Abstract
JC Virus (JCV) is a Polyomavirus that asymptomatically infects up to 80% of the worldwide human population and establishes latency in the kidney. In the case of host immunodeficiency, it can cause Progressive Multifocal Leukoencephalopathy (PML), which is a fatal demyelinating disease of the central nervous system.
In an attempt to understand better Progressive Multifocal Leukoencephalopathy pathogenesis and JCV infection, the presence of the JCV genome and expression of the early viral protein in the brain of deceased individuals, with and without HIV infection, was investigated. Sixty autopsy samples of brain tissues were collected from 15 HIV-positive Progressive Multifocal Leukoencephalopathy patients, 15 HIV-positive patients with other neurological diseases, 15 HIV-positive patients without neurological disorders, and 15 HIV-negative individuals who died from diseases unrelated to the central nervous system.
By means of specific Real Time Polymerase Chain Reaction, the JCV genome was detected in 14 of 15 Progressive Multifocal Leukoencephalopathy brains, three of 15 HIV-positive brains (with and without neurological diseases), and one of 15 HIV-negative brains. JCV genotyping was also performed. Expression of the early JCV protein T Antigen was verified by a specific immunohistochemistry assay, and it was found in the brain tissues from 12 Progressive Multifocal Leukoencephalopathy cases and one case with other neurological disease.
The data obtained demonstrate that infection of the brain with JCV can also be observed in the brains of HIV-negative individuals, without neurological disorders. However, viral protein expression was limited to Progressive Multifocal Leukoencephalopathy brains and to one brain from a patient with other neurological disease, suggesting that JCV can also be present in the brains of patients without Progressive Multifocal Leukoencephalopathy.
Keywords: JC Virus, brains, latency, immunocompetent individuals
INTRODUCTION
JC Virus (JCV) is a human Polyomavirus that infects asymptomatically about 80% of the worldwide healthy adult population during childhood [Padgett and Walker, 1973]. After the first infection, which most likely occurs via a respiratory route, the virus persists in the kidney and can be reactivated and excreted in the urine [Chesters et al., 1983; Rossi et al., 2007].
In cases of severe immunological deficits, JCV may replicate lytically into myelin-producing oligodendrocytes, causing focal demyelination of the white matter of the brain, which represents the main pathological feature of Progressive Multifocal Leukoencephalopathy (PML) [Walker and Padgett, 1983].
The reactivation process for JCV has not been understood completely, and where viral latency occurs and which pathway the virus utilizes to reach the brain are not well known.
Beyond kidney epithelial cells, several studies have suggested that B lymphocytes are the potential cells that harbor JCV in the latent state [Monaco et al., 1996; Sabath and Major, 2002], but the viral genome has also been detected in the brain, lung, spleen, liver, and tonsils [Ferrante et al., 1995; White et al., 1992; Elsner and Dorries, 1992; Caldarelli-Stefano et al., 1999; Monaco et al., 1998].
Interestingly, several investigations have found the JCV genome not only in the brain tissue of Progressive Multifocal Leukoencephalopathy patients but also in the brain tissue of immunocompetent individuals [Mori et al, 1991; Ferrante et al., 1995; White et al., 1992; Elsner et Dorries, 1992], which suggests that JCV could reach the Central Nervous System during primary infection and remain latent in the brain tissue, without being symptomatic.
Recently, two case studies of Progressive Multifocal Leukoencephalopathy that developed after treatment of Multiple Sclerosis with the new immunomodulatory drug Natalizumab (Tysabri, Biogen) were reported, along with one case of a patient with Crohn’s disease [Kleinschmidt-DeMasters and Tyler, 2005; Langer-Gould et al., 2005]
This finding emphasized the need to understand better the reactivation mechanism of JCV and the development of Progressive Multifocal Leukoencephalopathy. It could be important to assess if JCV reaches the brain after reactivation in the kidney or if it is already present in the brain and undergoes reactivation locally to induce demyelination, at least in a limited number of individuals. In the present study, JCV DNA and the early protein T Antigen expression were searched for in autoptic brain tissues collected from HIV-positive patients with Progressive Multifocal Leukoencephalopathy and other neurological diseases as well as from HIV-positive and HIV-negative individuals without any neurological disorders or brain tissue abnormalities.
