How did the study come about?
Highly active antiretroviral therapy (HAART) delays disease progression and death.1–4 However, the treatments incompletely control HIV replication,5–7 only partially restore immune function,8,9 have significant short- and long-term toxicities,10–14 and eventually fail in many patients with consequent development of HIV drug resistance.6 Thus, there is increasing need for information to guide HIV-infected patients and their providers in making decisions regarding optimal use of antiretroviral therapies. Although clinical trials provide valuable information about efficacy and side effects of antiretroviral treatment, they have limited size, duration and power to detect effects on clinical outcomes, focusing instead on surrogate endpoints such as virologic failure, treatment discontinuation or composite outcome measures.15
Outside the clinical trial setting, there is tremendous heterogeneity among HIV-infected patients. The prevalence and impact of important health conditions such as hepatitis C virus (HCV) co-infection, mental illness and substance abuse likely contribute to increased toxicity and decreased clinical effectiveness of HAART regimens among the broader spectrum of patients treated in routine care. Cohorts with significant diversity in HIV disease severity, comorbidities and demographic distributions are required to provide information regarding long-term outcomes and complications of HIV infection in the modern HAART era.
The Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) was created to better define the relationship between patient and treatment factors and long-term clinical outcomes among HIV-infected patients in the HAART era. The CFARs are a national network of centres of excellence for HIV care and research established by the National Institutes of Health (NIH) whose mission is to support a multi-disciplinary environment for basic, clinical, epidemiologic, behavioural and translational research in the prevention, detection and treatment of HIV infection and AIDS. There are 19 CFARs located at academic and research institutions throughout the United States. The objective of the CNICS project is to integrate clinical data from the large and diverse population of HIV-infected persons receiving care at CFAR sites to investigate questions related to HIV disease management that cannot be readily addressed through traditional randomized controlled clinical trials and other cohort studies. Investigators with expertise in basic, clinical, translational and epidemiologic research, in addition to medical informatics, are collaborating on the CNICS project.
The potential to build a comprehensive clinical data repository for HIV disease was greatly advanced by the work of CFAR investigators at the participating CNICS sites who had instituted point-of-care electronic medical record systems (EMRs) with the dual purpose of providing real-time clinical information to facilitate the delivery of HIV care and capturing standardized clinical data to support population-based HIV research. The initial four CNICS sites were Case Western Reserve University, University of Alabama, Birmingham, University of California, San Francisco and the University of Washington. Two other CFAR sites, the University of California, San Diego and Fenway Community Health Center of Harvard University, were successfully integrated into the CNICS project. Most recently, Johns Hopkins University was added as a seventh site, bolstering both the geographic and ethnic representation of the cohort. These seven sites currently constitute the CNICS cohort, providing data on >15 000 patients.
As a clinic-based research network, CNICS directly reflects the outcomes of clinical decisions made daily in the care of HIV-infected individuals. Unlike classical epidemiologic studies that collect data through structured interviews or retrospective medical record review, CNICS captures a broader range of information associated with the rapidly changing course of HIV disease management through prospective collection of data at the point-of-care. This continuous collection of clinically relevant information can be used to monitor trends, detect new events and address new research questions. The CNICS project has established links with specimen repositories at CNICS sites to support basic and translational research investigating HIV disease mechanisms and pathogenesis. The flexibility of this consortium enables CNICS to address scientific questions that cannot be answered through other collaborative cohorts with less comprehensive data and more limited accession of specimens.
