, demyelinating diseases, are associated with in imbalance between these two biologic processes. Thus, when immune cells proliferating against self-antigens are not destroyed, diseases ensue.
PCD is tightly regulated and mainly orchestrated by the activation of the aspartate-specific cysteine protease (caspase) cascade. The two main pathways leading to the activation of caspase depend upon the participation of mitochondria (receptor-independent) and includes pro- and anti-apoptotic members of the Bcl-2 family, or involve the interaction of a death receptor and its ligand, such as Fas/FasL [24]. This second, receptor-dependent pathway, could be more important in preventing the uncontrolled activation of lymphocytes associated with autoimmune disease. It will be extremely interesting to evaluate and compare these different processes in demyelinating diseases with diverse etiologies. Finally, further analyses will be needed to ascertain the possible clinical value of these results and to verify these data in the context of the cefalo- spinal compartment.
Table 1.
Non immunogenic peptides
| Protein and Position | Amino acid sequence | Score |
|---|---|---|
| MBP 10 | GSKYLATAS | 0 |
| MBP 51 | PKRGSGKDS | 0 |
| MBP 136 | AHKGFKGVD | 0 |
| MBP 249 | SWGAEGQRPGFGYGG | 0 |
Acknowledgments
This study was supported by grants from the Istituto Superiore di Sanita’ “Programma Nazionale di Ricerca sull’ AIDS”; NIH grant n. MH072528; the EMPRO and AVIP EC WP6 Projects; the Japan Health Science Foundation; 2007 Ricerca Finalizzata (Italian Ministry of Health); 2007 Ricerca Corrente (Italian Ministry of Health); 2007 FIRB RETI, CHEM-PROFARMA-NET (RBPR05NWWC)
References
- 1.Mayo J, Collazos J, Martínez E. Progressive multifocal leukoencephalopathy following initiation of highly active antiretroviral therapy. AIDS. 1998;12:1720–2. [PubMed] [Google Scholar]
- 2.Clifford DB, Yiannoutsos C, Glicksman M, Simpson DM, Singer EJ, Piliero PJ, Marra CM, Francis GS, McArthur JC, Tyler KL, Tselis AC, Hyslop NE. HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology. 1999;52:623–5. doi: 10.1212/wnl.52.3.623. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht H, Hoffmann C, Degen O, Stoehr A, Plettenberg A, Mertenskotter T, Eggers C, Stellbrink HJ. Highly active antiretroviral therapy significantly improves the prognosis of patients with HIV-associated progressive multifocal leukoencephalopathy. AIDS. 1998;12:1149–54. doi: 10.1097/00002030-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Antinori A, Cingolani A, Lorenzini P, Giancola ML, Uccella I, Bossolasco S, Grisetti S, Moretti F, Vigo B, Bongiovanni M, Del Grosso B, Arcidiacono MI, Fibbia GC, Mena M, Finazzi MG, Guaraldi G, Ammassari A, d’Arminio Monforte A, Cinque P, De Luca A Italian Registry Investigative Neuro AIDS Study Group. Clinical epidemiology and survival of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy: data from the Italian Registry Investigative Neuro AIDS (IRINA) J Neurovirol. 2003;9:47–53. doi: 10.1080/13550280390195388. [DOI] [PubMed] [Google Scholar]
- 5.Safdar A, Rubocki RJ, Horvath JA, Narayan KK, Waldron RL. Fatal immune restoration disease in human immunodeficiency virus type 1-infected patients with progressive multifocal leukoencephalopathy: impact of antiretroviral therapy-associated immune reconstitution. Clin Infect Dis. 2002;15:1250–7. doi: 10.1086/344056. [DOI] [PubMed] [Google Scholar]
- 6.Koralnik IJ. New insights into progressive multifocal leukoencephalopathy. Curr Opin Neurol. 2004;17:365–70. doi: 10.1097/00019052-200406000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Di Giambenedetto S, Vago G, Pompucci A, Scoppettuolo G, Cingolani A, Marzocchetti A, Tumbarello M, Cauda R, De Luca Fatal inflammatory AIDS-associated PML with high CD4 counts on HAART: a new clinical entity? Neurology. 2004;28:2452–3. doi: 10.1212/01.wnl.0000148585.41802.6c. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka M, Miyake Y. Apoptotic cell clearance and autoimmune disorder. Curr Med Chem. 2007;14:2892–7. doi: 10.2174/092986707782360006. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RM, Chan FKM, Lenardo MJ. The multifaceted role of Fas signaling in immune cells homeostasis and autoimmunity. Nature Immunology. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 10.Saresella M, Marventano I, Speciale L, Ruzzante S, Trabattoni D, Della Bella S, Filippi M, Fasano F, Cavarretta R, Caputo D, Clerici M, Ferrante P. Programmed cell death of myelin basic protein-specific T lymphocytes is reduced in patients with acute multiple sclerosis. J Neuroimmunol. 2005;166:173–9. doi: 10.1016/j.jneuroim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Delbue S, Sotgiu G, Fumagalli D, Valli M, Borghi E, Mancuso R, Marchioni E, Maserati R, Ferrante P. A case of a PML patient with four different JC virus TCR rearrangements in CSF, blood, serum and urine. J Neurovirol. 2005;11:51–57. doi: 10.1080/13550280590900382. [DOI] [PubMed] [Google Scholar]
- 12.Magri G, Clerici M, Dall’Ara P, Biasin M, Caramelli M, Casalone C, Giannino ML, Longhi R, Piacentini L, Della Bella S, Gazzuola P, Martino PA, Pollera C, Puricelli M, Servida F, Crescio I, Boasso A, Ponti W, Poli G. Decrease in pathology and progression of scrapie after immunisation with synthetic prion protein peptides in hamsters. Vaccine. 2005;23:2862–8. doi: 10.1016/j.vaccine.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 13.Lecoeur H, Ledru E, Prèvost MC, Gougeont ML. Strategies For Phenotyping apoptotic peripheral human lymphocytes comparing ISNT, annexin-V and 7-AAD cytofluorometric staining methods. J Immunol Methods. 1997;209:111–123. doi: 10.1016/s0022-1759(97)00138-5. [DOI] [PubMed] [Google Scholar]
- 14.Lecoeur H, Ledru E, Gougeont ML. A cytofluorimetric method for the imultaneous detection of both intracellular and surface antigens of apoptotic peripheral lymphocytes. J Immunol Methods. 1998;217:11–26. doi: 10.1016/s0022-1759(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 15.Lyons AB. Anlysing cell division in vivo and in vitro using flow cytometric measurement of CSFE dye diluition. J Immunol Methods. 2000;243:147–154. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 16.Crawford MP, Yan SX, Ortega SB, Mehta RS, Hewitt RE, Price DA, Stastny P, Douek DC, Koup RA, Racke MK, Karandikar NJ. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004 Jun 1;103(11):4222–31. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- 17.Maniati E, Potter P, Rogers NJ, Morley BJ. Control of apoptosis in autoimmunity. J Pathol. 2008;214:190–8. doi: 10.1002/path.2270. [DOI] [PubMed] [Google Scholar]
- 18.Berger JR, Major EO. Progressive multifocal leukoencephalopathy. Semin Neurol. 1999;19:193–200. doi: 10.1055/s-2008-1040837. [DOI] [PubMed] [Google Scholar]
- 19.Koralnik I. Progressive multifocal leukoencephalopathy revisited:has the disease outgrown its name? Ann Neurol. 2006;60:162–173. doi: 10.1002/ana.20933. [DOI] [PubMed] [Google Scholar]
- 20.Du Pasquier RA, Kuroda MJ, Schmitz JE, Zheng Y, Martin K, Peyerl FW, Lifton M, Gorgone D, Autissier P, Letvin NL, Koralnik IJ. Low frequency of cytotoxic T lymphocytes against the novel HLA-A*0201-restricted JC virus epitope VP1(p36) in patients with proven or possible progressive multifocal leukoencephalopathy. J Virol. 2003;77:11918–26. doi: 10.1128/JVI.77.22.11918-11926.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delbue S, Marchioni E, Sotgiu G, Saresella M, Tavazzi E, Colombo E, Guerini FR, Maserati R, Sinforiani E, Schifino MR, Ferrante P. Longitudinal study of two cases of progressive multifocal leukoencephalopathy with a clinical benign evolution. J Neurovirol. 2007;13:268–73. doi: 10.1080/13550280701291796. [DOI] [PubMed] [Google Scholar]
- 22.Langford TD, Letendre SL, Marcotte TD, Ellis RJ, McCutchan JA, Grant I, Mallory ME, Hansen LA, Archibald S, Jernigan T, Masliah E HNRC Group. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS. 2002;16:1019–29. doi: 10.1097/00002030-200205030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du Pasquier RA, Stein MC, Lima MA, Dang X, Jean-Jacques J, Zheng Y, Letvin NL, Koralnik IJ. JC virus induces a vigorous CD8+ cytotoxic T cell response in multiple sclerosis patients. J Neuroimmunol. 2006;176:181–6. doi: 10.1016/j.jneuroim.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann KC, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]