Tissue samples
A total of 60 formalin-fixed, paraffin-embedded autopsy samples of brain tissues were collected from the pathology files of Luigi Sacco Hospital, Milan, Italy.
The cases included 15 HIV-positive patients who died of Progressive Multifocal Leukoencephalopathy (group A), 15 HIV-positive patients who died of other neurological diseases (group B), 15 HIV-positive patients without neurological disorders (group C), and 15 HIV-negative patients who died of diseases unrelated to the central nervous system (group D).
DNA extraction and JCV-specific Real Time Polymerase Chain Reaction Assay
DNA was obtained according to standard procedures. Briefly, two 10 μm sections were deparaffined and incubated in digestion buffer, containing proteinase K (20 mg/ml), at 52°C overnight. After phenol-chloroform extraction and ethanol precipitation, the DNA was resuspended in water, and the amount was quantified with a spectrophotometer.
Extracted DNA was analyzed using Real Time PCR for the detection and quantification of the JCV genome using a 7000 Real-Time PCR System (Applied Biosystems, USA), following a published protocol [Delbue et al., 2005a]. Five hundred nanograms of DNA was used as a template in each reaction, and a 54-bp amplicon in the JCV T antigen region was detected. Each sample was analyzed in triplicate; samples that did not yield three positive reactions were repeated in triplicate, and the viral load results were given as the mean of the three positive reactions. Each run contained a negative control composed of the reaction mixture without DNA template. A positive control consisted of serial dilutions (range: 106 copies/μl – 1 copy/μl) of plasmid containing the entire JCV genome. The detection limit was two copies/μl, which is equivalent to 40 copies/μg of DNA extracted from the brain tissue.
In order to define the JCV genotype of the isolated viral strains, a 215-bp fragment of the JCV VP1 gene was amplified using a single set of primers, JLP15 and JLP16 [Pagani et al., 2003], and then direct automatic sequencing was performed using an ABI PRISM 310 Genetic Analyzer [Delbue et al., 2005b]. Sequence homology searches were performed using BLAST at the National Center for Biotechnology Information (USA), as described by Agostini et al. [1996].
Histological and Immunohistochemical Analysis
Five micron thick sections were obtained using disposable blades and stained routinely by haematoxylin-eosin; immunohistochemistry was performed using the streptavidin-biotin-peroxidase complex system with diaminobenzidine as the chromogen. In order to detect the JCV T Antigen, mouse monoclonal antibody for the detection of SV40 T Antigen which cross-reacts with JCV T Antigen (clone pAb416, 1:100 dilution, Oncogene Science, Boston, MA) was employed.
The data regarding the detection and quantification of JCV DNA in the brains are shown in Table I. Fourteen (93.3%) out of the 15 samples belonging to Group A were positive for JCV DNA, with a median viral load of 3.05E+03 copies/μg (range: 7.00E+01−3.70E+10 copies/μg). In Groups B and C, JCV DNA was found in three (20%) out of the 15 samples, with a median viral load of 6.21E+02 copies/μg (range: 2.74E+02−1.81E+03 copies/μg) and 6.70E+02 copies/μg (range: 6.50E+01−2.68E+03 copies/μg), respectively, whereas, in Group D, JCV DNA was detected in two (13.3%) of the 15 samples, with a median viral load of 1.13E+03 copies/μg (range: 6.24E+02−1.64E+03 copies/μg).
TABLE I.
Median viral load of JCV DNA and large T antigen expression in the brain tissues collected from the four studied groups of subjects. IH, immunohistochemistry.
| Patient diagnosis | Patient Group | Number of patients | JCV LT-Ag DNA positive | Median Viral load(copies/μg) | IH JCV LT-Ag positive |
|---|---|---|---|---|---|
| HIV+PML | A | 15 | 14 (93.3%) | 3.05E+03 | 12 (80%) |
| HIV+ OND | B | 15 | 3 (20%) | 6.21E+02 | 1 (6.7%) |
| HIV+ NND | C | 15 | 3 (20%) | 6.70E+02 | 0 |
| HIV−NND | D | 15 | 2 (13.3%) | 1.13E+03 | 0 |
The diagnosis at autopsy and the virological data for each of the patients with JCV DNA-positive brain tissue are shown in Table II.