The work of CNICS is organized into four major cores (Administrative, Data Management, Biostatistical and Research Coordination) and an Executive Committee (EC) that provide an infrastructure for efficiently identifying critical and timely scientific questions, and for conducting analyses to address the CNICS scientific agenda. The Administrative Core (AC) is located at the University of Alabama, Birmingham. The AC is responsible for the administrative and operational functions of CNICS, provides scientific leadership, and facilitates communication and linkages for CNICS sites through a programme of regular meetings, electronic communications and symposia. The Data Management Core (DMC) is located at the University of Washington, Seattle, and provides expertise to the CNICS sites and implementation of central data services. The DMC brings to bear extensive experience in the design and conduct of HIV cohort research, as well as in clinical and population health informatics. The DMC establishes standards for data quality at the CNICS sites prior to transmission of data, develops data submissions standards and metadata tables for mapping local site codes to CNICS standard labels, and informs decisions about the content and format of data to meet the scientific goals of this project. The DMC develops and supports scalable models of data integration, incorporating an extensible repository model to capture new data types such as HIV-1 resistance data, including genotype data at the codon level and patient-based measurements. The model also supports automated validation and integration procedures, allowing the methods to be scaled as the number of participating sites increases. The CNICS Biostatistical Core (BC) provides statistical input on data definitions, quality assurance of the CNICS data repository and guidance to all protocol and manuscript teams in the design, conduct, analysis and reporting of data from the CNICS repository. The BC helps link other CFAR statistical groups to participate in data analysis. In addition, the BC conducts methodological research to develop, evaluate and implement novel approaches to the design of studies and analysis of observational data. The Research Coordination Core (RCC) is responsible for developing, evaluating and prioritizing the scientific agenda of CNICS. It reviews all concept proposals for scientific merit, feasibility, resource use and overlap with existing projects, and makes recommendations to the EC about which projects should move forward. In addition, the RCC monitors the progress of ongoing studies and facilitates timely publication of results.
The EC provides leadership and general oversight for the CNICS project and includes the Principal Investigator (PI), Co-PI, the Administrative, Biostatistical, Data Management and Research Coordinating Core Chair and Vice Chair, and representatives from each of the participating CFAR sites. The EC assumes responsibility for the conduct of research performed and ensures that these studies are carried out efficiently and with the highest scientific standards. It approves concept sheets proposing secondary hypotheses/research questions and ancillary proposals for research from both internal and external investigators. Further, the EC oversees operational methods and procedures and reviews performance-monitoring reports.
What does it cover and who is in the sample?
The CNICS repository contains data from EMR systems used to provide HIV care and support clinical and translational HIV research at seven CFAR-affiliated medical centres (Table 1). The CNICS cohort includes HIV-infected individuals aged 18 years or older who initiated primary care at CNICS sites after January 1, 1995. As of May 30, 2007, the CNICS repository contains data on 15 126 patients, the vast majority of whom have been under care in the HAART era. As shown in Table 1, the CNICS cohort is a large and diverse population of patients with regard to sex, race, ethnicity, age, risk factor for HIV transmission and geographic distribution. Women make up 20% of the combined cohort, 46% of individuals are non-Hispanic white, 40% are African–American/black, and 9% are Hispanic. In addition, comprehensive clinical data on patients in the CNICS cohort are linked to specimen repositories located at CNICS sites that currently store over 127 000 specimens of plasma or cryopreserved peripheral blood mononuclear cells from over 4500 patients available for research studies.
