Abstract
Ovarian cancer is one of the leading causes of death from gynecological cancers in the United States. Conventional therapies are unlikely to control advanced stage ovarian cancers, thus requiring innovative alternative therapies. In the current study, we characterized the therapeutic effect of tumor cell-based vaccines combined with the adjuvant, α-Galactosylceramide (α-GalCer) using two different mouse models. Our data suggests that treatment with α-GalCer led to an increase in the IFN-γ serum levels in the presence or absence of irradiated mouse ovarian surface epithelial tumor cells (MOSEC). Furthermore, administration of irradiated MOSEC tumor cells with adjuvant α-GalCer generated significant protective and therapeutic antitumor effects against MOSEC tumors in vaccinated C57BL/6 mice. In addition, immune cells expressing CD4, CD8 or NK1.1 markers were found to be important for the protective anti-tumor effects generated by irradiated tumor cell-based vaccines combined with adjuvant α-GalCer. We also found that treatment of a spontaneous ovarian cancer murine model, the Müllerian inhibiting substance type II receptor T antigen (TgMISIIR-TAg) transgenic mice with ovarian tumor cell-based vaccines combined with adjuvant α-GalCer led to prolonged survival as well as increased numbers of tumor-specific CD8+ T cells. Therefore, irradiated tumor cell-based vaccines in combination with α-GalCer are capable of breaking immune tolerance and generating significant antitumor effects in two different mouse tumor models. Our study serves as a foundation for future clinical translation.
Keywords: α-Galactosyl Ceramide, tumor cell-based vaccines, adjuvant, MOSEC, ovarian
2.0 Introduction
Ovarian cancer is the sixth most common malignancy in women and the leading cause of death from all gynecological cancers in the United States [1]. Current therapies such as surgery, chemotherapy and radiotherapy usually fail to control advanced stages of the disease. Therefore, alternative approaches such as immunotherapeutic strategies to enhance immune responses may serve as an important method to control these intraperitoneal tumors.
Tumor-cell based vaccines have emerged as an attractive immunological approach for the treatment of ovarian cancer (for review, see [2, 3]). This vaccination strategy has an advantage over other strategies because the irradiated tumor cells are engineered to generate specific immune responses against tumor without the need to identify a specific immunodominant tumor-specific antigenic epitope tumor antigen. However, the efficacy of this approach relies on the ability of the vaccine to effectively induce specific immunity against tumors. Furthermore, multiple antigens relevant to the tumor can be targeted by tumor cell-based vaccines. However, unmodified tumor cell-based vaccines face limitations in their immunogenicity. This has led to the administration of adjuvants, such as cytokines or co-stimulatory molecules, along with tumor cell-based vaccines in order to enhance vaccine potency (for reviews, see[4, 5]). Therefore, strategies to enhance the efficiency of tumor-cell based vaccines such as the use of adjuvants are important for improving the treatment of ovarian tumors.
The glycosphingolipid, α-galactosylceramide (α-GalCer) represents a potential adjuvant for cancer immunotherapy using tumor cell-based vaccines. These molecules have been reported to induce significant antitumor immunity in mouse metastases models [6, 7]. α-GalCer can be presented by CD1d molecules of antigen-presenting cells, and is known to induce a potent natural killer T (NKT) cell-dependent cytotoxic response against tumor cells [8-10]. α-GalCer has also been shown to enhance the anti-tumor activity in mice when administered in combination with various types of vaccines [11-15]. Thus, α-GalCer represents a potential adjuvant for tumor cell-based vaccines.
In the current study, we hypothesized that irradiated tumor cell-based vaccine co-administered with α-GalCer may generate potent antitumor effects against ovarian cancer in vaccinated mice. We have employed a transplantable mouse ovarian surface epithelial carcinoma (MOSEC) model as well as a murine Müllerian inhibiting substance type II receptor T antigen (TgMISIIR-TAg) transgenic mouse model that is capable of developing ovarian cancer spontaneously. We found that treatment with α-GalCer leads to an increase in the IFN-γ serum levels in the presence or absence of irradiated MOSEC tumor cells. Furthermore, administration of irradiated MOSEC tumor cells with adjuvant α-GalCer generated significant protective and therapeutic antitumor effects against MOSEC tumors in vaccinated mice. We also found that treatment of the TgMISIIR-TAg transgenic mice with ovarian tumor cell-based vaccines combined with adjuvant α-GalCer led to prolonged survival as well as increased numbers of tumor-specific CD8+ T cells. Thus, α-GalCer represents an important adjuvant for improving the efficacy of tumor-cell based vaccines to treat ovarian cancer.
3.0 Materials and Methods
3.0.1 Mice and cell lines
Female C57BL/6 mice aged 5-7 weeks were purchased from the National Cancer Institute. Female TgMISIIR-TAg transgenic mice were obtained by breeding female C57BL/6 mice with male TgMISIIR-TAg transgenic mice and used at 6 weeks of age in the experiments. These mice spontaneously developed ovarian tumors with complete tissue penetration. The male MISIIR-TAg transgenic mice were obtained from Fox Chase Cancer Center (Philadelphia, PA, USA) [16]. All animals were maintained under specific pathogen-free conditions, and all procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
The MOSEC cell line was generated from C57BL/6 mouse ovarian surface epithelial cells as described previously [17]. Luciferase expressing MOSEC tumor cells (MOSEC-luc) were generated by transducing the MOSEC cells with retrovirus containing luciferase, pLucithy1.1, and flow cytometry sorting following the previously described protocol [18]. The MOVCAR (mouse ovarian carcinoma) cell line was obtained from Fox Chase Cancer Center (Philadelphia, PA, USA). It was derived from the ascites of a TgMISIIR-TAg transgenic mouse with ovarian tumors. All cell lines were maintained in RPMI 1640 complete medium supplemented with 10% heat-inactivated FBS (HyClone), 1% non-essential aminoacid, 2 mM L-glutamine, 50 U/ml penicillin, 50 mg/ml streptomycin, and 5 mM 2-ME (Invitrogen Life Technologies).
