Abstract
Skeletal muscle atrophy/wasting is a serious complication of a wide range of diseases and conditions such as aging, disuse, AIDS, chronic obstructive pulmonary disease, space travel, muscular dystrophy, chronic heart failure, sepsis, and cancer. Emerging evidence suggests that nuclear factor-kappa B (NF-κB) is one of most important signaling pathways linked to the loss of skeletal muscle mass in various physiological and pathophysiological conditions. Activation of NF-κB in skeletal muscle leads to degradation of specific muscle proteins, induces inflammation and fibrosis, and blocks the regeneration of myofibers after injury/atrophy. Recent studies employing genetic mouse models have provided strong evidence that NF-κB can serve as an important molecular target for the prevention of skeletal muscle loss. In this article, we have outlined the current understanding regarding the role of NF-κB in skeletal muscle with particular reference to different models of muscle-wasting and the development of novel therapy.
Keywords: Skeletal muscle atrophy, NF-κB, muscular dystrophy, cancer, COPD
1. INTRODUCTION
Skeletal muscle tissue constitutes about 40% of human body mass which ensures basic functions such as locomotion, metabolism, and respiration. Skeletal muscle exhibits very high level of plasticity. Resistance exercise and nutritional uptake leads to skeletal muscle hypertrophy which is characterized by increased muscle size, protein content, and strength [1, 2]. Conversely, prolonged inactivity that occurs during unloading, immobilization, microgravity, bed rest, or nerve injury results in the loss of skeletal muscle mass commonly known as skeletal muscle atrophy or wasting [3]. Skeletal muscle atrophy is also a devastating complication of several disease states such as cancer, AIDS, inflammatory bowel disease, sepsis, diabetes, chronic obstructive pulmonary disease (COPD), chronic heart failure (CHF), high dose glucocorticoid therapy, renal failure, burn injury, and cystic fibrosis [4, 5]. Skeletal muscle atrophy is characterized by the reduction in fiber cross-sectional area, protein content, and strength leading to increased fatigability and insulin resistance [1, 2].
Skeletal muscle atrophy can result from either enhanced protein degradation or reduced synthesis or both [1, 5, 6]. Diverse physiological and pathophysiological stimuli trigger muscle atrophy through distinct upstream mechanisms. For example, unloading initiates atrophy through mechanisms related to reduced tension [7], whereas elevated levels of proinflammatory cytokines, glucocorticosteroids, tumor-derived factors, and endotoxins are responsible for the induction of muscle atrophy during chronic diseases [4, 5]. In the recent years, considerable progress has been made towards understanding the biochemical signaling mechanisms involved in the regulation of skeletal muscle mass [2, 7]. Generally, the activation of phosphatidyl inosital 3-kinase (PI3K)/Akt pathway that occurs in response loading and growth factors such insulin and insulin-like growth factor (IGF) prevents the loss of skeletal muscle mass [8]. On the other hand, activation of nuclear factor-κB (NF-κB), activator protein-1 (AP-1), p53, Foxo, and p38 mitogen-activated protein kinase (MAPK) pathways lead to skeletal muscle atrophy [9].
Although muscle atrophy might involve differential activation of multiple cell signaling pathways, recent evidences suggest that NF-κB is one of the most important signaling systems, the activation of which leads to skeletal muscle loss. Proinflammatory cytokines, tumor-derived factors, and other mediators of atrophy function through the activation of NF-κB. Indeed, recent genetic and pharmacological studies in experimental animal models have provided unequivocal evidence that specific modulation of NF-κB activity can prevent skeletal muscle loss in Duchenne muscular dystrophy (DMD), several types of cancer, nerve injury, and unloading etc. [10-13]. In this article, we have discussed the most direct emerging evidences suggesting a pivotal role for NF-κB in skeletal muscle-wasting. We have also illustrated the possible mechanisms by which activation of NF-κB leads to skeletal muscle-wasting.
