Abstract
Background
Converging lines of evidence implicate the neurotrophin brain-derived neurotrophic factor (BDNF) in the pathophysiology of major depression. Recent studies have begun to explore the relationship between serum BDNF and depression.
Methods
We conducted meta-analyses of 11 studies examining differences in serum BDNF content between depressed and non-depressed subjects (total N = 748), and 8 studies comparing pre and post antidepressant treatment serum BDNF content (total N=220).
Results
The meta-analysis revealed strong evidence that BDNF levels were lower in depressed subjects than healthy controls (p < 6.8 ×10−8). Similarly, the second meta-analysis found significantly higher BDNF levels after antidepressant treatment (p = 0.003). There was no evidence of publication bias in the first (p = 0.376) or second (p = 0.571) meta-analysis and no evidence that either meta-analysis was unduly influenced by any one study.
Conclusions
These findings provide strong evidence to suggest that serum BDNF levels are abnormally low in patients suffering from major depressive disorder, and that the BDNF levels are elevated following a course of antidepressant treatment. Although the relationship of the present findings to pathophysiology of depression and the mechanism of drug action remains to be determined, the measure may have potential use as a biomarker for psychiatric disorders or as a predictor of antidepressant efficacy.
Keywords: Neurotrophic, Stress, Depressive, Mood
Over the past decade, increasing evidence has implicated neurotrophic factors in the pathophysiology of depression (1). Stress, an important precipitant of depression, has been repeatedly shown to robustly reduce neurogenesis and the expression of neurotrophic factor genes in the brain (2, 3). In contrast, antidepressant treatments almost universally promote neurogenesis and neurotrophic factor gene expression (3, 4). Further, depressed subjects show increased cellular atrophy in limbic and cortical areas of the brain, consistent with decreased neurotrophic activity (5).
Among neurotrophins, brain-derived neurotrophic factor (BDNF) has been most extensively studied in relation to depression. BDNF is a dimeric protein found throughout the brain, with particular abundance in the hippocampus and cerebral cortex (6). Depressed subjects show reduced levels of hippocampal and cortical BDNF in postmortem studies (7). This is of special interest considering hippocampal lesions have effects on anxiety and depressive behaviors (8), and the hippocampi of depressed patients are consistently found to be smaller than those of healthy individuals (9). In addition, a polymorphism in the BDNF gene has been associated with depression related traits in some but not all studies (10–13), and direct infusion of BDNF into the brains of animals has been shown to produce an ‘anti-depressant effect’ in learned helplessness and forced swim models of depression (14, 15).
Given the difficulty of studying BDNF levels in the brain directly, there has been great interest in the accurate assessment of BDNF activity peripherally. Peripheral measures of BDNF can be obtained relatively non-invasively from blood samples. Serum, plasma, and whole blood BDNF content are commonly measured using enzyme-linked immunosorbent assays (ELISA) commercially supplied by Promega (Walisellen, Switzerland) or R & D Systems (Minneapolis, Minnesota) with relatively high specificity and sensitivity. In 2002, Karege and colleagues (16) identified a correlation in rodents between cortical and serum BDNF and subsequently found that serum BDNF was lower in depressed patients than matched controls (17). In the years since this report, there have been multiple attempts to replicate this association.
Other studies have begun to explore serum BDNF through the comparison of levels in depressed patients before and after pharmacological antidepressant treatment. Some studies have found a significant difference in serum BDNF levels while others did not (18, 19).
A technique that has proven useful in resolving discrepancies between association studies is meta-analysis (20). Meta-analysis is a quantitative method of combining the results of independent studies and synthesizing summaries and conclusions. This method increases power to distinguish between small effects and no effect. Furthermore, it can help determine whether variation in effect between studies is due merely to expected random statistical fluctuation, or also due to variation between studies in the sample used or trait assessment.
In this report, we perform meta-analyses on studies of: 1) the comparison of serum BDNF levels between depressed patients and healthy controls 2) the comparison of serum BDNF levels in patients with depression before and after antidepressant treatment.