Table 1.
Demographics characteristics of CNICS cohort by site
| CWRUa | Fenway | JHUb | UABc | UCSDd | UCSFe | UWf | Total | |
|---|---|---|---|---|---|---|---|---|
| Cleveland, OH | Boston, MA | Baltimore, MD | Birmingham, AL | San Diego, CA | San Francisco, CA | Seattle, WA | All | |
| Sex | ||||||||
| Female | 296 (21) | 45 (4) | 1361 (33) | 462 (23) | 339 (12) | 257 (14) | 231 (14) | 2991 (20) |
| Male | 1094 (79) | 1089 (96) | 2775 (67) | 1564 (77) | 2599 (88) | 1645 (86) | 1369 (86) | 12 135 (80) |
| Race/ethnicity | ||||||||
| African–American/Black | 710 (51) | 85 (7) | 3121 (76) | 972 (48) | 387 (13) | 453 (24) | 319 (20) | 6047 (40) |
| American Indian | 0 (0) | 0 (0) | 0 (0) | 3 (<1) | 14 (<1) | 14 (1) | 45 (3) | 76 (1) |
| Asian/Pacific Islander | 6 (<1) | 12 (1) | 11 (<1) | 1 (<1) | 59 (2) | 84 (4) | 30 (2) | 203 (1) |
| White | 555 (40) | 840 (74) | 922 (22) | 1017 (50) | 1631 (56) | 957 (50) | 1062 (66) | 6984 (46) |
| Hispanic or Latino | 65 (5) | 76 (7) | 56 (1) | 22 (1) | 685 (23) | 366 (19) | 128 (8) | 1398 (9) |
| Multiracial | 0 (0) | 0 (0) | 0 (0) | 2 (<1) | 12 (<1) | 0 (0) | 7 (<1) | 21 (<1) |
| Other/unknown | 54 (4) | 121 (11) | 26 (1) | 9 (<1) | 150 (5) | 28 (1) | 9 (1) | 397 (3) |
| Age at first visit (years) | ||||||||
| 18–29 | 242 (17) | 144 (13) | 552 (13) | 376 (19) | 378 (13) | 249 (13) | 268 (17) | 2209 (15) |
| 30–39 | 571 (41) | 544 (48) | 1728 (42) | 852 (42) | 1235 (42) | 753 (40) | 725 (45) | 6408 (42) |
| 40–49 | 444 (32) | 366 (32) | 1391 (34) | 586 (29) | 961 (33) | 641 (34) | 461 (29) | 4850 (32) |
| 50–59 | 107 (8) | 63 (6) | 370 (9) | 184 (9) | 313 (11) | 220 (12) | 136 (9) | 1393 (9) |
| ≥60 | 26 (2) | 17 (1) | 95 (2) | 28 (1) | 51 (2) | 39 (2) | 10 (1) | 266 (2) |
| HIV transmission risk factor | ||||||||
| MSMg | 674 (49) | 595 (53) | 945 (23) | 903 (45) | 1822 (62) | 756 (40) | 824 (52) | 6519 (43) |
| Injection drug use | 35 (3) | 29 (3) | 1677 (41) | 135 (7) | 147 (5) | 188 (10) | 217 (14) | 2428 (16) |
| MSM and injection drug use | 20 (1) | 5 (<1) | 1268 (31) | 74 (4) | 244 (8) | 138 (7) | 275 (17) | 2024 (13) |
| Heterosexual | 476 (34) | 11 (1) | 83 (2) | 562 (28) | 484 (17) | 204 (11) | 225 (14) | 2045 (14) |
| Other/unknown | 185 (13) | 494 (44) | 163 (4) | 352 (17) | 241 (8) | 616 (32) | 59 (4) | 2110 (14) |
| Total | 1390 | 1134 | 4136 | 2026 | 2938 | 1902 | 1600 | 15 126 |
aCase Western Reserve University.
bJohns Hopkins University.
cUniversity of Alabama, Birmingham.
dUniversity of California, San Diego.
eUniversity of California, San Francisco.
fUniversity of Washington.
gMen who have sex with men.
How often have they been followed up and what is the attrition rate?
Data captured on the CNICS cohort reflect events associated with ongoing care provided at centres of excellence. Clinical cohorts, such as CNICS, differ from classical epidemiologic cohort studies in that the type and timing of data collected are determined by the health care services provided. Traditional epidemiologic cohort studies recruit participants outside of regular care and have specified follow-up intervals (e.g. semiannual). The frequency of follow-up for patients in the CNICS cohort is a clinic visit approximately every 3 months, however patients can be seen more or less often depending upon their unique clinical care situation. An advantage of the CNICS project is that data from individuals in the CNICS cohort are less subject to volunteer and non-response bias than classical epidemiologic cohort studies that rely on recruitment and willingness of participants to continue.16 CNICS is a dynamic cohort that has enrolled ∼1400 new patients each year, a median follow-up of 33 months and 10% of patients leave care annually.
What has been measured?
The sources of data within the CNICS repository are the EMR systems at each participating CNICS site. While the EMRs at the seven sites were developed independently, there is significant commonality among data elements generated during the provision of HIV care. The EMRs were designed to collect standardized data that meet clinical care needs and have the accuracy, specificity and completeness required for research. The CNICS consortium has established standards for terminology, format, data verification and quality assurance procedures that can be used by other sites as they develop local EMRs, and that facilitate both expansion of CNICS within the CFARs and data sharing with other cohorts.