TABLE II.
Real Time PCR results, JCV genotyping, and immunohistochemical analysis of brain tissues in JCV DNA positive patients, according to their pathology diagnosis (ND = not determined).
| Case no. | DIAGNOSIS | Q- PCR JCV LT-Ag (copies/μg) | JCV genotype | IMMUNOHISTOCHEMISTRY JCV LT-Ag |
|---|---|---|---|---|
| 1 | PML | 1.30E+06 | 1B | Plurimi foci of POSITIVITY in PML |
| 2 | PML | 1.90E+05 | 1B | Focal POSITIVITY in oligodendrocytes |
| 3 | PML | 2.13E+07 | 1A | Diffuse POSITIVITY in lytic PML |
| 4 | PML | 9.60E+02 | 4 | Diffuse POSITIVITY in lytic PML |
| 5 | PML | 6.00E+06 | 1A | NEGATIVE |
| 6 | PML | 1.20E+02 | 1B | Diffuse POSITIVITY in lytic PML |
| 7 | PML | 3.70E+09 | 1B and 4 | Diffuse POSITIVITY in lytic PML |
| 8 | PML | 1.80E+03 | 1B | Diffuse POSITIVITY in lytic PML |
| 9 | PML | 2.00E+03 | N.D. | NEGATIVE |
| 10 | PML | 3.60E+02 | N.D. | Focal POSITIVITY in PML |
| 11 | PML | 2.70E+03 | N.D. | Diffuse POSITIVITY in lytic PML |
| 12 | PML | 3.40E+03 | 1A | Focal POSITIVITY in cerebellar oligodendrocytes |
| 13 | PML | 7.00E+01 | N.D. | Diffuse POSITIVITY in lytic PML |
| 14 | PML | 5.70E+03 | N.D. | Diffuse POSITIVITY in lytic PML |
|
| ||||
| 15 | HIV related- encephalitis | 1.81E+03 | 1B | NEGATIVE |
| 16 | Lymphoma Cerebral | 6.21E+02 | N.D. | NEGATIVE |
| 17 | Toxoplasmosis and lymphoma | 2.74E+02 | N.D. | Focal POSITIVITY on the sides of the lesions |
|
| ||||
| 18 | Pulmonary edema | 6.70E+02 | 1A | NEGATIVE |
| 19 | Kaposi’s Sarcoma Pulmonary | 6.50E+01 | 1A | NEGATIVE |
| 20 | Pneumocystosis HIV related - lymphadenopathy | 2.68E+03 | 7C | NEGATIVE |
|
| ||||
| 21 | HIV- Myocardiopathy aterosclerotic and cerebral atrophia | 6.24E+02 | 1B | NEGATIVE |
| 22 | HIV-, Myocardial infarction | 1.64E+03 | 1B | NEGATIVE |
Using specific PCR and automatic sequencing analysis, it has also been observed that genotype 1 (both 1A and 1B) was most frequently detected. One Progressive Multifocal Leukoencephalopathy patient showed a double infection with genotypes 1B and 4, and one HIV-positive subject showed an infection with JCV genotype 7C (Table II).