3.0.2 α-GalCer Treatment Protocol
α-Galactosylceramide (α-GalCer) was purchased from Alexis Biochemicals (Lausen, Switzerland), and dissolved in 0.5% Tween 20 in PBS for a final concentration of 0.2 mg /ml, and diluted with PBS before use. Each mouse received 1 μg of α-GalCer by intraperitoneal injection with or without the cell-based vaccination.
3.0.3 Irradiated cells and cell-based vaccinations
The irradiated MOSEC and MOVCAR tumor cells were prepared using an irradiation dosage of 93 Gy/10min. In the murine ovarian tumor model, C57BL/6 mice were intraperitoneally (i.p.) injected with 1×106 /mouse of irradiated MOSEC cells. In the spontaneous tumor model, TgMISIIR-TAg transgenic mice were i.p. injected with 1×106 /mouse of irradiated MOVCAR cells. The cell-based vaccinations were given twice with a 1-wk interval (not including the ELISA).
3.0.4 ELISA
Naïve female C57BL/6 mice were injected once with 1 × 106 /mouse of the irradiated MOSEC tumor cells and/or 1 μg /mouse of α-GalCer. Five hrs after the injections with irradiated MOSEC tumor cells and/or αGal-Cer, sera were collected from the various groups of mice. The collected sera were assayed for the presence of IFN-γ using ELISA kits (Endogen, Woburn, MA) according to the manufacturer protocol.
3.0.5 Cytolytic activity assay
MOSEC-Luc as target tumor cells were plated at a concentration of 1 × 105 cells per 0.1 ml/well in 96-well microplates. Naïve female C57BL/6 mice were injected twice with 1 × 106 /mouse of the irradiated MOSEC tumor cells with a 1 wk interval and/or 1 μg /mouse of α-GalCer. A group of naive mice was used as a control. Two weeks after the last vaccination, the mice were challenged with 2 × 105 /mouse of live MOSEC cells for in vivo whole cell-based antigenic stimulation. One day later, splenocytes were obtained from the different groups of mice. Increasing amounts of splenocytes (1 × 105 or 3 ×105 in a final volume of 0.2 ml /well) were then co-cultured with the MOSEC-luc tumor cells at 37°C for 16 hrs, and luminescence was read on the IVIS Imaging System Series 200. Cytolytic activity was determined as described previously [19] after adding luciferin to the wells.
3.0.6 Tumor protection assay
Naïve female C57BL/6 mice were inoculated with 1×106 /mouse of the irradiated MOSEC tumor cells and/or 1 μg /mouse of α-GalCer twice with a 1-wk interval. Two weeks after the second vaccination, mice were challenged i.p. with 2×105 cells /mouse of live MOSEC-luc tumor cells. Detection of luminescence activity indicating relative tumor loading was performed by IVIS imaging system Series 200 (Xenogen, Cranbury, NJ, USA) and the luminescence activity was monitored using IVIS imaging once a week for 28 days. The survival rates of each group were monitored over the following 120 days after tumor challenge day.
3.0.7 Tumor treatment assay
Naïve female C57BL/6 mice were inoculated i.p. with 2×105 cells /mouse of live MOSEC-luc tumor cells. Five days later, the tumor-bearing mice were injected with 1×106 /mouse of the irradiated MOSEC tumor cells and/or 1 μg /mouse of α-GalCer twice with a 1 wk interval. Detection of luminescence activity indicating relative tumor loading was performed by IVIS Imaging System Series 200 (Xenogen, Cranbury, NJ, USA) and the luminescence activity was monitored using IVIS imaging once a week for 28 days. The survival rates of each group were monitored over 120 days after the first vaccination.
3.0.8 Depletion of lymphocyte subsets in vivo
Naïve female C57BL/6 mice were inoculated with 1×106 /mouse of the irradiated MOSEC tumor cells and/or 1 μg /mouse of α-GalCer twice with a 1 wk interval. One week after the last vaccination, depletion was performed using 200 μg of purified rat monoclonal antibody GK1.5 (anti-CD4), mAb 2.43 (anti-CD8), or mAb PK136 (anti-NK1.1) given i.p. every other day for three times. The depletion was maintained by continuing the antibody injections weekly three days after the last of the three initial injections. Two weeks after the second vaccination, mice were challenged i.p. with 2×105 cells/mouse of live MOSEC-luc tumor cells. One group of mice was not administered antibodies as a control. Differences in the luminescence activity of tumor growth were monitored by IVIS imaging system Series 200 once a week for 28 days.
3.0.9 Intracellular Cytokine Staining and Flow Cytometry Analysis
Female TgMISIIR-TAg transgenic mice were inoculated with 1×106 /mouse of the irradiated MOVCAR tumor cells and/or 1 μg /mouse of α-GalCer twice with a 1-wk interval. One week after the last vaccination, 5 × 106 splenocytes were harvested from each mouse and cultured in vitro with 2 × 105 live MOVCAR tumor cells for 16 hrs with 2 μg Golgistop (PharMingen, San Diego, CA). Cells were then washed once in FACScan buffer and stained with FITC-conjugated monoclonal rat anti-mouse IFN-γ antibody using the Cytofix/Cytoperm kit according to the manufacturer's instructions (PharMingen, San diego, CA). Flow analysis was performed with a Becton Dickinson FACScan with CELLQuest software (Becton Dickinson Immunocytometry System, Mountain View, CA).
3.1.0 Treatment of a MISIIR-TAg transgenic mouse model
Female TgMISIIR-TAg transgenic mice (5 per group) at 6 weeks of age were treated with 1×106 cells/mouse of irradiated MOVCAR tumor cells and/or 1 μg /mouse of α-GalCer twice with a 1-week interval. The survival rates of the each group were monitored over 180 days following from the first treatment.
3.1.1 Statistical Analysis
All data expressed as means ± standard error (s.e.) are representative of at least two different experiments. Comparisons between individual data points were made using a Student's t-test or ANOVA (analysis of variance). Differences in survival between experimental groups were analyzed using the Log rank test.