The CNICS data repository integrates demographic information [year of birth, sex, race and ethnicity categorized according to US Federal Health Resource and Services Administration (HRSA) standards]; risk factors for HIV transmission classified according to the Centers for Disease Control and Prevention (CDC) classification;17 laboratory data including results of CD4+ T cell counts, plasma HIV-1 RNA levels and hematologic and metabolic markers; antiretroviral medication data; diagnosis data classified as to source, reliability and completeness (AIDS-defining diagnoses defined by the CDC 1993 case definition18); and death data. Comorbid conditions, including diabetes mellitus, hypertension, cardiovascular disease, mental illness, substance abuse, liver and renal conditions, and causes of death (classified according to the CoDe19), are currently being integrated in the CNICS repository. Historical data regarding a patient's prior antiretroviral treatment and diagnosis history are collected at the time of their initial clinic visit, which coincides with enrollment into the cohort. HIV resistance data including all genotype and phenotype test results as raw codon sequences that permit flexibility in generating different types of resistance variables are now being incorporated into the repository through the NIH-Funded Roadmap Project: ‘Integrating HIV resistance data into the CNICS cohort’.
Prospective collection of patient-reported measures using validated instruments to assess body morphology abnormalities, depression and anxiety, current symptom burden, antiretroviral medication adherence, drug/alcohol/tobacco use, health-related quality of life and physical activity level20–29 is being implemented at all sites. The CNICS DMC developed a web-based survey software application to collect patient-based measures that uses tablet personal computers (PCs) with touch screens operating over a secure wireless network infrastructure. While there is the potential for reporting bias, the use of computer-based collection rather than interview-based may allow patients to feel more at ease reporting socially undesirable behaviours reducing social desirability bias.30 Patients complete these assessments approximately every 6 months.
The specimen repositories associated with each of the seven CNICS sites are a key strength of the CNICS project with individual specimens linked to data contained within the CNICS data repository. All CNICS participating sites have agreed to a minimum standard of plasma/serum and cell pellet collection at least every 6 months, beginning with the patient's first visit to the clinic whenever possible. In addition, events and features of particular interest in a patient's clinical course increase the level of priority for specimen collection including: (i) first visit of untreated patients, (ii) initiation of first antiretroviral regimen, (iii) antiretroviral regimen change, which is often defined by the time of resistance testing and (iv) unique clinical profiles, such as long-term non-progressors, immunological failures and elite controllers who maintain an undetectable viral load without antiretroviral treatment. CNICS is designed to expand with the network as the participating sites grow in size and number, and to manage increasingly complex clinical and biological specimen data.
Data validation and integrity checking occur in multiple stages, both at the contributing CFAR sites and at the centralized CNICS repository. At the sites, data quality monitoring includes procedures specific for each data type: (i) laboratory test results are uploaded directly from the Clinical Laboratory Systems at each site, and surveillance for coding changes and outliers is conducted; (ii) diagnoses are recorded prospectively in the EMR by the treating clinician using standardized, constrained vocabulary and verified through systematic review of clinician progress notes and other existing medical records, event driven audits, and verification of random samples of clinical events; (iii) medication data are entered into the EMR by clinicians or prescription fill/refill data are uploaded directly from institutional pharmacy dispensing systems and verified through medical record review. Data source coding in CNICS provides a hierarchy of indicators with increasing reliability for diagnosis and medication data and (iv) Each CNICS site maintains a death registry and regularly queries the United States Social Security Death Index to confirm mortality data.
At the CNICS repository level, all data are validated against the CNICS constrained vocabulary and coding standards, using an automated validation framework. Validation occurs: (i) prospectively, at the individual sites prior to the transmission of data to CNICS, (ii) synchronously, at the time of submission, as data are being parsed for insertion into the repository and (iii) asynchronously, after data are loaded into the CNICS repository, through the use of applications to monitor data quality. Synchronous validation implements first-order validation procedures to verify that data elements are reported using a valid format and value. First-order rules are applied twice, both prospectively and synchronously. Second-order validation procedures are applied asynchronously. They use rules specific for different data types and require a database query of other data for a patient to implement both temporal- and database-dependent business rules. For example, a patient without concurrent antiretroviral treatment would not be expected to have an undetectable viral load. The validation framework is comprised of a flexible business logic tier to implement synchronous rules and a suite of data quality reporting functions that run against the repository. The business logic tier generates notification to the appropriate data manager, while the data quality reporting functions generate web-accessible reports.