Materials and Methods
Studies
Studies were identified through PubMed at the National Library of Medicine using the search terms: 1) BDNF Depression 2) Brain Derived Neurotrophic Factor Depression. We subsequently checked the reference sections of the publications found through our search to identify additional studies that may have been missed. Among identified references, 13 studies comparing serum BDNF levels in depressed and control samples were identified. Of these studies, 2 were eliminated from our first analysis because of an overlap in sample with included studies. 8 studies comparing serum BDNF levels in depressed subjects before and after antidepressant treatment were identified and included for our second analysis. Although there are now a few recent studies suggesting plasma BDNF content is also decreased in depressed subjects (19, 21–23), this data was not included in this analysis in order to minimize potential confounds.
Statistical Analysis
For both analyses, we recorded the number of subjects, mean serum BDNF level and standard deviation for each group of subjects (Analysis 1: Depressed and Controls; Analysis 2: Pretreatment and Posttreatment) in each study included in our analysis. For analysis 2, investigators measured posttreatment BDNF levels at various times after antidepressants were started. For our meta-analysis, we used the BDNF levels from the last time point reported in each study. For both meta-analyses, we also recorded the gender and age compositions of the sample used in each study.
In order to have all studies on the same scale for our analyses, the raw score from each study group was converted to a T-score so that each study had an overall mean score of 50 ± 10. Random effects meta-analyses were performed with the diagnosis T-score and the drug treatment T-scores as the dependent variables. Age and gender composition of the study were included as covariates and each study group was weighted according to the inverse of its variance.
To ascertain if the results of our analyses were strongly influenced by any single study, sensitivity analyses were performed. The overall significance was recomputed after each study was individually deleted from the analysis (Figure 2).
Figure 2.
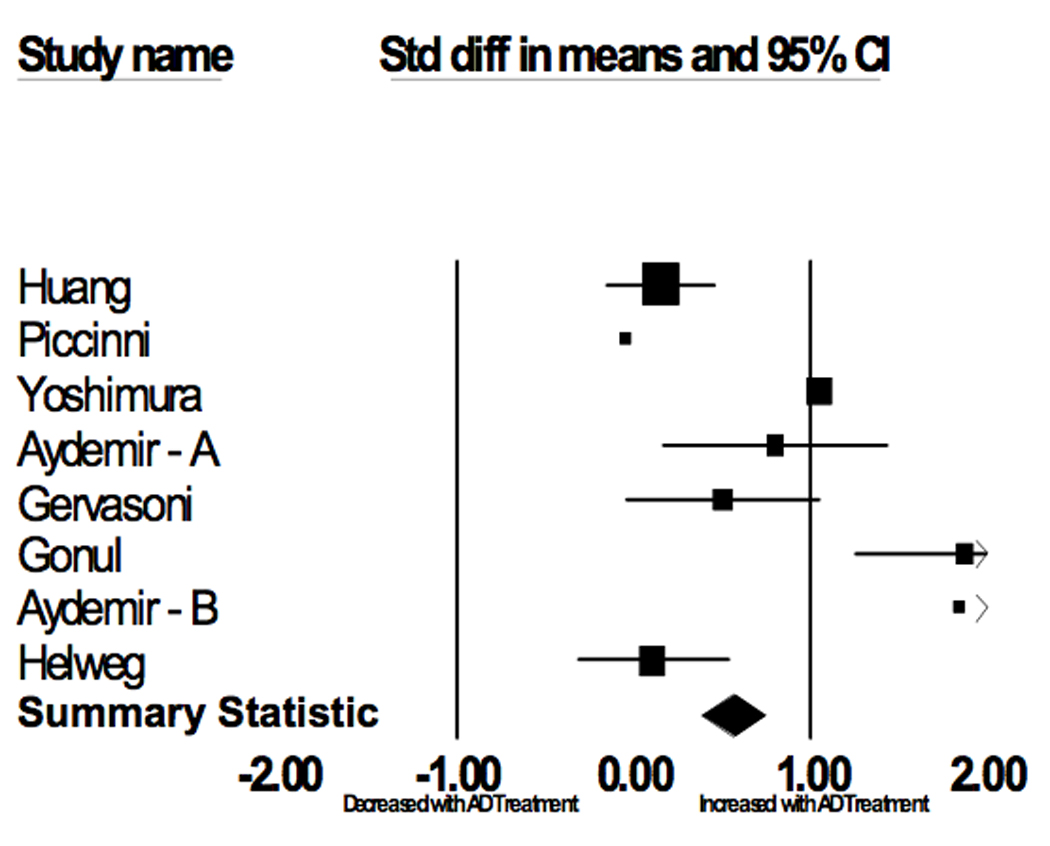
Serum BDNF Changes with AD Treatment
In order to assess for significant publication bias, the method described by Egger and colleagues was used (24). This method regresses the standard normal deviate (estimated regression coefficient divided by standard error) against the weight (1/variance) of the study. The regression coefficients were also used to test for the presence of heterogeneity among the results of the studies. All analyses were carried out in SPSS 15.0 (SPSS Inc., Chicago, Il, USA).