2. BRIEF OVERVIEW OF NF-κB SIGNALING PATHWAY
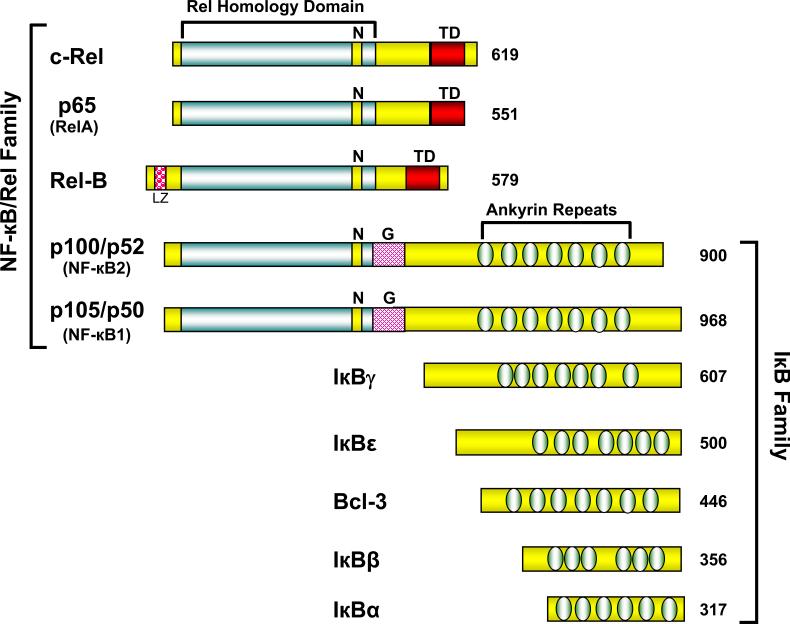
NF-κB refers to structurally related Rel family eukaryotic transcription factors which regulate a variety of cellular responses [14]. NF-κB family constitutes five members which can be further divided in two groups. One group includes RelA (p65), RelB and c-Rel, which are synthesized as mature protein, and are characterized by the presence of an N-terminal Rel homology domain (RHD) essential for dimerization and DNA binding, and a C-terminus transcriptional activation domain. The second group consists of the NF-κB1 (p50) and NF-κB2 (p52) proteins, which are synthesized as large precursor p105 and p100, respectively, containing an N-terminal RHD and a C-terminal ankyrin repeat domain. Proteolytic processing of p105 and p100 at the C-terminus gives rise to p50 and p52 proteins, respectively. Both p50 and p52 proteins contain the N-terminal RHD but lack transcriptional activation domains at the C-terminus end [14].
Different members of the NF-κB family dimerize to facilitate binding of NF-κB to DNA. Among them, p50 and p65 are the most prototypical hetrodimer present in almost all the cell types and are responsible for the increased expression of a number of proinflammatory and cell survival genes. However, homodimers or hetrodimers of p50 and p52 proteins, which lack transcriptional activation domains, can still bind to NF-κB consensus sites in the DNA, and act as transcriptional repressor by blocking the consensus sites [15]. Before activation, most of the NF-κB dimers are retained in the cytoplasm by binding to specific inhibitors-the inhibitors of NF-κB (IκBs). There are seven mammalian IκBs, including IκBα, IκBβ, IκBε and IκBγ, BCL-3 and the precursor proteins, p100 and p105, which contain five to seven C-terminal ankyrin repeats (Figure 1). The interaction with IkBs masks the nuclear localization sequence in the NF-κB complex, thus preventing nuclear translocation and maintaining NF-κB in an inactive state in the cytoplasmic compartment [15].
Figure 1. Diagrammatic representation of NF-κB/IκB family proteins.
The protein of NF-κB and IκB families along with their structural features are depicted here. The number of amino acids in each human protein is shown on right. TD, transactivation domain; N, nuclear localization sequence; LG, leucine zipper motif; and G, Glycine rich repeat.
