Abstract
Objectives
Intraductal mucinous papillary neoplasm (IPMN) is being recognized with increasing frequency around the world. The true incidence, however, remains unknown. Our goal was to determine the incidence of IPMN in a population study.
Methods
We used the records-linkage system of the Rochester Epidemiology Project to ascertain age- and sex-adjusted incidence rates of IPMN in Olmsted County, Minn, from January 1, 1984, to December 31, 2005. We also evaluated the number of prevalent cases as of December 31, 2005.
Results
We identified 28 incident cases of IPMN. The age- and sex-adjusted cumulative incidence for IPMN in Olmsted County is 2.04 cases per 100,000 persons (95% confidence interval [CI], 1.28–2.80) from 1984 to 2005. Point prevalence on December 31, 2005, was 25.96 cases per 100,000 persons (95% CI, 14.53–37.38 cases) or 1 per 3852. Restricting to county residents 60 years and older, the point prevalence is 99.10 cases per 100,000 persons (95% CI, 54.40–143.79 cases) or one per 1009 persons. Thirty-two percent of patients were treated surgically. The 5-year survival rate after diagnosis was 59.6%.
Conclusions
The incidence of IPMN in Olmsted County is low but increasing. Most patients do not die of complications related to the disease.
Keywords: IPMN, incidence, mucinous cystic neoplasm, prevalence, IPMT, intraductal papillary-mucinous neoplasm
Intraductal mucinous pancreatic neoplasm was first described in 1982 by Ohhasi et al1 and has since had a number of names ultimately culminating in the term intraductal papillary mucinous neoplasm (IPMN).
IPMN is a spectrum characterized by cystic dilation of the main pancreatic duct and/or its side branches.2,3 Currently, IPMN is reported to account for approximately 0.5% of all pancreatic neoplasms found at autopsy and 7.5% of all clinically diagnosed pancreatic lesions.4 This pathological process usually presents in elderly patients and can be associated with pancreatitis. IPMN is not thought to have a familial genetic component, but patients who harbor IPMN are believed to be at an increased risk for pancreatic cancer and other malignancies such as colon cancer.5–7
IPMN is being recognized with increasing frequency around the world.8,9 The number of pancreatic resections for IPMN has risen sharply in the last decade with up to 20% of pancreatic resections due to IPMN.10 IPMN was the diagnosis of 5% of all pancreaticoduodenectomies in a series of 1000 patients at Johns Hopkins Hospital over the last 34 years.11 Despite the increase in familiarity with this lesion, the incidence rate and natural history of IPMN remain unknown. Therefore, a debatable and aggressive approach to the treatment of IPMN is currently practiced. The International Association of Pancreatology recently released a consensus statement that gives formal recommendations to guide the evaluation and management process of this entity.12
The goal of this study was to take advantage of resources available through the Rochester Epidemiology Project (REP)13 to determine the incidence, prevalence, and the most common presenting signs and symptoms, preoperative evaluation, treatment options, and long-term outcomes of IPMN in Olmsted County, Minn. This study will be the first population-based study to determine the incidence and prevalence of IPMN. These data have implications for funding future medical research and allocation of funds for clinical evaluation.
MATERIALS AND METHODS
Study Population
Olmsted County, Minn, is 654 m2 and has a population of 124,277. According to the year 2000 US Census, the majority of its residents are white. Olmsted County is south of Minneapolis and includes the city Rochester, which is home to 75% of the county’s residents (Fig. 1). Olmsted County is served by a largely unified medical care system that has accumulated comprehensive clinical records since the early 1900s. The REP has developed an index for the records of virtually all providers of medical care to Olmsted County residents.
FIGURE 1.
Relationship of Olmsted County to city of Rochester and state of Minnesota. Each dot represents the center of each city (Minneapolis and Rochester). MSA refers to the Metropolitan Statistical Area which includes parts of Minnesota and Wisconsin as MSA is determined by counties. The center of Rochester, Minn, is 150 km from the center of Minneapolis/St Paul MSA.
