Abstract
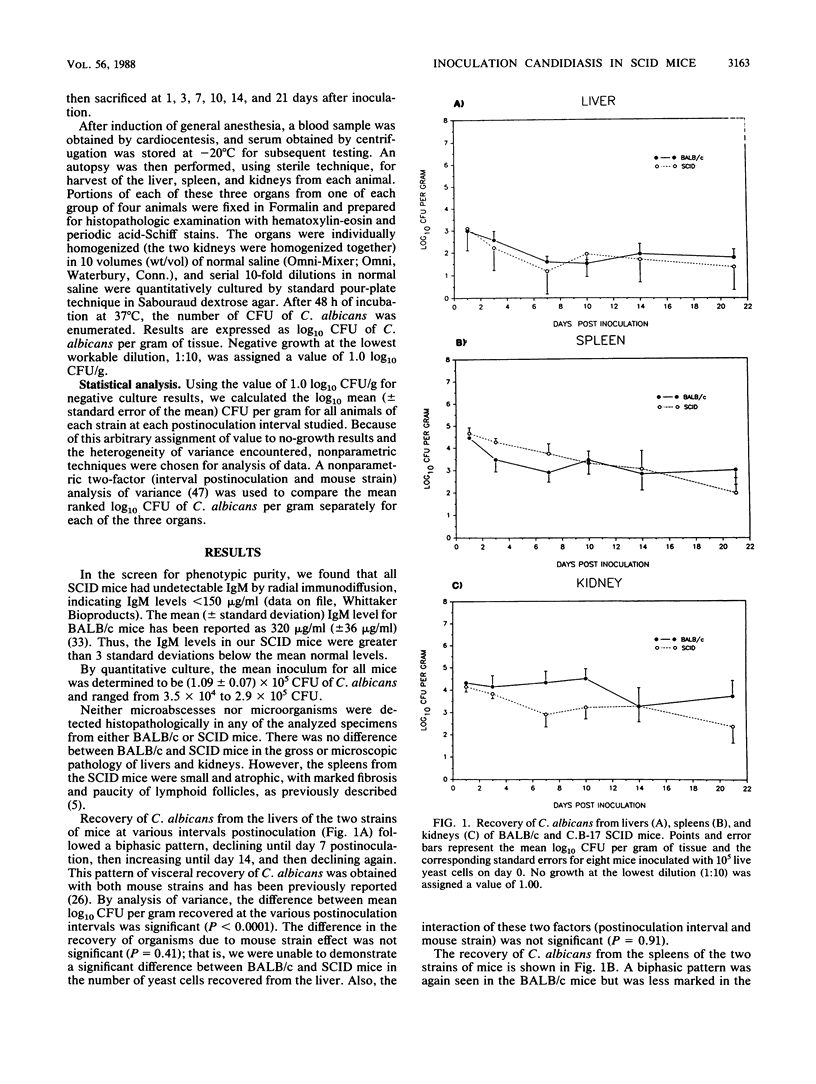
To further elucidate the importance of T- and B-lymphocyte-mediated responses in host defense against systemic infection with Candida albicans, we studied this infection in a murine model of severe combined immunodeficiency (SCID). The course of inoculation candidiasis in these mice, which lack functional T and B lymphocytes, was compared with that in immunologically normal BALB/c mice. Mice were inoculated intravenously with 10(5) yeast cells. Quantitative cultures of liver, spleen, and kidneys were performed with necropsy specimens obtained 1, 3, 7, 10, 14, and 21 days after this intravenous inoculation. The differences in the time courses of recovery of organisms from liver and spleen specimens were not significantly different in the SCID mice compared with the BALB/c mice. The recovery of C. albicans from the kidneys was significantly lower in the SCID mice, indicating less persistence of the organism in the kidneys of the SCID mice than in those of the BALB/c mice. These data indicate that defense mechanisms other than T- and B-lymphocyte-mediated mechanisms are primarily responsible for host defense against inoculation candidiasis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. B. Mouse candidiasis. II. Host responses are T-cell dependent and regulated by genes in the major histocompatibility complex. Immunogenetics. 1987;25(3):200–203. doi: 10.1007/BF00344035. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Bosma M. J., Bosma G. C., Unanue E. R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J Immunol. 1986 Jul 1;137(1):4–9. [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Custer R. P., Bosma G. C., Bosma M. J. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985 Sep;120(3):464–477. [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Diamond R. D., Haudenschild C. C. Monocyte-mediated serum-independent damage to hyphal and pseudohyphal forms of Candida albicans in vitro. J Clin Invest. 1981 Jan;67(1):173–182. doi: 10.1172/JCI110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E. Intragastric colonization of infant mice with Candida albicans induces systemic immunity demonstrable upon challenge as adults. J Infect Dis. 1988 May;157(5):950–958. doi: 10.1093/infdis/157.5.950. [DOI] [PubMed] [Google Scholar]
- Domer J. E., Moser S. A. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1978 Apr;20(1):88–98. doi: 10.1128/iai.20.1.88-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elin R. J., Edelin J. B., Wolff S. M. Infection and immunoglobulin concentrations in Chediak-Higashi mice. Infect Immun. 1974 Jul;10(1):88–91. doi: 10.1128/iai.10.1.88-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., Moser S. A., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses in T-lymphocyte-depleted mice. Infect Immun. 1978 Sep;21(3):729–737. doi: 10.1128/iai.21.3.729-737.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett J., Jr, Bosma G. C., Bosma M. J., Bennett M., Kumar V. Transplantable progenitors of natural killer cells are distinct from those of T and B lymphocytes. Proc Natl Acad Sci U S A. 1986 May;83(10):3427–3431. doi: 10.1073/pnas.83.10.