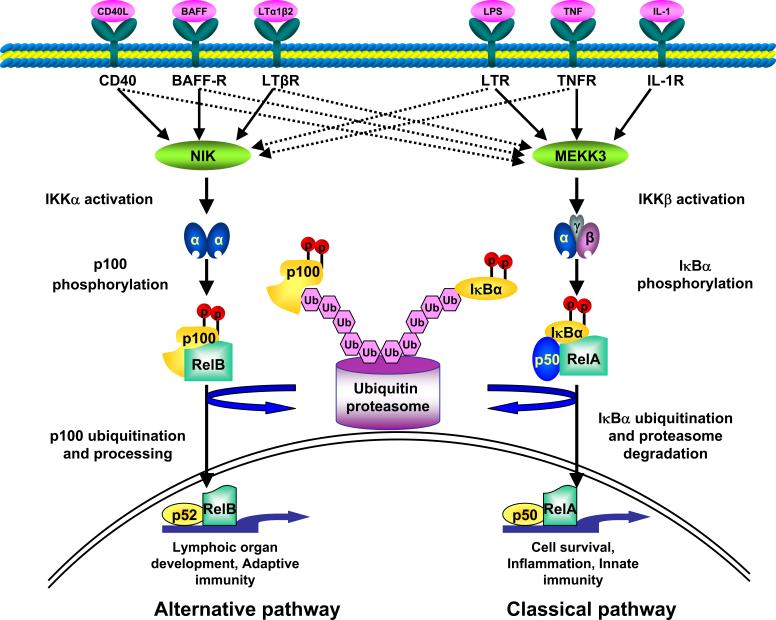
The NF-κB complex is activated in response to a variety of stimuli, including viral and bacterial infection, exposure to proinflammatory cytokines, mitogens, growth factors, and oxidative and biomechanical stresses [16]. Different stimuli initiate different downstream signal transduction pathways that involve distinct adaptor and signaling proteins such as NF-κB-inducing kinase (NIK), mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1, Akt kinase, IL-1 receptor-associated kinases, MyD88, receptor-interacting protein, TNF receptor (TNFR)-associated death domain, TNFR-associated factors, double-stranded (ds) RNA-dependent protein kinase, protein kinase C, TGF-β-activated kinase-1 (TAK1), etc. [15, 17]. Most of these signaling pathways converge on the IκB kinase (IKK) complex that is composed of two catalytic subunits (IKK-α and IKK-β) and a regulatory subunit (IKK-γ/NEMO). Depending on which subunits of IKK complex are involved, NF-κB activation can occur via either the classical or alternative pathway (Figure 2). The classical pathway is IKKβ and IKKγ-dependent and NF-κB activation occurs through the degradation of IκB proteins. The alternative pathway depends on IKK-α and NF-κB-inducing kinase (NIK) and involves regulated processing of the p100 precursor protein [15, 17, 18]. Recent findings suggest that depending on the type of stimuli, both classical and alternative pathways are activated in skeletal muscle and activation of either of these two NF-κB pathways can cause skeletal muscle atrophy [10, 11, 19].
Figure 2. Schematic representation of two NF-κB signaling pathways.
Activation of NF-κB can occur by classical or alternative pathway. Classical pathway involves the activation of IKKβ leading to phosphorylation and degradation of IκBs. The alternative pathway involves IKKα and leads to the phosphorylation of processing of p100, generating p52:relB heterodimers. NIK, NF-κB-inducing kinase; Ub, ubiquitin; MEKK3, mitogen-activated protein kinase kinase kinase 3.
Activated IKK phosphorylates NF-κB-bound IκB proteins and targets them for polyubiquitination and rapid degradation by creating a binding site for the SCFβ-TrCP ubiquitin ligase complex [15, 18]. Classical stimuli such as proinflammatory cytokines activate NF-κB through IKKβ-mediated site-specific phosphorylation and subsequent ubiquitination and degradation of inhibitory protein IκBα by the 26S proteasome pathways. NF-κB complexes liberated from IκB inhibitory proteins then translocate to the nucleus leading to transcriptional activation of several target genes, many of which also depend on the activity of other transcription factors such as activator protein-1 (AP-1), CCAAT enhancer binding protein (C/EBP), and Signal transducers and activators of transcription (STAT) family transcription factors [14]. In addition to this classical activation mechanism by IκB degradation, post-translational modifications of p65 by phosphorylation, acetylation, and ubiquitination have been shown to modulate the transactivation potential of NF-κB [15, 18]. Activated NF-κB can be down-regulated through multiple mechanisms including a well-characterized feedback loop involving newly synthesized IκBα, which binds to nuclear NF-κB and thus removes it from DNA and exports it to the cytosol [14].
NF-κB controls the expression of a large number of genes, including those encoding cytokines, chemokines, cell adhesion molecules, growth factors, immunoregulatory molecules, acute-phase and stress response proteins, cell surface receptors, transcription factors, and several enzymes including those involved in protein degradation by ubiquitin-proteasome system [16]. In addition, recent in vitro and in vivo studies using genetic knockout mice have revealed that the activation of NF-κB provides protection against apoptotic insults both by inducing the expression of anti-apoptotic molecules and by repressing the activity of proapoptotic proteins [14, 16]. Furthermore, NF-κB can also augment the transcription of its own family members RelB, c-Rel, and p105 [18].