All diagnoses associated with outpatient visits (clinic and emergency room visits), inpatient visits (hospital admission, surgical procedures), autopsy, and nursing home care are indexed for computerized retrieval and are available for study under the auspices of the REP. The REP has been used to study the epidemiology of many disorders including gastrointestinal diseases for over 30 years which has resulted in more than 1000 publications to date.13
Case Ascertainment
The REP records linkage system was queried for all Olmsted County patients with the following codes of the Hospital Adaptation of the International Classification of Diseases (H-ICDA),14 adapted for indexing of hospital records and operation classification based on the International Classification of Diseases (ICD) version 8 codes: 01790453, 01790630, 01790860, 01790861, 01790870, 01791930, 01792430, 01792761, 01790210, 01792840, 01792830, 01792640, 02290890, 02290940, 02290430, 02399220, 01570110, 01579110, 01578000, 01578110, 01578210, 01578310, 01578410, 34087110, 02116110, and 02306110, and ICD codes 157, 211.6, 230.9, and 239.0 for the years 1976–2005. An experimental tool that matches text written in electronic notes called the Mayo Clinic Life Sciences Systems was also used but was available only for patients seen at Mayo Clinic hospitals after 1996. The Mayo Clinic Life Sciences Systems searched for the terms “IPMT,” “IPMN,” “intraductal papillary, mucin,” “intraductal papillary mucin,” “intraductal Papillary-Mucinous tumor,” “intrapapillary mucin,” “papillary mucin,” “papillary mucous,” “intrapapillary mucinous, “mucinous tumor,” “serous cystadenoma,” “mucous cystadenoma,” “mucinous type,” “intrapapillary mucus,” “papillary mucus,” “mucin producing tumor,” mucinous papillary cystic tumor,” “mucinous papillary cystic,” “mucin-hypersecreting tumor,” “mucin villous adenomatosis,” “mucinous cystadenoma,” “serous cyst adenoma,” and “papillary ductal mucin.”
We identified 357 patients from 1976 to 2005 that matched any or all of the terms or ICD codes cited above. All 357 charts were then reviewed by a physician to confirm the diagnosis of IPMN (Table 1). Because IPMN is a relatively new pathological diagnosis, each available slide (n = 56) from patients carrying the above listed diagnoses was reviewed by independent pathologists (n = 2) with an interest in IPMN. This was done to ensure that the slides contained findings consistent with IPMN or to diagnose IPMN that was not previously identified after pathological review. The first confirmed case of IPMN was noted in 1984, and as up-to-date imaging techniques were not available before this date, IPMN cases may have been missed. Therefore, we limited all further analyses to the years 1984 through 2005.
TABLE 1.
Diagnosis Obtained After Chart and Slide Review of 357 Patients
| Pancreatic adenocarcinoma | 269 |
|---|---|
| No pancreatic diagnosis in chart | 17 |
| Mucinous cystic neoplasm | 16 |
| IPMN* | 28 |
| Microcystic adenocarcinoma | 6 |
| IPMN outside Olmsted County | 4 |
| Ovarian carcinoma | 4 |
| Neuroendocrine tumor | 3 |
| Breast cancer | 2 |
| Ampullary carcinoma | 2 |
| Ampullary stricture | 1 |
| Cholangiocarcinoma | 1 |
| Chronic pancreatitis | 1 |
| Cystadenocarcinoma | 1 |
| Pancreatic transplant | 1 |
| Pseudocyst | 1 |
| Total | 357 |
Includes patients with IPMN adenoma, IPMN borderline, IPMN carcinoma. Diagnosis based on radiographic and pathological criteria.