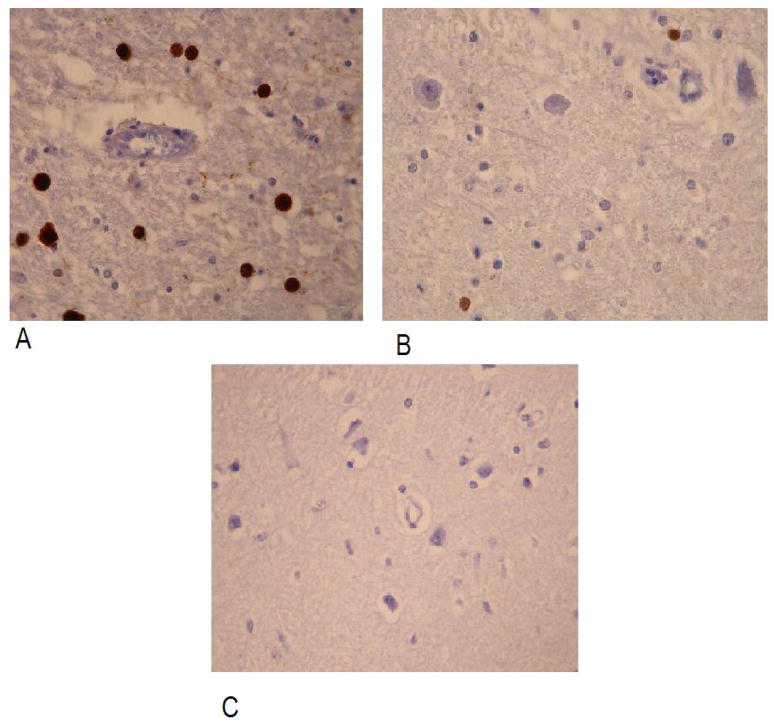
To investigate the expression of JCV large T (LT) antigen, immunohistochemistry was performed, and LT antigen was detected in 12 (80%) out of the 15 samples collected from HIV-positive Progressive Multifocal Leukoencephalopathy patients. LT antigen was detected in one tissue collected from a HIV-positive patient with other neurological disease, whereas there was no expression in the brains of all other individuals, including those that were positive for JCV DNA. Examples of positive and negative staining for JCV LT antigen are shown in Figure 1, panels A, B and C.
Immunohistochemical evidence of JCV infection of a large number (panel A) and rare (panel B) of oligodendroglial cells in brain of HIV positive subjects with OND and PML. In panel C immunohistochemical analysis of an autopsy sample of brain with cerebrovascular diesase used as negative control of immunohistochemistry is shown: no cells were stained. Immunoperoxidase, with light hematoxilyn counterstaining, original magnification 400x
DISCUSSION
Primary infection with JCV occurs during childhood and is followed by a lifelong viral persistence in the host and periodic viral reactivation leading to an intermittent JCV viruria. Latency of the virus in the human kidney has been recognized for a long time, as was urinary excretion of JCV, both in immunocompetent individuals and during immunosuppression or pregnancy [Chesters et al., 1983]. However, little is known about other possible latency sites, even if many studies have reported finding the viral genome in several human tissues, such as liver, lung, spleen, bone marrow, and lymph nodes of both Progressive Multifocal Leukoencephalopathy patients and immunocompetent individuals [Monaco et al., 1998; Newman et al., 1997; Caldarelli-Stefano et al., 1999].
It is well known that JCV infects the central nervous system and causes Progressive Multifocal Leukoencephalopathy as a consequence of its active replication in oligodendrocytes. The traditional view of Progressive Multifocal Leukoencephalopathy pathogenesis is that, as a consequence of immunosuppression or severe diseases, such as hematopoietic tumours, the JCV already latent in the kidney reactivates and reaches the central nervous system through the blood. Moreover, several reports have shown that two different variants of the virus infect the kidneys and brain: the archetypal strain that is mostly isolated from the urine [Agostini et al., 1996; Yogo et al., 1990] and rearranged forms of JCV that are isolated from the brain and Cerebrospinal Fluid (CSF) of Progressive Multifocal Leukoencephalopathy patients [Frisque et al., 1984; Kato et al., 1994]. The most accredited hypothesis is that the archetypal form of the virus persists in the kidneys after primary infection and is subjected to genomic rearrangements during the transfer from the kidney to the brain, which occurs by means of B lymphocytes [Sabath and Major, 2002].
However, due to the fact that several research groups have isolated JCV DNA and demonstrated protein expression in the brains of non-Progressive Multifocal Leukoencephalopathy subjects [Caldarelli-Stefano et al., 1999; Elsner and Dorries, 1992; Chesters et al., 1983; White et al., 1992, Mori et al., 1991; Vago et al., 1996], it could also be postulated that JCV reaches the brain during or immediately after the primary infection and persists without causing any clinical symptoms or brain lesions.