4.0 Results
4.0.1 Treatment with α-GalCer leads to an increase in the IFN-γ serum levels in the presence or absence of irradiated MOSEC tumor cells
It has been established that NKT cells quickly respond to α-Galactosylceramide (α-GalCer) by IFN-γ production [20]. In order to determine if administration of α-GalCer will lead to IFG-γ secretion in vaccinated mice, we performed ELISA assays to check the IFN-γ serum levels. C57BL/6 mice were injected with irradiated MOSEC tumor cells and/or α-GalCer. Five hours after injection, sera were collected from the different groups of mice and assayed for IFN-γ production by ELISA analysis. As shown in Figure 1, the levels of IFN-γ were significantly higher in sera collected from the mice injected with α-GalCer treated with or without irradiated MOSEC cells compared to the sera from the control or mice treated with irradiated MOSEC tumor cell alone. Thus, our data suggest that administration of α-GalCer with or without irradiated MOSEC tumor cells leads to increased levels of IFN-γ in the serum of vaccinated mice.
Figure 1. ELISA assay characterizing the levels of IFN-γ in the serum of vaccinated mice.
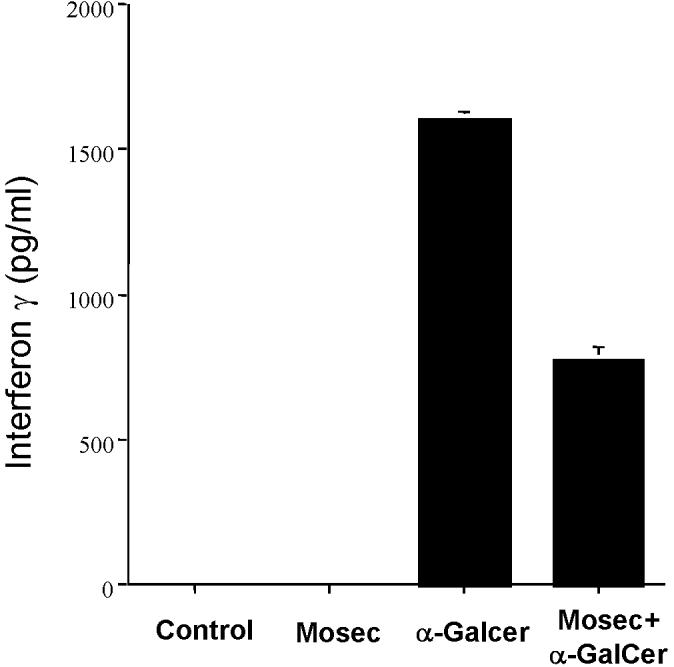
Groups of C57BL/6 mice (5 per group) were vaccinated intraperitoneally with 1 × 106/mouse of irradiated MOSEC tumor cells together with 1 μg/mouse of α-GalCer or each component alone. A group of untreated mice were used as a control. Five hours after the injection, sera were collected from the naïve and vaccinated mice and assayed for levels of IFN-γ using ELISA. The bar graph shows the levels of IFN-γ in picograms /ml. The data shown is from one representative experiment of two performed.
4.0.2 Administration of irradiated MOSEC tumor cells with adjuvant α-GalCer generates cytotoxic T lymphocytes against MOSEC tumor cells in vaccinated mice
In order to determine if administration of irradiated MOSEC tumor cells with adjuvant α-GalCer can generate cytotoxic T lymphocytes against MOSEC tumor cells in vaccinated mice, we performed in vitro cytotoxicity assays using splenocytes from mice vaccinated with irradiated MOSEC tumor cells and/or α-GalCer. A group of naïve mice was used as a control. The harvested splenocytes were incubated with the luciferase expressing MOSEC tumor cells (MOSEC-luc). The bioluminescent intensity correlates directly with number of viable tumor cells. As shown in Figure 2, splenocytes from mice vaccinated with irradiated MOSEC tumor cells and α-GalCer demonstrated the highest cytotoxic activity against MOSEC-luc tumor cells compared to splenocytes from mice vaccinated with irradiated MOSEC tumor cells alone or α-GalCer alone. Thus, our results indicate that vaccination with irradiated MOSEC tumor cells together with α-GalCer were capable of generating a significant number of cytotoxic T lymphocytes against MOSEC tumor cells compared to vaccination with either irradiated MOSEC tumor cells or α-GalCer alone.
Figure 2. In vitro cytoxicity assay with splenocytes from vaccinated mice.
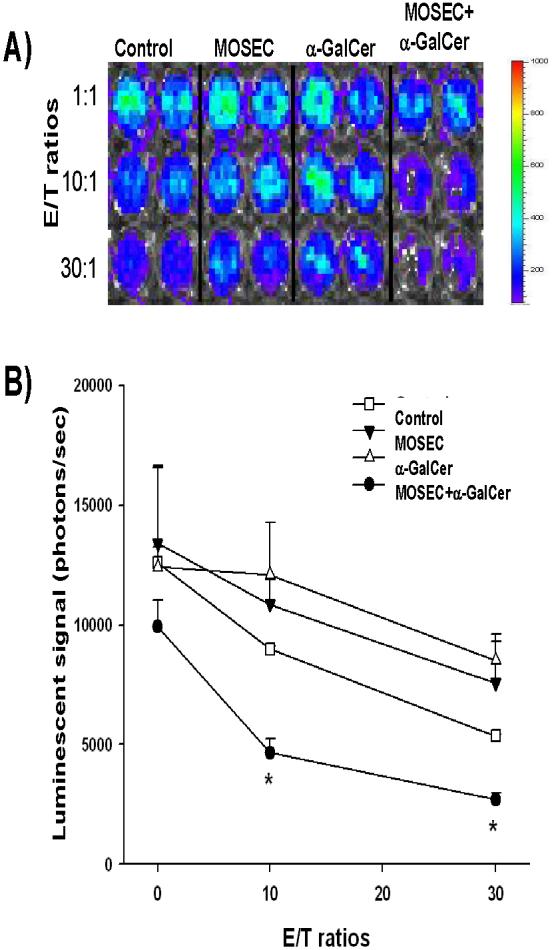
Groups of C57BL/6 mice (5 per group) were vaccinated intraperitoneally with 1 × 106 /mouse of irradiated MOSEC tumor cells and/or 1μg/mouse of α-GalCer twice with a one-week interval. A group of naïve mice was used as a control. Two weeks after the last vaccination, the mice were challenged intraperitoneally with 2 × 105 /mouse of MOSEC tumor cells for in vivo antigenic stimulation. One day after tumor challenge, splenocytes were harvested from the various groups of mice and plated in 96-well plates with luciferase expressing MOSEC tumor cells (MOSEC-luc) in increasing effector: target cell ratios (E:T ratios). Cell viability was demonstrated using luciferase activity and detected using the IVIS Imaging System Series 200. Bioluminescence signals were acquired for one minute. (A) Representative figures of wells showing the luciferase expression in MOSEC-luc cells incubated with splenocytes from the various groups of mice. (B) Line graph quantifying the luminescent activity of the MOSEC-luc cells incubated with splenocytes from the vaccinated mice (mean±s.e.). (* indicates p < 0.05 as compared to control).