3. MECHANISMS OF ACTION OF NF-κB IN SKELETAL MUSCLE
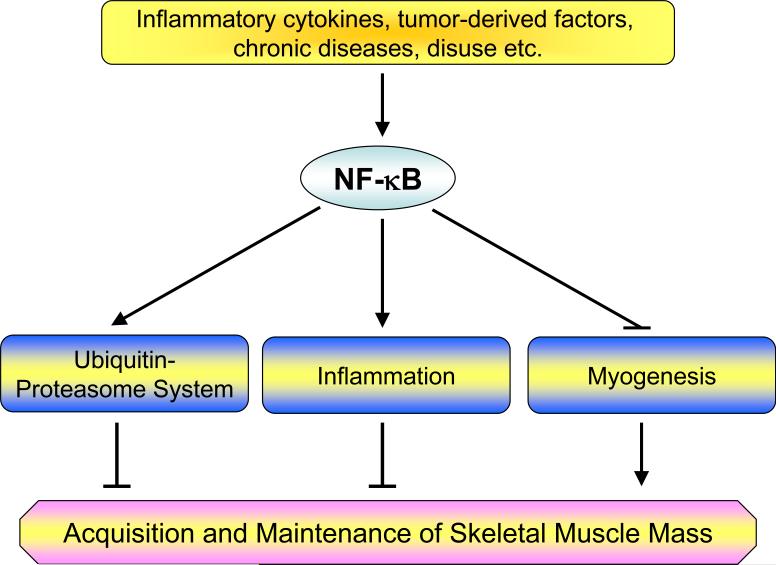
Research in the past decade has elucidated several important functions of NF-κB in the regulation of skeletal muscle mass especially in disease conditions. Evidently, there are three potential mechanisms by which increased activity of NF-κB leads to skeletal muscle atrophy: 1) NF-κB can augment the expression of several proteins of the ubiquitin-proteasome system that are involved in degradation of specific muscle proteins during atrophy; 2) NF-κB may increase the expression inflammation-related molecules which directly or indirectly promote muscle-wasting; and 3) NF-κB can also interfere with the process of myogenic differentiation that may be required for regeneration of atrophied skeletal muscles (Figure 3). In this section, we discuss these three functions of NF-κB in greater detail.
Figure 3. Putative mechanisms of action of NF-κB in skeletal muscle metabolism.
Activation of NF-κB leads to muscle-wasting by multiple mechanisms. NF-κB can augment the expression of ubiquitin-proteasome system proteins (e.g. MURF1) and/or proinflammatory cytokines, chemokines, and tissue-degrading enzymes which leads to skeletal muscle-wasting. Activation of NF-κB can also induce muscle-wasting by blocking the process of myogenic differentiation possibly by modulating the levels of MyoD.
3.1 NF-κB and Ubiquitin-Proteasome System
The ubiquitin-proteasome system is the primary regulator of protein breakdown which provides a mechanism for selective degradation of regulatory and structural proteins [20]. Although the ubiquitin-proteasome system involves the concerted action of many proteins, the key enzyme in this system is E3 (ubiquitin ligase), which couples activated ubiquitin to lysine residues on the protein substrate and confers specificity to the system. Recently, two inducible E3 ubiquitin ligases i.e. atrogin-1 (also known as MAFbx) and muscle RING finger protein 1 (MuRF1) have been identified which are responsible for the degradation of bulk of skeletal muscle protein in various atrophying conditions [21, 22]. Increased expression of atrogin-1 and/or MuRF1 has been reported in animal models of muscle atrophy induced by immobilization, denervation, hind limb suspension, starvation, tumor load, lipopolysaccharide (LPS), and glucocorticoids [20-22]. Furthermore, targeted deletion of atrogin-1 and/or MuRF1 resulted in reduced muscle atrophy in response to denervation and hind limb suspension confirming the role of these ubiquitin ligases in skeletal muscle-wasting [21, 22].
Although the activation of NF-κB itself requires proteolytic processing of NF-κB and IκB family proteins (IκBs, p105, p100) by the ubiquitin-proteasome system [15, 18], there are reports suggesting that activated NF-κB can induce expression of a few proteins of the ubiquitin-proteasome system that are involved in skeletal muscle protein degradation. Cai et al. [10] demonstrated that the transgenic expression of constitutively active IKKβ causes a drastic increase in the expression of the MuRF1 gene. The muscle-wasting in IKKβ-expressing mice was significantly reduced by crossing them with MuRF1-knockout mice [10]. Mourkioti et al. [12] used a reverse approach to study the role of NF-κB in MuRF1 expression. They found that skeletal muscle-specific deletion of IKKβ in mice prevented the expression of MuRF1 gene and atrophy in response to nerve injury. Taken together, these two reports suggest that the activation of NF-κB induces muscle-wasting, at least in part, through the increased expression of MuRF1 E3 ubiquitin ligase in skeletal muscle [10, 12].