Diagnostic Criteria
Only patients residing in Olmsted Medical County, Minn, who had clinical diagnoses of main duct, side-branch, or mixed-type IPMN were included in this study. Diagnosis was made after radiographic imaging (computed tomography [CT], endoscopic ultrasound [EUS], ultrasound [US], and endoscopic retrograde cholangiopancreatography [ERCP]) or pathological assessment of a biopsy specimen or surgically resected specimen. The classification as recommended by the World Health Organization was used.15 Patients with other cystic pancreatic lesions, chronic pancreatitis with pseudocyst, and pancreatic adenocarcinoma were excluded. Each chart was then abstracted for demographic information, presenting signs and symptoms, radiographic and pathological diagnosis, medical versus surgical management, surgical outcomes, long-term follow-up data, and survival data. Death and cause of death were assessed by chart review and available death registries.
Data Analysis
The characteristics of the IPMN cases are reported using standard descriptive statistics, number (percent) for the discrete factors, and the mean (SD) or median (range), as appropriate, for continuous factors.
Incident cases were identified among residents of Olmsted County 20 years or older having an IPMN diagnosis from January 1, 1984 to December 31, 2005. The incidence rate of IPMN was estimated as the number of incident IPMN cases divided by the number of person-years at risk for the population (per census data) over the study period, with direct standardization to the age and sex distribution of the 2000 US white population. Person-years for the population at risk was estimated from the decennial census figures using linear interpolation between censuses. Crude as well as age-adjusted sex-specific incidence and age-and sex-adjusted incidence rates were estimated along with the 95% confidence intervals (CIs), assuming that the number of incident cases follows a Poisson distribution. Incidence rates are reported as per 100,000 person-years of observation.16
The point prevalence of IPMN was calculated using the estimated Olmsted County population on December 31, 2005, as the denominator. Overall survival among the IPMN patients was estimated using the Kaplan-Meier survival method.
RESULTS
We abstracted information from 357 charts and identified 28 cases of IPMN in Olmsted County (Table 1). Five of these patients were identified after slide review by our pathologists, after an initial diagnosis of either “mucinous tumor,” “mucinous adenoma,” or “mucinous adenocarcinoma.” The earliest IPMN diagnosis was made in 1984. This patient had a “cystic malignancy” and was treated with a Whipple procedure. On later review, she was found to have IPMN with dysplastic changes in the setting of a mucinous adenocarcinoma. All of the other patients were diagnosed in or after the 1990s. Pathology information was available for 10 of the 28 IPMN patients. One patient had a diagnosis that was based on a biopsy that revealed IPMN adenoma. Of the 28 patients, 23 (82.1%) had a diagnosis of IPMN adenoma, 2 patients (3.4%) had a diagnosis of IPMN borderline, and 3 patients (10.7%) had a diagnosis of intraductal papillary-mucinous carcinoma. Five of these patients were identified after slide review by our pathologist; their initial diagnosis of mucinous cystic neoplasm was made in 1984, 1993, 1995, 1997, and 1999.
Incidence and Prevalence of IPMN
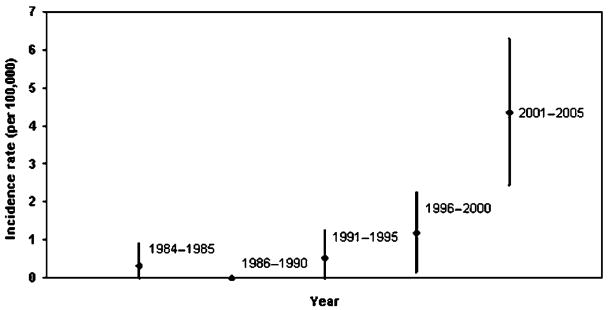
The age- and sex-adjusted cumulative incidence of IPMN in Olmsted County was 2.04 (95% CI, 1.28–2.80) per 100,000 person-years from 1984 to 2005 (Table 2). The incidence increased with time but was not statistically analyzed given the small number in each year (Fig. 2).
TABLE 2.