Results
Analysis 1: Depressed vs. Controls
Across 11 included studies investigating depression status and BDNF levels (depressed N = 366; control N = 382), the weighted diagnosis T-scores were (mean ± SD): depressed, 45.7 ± 9.5; control, 54.1 ± 10.5. The meta-analysis showed strong evidence for an association between Serum BDNF and Depression status (p < 6×10−8). Neither the age (p = 0.671) nor gender (p = 0.274) composition of the studies was a significant covariate in the analysis. There was no evidence for heterogeneity among the studies (p = 0.919).
To determine if an individual study was responsible for the presence or absence of an association in each of the tests, we performed a series of sensitivity analyses (Table 2). Each study was individually excluded and the significance of the analysis was recomputed. The result remained highly significant in each of these analyses.
Table 2.
Serum BDNF Levels (µg/ml) Before and After Antidepressant Medications – Included Studies
| Author | Age | Gender (Female) | Antidepressant | Pretreatment | Posttreatment | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Serum BDNF | SD | N | Serum BDNF | SD | ||||
| Huang(47) | 37.3 | 0.77 | SSRIs and Venlafaxine | 79 | 10.7 | 8.9 | 79 | 12 | 8.9 |
| Piccinni(19) | 47.0 | 0.87 | SSRIs and TCAs | 15 | 19.3 | 8.8 | 7 | 18.8 | 4.1 |
| Yoshimura(18) | 46.5 | 0.62 | Paroxetine and Milnacipran | 42 | 9.5 | 7.8 | 42 | 17.7 | 8.1 |
| Aydemir – A(45) | 35.6 | 1 | Escitalopram | 20 | 27.7 | 13.7 | 20 | 38.6 | 15.3 |
| Gervasoni(44) | 40.5 | 0.58 | Paroxetine, Venlafaxine, Clomipramine and Lithium | 26 | 22.6 | 3.6 | 26 | 24.4 | 3.6 |
| Gonul(48) | 35.5 | 0.75 | SSRIs and Venlafaxine | 28 | 20.8 | 6.7 | 28 | 33.3 | 9.9 |
| Aydemir – B(46) | 31.8 | 0.80 | Venlafaxine | 10 | 17.9 | 9.1 | 10 | 34.6 | 7.1 |
| Hellweg(50) | 50.5 | 0.78 | Amitriptyline and Paroxetine | 40 | 13.1 | 4.4 | 40 | 13.6 | 5.4 |
The publication bias statistic of Egger and colleagues was not significant (p = 0.376).
Analysis 2: Pretreatment vs. Posttreatment
Among studies comparing serum BDNF levels in patients before and after antidepressant treatment (N = 220), the weighted drug treatment T-scores were (mean ± SD): pretreatment, 46.9 ± 9.9; posttreatment, 53.1 ± 10.1. The meta-analysis showed evidence for an association between Serum BDNF and Depression status (p = 0.003). Neither the age (p = 0.990) nor gender (p = 0.785) composition of the studies was a significant covariate in the analysis. There was no evidence for heterogeneity among the studies (p = 0.19). Sensitivity analysis showed that the significant result of this meta-analysis was not unduly influenced by any one study. The publication bias statistic of Egger and colleagues was not significant (p = 0.571) indicating that it was unlikely that publication bias was responsible for this association.