Several tumor-derived products and catabolic cytokines have also been reported to augment the expression of various ubiquitin-proteasome proteins through the activation of NF-κB. Treatment of cultured myotubes with proteolysis-inducing factor has been found to increase the activation of NF-κB, expression of ubiquitin-conjugating enzyme E2 (14k), and the degradation of specific muscle proteins [23]. The PIF-induced expression of ubiquitin-proteasome-related proteins and degradation of muscle protein was blocked by over-expression of a degradation resistant mutant of IκBα protein [23].
3.2. Role of NF-κB in Muscle Inflammation
Systematic inflammation is the primary cause of skeletal muscle wasting and fatigue that is characteristic of normal aging, AIDS, chronic heart failure, COPD, and cancer cachexia. Proinflammatory cytokines such as TNF-α, TWEAK, IL-1β, IFN-γ, and IL-6 are some of the most important inducers of muscle-wasting in chronic disease states [24]. Elevated serum levels of several of these proinflammatory cytokines has been consistently observed in humans and animal models of chronic disorders involving muscle-wasting [24-27]. Increased levels of TNF-α or IL-1β has been shown to inhibit the expression and activity of hormones such as growth hormone and IGF-I, the two most important contributors to postnatal growth [28, 29]. In addition, expression of several chemokines, cell adhesion molecules, and extracellular proteases can augment the infiltration of macrophages, lymphocytes, and neutrophils in skeletal muscle tissues. Increased chemokine expression, leukocyte infiltration, and weakness have been observed in the diaphragm and other muscles of mice suffering from muscular dystrophy [30]. Interestingly, NF-κB regulates expression of a number of inflammatory molecules including cytokines, chemokines, cell adhesion molecules, and tissue-degrading enzymes such as matrix metalloproteinases (MMPs) [16]. In addition, several NF-κB-regulated molecules especially proinflammatory cytokines (e.g. TNF-α, IL-1β, IL-6 etc) are also the potent activators of NF-κB thus establishing a positive feed-back loop resulting in the over-stimulation of NF-κB and the development of muscular abnormalities [16]. Recently, Mourkioti et al. [12] provided genetic evidence that the inhibition of NF-κB activity can promote skeletal muscle regeneration. Their study demonstrated that specific deletion of IKKβ augments skeletal muscle regeneration by limiting the inflammatory response and fibrosis [12].
Skeletal muscle atrophy involves both intracellular and extracellular alterations. To understand the extracellular mechanisms in muscle atrophy, we recently investigated the effects of TNF-α, a well known muscle-wasting cytokine, on the production of extracellular proteases in skeletal muscle. Our study showed that the TNF-α-induced expression of metalloproteinase-9 (MMP-9) from myotubes was completely blocked by over-expression of a dominant-negative inhibitor of NF-κB (i.e., IκBαΔN) suggesting that NF-κB mediates the production of MMP-9 in skeletal muscle cells in response to TNF-α [31]. Because MMP-9 is a key protease that can degrade several components of extracellular matrix-cytoskeleton linkage in skeletal muscle, its increased production through NF-κB-dependent mechanisms might play an important role in extracellular alterations and myopathy in various pathological conditions.
3.3. Role of NF-κB in Myogenesis
Skeletal muscle formation or myogenesis is a highly regulated process that involves the determination of multipotential mesodermal cells to give rise to myoblasts, exit of these myoblasts from the cell cycle, and their differentiation into muscle fibers [32]. Myogenesis is required not only for growth but also for the maintenance and repair of myofibers. Impairment in the process of muscle regeneration has been proposed as one of the important determinants of skeletal muscle-wasting especially in chronic disease states such as cancer, heart failure, COPD etc. [8, 24, 33, 34].