Incident Cases of IPMN in Olmsted County
| Incident Cases
|
Olmsted County Population
|
Incidence Rate/100,000 (95% CI)† |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Age,* yrs | F | M | Total | F | M | Total | F | M | Total |
| 20–29 | 0 | 0 | 0 | 190,924 | 176,180 | 367,105 | 0.0 | 0.0 | 0.0 |
| 30–39 | 0 | 0 | 0 | 216,660 | 211,770 | 428,430 | 0.0 | 0.0 | 0.0 |
| 40–49 | 1 | 1 | 2 | 184,726 | 176,503 | 361,229 | 0.54 | 0.57 | 0.55 |
| 50–59 | 0 | 1 | 1 | 122,909 | 118,300 | 241,209 | 0.0 | 0.85 | 0.41 |
| 60–69 | 5 | 3 | 8 | 84,584 | 76,053 | 160,637 | 5.91 | 3.94 | 4.98 |
| 70–79 | 5 | 7 | 12 | 65,940 | 46,806 | 112,746 | 7.58 | 14.96 | 10.64 |
| 80–89 | 3 | 1 | 4 | 42,124 | 19,411 | 61,535 | 7.12 | 5.15 | 6.50 |
| 90+ | 1 | 0 | 1 | 11,358 | 2726 | 14,083 | 8.80 | 0.0 | 7.10 |
| All ages | 15 | 13 | 28 | 919,225 | 827,749 | 1,746,974 | |||
| Crude | 1.63 | 1.57 | 1.60 | ||||||
| Adjusted | 1.83 (0.90–2.77) | 2.27 (1.02–3.53) | 2.04‡ (1.28–2.80) | ||||||
Age at diagnosis.
Corrected incidence with prevalent cases removed from denominator the same as crude.
Age adjusted to US white population in 2000. The cumulative incidence when age and sex adjusted to US white population in 2000 is 2.04 per 100,000 (1.28–2.80) person-years. Population of Olmsted County based on US census in year 2000.
FIGURE 2.
Incidence rate and 95% CI for IPMN in Olmsted County from 1984 to 2005.
The point prevalence of IPMN on December 31, 2005, was 25.96 cases per 100,000 persons (95% CI, 14.53–37.38 cases). Point prevalence for age 60 years and older is 99.10 cases per 100,000 persons (95% CI, 54.4–143.8 cases) or 1 per 1009 persons (Table 3). Patients who were deceased at the time prevalence was calculated (December 31, 2005, n = 8) were excluded, hence the difference in total number of cases seen in Tables 2 and 4 versus Table 3.
TABLE 3.
Prevalent Cases of IPMN in Olmsted County
| Age-Specific Prevalence of IPMN in Olmsted County, 12/31/2005
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalent Cases
|
Olmsted County Population
|
Prevalence/100,000 (95% CI)
|
|||||||
| Age, yrs | F | M | Total | F | M | Total | F | M | Total |
| 20–29 | 0 | 0 | 0 | 8978 | 8632 | 17,609 | 0.0 | 0.0 | 0.0 |
| 30–39 | 0 | 0 | 0 | 11,019 | 10,923 | 21,942 | 0.0 | 0.0 | 0.0 |
| 40–49 | 0 | 1 | 1 | 11,214 | 10,731 | 21,945 | 0.0 | 9.32 | 4.56 |
| 50–59 | 0 | 0 | 0 | 7400 | 6898 | 14,298 | 0.0 | 0.0 | 0.0 |
| 60–69 | 5 | 3 | 8 | 4629 | 4283 | 8912 | 108.01 | 70.05 | 89.77 |
| 70–79 | 4 | 4 | 8 | 3579 | 2756 | 6336 | 111.75 | 145.12 | 126.27 |
| 80–89 | 2 | 1 | 3 | 2368 | 1188 | 3556 | 88.45 | 84.17 | 84.36 |
| 90+ | 0 | 0 | 0 | 662 | 171 | 833 | 0.0 | 0.0 | 0.0 |
| Total | 11 | 9 | 20 | 49,848 | 45,582 | 95,431 | |||
| Crude | 22.07 | 19.74 | 20.96 | ||||||
| Adjusted | 25.63 (10.41–40.85) | 26.49 (8.91–44.08) | 25.96* (14.53–37.38) | ||||||
Age and sex adjusted.