Issues of central importance to the CNICS project include protecting patient privacy, obtaining consent for electronic databases, and maintaining linkages between clinical data and biological specimens. CNICS has adopted strong protections for research participants and has secured consent from the governing institutional review boards and also secured a Certificate of Confidentiality from the NIH. The submitting CFAR site generates Patient Identification values (IDs) stripped of any identifiable characteristics that could be used by the CNICS DMC to determine patients’ identity. All CNICS data are transmitted via secure, encrypted FTP to the CNICS repository and require FTP password authentication and IP address validation by the University of Washington host-based firewall for access.
What has been found? Key findings and publications
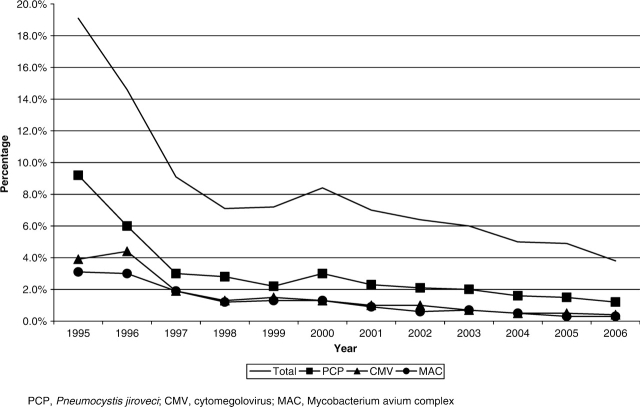
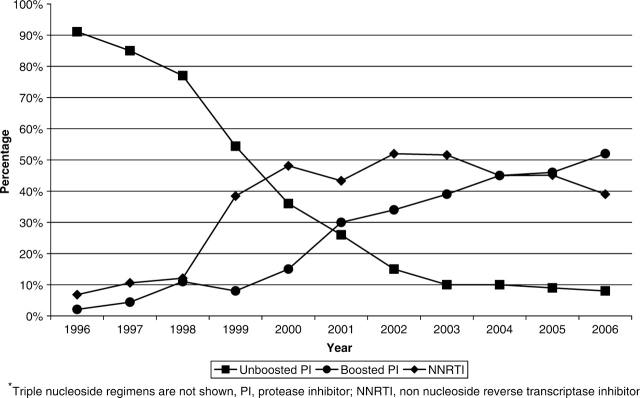
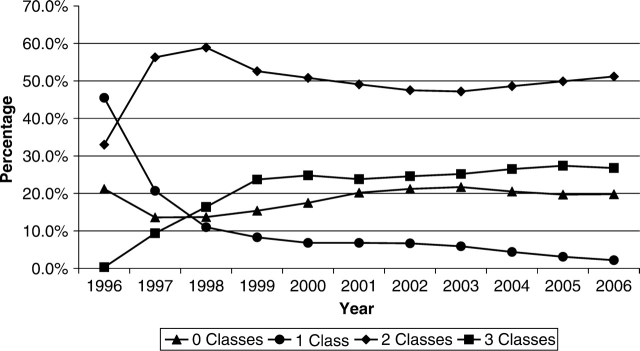
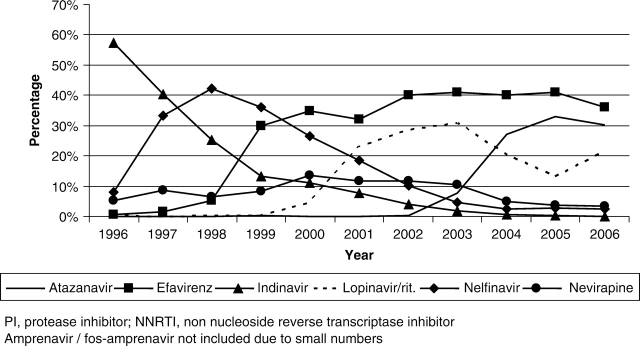
The CNICS repository contains longitudinal data on over 15 000 HIV-infected patients in care between January 1, 1995 and May 30, 2007 that are available for research, including studies examining the determinants of long-term clinical outcomes in the HAART era. There were 2270 unique AIDS-defining events observed during this time period at six clinical CNICS-participating sites (Johns Hopkins University, the seventh site, has newly joined CNICS and is not included in the following results). As shown in Figure 1, the incidence of clinical AIDS-defining diagnoses decreased significantly in 1996 after the introduction of HAART, and continues to decline slowly in the most recent time period. Figure 2 depicts the trends in type of antiretroviral regimens used as initial therapy over time and shows the early predominance of PI-based regimens, followed by a substantial increase in non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimens in 1999, and the transition from unboosted to boosted PI-based regimens. Figure 3 shows the cumulative exposure to zero, one, two or three classes of antiretroviral medications among the cohort, demonstrating the significant drop in the number of patients exposed to a single class of agents and a fairly steady proportion exposed to two and three classes of antiretroviral medications over time. Figure 4 shows the trends in the type of individual antiretroviral medications used as initial therapy during the study period.