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector R. F., Domer J. E., Carrow E. W. Immune responses to Candida albicans in genetically distinct mice. Infect Immun. 1982 Dec;38(3):1020–1028. doi: 10.1128/iai.38.3.1020-1028.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helstrom P. B., Balish E. Effect of oral tetracycline, the microbial flora, and the athymic state on gastrointestinal colonization and infection of BALB/c mice with Candida albicans. Infect Immun. 1979 Mar;23(3):764–774. doi: 10.1128/iai.23.3.764-774.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayoshi K., Nishikawa S., Kina T., Hatanaka M., Habu S., Nomura T., Katsura Y. Immunoglobulin heavy chain gene diversification in the long-term bone marrow culture of normal mice and mice with severe combined immunodeficiency. Eur J Immunol. 1987 Jul;17(7):1051–1057. doi: 10.1002/eji.1830170723. [DOI] [PubMed] [Google Scholar]
- Hurtrel B., Langrange P. H., Michel J. C. Absence of correlation between delayed-type hypersensitivity and protection in experimental systemic candidiasis in immunized mice. Infect Immun. 1981 Jan;31(1):95–101. doi: 10.1128/iai.31.1.95-101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. M. Quantitation of antibody against cell wall mannan and a major cytoplasmic antigen of Candida in rabbits, mice, and humans. Infect Immun. 1980 Oct;30(1):78–89. doi: 10.1128/iai.30.1.78-89.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. H., Rodey G. E., Good R. A., Chilgren R. A., Quie P. G. Defective candidacidal capacity of polymorphonuclear leukocytes in chronic granulomatous disease of childhood. J Pediatr. 1969 Aug;75(2):300–303. doi: 10.1016/s0022-3476(69)80403-8. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- Lauzon R. J., Siminovitch K. A., Fulop G. M., Phillips R. A., Roder J. C. An expanded population of natural killer cells in mice with severe combined immunodeficiency (SCID) lack rearrangement and expression of T cell receptor genes. J Exp Med. 1986 Nov 1;164(5):1797–1802. doi: 10.1084/jem.164.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. W., Balish E. Systemic candidosis in germfree, flora-defined and conventional nude and thymus-bearing mice. J Reticuloendothel Soc. 1981 Jan;29(1):71–77. [PubMed] [Google Scholar]
- Lee K. W., Balish E. Systemic candidosis in silica-treated athymic and euthymic mice. Infect Immun. 1983 Sep;41(3):902–907. doi: 10.1128/iai.41.3.902-907.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louria D. B., Smith J. K., Brayton R. G., Buse M. Anti-Candida factors in serum and their inhibitors. I. Clinical and laboratory observations. J Infect Dis. 1972 Feb;125(2):102–114. doi: 10.1093/infdis/125.2.102. [DOI] [PubMed] [Google Scholar]
- Miyake T., Takeya K., Nomoto K., Muraoka S. Cellular elements in the resistance to candida infection in mice. I. Contribution of T lymphocytes and phagocytes at various stages of infection. Microbiol Immunol. 1977;21(12):703–725. doi: 10.1111/j.1348-0421.1977.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Morelli R., Rosenberg L. T. Role of complement during experimental Candida infection in mice. Infect Immun. 1971 Apr;3(4):521–523. doi: 10.1128/iai.3.4.521-523.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad S., Friedman L. Passive immunization of mice against Candida albicans. Sabouraudia. 1968 Feb;6(2):103–105. [PubMed] [Google Scholar]