TABLE 4.
Patient Characteristics
| n | % | |
|---|---|---|
| Sex | ||
| Female | 15 | 46.4 |
| Male | 13 | 54.6 |
| Age, yrs | ||
| 20–29 | 0 | 0 |
| 30–39 | 0 | 0 |
| 40–49 | 2 | 7.1 |
| 50–59 | 1 | 3.6 |
| 60–69 | 8 | 28.6 |
| 70–79 | 12 | 42.9 |
| 80–89 | 4 | 14.3 |
| 90+ | 1 | 3.6 |
| Race/Ethnicity | ||
| White | 25 | 89.3 |
| African American | 1 | 3.6 |
| Hispanic | 0 | 0 |
| Asian | 2 | 7.1 |
| Native American | 0 | 0 |
| Risk factors | ||
| Smoking | 11 | 39.3 |
| Alcohol use | 10 | 35.7 |
| Family history IPMN or pancreatic cancer | 1 | 3.6 |
| Personal history any cancer | 13 | 46.4 |
| IPMN location | ||
| Head | 9 | 32.1 |
| Body/Tail | 4 | 14.3 |
| Body | 3 | 10.7 |
| Entire pancreas | 3 | 10.7 |
| Other/Unknown | 9 | 32.1 |
| IPMN Type | ||
| Main duct | 3 | 10.7 |
| Branch type | 19 | 67.9 |
| Mixed type | 3 | 10.7 |
| Unknown | 3 | 10.7 |
| Pathology* | ||
| IPMN adenoma | 5 | 50.0 |
| IPMN borderline | 2 | 20.0 |
| Intraductal papillary-mucinous carcinoma | 3 | 30.0 |
Available for 10 patients (9 surgical patients and 1 medical patient).
Patient Characteristics
The median age of patients diagnosed with IPMN in Olmsted County was 73.1 years (range, 41.0–92.4 years). Women (n = 15) and men (n = 13) were equally represented (P = 0.7), and white (n = 14, 89.3%) was the predominant racial group (Table 4). The principal reason for diagnosis was an incidental finding (n = 17, 63.0%) on an abdominal imaging study in an asymptomatic patient. Of the 11 patients who were symptomatic, 8 had abdominal pain, 1 had an increasing abdominal girth, and 2 patients presented with jaundice requiring further evaluation. A number of patients possessed known risk factors for pancreatic cancer: 11 patients (39.3%) smoked, and 10 (35.7%) had a history of alcohol abuse. One patient’s mother had a history of pancreatic cysts that were never resected. A personal history of cancer was found in 13 (46.4%) of the 28 patients, with colon cancer being the more frequent type (5/28, 17.9%).
Mean cyst size was 17.4 mm (SD, 12.6 mm). The location of tumors on preoperative evaluation varied; 9 patients (32.1%) had IPMN isolated to the head of the pancreas, whereas 4 (14.3%) had IPMN in the body and tail. The third largest areas of occurrence were in the body (n = 3, 10.7%) or in the entire pancreas (n = 3, 10.7%).
Diagnostic Studies
The most frequent radiographic evaluation tool was CT (23/28, 82.1%), followed by EUS (20/28, 71.4%). Endoscopic retrograde cholangiopancreatography was performed independently in 1 patient and was used in another 2 patients in combination with CT and EUS. Abdominal ultrasound alone was used in 1 case. Information on intracellular molecular markers was rarely reported (2/28, 7.1%) in this population. This was limited to carcinoembryonic antigen (n = 3) and Cerb-2 (n = 1). Mucin was seen extruding from the ampulla in 1 of the 3 patients that was studied with ERCP and was confirmed with a positive stain.