Figure 1.
Incidence of AIDS-defining illness within the CNICS cohort by year (1995–2006)
Figure 2.
Type of initial HAART regimen by year of initiation (1996–2006)
Figure 3.
Distribution of antiretroviral class exposure by year (1996–2006)
Figure 4.
Distribution of individual PIs and NNRTIs
The distribution of CD4+ T cell counts among patients initiating care at CNICS-affiliated clinics during 2000–06 was as follows: 25% were between 0 and 199 cells/mm3, 45% between 200 and 349 cells/mm3 and 30% had >350 cells/mm3. Among untreated patients, higher presenting plasma HIV-1 RNA levels were associated with greater subsequent CD4+ T cell decline.31 However, only a small proportion of CD4+ T cell loss variability (4–6%) could be explained by presenting plasma HIV RNA level.31 Other factors, as yet undefined, likely drive CD4+ T cell losses in HIV infection. These findings have implications for treatment decisions in HIV infection and for understanding the pathogenesis of progressive immune deficiency. The overall prevalence of HCV co-infection was 17% during the period between 2001 and 2006, and ranged from ∼4–28% across sites. The prevalence of HCV co-infection observed in the CNICS population underscores the importance of conducting studies to understand the effect of this comorbidity on treatment-related complications and disease progression to develop better strategies for use of HAART among these patients.
What are the main strengths and weaknesses?
Classical epidemiological cohorts collect data from patients at set intervals using standardized forms and interviews, relying heavily on patients’ self-report of diagnoses made and treatment received in the clinical setting. Clinical cohorts such as those in CNICS capture data generated in the care of patients at the time it is provided largely collected through medical records. A limitation of clinical cohort data is that medical records are intended for use in patient care not research and the quality of information obtained depends on clinician documentation.16 While paper records suffer from illegibility and missing reports, the shift toward EMR can improve the quality of data capture through use of standardized forms at intake, structured diagnosis and medication lists and coding, required fields for completion, and integrated data such as comprehensive laboratory results. EMRs designed to standardize clinician documentation at the time care is provided improve the accuracy and completeness of clinical data required for research.
CNICS was designed to address scientific questions that cannot be answered through other collaborative cohorts. The features that facilitate this include: (i) rich longitudinal data generated over a 12-year period at geographically and ethnically diverse centres of excellence in HIV research and care in the United States, using EMR point-of-care systems that reflect the daily decisions made in real clinical practice; (ii) a CNICS scientific agenda that was conceived and developed by investigators with expertise in clinical care, HIV pathogenesis, medical informatics, outcomes research and bioethics; (iii) ongoing collection of patient-reported measures using a standardized set of questionnaires across sites that provides a unique resource for researchers investigating antiretroviral medication adherence, substance abuse, mental illness and body morphology changes among a large well-characterized HIV cohort; (iv) the integration of these clinical outcomes data with linked specimens, thereby allowing cross disciplinary analyses of basic, clinical and behavioural sciences; (v) the systematic development of procedures for data quality assurance at each CNICS site through rigorous, ongoing standardized review and verification strategies of the clinical data; (vi) a flexible, robust data system that can accommodate new data elements, new clinical sites and new research agendas that are responsive to the changing needs of the epidemic; and (vii) data analyses that will complement other sources of data analyses such as clinical trials and classical epidemiologic cohorts, synergistically to address emerging clinical questions. Taken together, CNICS is a unique resource for HIV clinical, translational and basic research, poised to address the challenging and evolving issues in HIV care and research.
How can I collaborate? Where can I find out more?