Accumulating literature suggests that NF-κB is essential for myoblast proliferation and for their maintenance in undifferentiated state. NF-κB inhibits myogenic differentiation presumably by stimulating cyclin D1 accumulation and cell cycle progression [35, 36]. In an earlier report, Lehtinen et al. [37] demonstrated that the DNA-binding activity of NF-κB in cultured myoblasts was significantly decreased within 12−24h of their incubation in differentiation medium. The down-regulation of NF-κB activity preceded the activation of muscle-specific promoters and myoblasts fusion [37]. On the other hand, pharmacological inhibitors of myoblast differentiation augmented the activation of NF-κB in myoblasts incubated in differentiation medium [37]. Recently, Wang et al. [38] reported that one of the mechanisms by which NF-κB inhibits myogenesis is through the increased expression of YinYang1 (YY1) transcription factor [38]. YY1 is a negative regulator of myogenesis which acts by directly repressing the synthesis of late-stage differentiation genes such as skeletal-α actin, muscle creatine kinase, and myosin heavy chain IIb [38]. Thaloor et al [39] demonstrated that systematic administration of curcumin, an inhibitor of NF-κB, augments the regeneration of myofibers after cryoinjury. However, whether the beneficial effect of curcumin on muscle regeneration was only due to inhibition of NF-κB or it also affected the activity of other regulators remains unclear. This is because curcumin has also been reported to inhibit the activation of many other transcription factors (e.g. AP-1, Egr-1, β-catenin, and PPAR-γ) in some other cell types [40]. Nevertheless, the inhibitory role of NF-κB in skeletal muscle regeneration has been recently confirmed by Mo et al [12] demonstrating that the muscle-specific deletion of IKK2 promotes myofiber regeneration in response to cardiotoxin injury.
Proinflammatory cytokines such as TNF-α, IL-1β, IFN-γ, and TWEAK are some of the important mediators of skeletal muscle-wasting in chronic disorders [24, 41]. Langen et al [34] demonstrated that TNF-α and IL-1β blocks the differentiation of cultured myoblasts into myotubes through the activation of NF-κB transcription factor. Inhibition of NF-κB activity through overexpression of a degradation resistant mutant of NF-κB inhibitory protein, IκBα protein (i.e. IκBαSR), was sufficient to reverse the inhibitory effects of TNF-α or IL-1β on myoblast differentiation [34]. The study also showed that activation of NF-κB through overexpression of IKKβ was equally effective in blocking the differentiation of myoblasts into myotubes [34]. In a recent report from our laboratory, we have also shown that TWEAK, a structural homologue of TNF-α, can inhibit myoblast terminal differentiation through constitutive activation of the canonical NF-κB signaling pathway [42]. In terms of the mechanisms by which NF-κB affects myogenic differentiation, it has been suggested that activation of NF-κB reduces the cellular levels of MyoD protein by post-transcriptional mechanisms. MyoD is a major myogenic transcription factor which plays specific roles in the muscle progenitor cell activation as well as in the transition from proliferation to differentiation that occurs upstream of terminal differentiation [43]. Earlier reports have provided strong evidence increased activation of NF-κB in response to proinflammatory cytokines leads to the destabilization of MyoD mRNA in the skeletal muscle cells [44-46]. Recently, Marco et al. [46] reported that NF-κB causes MyoD transcript decay by regulating the binding of MyoD-stabilizing RNA binding protein HuR. Activation of NF-κB augments the expression of inducible nitric oxide synthase (iNOS) in muscle cells which sequesters HuR away from the MyoD transcript, thereby causing MyoD decay [46].
Although NF-κB has been widely believed to be an inhibitor of myogenesis, there are also reports suggesting that insulin growth factor-II (IGF-II) stimulates the activation of NF-κB [47, 48]. Treatment of L6E9 myoblasts with IGF-II causes a transient increase in NF-κB activity which is required for the terminal differentiation in the presence of IGF-II [47, 48]. It has been found that the activation of NIK and IKKα but not IKKβ is essential for IGF-II-induced differentiation of L6E9 myoblasts [48]. Why the activation of NF-κB promotes myogenic differentiation in response to IGF-II whereas its activation in response to proinflammatory cytokines inhibits myogenic differentiation remains enigmatic. It is possible that cellular context, type of stimuli, level and length of NF-κB activation, and composition of activated NF-κB complex are all critical for the inhibitory or stimulatory roles of NF-κB in myogenesis. It is important to recognize that although IGF-II activated NF-κB in L6E9 myoblasts, the level of activation of NF-κB was not very high [47, 48]. Furthermore, IGF-II predominantly activated alternative NF-κB signaling pathway that involves the activation of NIK and IKKα and proteolytic processing of p100 subunit of NF-κB [47, 48]. On the other hand, proinflammatory cytokines causes constitutive and sustained activation of classical NF-κB pathway (involving IKKβ) in C2C12 myoblasts [34, 42]. The incongruity between anti- and promyogenic functions of NF-κB was also recently addressed Bakker et al [49]. Their study showed that constitutive activation of the classical NF-κB pathway functions to inhibit myogenic differentiation. However, late during myogenic differentiation, NF-κB signaling switches to the alternative pathway to promote skeletal muscle homeostasis [49].