Treatment and Outcomes
Surgery was offered to 9 patients (32.1%), whereas 19 (67.9%) were followed up medically. In the medical group, 79% had a diagnosis of asymptomatic branch-type IPMN. Of the remaining 4 patients, 2 had main duct disease but were not operative candidates, and 2 others were diagnosed via EUS with mixed-type IPMN but one refused surgery and the other was not an operative candidate. Three of the surgical patients were initially treated medically for a mean time of 14 months. The median time to surgery was 8 days (range, 0 day to 6.5 years). The primary reason for the decision to operate was symptoms (abdominal pain) (n = 4,44.4%). Surgical management included distal pancreatectomy with splenectomy (n = 4), pylorus-preserving pancreaticoduodenectomy (n = 2), pancreaticoduodenectomy (n = 1), total pancreatectomy (n = 1), and central pancreatectomy for a lesion that primarily involved the neck of the pancreas (n = 1).
Pathological finding was available for 10 patients, including the 9 patients who proceeded to surgery. On pathological findings, 5 (50%) had a diagnosis of IPMN, 2 patients (20%) had a diagnosis of IPMN with dysplasia, and 3 patients (30%) had a diagnosis of mucinous ductal adenocarcinoma.
Twenty patients (76.9%) had an annual follow-up documented. The median time for follow-up was 1.9 years (range, 0.1–14.5 year). Nine deaths were observed by 2005, 4 from the surgical group (3 of which died of pancreatic cancer) and 5 from the medical group. The causes of death included heart disease (n = 2) and pancreatic cancer (n = 4), and 1 patient died of complications related to a stroke, whereas another died of disseminated amyloidosis. The survival plot shows that 5-year survival of patients diagnosed with IPMN in Olmsted County is 59.6% (Fig. 3).
FIGURE 3.
Survival of cohort over time.
DISCUSSION
Our review from 1976 to 2005 revealed that in 1984 the first patient was diagnosed with IPMN in Olmsted County, Minn, the setting of multifocal pancreatic cancer. The time period is consistent with other publications that report their first diagnosis of IPMN in the late 1980s to early 1990s.2,17,18 We expected to see an increase in the annual incidence over time in our population but did not, as the incidence of IPMN was stable between 1993 and 2005. This suggests that the incidence of IPMN has not dramatically increased in the past 10 years. However, when we analyzed the data in 5-year increments, there was an increasing of the incidence since 2000. This trend would, however, have to be evaluated in a larger population, as this study was underpowered to detect an increase over time.
Our findings from this population-based study contradict many observational reports from institutions that have observed an increase in IPMN diagnoses over the last 2 decades. For example, a paper from Johns Hopkins Hospital reported that its first case of IPMN was seen in 1988. They observed an increase of IPMN cases in the year 1999 with a peak in cases (n = 37) during the year 2001. If we examine all patients who visit a referral center such as Johns Hopkins, the incidence undoubtedly seems to be increasing likely because of the increased recognition and increased consensus of a common definition of this entity.19
Certain limitations do exist. First, our incident cases are based only on patients who have been clinically evaluated and rendered a diagnosis. Given that this can be a silent disease (most of our patients were diagnosed incidentally), we may be missing a number of asymptomatic patients who harbor these changes within their pancreas. Second, our search for IPMN in medical records is difficult because searches in REP are based on ICD-9 codes or H-ICDA codes, and IPMN does not have a unique ICD-9 or H-ICDA code. An autopsy study or a prospective imaging study on all county residents would be required to address these limitations but is prohibitive because of cost.
A similar clinic-based study from Mayo Clinic was also able to detect IPMN in specimens before 1980.20 Tollefson et al20 reviewed 4000 pathology reports which used the terms “mucinous,” “cystic,” and “papillary” from 1960 to 1980 to see if IPMN was detected in any of the specimens before it being a recognized entity. They found 84 cases that met criteria and after review identified 21 patients with IPMN as defined by the World Health Organization during the study period. This report, however, included all patients seen at Mayo Clinic during that time period and was not limited to Olmsted County patients.
The dramatic increase in the use of CT and other imaging modalities in the last decade as a surveillance tool might also have caused the detection of IPMN in our patient population as most were diagnosed after an incidental finding. Other population-based incidence studies are still required.