4.0.3 Administration of irradiated MOSEC tumor cells with adjuvant α-GalCer generates significant protective antitumor effects against MOSEC tumors in vaccinated mice
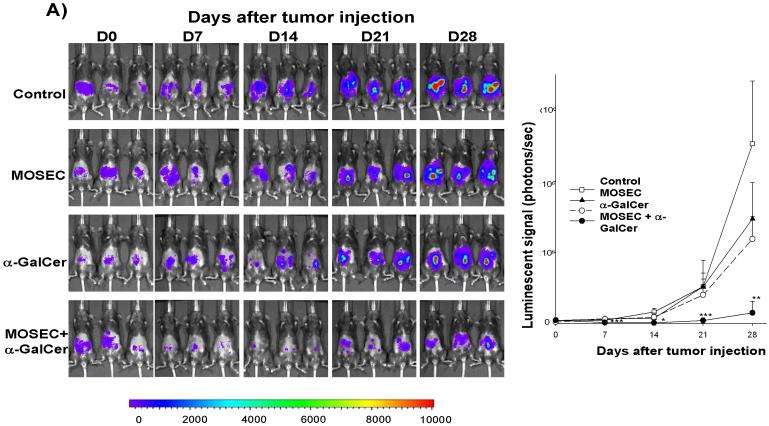
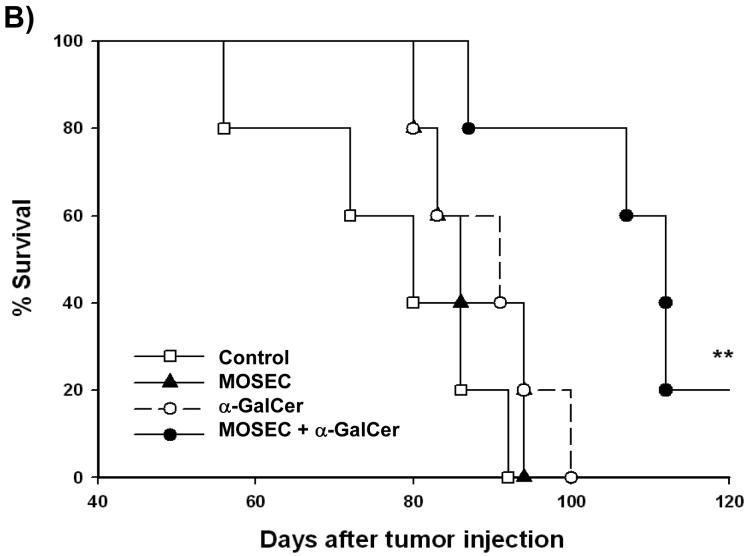
In order to determine whether administration of irradiated MOSEC tumor cells with adjuvant α-GalCer generates protective antitumor effects against MOSEC tumors in vaccinated mice, we performed in vivo tumor protection experiments. C57BL/6 mice (5 per group) were vaccinated with irradiated MOSEC tumor cells and/or α-GalCer twice with a 1-week interval. A group of unvaccinated mice was used as a control. Five days after the last vaccination, the mice groups were challenged with MOSEC-luc tumor cells. The luciferase activity has been shown to directly correlate with the tumor load [21, 22]. As shown in Figure 3A, mice vaccinated with irradiated MOSEC tumor cells and α-GalCer prior to tumor challenge showed significantly decreased tumor growth compared to mice vaccinated with irradiated MOSEC tumor cells or α-GalCer alone. We also characterized the survival of the vaccinated mice after tumor challenge. As shown in Figure 3B, mice vaccinated prior to tumor challenge with irradiated MOSEC tumor cells and α-GalCer exhibited improved survival compared to mice vaccinated with irradiated MOSEC tumor cells or α-GalCer alone. Thus, our data suggest that vaccination with irradiated MOSEC tumor cells combined with the adjuvant α-GalCer can generate potent protective anti-tumor effects in vaccinated mice.
Figure 3. In vivotumor prevention experiments.
C57BL/6 mice (5 per group) were vaccinated intraperitoneally with 1 × 106 /mouse of irradiated MOSEC tumor cells and/or 1 μg /mouse of α-GalCer twice with a one-week interval. A group of naïve mice was used as a control. Two weeks after the last vaccination, the mice were challenged i.p. with 2 × 105 /mouse of MOSEC-luc tumor cells (day 0). Mice were imaged on days 0, 7, 14, 21 and 28 after tumor challenge using the IVIS Imaging System Series 200. Bioluminescence signals were acquired for one minute. (A) Left panel; representative images of the various groups of mice vaccinated with irradiated MOSEC cells and/or α-GalCer prior to tumor challenge with MOSEC-luc. Right panel; line graph depicting the kinetic expression of luciferase (mean±s.e.) in the various groups of mice vaccinated with irradiated MOSEC cells and/or α-GalCer prior to tumor challenge with MOSEC-luc. (B) Kaplan & Meier survival analysis of mice vaccinated with irradiated MOSEC cells and/or α-GalCer prior to tumor challenge with MOSEC-luc. (* indicates p < 0.05; ** indicates p < 0.01; *** indicates p < 0.001 as compared to the control). The data shown is from one representative experiment of two performed.