4. ACTIVATION OF NF-κB AND SKELETAL MUSCLE ATROPHY
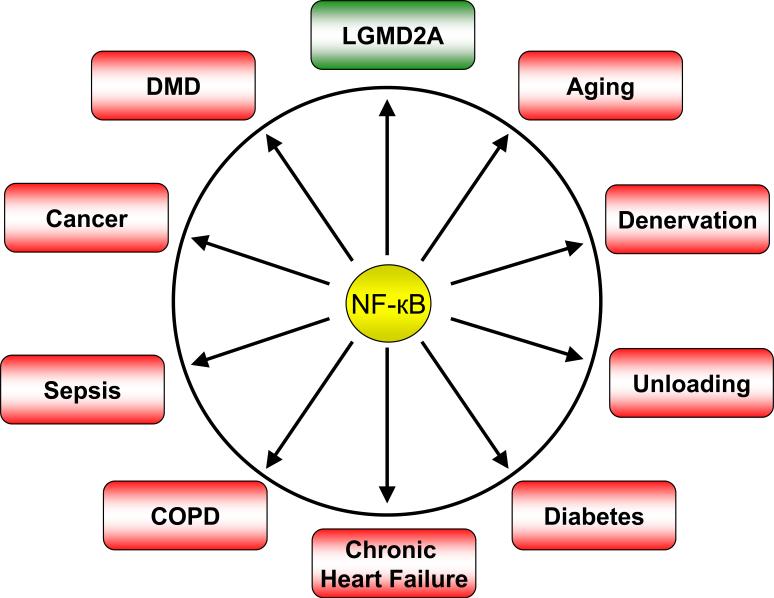
Abundant evidences from in vitro studies and experimental models of several human diseases and conditions suggest that aberrant activation of the NF-κB in skeletal muscle may lead to skeletal muscle atrophy (Figure 4). In this section, we provide a succinct review of the evidences for abnormal NF-κB regulation in some important muscle-wasting diseases.
Figure 4. Role of NF-κB in different muscle-related disorders.
Numerous studies have shown that the activation of NF-κB causes muscle-wasting in many disease states and conditions (shown in red). In LGMD2A, the NF-κB is essential for the survival of muscle fibers (shown in green). COPD, Chronic obstructive pulmonary disorder; DMD, Duchenne muscular dystrophy; LGMD2A, Limb-girdle muscular dystrophy type 2A.
4.1 Muscular Dystrophy
Muscular dystrophy is an inherited group of muscle disorders, which causes a slow but progressive degeneration of skeletal muscle fibers leading to lifelong pain, disability, and eventually death [50]. Duchenne muscular dystrophy (DMD) is the most prevalent form of muscular dystrophy that is caused due to total or partial deficiency of dystrophin [51]. Dystrophin is a component of the multiprotein complex known as dystrophin-glycoprotein complex (DGC) which links cytoskeleton of the muscle fibers to the extracellular matrix [50]. In the absence of dystrophin, the complex is functionally impaired, and the mechanical stress associated with contraction progressively leads to the degeneration of skeletal muscle fibers, muscle-wasting, progressive impairment of movements, and eventually paralysis and death [50].
Several studies have demonstrated that secondary events such as inflammation, regeneration of myofibers after necrosis, and fibrosis actively contribute to the pathogenesis of DMD [52-54]. In an earlier report, we demonstrated that NF-κB was highly activated in the diaphragm muscle of dystrophin-deficient mdx mice even before the onset of muscle necrosis [55]. We also found that the mRNA levels of NF-κB-regulated proinflammatory cytokines IL-1β and TNF-α were significantly higher in the diaphragm muscle of mdx mice compared to normal mice even at prenecrotic stages [55]. Based on our findings in mdx mice, we suggested that the activation of NF-κB may lead to the accumulation of muscle catabolic cytokines such as TNF-α and IL-1β in skeletal muscle of mdx mice. Persistent stimulation of skeletal muscle fibers by mechanical stress or positive feedback loops that could involve proinflammatory cytokines such TNF-α and IL-1β may result in the over-stimulation of NF-κB leading to skeletal muscle-wasting [55]. Similar to mdx mice, higher activation of NF-κB has also been reported in muscle biopsies of DMD patients suggesting that similar mechanisms might be involved in the activation of NF-κB in DMD patients [56].