CNICS has policies to ensure that multi-centre research findings are developed collaboratively, efficiently and fairly. The following principles previously outlined by other cohort collaborations32,33 guide our scientific research policies: (i) ownership of individual cohort data remains with the contributing CFAR cohort, as an entity, CNICS will not own any data; (ii) data transmitted to and residing at the DMC will be de-identified of all personal health identifiers (PHI) as defined by Health Insurance Portability and Accountability Act (HIPAA) criteria; (iii) ownership of individual repository specimens remains with the contributing cohort; (iv) an individual cohort can choose to participate or not in any scientific aim or sub-aim; and (v) all cohorts will have input and one vote in the EC irrespective of contributing data to a specific scientific aim or question.
Process for new collaborators
Collaborators who are not members of CNICS or partner institutions are encouraged to be involved in studies/data analyses. These collaborators will be required to acknowledge that CNICS data and specimens are the property of the individual CNICS sites. Collaborating investigators will be encouraged to pose relevant scientific questions and to complete a concept proposal. Non-CNICS investigators should name a CNICS contact person whose responsibility is to ensure the lead investigator has had the opportunity to share his/her ideas with the working groups. Proposals from non-CNICS collaborators will follow the same review processes and publication policies as CNICS investigators. Submissions dealing with the primary CNICS focus areas will have priority both in terms of timing and in use of study resources for data analysis. Concept proposals will be reviewed by experts in the RCC, the appropriate Working Group, the BC, and then will be discussed by the EC. After addressing any concerns through revision, the EC will vote on approval of a proposal. If approved, each cohort will decide through its own established procedures whether it wishes to participate in the specific proposal and whether it can participate (i.e. has the required data elements). Potential users of the CNICS cohort include: (i) projects that comprise part of the CNICS scientific agenda; (ii) projects from CFAR sites that are not part of the CNICS scientific agenda; (iii) projects from academic sites or government agencies not associated with CFAR and (iv) investigators from industry seeking collaboration. For additional information on collaboration, those interested can contact Michael Saag, Principal Investigator, UAB Center for AIDS Research, BBRB 256G, 845 19th Street South, Birmingham, AL 35294-2170, E-mail: msaag@uab.edu
Process for adding new collaborating cohorts
New collaborating cohorts will be added as needed in order to augment CNICS geographic and ethnic diversity, as well as to add to our scientific expertise. Johns Hopkins University is the cohort most recently added to CNICS. All CFAR sites will be considered for inclusion, but preference will be given to sites that have existing databases and EMRs. These sites have significant expertise in clinical research and the ability to maintain the homogeneity of the CNICS cohort in terms of quality data collection, implementation of standardized questionnaires, as well as the presence of a specimen repository. Addition of cohorts must be approved by the EC. Once approved, the cohort will be represented on the EC equal to the existing CFAR sites. Researchers interested in having their site considered for inclusion should contact Michael Saag (contact information provided above). CNICS is flexible and specifically designed to accommodate the planned expansion for new sites and the addition of new data elements necessary for the evolving HIV research agenda that will provide a unique and powerful new resource for HIV research.
Acknowledgements
CNICS receives funding from the National Institute of Allergy and Infectious Diseases (R24 AI067039-1), as well as the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, and the National Heart, Lung and Blood Institute. CNICS is funded in part by six Centers for AIDS Research (CFAR) sites (P30 AI027767, P30 AI27757, P30 AI027763, P30 AI036214, P30 AI036219 and P30 AI060354). CNICS also receives support from two additional grants: HHSN268200425213C and K24 AI064086.