4.0.4 Administration of irradiated MOSEC tumor cells with adjuvant α-GalCer generates significant therapeutic antitumor effects against MOSEC tumors in vaccinated mice
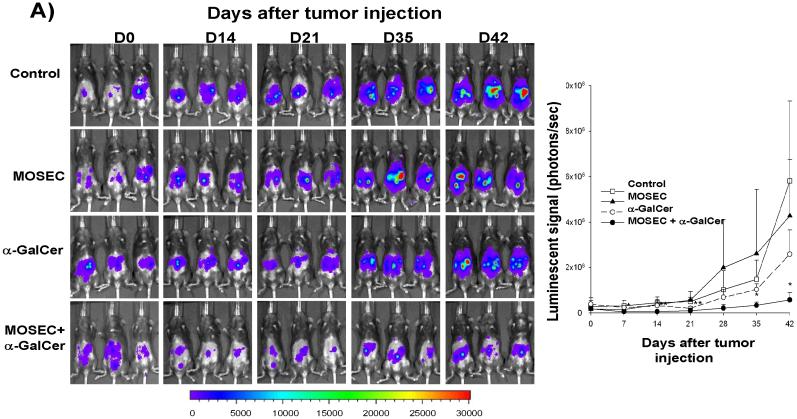
In order to determine whether administration of irradiated MOSEC tumor cells with adjuvant α-GalCer generates therapeutic antitumor effects against MOSEC tumors in vaccinated mice, we performed in vivo tumor treatment experiments using C57BL/6 mice challenged with MOSEC-luc tumors. Five days after the challenge, the mice were treated with irradiated MOSEC tumor cells and/or α-GalCer twice with a 1-week interval. A group of tumor-bearing mice was left untreated as a control. As shown in Figure 4A, treatment of mice with irradiated MOSEC tumor cells and α-GalCer led to significantly decreased tumor growth over time compared to treatment with irradiated MOSEC tumor cells alone or α-GalCer alone. We also characterized the survival of MOSEC-luc tumor-bearing mice after the different treatments. As shown in Figure 4B, MOSEC-luc tumor-bearing mice treated with irradiated MOSEC tumor cells and α-GalCer exhibited improved survival compared to the other treatment groups. Thus, our results suggest that administration of irradiated MOSEC tumor cells with adjuvant α-GalCer can generate significant therapeutic antitumor effects in MOSEC-luc tumor-bearing mice.
Figure 4. In vivotumor treatment experiments.
C57BL/6 mice (5 per group) were challenged i.p. with 2 × 105 /mouse of MOSEC-luc tumor cells on day 0. Five days after tumor challenge, the mice were treated i.p. with 1 × 106 /mouse of irradiated MOSEC tumor cells and/or 1 μg /mouse of α-GalCer twice with a 1-week interval. A group of untreated tumor-bearing mice was used as a control. Tumor-bearing mice were imaged on days 0, 7, 14, 21 and 28 after tumor challenge using the IVIS Imaging System Series 200. Bioluminescence signals were acquired for one minute. (A) Left panel; representative bioluminescence images of MOSEC-luc tumor-bearing mice treated with the various combinations of α-GalCer and irradiated MOSEC tumor cells. Right panel; line graph depicting the kinetic expression of luciferase in MOSEC-luc tumor-bearing mice treated with the various combinations of α-GalCer and irradiated MOSEC tumor cells over time (mean±s.e.). (B) Kaplan & Meier survival analysis of MOSEC-luc tumor-bearing mice treated with irradiated MOSEC tumor cells and/or α-GalCer. (* indicates p < 0.05; ** indicates p < 0.01 as compared to the control).
4.0.4 Immune cells expressing CD4, CD8 or NK1.1 markers are important for the protective anti-tumor effects generated by irradiated tumor cell-based vaccines combined with adjuvant α-GalCer
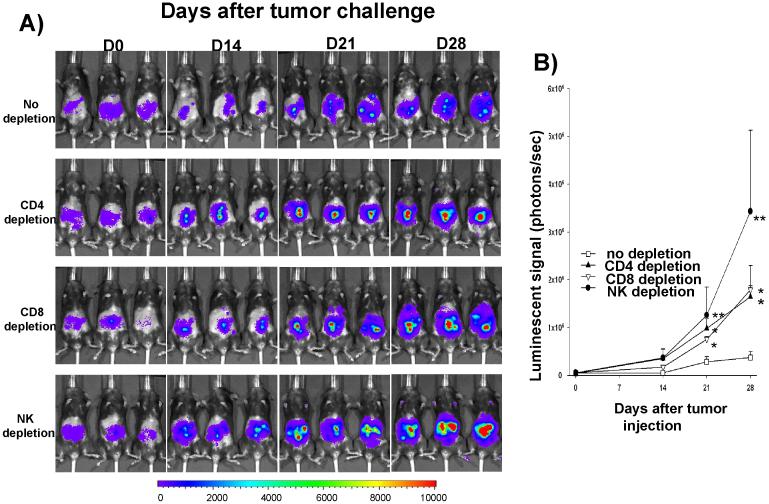
In order to determine the subsets of lymphocytes important for the protective anti-tumor effects seen in mice vaccinated with irradiated MOSEC-luc tumor cells combined with adjuvant α-GalCer, we performed in vivo antibody depletion experiments using monoclonal antibodies specific for immune cells expressing CD4, CD8 or NK1.1 markers. C57BL/6 mice were first vaccinated with irradiated MOSEC-luc tumor cells combined with α-GalCer twice with a 1-week interval. Antibody depletion was initiated one week after the last vaccination. A group of vaccinated mice without depletion of lymphocytes was used as a control. Two weeks after the last vaccination, the mice were challenged with MOSEC-luc tumor cells. Tumor growth was monitored using bioluminescent imaging systems. As shown in Figure 5, we observed that vaccinated mice depleted of immune cells expressing CD4, CD8 or NK1.1 markers exhibited a significantly higher tumor load compared to the vaccinated mice without depletion. Thus, our data suggest that the immune cells expressing CD4, CD8 or NK1.1 markers are important for protective anti-tumor immunity observed in mice vaccinated with irradiated MOSEC-luc cells combined with adjuvant α-GalCer.