CONCLUSIONS
The incidence of IPMN in Olmsted County is low but appears to be increasing. Most patients with this disease do not die of complications related to the disease. Although our data do not depict an epidemic, more epidemiological studies from other states and countries are required.
References
- 1.Ohhasi K, Murakami Y, Yakekoshi T, et al. Four cases of mucin-producing cancer of the pancreas on specific findings of the papilla of Vater. Prog Dig Endosc. 1982;20:348–351. [Google Scholar]
- 2.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized pathologic entity. Ann Surg. 2001;234:313–322. doi: 10.1097/00000658-200109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longnecker DS. Observations on the etiology and pathogenesis of intraductal papillary-mucinous neoplasms of the pancreas. Hepatogastroenterology. 1998;45:1973–1980. [PubMed] [Google Scholar]
- 4.Furuta K, Watanabe H, Ikeda S. Differences between solid and ductectatic types of pancreatic ductal carcinomas. Cancer. 1992;69:1327–1333. doi: 10.1002/1097-0142(19920315)69:6<1327::aid-cncr2820690605>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Kobayashi K, Mizumoto K, et al. Clinical aspects of intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol. 2005;4:669–675. doi: 10.1007/s00535-005-1646-4. [DOI] [PubMed] [Google Scholar]
- 6.Eguchi H, Ishikawa O, Ohigashi H, et al. Patients with pancreatic intraductal papillary mucinous neoplasms are at high risk of colorectal cancer development. Surgery. 2006;139:749–754. doi: 10.1016/j.surg.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Choi MG, Kim SW, Han SS, et al. High incidence of extrapancreatic neoplasms in patients with intraductal papillary mucinous neoplasms. Arch Surg. 2006;141:51–69. doi: 10.1001/archsurg.141.1.51. [DOI] [PubMed] [Google Scholar]
- 8.Wada K, Kozarek RA, Traverso LW. Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am J Surg. 2005;189:632–636. doi: 10.1016/j.amjsurg.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Reddy R, Chari ST. IPMN: not a new kid on the block anymore. Gastroenterology. 2004;127:1853–1855. doi: 10.1053/j.gastro.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 10.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–799. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ., 3rd History of the Rochester epidemiology project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.H-ICDA, Hospital Adaptation of ICDA. Commission on Professional and Hospital Activities. 2. Ann Arbor: MI; 1973. [Google Scholar]
- 15.Longnecker DS, Adler G, Hruban R, et al. Intraductal papillary mucinous neoplasms of the pancreas. In: Hamilton SR, Analtonen LA, editors. World Health Organization Classification of Tumors. Tumors of the Digestive System. Oxford: IARC Press; 2000. pp. 237–240. [Google Scholar]
- 16.Bergstralh EJ, Offord KP, Chu CP, et al. Technical Report Series #49. Rochester, MN: Department of Health Science Research, Mayo Clinic; 1992. Calculating incidence, prevalence, and mortality rates for Olmsted County, Minnesota: an update. [Google Scholar]
- 17.Traverso LW, Peralta EA, Ryan JA, Jr, et al. Intraductal neoplasms of the pancreas. Am J Surg. 1998;175:426–432. doi: 10.1016/s0002-9610(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 18.Shyr YM, Su CH, Tsay SH, et al. Mucin-producing neoplasms of the pancreas. Intraductal papillary and mucinous cystic neoplasms. Ann Surg. 1996;223:141–146. doi: 10.1097/00000658-199602000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longnecker DS, Adsay NV, Fernandez-del Castillo C, et al. Histopathological diagnosis of pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms: interobserver agreement. Pancreas. 2005;31:344–349. doi: 10.1097/01.mpa.0000186245.35716.18. [DOI] [PubMed] [Google Scholar]
- 20.Tollefson MK, Libsch KD, Sarr MG, et al. Intraductal papillary mucinous neoplasm: did it exist prior to 1980? Pancreas. 2003;26:E55–E58. doi: 10.1097/00006676-200304000-00023. [DOI] [PubMed] [Google Scholar]