In the present study, using the highly sensitive Real Time PCR method, JCV DNA has been successfully amplified and identified in the brains of HIV-positive individuals who died from Progressive Multifocal Leukoencephalopathy, other neurological diseases (HIV-encephalitis, lymphoma, and cerebral toxoplasmosis), and without any neurological disorders (Kaposi’s Sarcoma, pulmonary pneumocytosis and HIV-related lymphadenopathy), as well as in the brains of HIV- immunocompetent individuals who died from causes unrelated to neurological complications (myocardiopathy and infarction).
JCV DNA was found in 14 out of 15 patients affected with Progressive Multifocal Leukoencephalopathy, confirming the high sensitivity of the technique (detection limit: two copies/μl) [Bossolasco et al., 2005]; the negativity of one case could be due to the particular slide or because the viral amount was under the limit of detection. In addition, JCV was isolated in three HIV-positive patients with other neurological diseases and three patients without any neurological disorders, demonstrating that the virus can be present within the brains of non-Progressive Multifocal Leukoencephalopathy affected patients without being symptomatic. More surprisingly, JCV DNA was also detected in the tissue of an HIV- immunocompetent subject. In contrast to a previous report [White et al., 1992], no significant differences were noted in the median viral load of the virus between the different groups, even if the range of viral load in the group of patients with Progressive Multifocal Leukoencephalopathy was much wider than the viral load range for the other two groups.
JCV 1, Subtypes 1A and 1B, was the most commonly identified genotype in JCV-positive autopsy samples. These findings are in line with the results of previous epidemiological reports, in which genotype 1 was found to be the most prevalent genotype in an overall Italian healthy population [Pagani et al., 2003] and a population affected with brain tumours [Delbue et al., 2005b]. On the contrary, the obtained data did not confirm the possible association between JCV genotype 2 and Progressive Multifocal Leukoencephalopathy [Ferrante et al., 2001; Agostini et al., 1998].
In order to verify if the viral genome was expressed in brain tissue, immunohistochemistry was performed using a JCV T Antigen-specific antibody, and a positive signal was found in 12 out of 15 Progressive Multifocal Leukoencephalopathy patients. JCV is able to replicate lytically in oligodendrocytes, such that finding of the early protein in Progressive Multifocal Leukoencephalopathy patients is expected. The presence of JCV DNA in two tissues concomitant with the absence of JCV early protein in the same sections could be due to the low sensitivity of the immunohistochemistry assay or the specific tissue section or because the virus was not replicating. Early JCV protein was also expressed in tissue from an HIV-positive patient with other neurological diseases, but none of the other studied subjects had JCV LT antigen expressed in brain tissue, even if those tissues were positive for JCV DNA by Real Time-PCR. However, the negativity of these cases could be due to the fact that PCR is a more sensitive technique than the immunohistochemistry assay [Patel et al., 2008].
These data indicate that JCV infection could be associated with the penetration and presence of the virus in the central nervous system in the absence of Progressive Multifocal Leukoencephalopathy or relevant neurological symptoms. According to this hypothesis, the virus can remain in the brain in a latent state without translation of the viral proteins, even those encoded by the early genes; viral reactivation can occur if the infected subjects undergo severe immunosuppression, leading to neurological disorders, including Progressive Multifocal Leukoencephalopathy.
Some conclusions can be drawn in light of these results. The finding of JCV DNA in normal brains or in brains without Progressive Multifocal Leukoencephalopathy suggests that at least some cases of Progressive Multifocal Leukoencephalopathy could be due to the reactivation of latent JCV in the central nervous system, where it entered after the primary infection, typically during childhood. Reactivation of the latent virus in the brain could be easily triggered by an immunosuppressive state and enhanced by co-infection with HIV.
In this regard, a critical issue is the involvement of JCV in patients treated with a new group of biologically active drugs, including a large number of antibodies against regulatory factors, which are aimed to reduce the autoimmune activity of immunocompetent cells. This mechanism occurs when Natalizumab is used in treating Multiple Sclerosis treatment. Natalizumab is a humanized monoclonal antibody against alpha-integrin 4, and its mechanism of action is based on the prevention of peripheral blood (PB) cell adhesion and extravasion [Elices, 2003]. The efficacy of this drug is due to the fact that, in Multiple Sclerosis patients, many immune cells of the peripheral blood are self-reactive, particularly against myelin basic protein. However, blocking the passage of peripheral blood mmunologically active cells means that cell trafficking between the peripheral blood compartment and central nervous system decreases, reducing local immunosurveillance.