Figure 5. In vivoantibody depletion experiment.
Groups of C57BL/6 mice (5 per group) were vaccinated i.p. with 1 × 106 /mouse of irradiated MOSEC tumor cells and 1 μg /mouse α-GalCer twice with a 1-wk interval. One week after the last vaccination, mice were depleted of immune cells expressing CD4, CD8 or NK1.1 markers using 200 μg /mouse of purified rat monoclonal antibodies GK1.5 (anti-CD4), mAb 2.43 (anti-CD8), or mAb PK136 (anti-NK1.1). The mice were injected with antibodies 3 times for the first week and then once every week. One group of vaccinated mice without depletion was used as a control. Two weeks after the last vaccination, the non-depleted and depleted mice were challenged i.p. with 2 × 105 /mouse of MOSEC-luc tumor cells. The growth of the tumor in mice was imaged on days 0, 14, 21 and 28 after tumor challenge using the IVIS Imaging System Series 200. Bioluminescence signals were acquired for one minute. (A) Representative images of MOSEC-luc tumor challenged mice with no depletion, CD4 depletion, CD8 depletion or NK1.1 depletion. (B) Line graph depicting the kinetic expression of luciferase in MOSEC-luc tumor challenged mice with or without depletion (mean±s.e.). (* indicates p < 0.05; ** indicates p < 0.01 as compared to the control).
4.0.5 Administration of irradiated MOVCAR tumor cells combined with adjuvant α-GalCer prolongs the survival of TgMISIIR-TAg transgenic mice
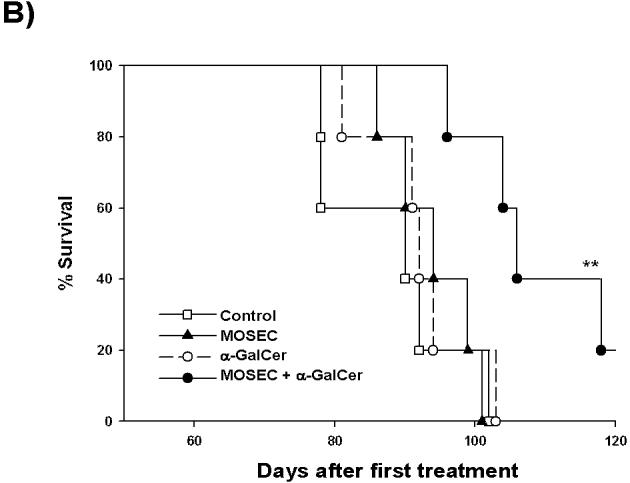
In order to determine if administration of irradiated tumor cells combined with α-GalCer can prolong the survival of TgMISIIR-TAg transgenic mice, we used the mouse ovarian carcinoma (MOVCAR) tumor cells for vaccine development. In general, TgMISIIR-TAg transgenic mice spontaneously develop ovarian carcinomas within 4 months. The MOVCAR cell line is a tumor cell line derived from the malignant ascites of TgMISIIR-TAg transgenic mouse with ovarian cancer. The TgMISIIR-TAg transgenic mice (5 per group) at the age of 6 weeks were injected with irradiated MOVCAR tumor cells and/or adjuvant α-GalCer twice with a 1-week interval. A group of untreated mice was used as a control. As shown in Figure 6, mice administered irradiated MOVCAR cells combined with α-GalCer showed significantly prolonged survival compared to the other groups of mice. Thus, our data suggest that the co-administration of irradiated MOVCAR tumor cells combined with adjuvant α-GalCer are capable of prolonging the survival of the TgMISIIR-TAg transgenic mice.
Figure 6. Survival analysis after treatment in a spontaneous tumor model.
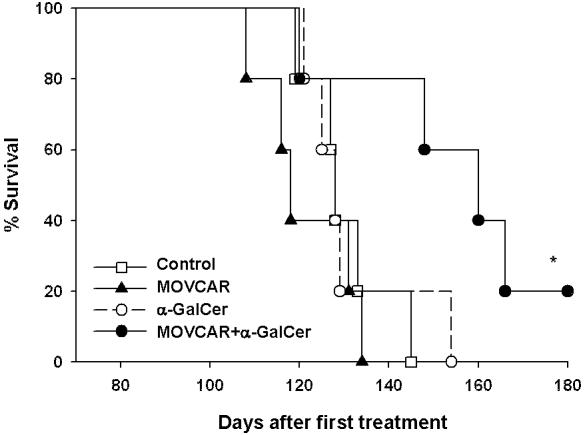
Kaplan & Meier analysis showing the survival of the MISIIR-TAg transgenic mice treated with irradiated MOVCAR tumor cells and/or α-GalCer. Female MISIIR-TAg transgenic mice (5 per group) were injected with 1 × 106 /mouse of irradiated MOVCAR tumor cells and/or 1 μg /mouse of α-GalCer. A group of mice was left untreated as a control. (* indicates p < 0.05 as compared to the control).