Conflict of interest: None declared.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Whitman S, Murphy J, Cohen M, Sherer R. Marked declines in human immunodeficiency virus-related mortality in Chicago in women, African Americans, Hispanics, young adults, and injection drug users, from 1995 through 1997. Arch Intern Med. 2000;160:365–69. doi: 10.1001/archinte.160.3.365. [DOI] [PubMed] [Google Scholar]
- 3.Hickman M, Bardsley M, De Angelis D, Ward H. Impact of HIV on adult (15–54) mortality in London: 1979–96. Sex Transm Infect. 1999;75:385–88. doi: 10.1136/sti.75.6.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Deeks SG, Hecht FM, Swanson M, et al. HIV RNA and CD4 cell count response to protease inhibitor therapy in an urban AIDS clinic: response to both initial and salvage therapy. AIDS. 1999;13:F35–43. doi: 10.1097/00002030-199904160-00001. [DOI] [PubMed] [Google Scholar]
- 6.May MT, Sterne JA, Costagliola D, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451–58. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 7.Knobel H, Guelar A, Carmona A, et al. Virologic outcome and predictors of virologic failure of highly active antiretroviral therapy containing protease inhibitors. AIDS Patient Care STDS. 2001;15:193–99. doi: 10.1089/10872910151133729. [DOI] [PubMed] [Google Scholar]
- 8.Yeni P, Cooper DA, Aboulker JP, et al. Virological and immunological outcomes at 3 years after starting antiretroviral therapy with regimens containing non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or both in INITIO: open-label randomised trial. Lancet. 2006;368:287–98. doi: 10.1016/S0140-6736(06)69074-0. [DOI] [PubMed] [Google Scholar]
- 9.Dragsted UB, Mocroft A, Vella S, et al. Predictors of immunological failure after initial response to highly active antiretroviral therapy in HIV-1-infected adults: a EuroSIDA study. J Infect Dis. 2004;190:148–55. doi: 10.1086/420786. [DOI] [PubMed] [Google Scholar]
- 10.Visnegarwala F, Krause KL, Musher DM. Severe diabetes associated with protease inhibitor therapy. Ann Intern Med. 1997;127:947. doi: 10.7326/0003-4819-127-10-199711150-00016. [DOI] [PubMed] [Google Scholar]
- 11.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Behrens G, Dejam A, Schmidt H, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13:F63–70. doi: 10.1097/00002030-199907090-00001. [DOI] [PubMed] [Google Scholar]
- 13.Walli R, Herfort O, Michl GM, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12:F167–73. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Mulligan K, Grunfeld C, Tai VW, et al. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr. 2000;23:35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Hughes MD. Initial treatment of HIV infection: randomized trials with clinical end points are still needed. J Infect Dis. 2006;194:542–44. doi: 10.1086/506369. [DOI] [PubMed] [Google Scholar]
- 16.Lau B, Gange SJ, Moore RD. Interval and clinical cohort studies: epidemiological issues. AIDS Res Hum Retroviruses. 2007;23:769–76. doi: 10.1089/aid.2006.0171. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. HIV/AIDS surveillance report. 2001;Vol. 13(No. 1) Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/#surveillance. [Google Scholar]
- 18.Centers for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 19.CoDe Working Group. Protocol: coding causes of death in HIV CoDe. v1.0: www.cphiv.dk/CoDe, last accessed date June 1, 2007.
- 20.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol use disorders identification test. Arch Intern Med. 1998;158:1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 23.Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female veterans affairs patient population. Arch Intern Med. 2003;163:821–29. doi: 10.1001/archinte.163.7.821. [DOI] [PubMed] [Google Scholar]
- 24.Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24:217–26. doi: 10.1080/09595230500170266. [DOI] [PubMed] [Google Scholar]
- 25.WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183–94. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 26.Gripshover B, Tien PC, Saag MS, Osmond D, Bacchetti P, Grunfeld C. Lipoatrophy is the dominant feature of the lipodystrophy syndrome in HIV-infected men. Paper presented at: 10th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2003. [Google Scholar]
- 27.Tien PC, Cole SR, Williams CM, et al. Incidence of Lipoatrophy and Lipohypertrophy in the Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2003;34:461–66. doi: 10.1097/00126334-200312150-00003. [DOI] [PubMed] [Google Scholar]
- 28.Bacchetti P, Gripshover B, Grunfeld C, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–31. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathews WC, May S. EuroQol (EQ-5D) measure of quality of life predicts mortality, emergency department utilization, and hospital discharge rates in HIV-infected adults under care. Health Qual Life Outcomes. 2007;5:5. doi: 10.1186/1477-7525-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kissinger P, Rice J, Farley T, et al. Application of computer-assisted interviews to sexual behavior research. Am J Epidemiol. 1999;149:950–54. doi: 10.1093/oxfordjournals.aje.a009739. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez B, Sethi AK, Cheruvu VK, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296:1498–506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 32.Gange SJ, Kitahata MM, Saag MS, et al. Cohort Profile: The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007;36:294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ART-CC. ART cohort collaboration. [last accessed date June 1, 2007]; http://www.art-cohort-collaboration.org,