Discussion
In this study, we report the results of meta-analyses that confirm significant associations between serum BDNF levels and both depression status and pharmacological antidepressant treatment. In the first analysis, healthy subjects had serum BDNF levels 0.84 standard deviations greater than depressed subjects. Given the median BDNF levels (22.2 µg/ml) of the included studies, this translates to 6.3 µg/ml serum BDNF levels. In the second analysis, subjects had serum BDNF levels 0.62 standard deviations greater after antidepressant treatment. This translates to an increase of 4.7 µg/ml serum BDNF levels after pharmacological antidepressant treatment. For both meta-analyses, there was no evidence for publication bias. Similarly, our sensitivity analyses demonstrate that the results of the meta-analyses were not due to the influence of any one study.
What is source of the serum BDNF?
Despite the name, BDNF is expressed in most tissues of the body at relatively high levels (25). BDNF in the blood could be derived from a number of these peripheral tissues, as well as from the brain. BDNF in the blood is stored at relatively high levels in platelets (23), but there is little or no information about the source of BDNF in the blood or its regulation by stress, endocrine factors or environmental stimuli. In the brain, many different types of stress, as well as adrenal-steroids, decrease the expression of BDNF in limbic brain regions in rodent, preclinical studies (5). Future studies will be required to determine if the reduction in serum BDNF levels results from decreased levels of BDNF in the brain, peripheral tissues, or both.
What is the utility of the measure as a biomarker?
Identifying biomarkers that could be used to assist in the diagnosis of major depressive disorder has remained a goal of clinicians and scientists for more than a half of a century. Despite the uncertainty surrounding the source of the serum BDNF, the relative simplicity of the test and the highly consistent findings of reduced serum BDNF levels in individuals with major depressive disorder suggest that the measure may prove to be a clinically useful biomarker. However, the apparent lack of diagnostic specificity is likely to be a major drawback limiting the true clinical utility of the measure. In addition to major depression, reduced serum and plasma BDNF levels have also recently been reported in several other disorders including; schizophrenia (26), bipolar disorder (27), eating disorders such as bulimia nervosa and anorexia nervosa (28), Huntington's disease (29), Alzheimer’s disease (30), autism (31) and even lower respiratory infections (32). Interestingly, there are also several recent reports suggesting that BDNF levels are decreased in individuals with type-2 diabetes (33, 34) and purporting a link between reduced circulating BDNF levels and metabolic syndrome (35, 36). Although the seemingly non-specific association of reduced serum BDNF levels to a broad range of disorders detracts from its appeal as a specific diagnostic biomarker, it may inform us about a common pathophysiological mechanism that is shared by several different disease processes. In addition, it may also provide some insight into the high rates of comorbidity that exist between many of the disorders.
A second potentially interesting application of serum BDNF measures could be as a surrogate biomarker of antidepressant efficacy. There is now strong evidence that serum BDNF levels increase following treatment with antidepressant medications, similar to what is seen with BDNF expression in specific brain regions in rodent models (5). This suggests that the measure may be used to screen for novel antidepressant agents or possibly even predict an individual’s response to an antidepressant treatment at an early time point after treatment initiation if a relationship can be established between the change in BDNF levels and clinical response. However, it is important to recognize that plasma and serum BDNF levels have also been shown to correlate with other events and activities such as food intake (37), stress (38), and exercise (39), that are likely to complicate the interpretation of the findings and possibly limit the true utility of the measure. On the other hand, it is worthy of note that some of these conditions are known to either exacerbate or precipitate depression (e.g., stress), or produce an antidepressant response (5). It will be interesting to see what effect non-pharmacological antidepressant treatments have on serum BDNF levels in depressed subjects.
What is the functional impact of serum BDNF?
In addition to the importance of BDNF as a potential biomarker of depression and antidepressant responsiveness, it is also possible that serum BDNF has functional effects on the brain. Although the blood brain barrier prohibits the passage of most proteins, there is now evidence for active transport of a number of regulatory factors, including peripheral growth factors (40). The best example is insulin-like growth factor 1 (IGF-1). IGF-1 is made predominantly in the liver, but is actively transported into the brain where it has been shown to regulate neuronal function, survival, neurogenesis and behavior (41, 42). Studies of BDNF uptake or transport have been mixed, and additional work will be required to determine if there is an active transport system for uptake of BDNF into the brain.