4.0.6 Administration of irradiated MOVCAR tumor cells with adjuvant α-GalCer leads to increased numbers of tumor-specific CD8+ T cells in TgMISIIR-TAg transgenic mice
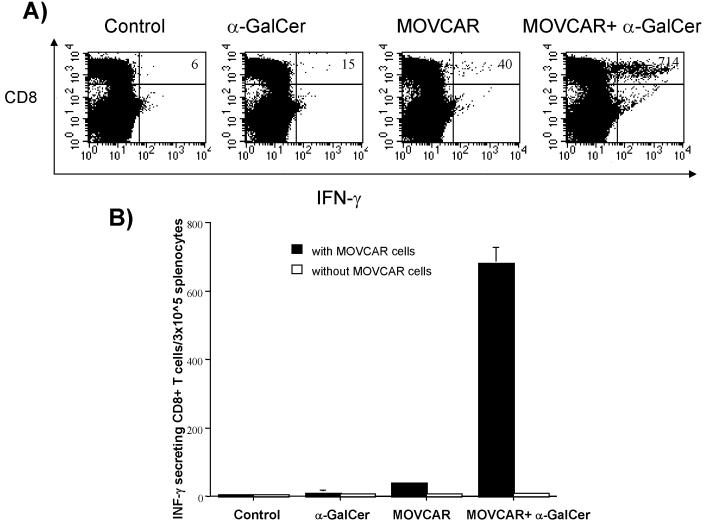
We further characterized the tumor-specific CD8+ T cell immune responses in TgMISIIR-TAg transgenic mice vaccinated with irradiated MOVCAR tumor cells and/or adjuvant α-GalCer twice with a 1-week interval. Unvaccinated mice were used as a control. One week after treatment, splenocytes from vaccinated mice were harvested, cultured in vitro with or without live MOVCAR cells and stained for intracellular IFN-γ using intracellular cytokine staining followed by flow cytometry analysis. As shown in Figure 7, MISIIR-TAg transgenic mice treated with irradiated MOVCAR cells combined with adjuvant α-GalCer generated the highest levels of IFNγ-secreting CD8+ T cells compared to TgMISIIR-TAg transgenic mice treated with α-GalCer alone or irradiated MOVCAR cells alone. Thus, our results suggest that vaccination with irradiated MOVCAR tumor cells combined with adjuvant α-GalCer is capable of generating significant levels of tumor-specific CD8+ T cell immune response in TgMISIIRTAg transgenic mice.
Figure 7. Characterization of tumor-specific CD8+ T cells in vaccinated MISIIR-TAg transgenic mice.
Female MISIIR-TAg transgenic mice (5 per group) at the age of 6 weeks were treated with 1 × 106 /mouse of irradiated MOVCAR tumor cells and/or 1 μg /mouse of α-GalCer. A group of mice was left untreated as a control. One week after the last treatment, splenocytes were harvested from the vaccinated mice. Pooled splenocytes were cultured in vitro for 16 hrs with or without 2 × 105 live MOVCAR cells with Golgistop and then stained for IFN-γ using intracellular cytokine staining followed by flow cytometry analysis. (A) Representative flow cytometry data for each group of treated transgenic mice. The numbers in the upper right hand corner represent the number of IFN-γ-secreting, CD8+ T cells per 3×105 harvested splenocytes. (B) Bar graph showing the levels of IFN-γ-secreting, CD8+ T cells per 3×105 harvested splenocytes (mean±s.e.).
5.0 Discussion
In the current study, we characterized the antitumor effects generated by co-administration of irradiated tumor cell-based vaccines with α-GalCer against ovarian cancer using two different mouse models. We observed that treatment with α-GalCer leads to an increase in the IFN-γ serum levels in the presence or absence of irradiated MOSEC tumor cells. Administration of irradiated MOSEC tumor cells with adjuvant α-GalCer generated significant protective and therapeutic antitumor effects against MOSEC tumors in vaccinated mice. Furthermore, treatment with ovarian tumor cell-based vaccines combined with adjuvant α-GalCer led to prolonged survival as well as increased numbers of tumor-specific CD8+ T cells in TgMISIIR-TAg transgenic mice. Thus, administration of α-GalCer as an adjuvant can significantly enhance the efficacy of tumor-cell based vaccines for the treatment of ovarian cancer.
In this study, we observed that the co-administration of irradiated MOVCAR tumor cells combined with α-GalCer could generate a strong tumor-specific CD8+ T cell immune response and could prolong the survival of TgMISIIRTAg transgenic mice. Thus, the vaccination regimen is capable of breaking immune tolerance, thus resulting in significant antitumor immune responses. However, it is not clear against which particular tumor antigen the observed CD8+ T cell immune responses were directed. The transgenic mice are engineered to express the simian virus 40 (SV40) T antigen under control of the murine Müllerian inhibiting substance type II receptor (MISIIR) gene promoter [16]. Thus, one potential possibility could be that the CD8+ T cell immune responses are directed against the SV40 T Ag. However, we found that the tumor-specific CD8+ T cell immune responses were not targeted against the SV40 T Ag (data not shown). We also found that the tumor-specific CD8+ T cells from the vaccination with irradiated MOVCAR cells were capable of killing MOSEC tumors (data not shown). Taken together, this information suggests that the tumor-specific CD8+ T cell immune responses are likely to be against a murine tumor antigen(s) shared between the MOSEC and MOVCAR tumors. It would be of interest to identify and characterize the specific tumor antigen(s) and to identify a human counterpart of this antigen. This would be greatly beneficial for the further development of effective therapies against ovarian cancer.
The results of our study suggest that immune cells expressing CD4, CD8 or NK1.1 markers play a role in the generation of anti-tumor effects by irradiated tumor cell-based vaccines combined with adjuvant α-GalCer. This may be due to an induction of NKT-mediated immunity by the adjuvant α-GalCer. Since NKT cells also express the surface molecules, NK1.1 and CD4, depletion of CD4+ T cells and/or NK1.1 positive cells may also lead to the depletion of NKT cells. Previous studies have suggested that α-GalCer induces a potent natural killer T (NKT) cell-dependent cytotoxic response against tumor cells [8-10]. Activated NKT cells rapidly release large amounts of proinflammatory cytokines into the serum including IL-4, IL-12 and IFN-γ [23-26]. The proinflammatory cytokines, in turn, regulate the activation of the immune cells including NK cells, CD4+ T cells, CD8+ T cells, B cells and dendritic cells, which function later in the immune response. Thus, the results of our current study are in agreement with the results of previous studies.