Many hypotheses have been proposed to explain the development of Progressive Multifocal Leukoencephalopathy in two Multiple Sclerosis and one Crohn’s disease patient treated with Natalizumab [Kleinschmidt-DeMasters and Tyler, 2005; Langer-Gould et al., 2005]. The data of the present study, particularly the presence of JCV DNA in the brain of HIV-negative subjects without Progressive Multifocal Leukoencephalopathy, suggest that the reduction of cell trafficking, at least in a limited number of subjects, leads to the reactivation of JCV that is already present in a latent status in the brain and, furthermore, to the development of Progressive Multifocal Leukoencephalopathy. These data also suggest that the widespread use of biologically active drugs in the near future for the treatment of various autoimmune diseases will probably increase Progressive Multifocal Leukoencephalopathy incidence and, thus, the need for predictive markers is critical.
Acknowledgments
This work was supported by NIH grant no. MH072528 and by a grant from the Italian Minister of Health (ISS 2006) to PF.
References
- Agostini HT, Ryschkewitsch CF, Stoner GL. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J Clin Microbiol. 1996;34:159–164. doi: 10.1128/jcm.34.1.159-164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini HT, Ryschkewitsch CF, Singer EJ, Baumhefner RW, Stoner GL. JC virus type 2B is found more frequently in brain tissue of progressive multifocal leukoencephalopathy patients than in urine from controls. J Hum Virol. 1998;1:200–206. [PubMed] [Google Scholar]
- Bossolasco S, Calori G, Moretti F, Boschini A, Bertelli D, Mena M, Gerevini S, Bestetti A, Pedale R, Sala S, Sala S, Lazzarin A, Cinque P. Prognostic significance of JC virus DNA levels in cerebrospinal fluid of patients with HIV-associated progressive multifocal leukoencephalopathy. Clin Infect Dis. 2005;40:738–744. doi: 10.1086/427698. [DOI] [PubMed] [Google Scholar]
- Caldarelli-Stefano R, Vago L, Omodeo-Zorini E, Mediati M, Losciale L, Nebuloni M, Costanzi G, Ferrante P. Detection and typing of JC virus in autopsy brains and extraneural organs of AIDS patients and non-immunocompromised individuals. J Neurovirol. 1999;5:125–133. doi: 10.3109/13550289909021994. [DOI] [PubMed] [Google Scholar]
- Chesters PM, Heritage J, McCance DJ. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseases tissues. J Infect Dis. 1983;147:676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- Delbue S, Sotgiu G, Fumagalli D, Valli M, Borghi E, Mancuso R, Marchioni E, Maserati R, Ferrante P. A case of a PML patient with four different JC virus TCR rearrangements in CSF, blood, serum and urine. J Neurovirol. 2005a;11:51–57. doi: 10.1080/13550280590900382. [DOI] [PubMed] [Google Scholar]
- Delbue S, Pagani E, Guerini FR, Agliardi C, Mancuso R, Borghi E, Rossi F, Boldorini R, Veggiani C, Car PG, Ferrante P. Distribution, characterization and significance of polyomavirus genomic sequences in tumors of the brain and its covering. J Med Virol. 2005b;77:447–454. doi: 10.1002/jmv.20474. [DOI] [PubMed] [Google Scholar]
- Elices MJ. Natalizumab. Elan/Biogen. Curr Opin Investig Drugs. 2003;4:1354–1362. [PubMed] [Google Scholar]
- Elsner C, Dorries K. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology. 1992;191:72–80. doi: 10.1016/0042-6822(92)90167-n. [DOI] [PubMed] [Google Scholar]
- Ferrante P, Caldarelli-Stefano R, Omodeo-Zorini E, Vago L, Boldorini R, Costanzo G. PCR detection of JC virus DNA in brain tissue from patients with and without PML. 1995. J Med Virol. 1995;47:72–80. doi: 10.1002/jmv.1890470306. [DOI] [PubMed] [Google Scholar]