However, in recent preliminary studies we have found that peripheral administration of BDNF produces both cellular effects in the brain and behavioral actions (Schmidt and Duman, unpublished observations). BDNF infusion over a period of 2 weeks increases neurogenesis in the adult hippocampus and produces anxiolytic and antidepressant effects in rodent behavioral models. It is possible that these effects of peripheral BDNF infusions could result from direct actions of BDNF that is transported into the brain, or from indirect effects of BDNF on peripheral tissue, resulting in release of other factors that are transported into the brain (e.g., IGF-1) or even metabolic effects. Regardless of the mechanism, these preliminary studies demonstrate that BDNF in the blood is capable of producing central actions at both the cellular and behavioral levels in animal models.
Summary and Conclusions
In sum, there is now overwhelming evidence demonstrating that serum BDNF levels are reduced in individuals suffering with major depressive disorder. There is also very strong and consistent evidence suggesting that these levels normalize following antidepressant treatment. These findings are of great interest to the field considering the growing evidence of neurotrophic factor involvement in the pathophysiology of the disorder and the mechanism of antidepressant drug action. Although the extent to which the serum BDNF levels reflect brain content remains in question, there is now reason to believe that peripheral BDNF may have effects on brain function and behavior. Lastly, the potential utility of serum BDNF levels as a biomarker for illness and antidepressant efficacy has stirred significant interest. While the reduced levels of serum BDNF may reflect a common pathophysiological phenomenon shared by many illnesses, the lack of specificity under the current diagnostic system severely limits the clinical utility of the measure at present. Future studies are required to determine if the measure can be used in a way to guide clinical decision-making and/or drug development.
Figure 1.
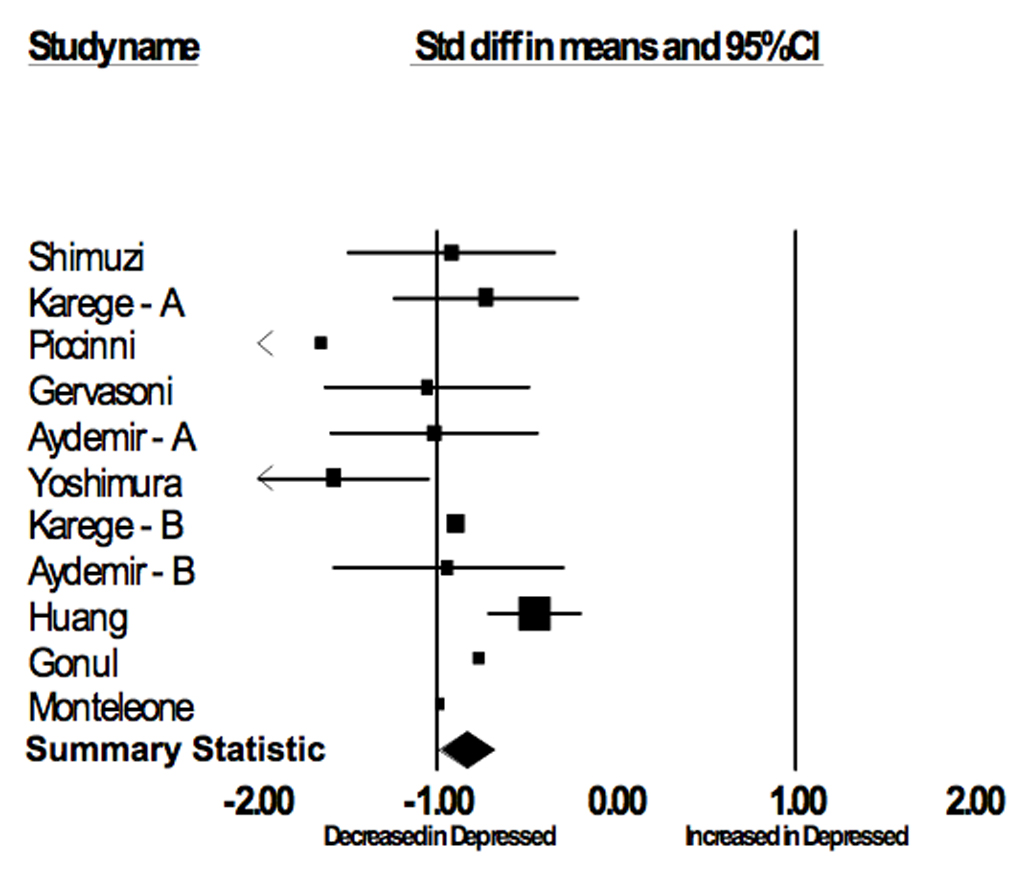
Serum BDNF in Depressed vs. Control Subjects
Table 1.