In summary, our study has demonstrated that ovarian tumor cell-based vaccines in conjunction with α-GalCer are capable of breaking immune tolerance and generating potent tumor-specific immune responses using two different mouse models. Our results potentially serve as an important foundation for future clinical translation. Furthermore, similar strategies may potentially be applied to other cancer systems.
6.0 Acknowledgements
We would like to thank Dr. T.-C. Wu for critical review of the manuscript. This work was supported by ovarian cancer grants from the Alliance for Cancer Gene Therapy (ACGT), the NCDGG (1U19 CA113341-01) and the American Cancer Society (ACS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7.0 References
- [1].Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000 Jan-Feb;50(1):7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- [2].Coukos G, Conejo-Garcia JR, Roden RB, Wu TC. Immunotherapy for gynaecological malignancies. Expert Opin Biol Ther. 2005 Sep;5(9):1193–210. doi: 10.1517/14712598.5.9.1193. [DOI] [PubMed] [Google Scholar]
- [3].Thompson PL, Dessureault S. Tumor cell vaccines. Adv Exp Med Biol. 2007;601:345–55. doi: 10.1007/978-0-387-72005-0_37. [DOI] [PubMed] [Google Scholar]
- [4].Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev. 2002 Oct;188:147–54. doi: 10.1034/j.1600-065x.2002.18813.x. [DOI] [PubMed] [Google Scholar]
- [5].Antonia SJ. B7-1 gene-modified tumor cell vaccines. Curr Opin Mol Ther. 1999 Feb;1(1):50–6. [PubMed] [Google Scholar]
- [6].Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7(1011):529–34. [PubMed] [Google Scholar]
- [7].Nakagawa R, Motoki K, Ueno H, Iijima R, Nakamura H, Kobayashi E, et al. Treatment of hepatic metastasis of the colon26 adenocarcinoma with an alpha-galactosylceramide, KRN7000. Cancer Res. 1998 Mar 15;58(6):1202–7. [PubMed] [Google Scholar]
- [8].Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, et al. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J Immunol. 1998 Oct 1;161(7):3271–81. [PubMed] [Google Scholar]
- [9].Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998 Oct 19;188(8):1529–34. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997 Nov 28;278(5343):1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- [11].Chung Y, Qin H, Kang CY, Kim S, Kwak LW, Dong C. An NKT-mediated autologous vaccine generates CD4 T cell-dependent potent anti-lymphoma immunity. Blood. 2007 Jun 20; doi: 10.1182/blood-2006-12-061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shimizu K, Goto A, Fukui M, Taniguchi M, Fujii S. Tumor cells loaded with alpha-galactosylceramide induce innate NKT and NK cell-dependent resistance to tumor implantation in mice. J Immunol. 2007 Mar 1;178(5):2853–61. doi: 10.4049/jimmunol.178.5.2853. [DOI] [PubMed] [Google Scholar]
- [13].Youn HJ, Ko SY, Lee KA, Ko HJ, Lee YS, Fujihashi K, et al. A single intranasal immunization with inactivated influenza virus and alpha-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine. 2007 Jul 9;25(28):5189–98. doi: 10.1016/j.vaccine.2007.04.081. [DOI] [PubMed] [Google Scholar]
- [14].Nagaraj S, Ziske C, Strehl J, Messmer D, Sauerbruch T, Schmidt-Wolf IG. Dendritic cells pulsed with alpha-galactosylceramide induce anti-tumor immunity against pancreatic cancer in vivo. Int Immunol. 2006 Aug;18(8):1279–83. doi: 10.1093/intimm/dxl059. [DOI] [PubMed] [Google Scholar]
- [15].Silk JD, Hermans IF, Gileadi U, Chong TW, Shepherd D, Salio M, et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004 Dec;114(12):1800–11. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, et al. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res. 2003 Mar 15;63(6):1389–97. [PubMed] [Google Scholar]
- [17].Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000 Apr;21(4):585–91. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- [18].Hung CF, Calizo R, Tsai YC, He L, Wu TC. A DNA vaccine encoding a single-chain trimer of HLA-A2 linked to human mesothelin peptide generates anti-tumor effects against human mesothelin-expressing tumors. Vaccine. 2007 Jan 2;25(1):127–35. doi: 10.1016/j.vaccine.2006.06.087. [DOI] [PubMed] [Google Scholar]
- [19].Hung CF, Tsai YC, He L, Wu TC. Control of mesothelin-expressing ovarian cancer using adoptive transfer of mesothelin peptide-specific CD8(+) T cells. Gene Ther. 2007 Jun;14(12):921–9. doi: 10.1038/sj.gt.3302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hayakawa Y, Takeda K, Yagita H, Kakuta S, Iwakura Y, Van Kaer L, et al. Critical contribution of IFN-gamma and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of alpha-galactosylceramide. Eur J Immunol. 2001 Jun;31(6):1720–7. [PubMed] [Google Scholar]
- [21].Chang CL, Wu TC, Hung CF. Control of human mesothelin-expressing tumors by DNA vaccines. Gene Ther. 2007 Aug;14(16):1189–98. doi: 10.1038/sj.gt.3302974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim D, Hung CF, Wu TC. Monitoring the Trafficking of Adoptively Transferred Antigen- Specific CD8-Positive T Cells In Vivo, Using Noninvasive Luminescence Imaging. Hum Gene Ther. 2007 Jun 18; doi: 10.1089/hum.2007.038. [DOI] [PubMed] [Google Scholar]
- [23].Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002 Sep;3(9):867–74. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- [24].Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci U S A. 2003 Sep 16;100(19):10913–8. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen H, Paul WE. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-gamma upon activation by anti-CD3 or CD1. J Immunol. 1997 Sep 1;159(5):2240–9. [PubMed] [Google Scholar]
- [26].Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005 Sep 1;175(5):3309–17. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]