- Natsuume-Sakai S., Motonishi K., Migita S. Quantitative estimations of five classes of immunoglobulin in inbred mouse strains. Immunology. 1977 Jun;32(6):861–866. [PMC free article] [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Human lymphocyte-activating properties of a purified polysaccharide from Candida albicans: B and T cell cooperation in the mitogenic response. J Immunol. 1980 Nov;125(5):2082–2088. [PubMed] [Google Scholar]
- Reitnauer P. J., Brown L. R., Hank J. A., Sondel P. M. Presentation of Candida albicans and purified protein derivative soluble antigens by Epstein-Barr virus-transformed human lymphoblastoid B-cell lines. Cell Immunol. 1986 Apr 15;99(1):196–208. doi: 10.1016/0008-8749(86)90228-5. [DOI] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Dharakul T., Kowalski E., Michalak S., Ogra P. L. Persistent rotavirus infection in mice with severe combined immunodeficiency. J Virol. 1987 Oct;61(10):3345–3348. doi: 10.1128/jvi.61.10.3345-3348.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Effect of systemic candidiasis on blastogenesis of lymphocytes from germfree and conventional rats. Infect Immun. 1978 Apr;20(1):142–150. doi: 10.1128/iai.20.1.142-150.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Immunity to Candida albicans. Microbiol Rev. 1980 Dec;44(4):660–682. doi: 10.1128/mr.44.4.660-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Immunity to experimental renal candidiasis in rats. Infect Immun. 1978 Feb;19(2):737–740. doi: 10.1128/iai.19.2.737-740.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Sinha B. K., Prasad S., Monga D. P. Studies of experimental candidiasis in T-cell-deficient mice. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Jun;265(1-2):203–209. doi: 10.1016/s0176-6724(87)80167-0. [DOI] [PubMed] [Google Scholar]
- Sohnle P. G., Frank M. M., Kirkpatrick C. H. Mechanisms involved in elimination of organisms from experimental cutaneous Candida albicans infections in guinea pigs. J Immunol. 1976 Aug;117(2):523–530. [PubMed] [Google Scholar]
- Trier J. S., Bjorkman D. J. Esophageal, gastric, and intestinal candidiasis. Am J Med. 1984 Oct 30;77(4D):39–43. [PubMed] [Google Scholar]
- Witte P. L., Burrows P. D., Kincade P. W., Cooper M. D. Characterization of B lymphocyte lineage progenitor cells from mice with severe combined immune deficiency disease (SCID) made possible by long term culture. J Immunol. 1987 Apr 15;138(8):2698–2705. [PubMed] [Google Scholar]
- Zunino S. J., Hudig D. Interactions between human natural killer (NK) lymphocytes and yeast cells: human NK cells do not kill Candida albicans, although C. albicans blocks NK lysis of K562 cells. Infect Immun. 1988 Mar;56(3):564–569. doi: 10.1128/iai.56.3.564-569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Doory Y. An immune factor in baboon anti-candida serum. Sabouraudia. 1970 May;8(1):41–47. doi: 10.1080/00362177085190621. [DOI] [PubMed] [Google Scholar]