Serum BDNF Levels (µg/ml) in Depressed vs. Healthy Subjects - Included Studies
| Author | Age | Gender (% female) | Control | Depressed | ||||
|---|---|---|---|---|---|---|---|---|
| N | Serum BDNF | SD | N | Serum BDNF | SD | |||
| Shimizu(43) | 41.6 | 0.42 | 50 | 27.7 | 11.4 | 16 | 17.6 | 9.6 |
| Karege-A(17) | 37.0 | 0.50 | 30 | 26.5 | 7.0 | 30 | 22.6 | 3.0 |
| Piccinni(19) | 42.0 | 0.83 | 15 | 33.6 | 8.6 | 15 | 19.3 | 8.8 |
| Gervasoni(44) | 40.1 | 0.54 | 26 | 26.4 | 3.6 | 26 | 22.6 | 3.6 |
| Aydemir – A(45) | 32.6 | 0.74 | 26 | 31.4 | 8.8 | 24 | 21.2 | 11.3 |
| Yoshimura(18) | 45.6 | 0.64 | 30 | 23.4 | 10.1 | 42 | 9.5 | 7.8 |
| Karege-B(23) | 33.8 | 0.55 | 34 | 12.2 | 2.4 | 43 | 10.1 | 2.3 |
| Aydemir - B(46) | 35.1 | 1.00 | 20 | 41.2 | 15.1 | 20 | 27.7 | 13.7 |
| Huang(47) | 32.5 | 0.71 | 107 | 14.1 | 7.0 | 111 | 10.9 | 7.1 |
| Gonul(48) | 35.6 | 0.72 | 18 | 26.8 | 9.3 | 28 | 20.8 | 6.7 |
| Monteleone(49) | 42.0 | 0.70 | 22 | 42.5 | 12.5 | 11 | 29.0 | 15.9 |
Table 3.
Sensitivity Analysis - Serum BDNF and Depression Status
Table 4.
Sensitivity Analysis - Serum BDNF and Antidepressant Treatment
Acknowledgments
(GS) NIMH K02 MH076222-01, (SS, RD, GS) VA REAP and the Connecticut Mental Health Center, (RSD) NIMH MH045481 and MH025642, (SS) Donaghue Foundation, (SS) Daniel X., and Mary Freedman Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Sen has no biomedical financial interests or potential conflicts of interest. Dr. Duman has received consulting and/or research funds from Taisho, Lilly, Wyeth-Ayerst, Roche, Sepracor, Psychogenics, Takeda, and Organon. Dr. Sanacora has received consulting and/or research funds from Abbott, AstraZeneca, Bristol-Myers Squibb, Lilly, Lundbeck, Pfizer, Roche, Ruxton, and Sepracor.