- Ferrante P, Mediati M, Caldarelli-Stefano R, Losciale L, Mancuso R, Cagni AE, Macerati R. Increased frequency of JC Virus type 2 and of dual infection of JC Virus type 1 and 2 in Italian Progressive Multifocal Leukoencephalopathy patients. J Neurovirol. 2001;7:35–42. doi: 10.1080/135502801300069638. [DOI] [PubMed] [Google Scholar]
- Frisque RJ, Bream GL, Cannella MT. Human polyomavirus JC virus genome. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Guo J, Taguchi F, Daimaru O, Tajima M, Haibara H, Matsuda J, Sumiya M, Yogo Y. Phylogenetic comparison between archetypal and disease-associated JC virus isolates in Japan. Jpn J Med Sci Biol. 1994;47:167–178. doi: 10.7883/yoken1952.47.167. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive Multifocal Leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A, Atlas SW, Bollen AW, Pelletier D. Progressive Multifocal Leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;35:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- Monaco MC, Atwood WJ, Gravell M, Tornatore CS, Major EO. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J Virol. 1996;70:7004–7012. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol. 1998;72:9918–9923. doi: 10.1128/jvi.72.12.9918-9923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Kurata H, Tajima M, Shimada H. JC virus detection by in situ hybridization in brain tissue from elderly patients. Annals of Neurology. 1991;29:428–432. doi: 10.1002/ana.410290414. [DOI] [PubMed] [Google Scholar]
- Newman JT, Frisque RJ. Detection of archetype and rearranged variants of JC virus in multiple tissues from a pediatric PML patient. J Med Virol. 1997;52:243–252. doi: 10.1002/(sici)1096-9071(199707)52:3<243::aid-jmv2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Padgett BL, Walker DL. Prevalence of antibodies in human sera against JC viruria, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973;127:467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Pagani E, Delbue S, Mancuso R, Borghi E, Tarantini L, Ferrante P. Molecular analysis of JC virus genotypes circulating among the Italian healthy population. J Neurovirol. 2003;9:559–566. doi: 10.1080/13550280390241269. [DOI] [PubMed] [Google Scholar]
- Patel NC, Vilchez RA, Killen DE, Zanwar P, Sroller V, Eldin KW, Lopez-Terrada D, Butel JS. Detection of polyomavirus SV40 in tonsils from immunocompetent children. J Clin Microbiol. 2008 doi: 10.1016/j.jcv.2008.04.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Delbue S, Mazziotti R, Valli M, Borghi E, Mancuso R, Calvo MG, Ferrante P. Presence, quantitation and characterization of JC virus in the urine of Italian immunocompetent subjects. J Med Virol. 2007;79:408–412. doi: 10.1002/jmv.20829. [DOI] [PubMed] [Google Scholar]
- Sabath BF, Major EO. Traffic of JC virus from sites of initial infection to the brain: the path to progressive multifocal leukoencephalopathy. J Infect Dis. 2002;(Suppl2):S180–186. doi: 10.1086/344280. [DOI] [PubMed] [Google Scholar]
- Vago L, Cinque P, Sala E, Nebuloni M, Caldarelli R, Racca S, Ferrante P, Trabattoni G, Costanzi G. JCV-DNA and BKV-DNA in the CNS tissue and CSF of AIDS patients and normal subjects. Study of 41 cases and review of the literature. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;1;12(2):139–46. doi: 10.1097/00042560-199606010-00006. [DOI] [PubMed] [Google Scholar]
- Walker DL, Padgett BL. The epidemiology of human polyomaviruses. In: Sever JL, Madden D, editors. Polyomaviruses and human neurological disease. Alan R. Liss; New York: 1983. pp. 99–106. [Google Scholar]
- White FA, Ishaq M, Stoner GL, Frisque RJ. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J Virol. 1992;66:5726–5734. doi: 10.1128/jvi.66.10.5726-5734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y, Kitamura T, Sugimoto C, Ueki T, Aso Y, Hara K, Taguchi F. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990;64:3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]