References
- 1.Tanis KQ, Newton SS, Duman RS. Targeting neurotrophic/growth factor expression and signaling for antidepressant drug development. CNS Neurol Disord Drug Targets. 2007;6(2):151–160. doi: 10.2174/187152707780363276. [DOI] [PubMed] [Google Scholar]
- 2.Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56(3):140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 3.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. Journal of Neuroscience. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. Journal of Neuroscience. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Altar CA. Neurotrophins and depression. Trends Pharmacol Sci. 1999;20(2):59–61. doi: 10.1016/s0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- 7.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 8.Bannerman DM, Lemaire M, Yee BK, Iversen SD, Oswald CJ, Good MA, Rawlins JN. Selective cytotoxic lesions of the retrohippocampal region produce a mild deficit in social recognition memory. Exp Brain Res. 2002;142(3):395–401. doi: 10.1007/s00221-001-0938-z. [DOI] [PubMed] [Google Scholar]
- 9.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 10.Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim YM, Tsan G, Schaffner S, Kirov G, Jones I, Owen M, Craddock N, DePaulo JR, Lander ES. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus Brain-derived neutrophic factor. Mol Psychiatry. 2002;7(6):579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- 11.Sen S, Nesse RM, Stoltenberg SF, Li S, Gleiberman L, Chakravarti A, Weder AB, Burmeister M. A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology. 2003;28(2):397–401. doi: 10.1038/sj.npp.1300053. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59(8):673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacology, Biochemistry & Behavior. 1997;56(1):131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 15.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328(3):261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- 17.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura R, Mitoma M, Sugita A, Hori H, Okamoto T, Umene W, Ueda N, Nakamura J. Effects of paroxetine or milnacipran on serum brain-derived neurotrophic factor in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(5):1034–1037. doi: 10.1016/j.pnpbp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Piccinni A, Marazziti D, Catena M, Domenici L, Del Debbio A, Bianchi C, Mannari C, Martini C, Da Pozzo E, Schiavi E, Mariotti A, Roncaglia I, Palla A, Consoli G, Giovannini L, Massimetti G, Dell'sso L. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord. 2007 doi: 10.1016/j.jad.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33(2):177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- 21.Grassi-Oliveira R, Stein LM, Lopes RP, Teixeira AL, Bauer ME. Low Plasma Brain-Derived Neurotrophic Factor and Childhood Physical Neglect Are Associated with Verbal Memory Impairment in Major Depression-A Preliminary Report. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Lee BH, Kim H, Park SH, Kim YK. Decreased plasma BDNF level in depressive patients. J Affect Disord. 2007;101(1–3):239–244. doi: 10.1016/j.jad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57(9):1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lommatzsch M, Quarcoo D, Schulte-Herbruggen O, Weber H, Virchow JC, Renz H, Braun A. Neurotrophins in murine viscera: a dynamic pattern from birth to adulthood. Int J Dev Neurosci. 2005;23(6):495–500. doi: 10.1016/j.ijdevneu.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda Y, Yahata N, Ito I, Nagano M, Toyota T, Yoshikawa T, Okubo Y, Suzuki H. Low serum levels of brain-derived neurotrophic factor and epidermal growth factor in patients with chronic schizophrenia. Schizophr Res. 2008 doi: 10.1016/j.schres.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Machado-Vieira R, Dietrich MO, Leke R, Cereser VH, Zanatto V, Kapczinski F, Souza DO, Portela LV, Gentil V. Decreased plasma brain derived neurotrophic factor levels in unmedicated bipolar patients during manic episode. Biol Psychiatry. 2007;61(2):142–144. doi: 10.1016/j.biopsych.2006.03.070. [DOI] [PubMed] [Google Scholar]
- 28.Nakazato M, Hashimoto K, Shimizu E, Kumakiri C, Koizumi H, Okamura N, Mitsumori M, Komatsu N, Iyo M. Decreased levels of serum brain-derived neurotrophic factor in female patients with eating disorders. Biol Psychiatry. 2003;54(4):485–490. doi: 10.1016/s0006-3223(02)01746-8. [DOI] [PubMed] [Google Scholar]
- 29.Ciammola A, Sassone J, Cannella M, Calza S, Poletti B, Frati L, Squitieri F, Silani V. Low brain-derived neurotrophic factor (BDNF) levels in serum of Huntington's disease patients. Am J Med Genet B Neuropsychiatr Genet. 2007;144(4):574–577. doi: 10.1002/ajmg.b.30501. [DOI] [PubMed] [Google Scholar]
- 30.Yasutake C, Kuroda K, Yanagawa T, Okamura T, Yoneda H. Serum BDNF, TNF-alpha and IL-1beta levels in dementia patients: comparison between Alzheimer's disease and vascular dementia. Eur Arch Psychiatry Clin Neurosci. 2006;256(7):402–406. doi: 10.1007/s00406-006-0652-8. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, Sekine Y, Suzuki K, Minabe Y, Takei N, Iyo M, Mori N. Reduced serum levels of brain-derived neurotrophic factor in adult male patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(8):1529–1531. doi: 10.1016/j.pnpbp.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Lommatzsch M, Niewerth A, Klotz J, Schulte-Herbruggen O, Zingler C, Schuff-Werner P, Virchow JC. Platelet and plasma BDNF in lower respiratory tract infections of the adult. Respir Med. 2007;101(7):1493–1499. doi: 10.1016/j.rmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AM, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H, Pedersen BK. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50(2):431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 34.Fujinami A, Ohta K, Obayashi H, Fukui M, Hasegawa G, Nakamura N, Kozai H, Imai S, Ohta M. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: Relationship to glucose metabolism and biomarkers of insulin resistance. Clin Biochem. 2008 doi: 10.1016/j.clinbiochem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Hristova M, Aloe L. Metabolic syndrome--neurotrophic hypothesis. Med Hypotheses. 2006;66(3):545–549. doi: 10.1016/j.mehy.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 36.Geroldi D, Minoretti P, Emanuele E. Brain-derived neurotrophic factor and the metabolic syndrome: more than just a hypothesis. Med Hypotheses. 2006;67(1):195–196. doi: 10.1016/j.mehy.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Stanek K, Gunstad J, Leahey T, Glickman E, Alexander T, Spitznagel MB, Juvancic Heltzel J, Murray L. Serum Brain-derived Neurotrophic Factor is Associated with Reduced Appetite in Healthy Older Adults. J Nutr Health Aging. 2008;12(3):183–185. doi: 10.1007/BF02982616. [DOI] [PubMed] [Google Scholar]
- 38.Mitoma M, Yoshimura R, Sugita A, Umene W, Hori H, Nakano H, Ueda N, Nakamura J. Stress at work alters serum brain-derived neurotrophic factor (BDNF) levels and plasma 3-methoxy-4-hydroxyphenylglycol (MHPG) levels in healthy volunteers: BDNF and MHPG as possible biological markers of mental stress? Prog Neuropsychopharmacol Biol Psychiatry. 2007 doi: 10.1016/j.pnpbp.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci Lett. 2008;431(1):62–65. doi: 10.1016/j.neulet.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Kastin AJ, Pan W, Maness LM, Banks WA. Peptides crossing the blood-brain barrier: some unusual observations. Brain Res. 1999;848(1–2):96–100. doi: 10.1016/s0006-8993(99)01961-7. [DOI] [PubMed] [Google Scholar]
- 41.Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20(8):2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21(5):1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54(1):70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 44.Gervasoni N, Aubry JM, Bondolfi G, Osiek C, Schwald M, Bertschy G, Karege F. Partial normalization of serum brain-derived neurotrophic factor in remitted patients after a major depressive episode. Neuropsychobiology. 2005;51(4):234–238. doi: 10.1159/000085725. [DOI] [PubMed] [Google Scholar]
- 45.Aydemir O, Deveci A, Taskin OE, Taneli F, Esen-Danaci A. Serum brain-derived neurotrophic factor level in dysthymia: a comparative study with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(5):1023–1026. doi: 10.1016/j.pnpbp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Aydemir C, Yalcin ES, Aksaray S, Kisa C, Yildirim SG, Uzbay T, Goka E. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1256–1260. doi: 10.1016/j.pnpbp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 47.Huang TL, Lee CT, Liu YL. Serum brain-derived neurotrophic factor levels in patients with major depression: Effects of antidepressants. J Psychiatr Res. 2007 doi: 10.1016/j.jpsychires.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Gonul AS, Akdeniz F, Taneli F, Donat O, Eker C, Vahip S. Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):381–386. doi: 10.1007/s00406-005-0578-6. [DOI] [PubMed] [Google Scholar]
- 49.Monteleone P, Serritella C, Martiadis V, Maj M. Decreased levels of serum brain-derived neurotrophic factor in both depressed and euthymic patients with unipolar depression and in euthymic patients with bipolar I and II disorders. Bipolar Disord. 2008;10(1):95–100. doi: 10.1111/j.1399-5618.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- 50.Hellweg R, Ziegenhorn A, Heuser I, Deuschle M. Serum Concentrations of Nerve Growth Factor and Brain-Derived Neurotrophic Factor in Depressed Patients before and after Antidepressant Treatment. Pharmacopsychiatry. 2008;41(2):66–71. doi: 10.1055/s-2007-1004594. [DOI] [PubMed] [Google Scholar]