In the recent years, several research groups have demonstrated that the inhibition of NF-κB can prevent muscle pathogenesis, at least, in the mouse models of DMD. Carlson et al. [57] reported that chronic administration of pyrrolidine dithiocarbamate (PDTC), an agent which inhibits NF-κB activation by stabilizing cytosolic IκBα protein, reduces the loss of triangularis sterni (TS) muscle and provides functional gain in TS fibers in mdx mice. Similar beneficial effects of PDTC in mdx mice were also reported by Messina et al. [58]. Their study showed that PDTC treatment reduces the activation of NF-κB, muscle necrosis, and fatigue in mdx mice [58]. In addition, in vivo administration of IRFI-042, a potent antioxidant and inhibitor of lipid peroxidation, has been found to block the NF-κB activation and muscle degeneration and provide functional gain in mdx mice [59].
The role of NF-κB in muscle pathogenesis of DMD was recently investigated using genetic mouse models. Acharyya et al. [13] demonstrated that the activation of NF-κB was related to the severity of muscle disease in the mouse models of DMD. There was a considerably increase in the DNA-binding activity of NF-κB in the myofibers of mdx/utrophin double knockout mice, which when compared to the mdx mice, more closely resemble the clinical signs and early lethality of DMD patients. NF-κB activity was increased not only in the skeletal muscle tissues but also in the macrophages of mdx mice [13]. Heterozygous deletion of p65 subunit of NF-κB in the mdx mice resulted in amelioration in muscle pathogenesis and enhanced regeneration of myofibers [13]. Similarly, genetic deletion of IKKβ in macrophages, skeletal muscle, or pharmacological inhibition of IKKβ using NEMO-binding domain (NBD) attenuated muscle pathology and augmented skeletal muscle regeneration in the mdx mice [13]. While the effectiveness of NF-κB inhibitory drugs for treatment of DMD patients will be evaluated in future clinical trails, it is important to recognize that at present glucocorticoid therapy (prednisone) is the only effective pharmacologic treatment that increases muscle strength and function, improves pulmonary function, and significantly slows the progression of weakness in DMD patients. Interestingly, glucocorticoids are also one of the most important known inhibitors of NF-κB [60].
Although the inhibition of NF-κB was found to suppress muscle pathogenesis in the mdx mice, there are reports suggesting that some level of NF-κB activity may be essential for the survival of muscle fibers, at least, in a few other types of muscular dystrophy. The prosurvival function of NF-κB in muscular dystrophy is not surprising because NF-κB is well-known inducer of several anti-apoptotic genes in several other cells and tissues [14, 16]. Limb-girdle muscular dystrophy type 2A (LGMD2A) is a recessive genetic disorder caused by mutations in the cysteine protease calpain-3 leading to selective muscle-wasting [50]. Calpain-3 seems to be required for the degradation of IκBα protein and hence the activation of NF-κB and suppression of apoptosis in skeletal muscle [61]. Loss of calpain-3 leads to increased apoptotic myonuclei in skeletal muscle of LGMD2A patients [62] and in calpain 3-knockout mice [61]. Recently, it has been shown that the expression of cellular-FLICE inhibitory protein (c-FLIP), a NF-κB regulated cell survival protein, is down-regulated in LGMD2A muscle biopsies [63]. Therefore, depending on the etiology of muscular dystrophy, activation of NF-κB may lead to skeletal muscle-wasting or promote muscle cell survival.
4.2. Cancer
Muscle-wasting is the most important phenotypic feature of cancer cachexia causing functional impairment, fatigue, and respiratory complications, attributed to the increased activation of muscle proteolytic pathways. Although the pathophysiology of cancer cachexia is not completely understood, it is believed that alteration in the muscle protein metabolism arises from the presence of tumor-derived factors such as proteolysis-inducing factor (PIF), parathyroid hormone-related peptides, or due to the increased production of humoral factors such as proinflammatory cytokines, many of which act through the activation of NF-κB and related transcription factors [64].
Functional significance of NF-κB in cancer cachexia has been highlighted by recent studies employing cell culture techniques or experimental animal models. NF-κB has been found to be involved in PIF-induced muscle protein degradation and the activation of ubiquitin-proteasome proteolytic pathway in cultured myotubes. Weight loss and skeletal muscle protein degradation in mice bearing MAC16 adenocarcinoma were significantly attenuated by in vivo administration of resveratrol, a pharmacological blocker of NF-κB [65]. Treatment of Yoshida AH-130 hepatoma bearing rats with SP100030, an inhibitor of NF-κB and activator protein-1 (AP-1), was also found to be effective in preventing skeletal muscle-wasting in this model [66]. Cancer cachexia is a prominent feature in prostate cancer patients [67]. Conditioned medium from a prostate cancer cell line PC3 was able to block the differentiation of mouse primary myoblasts into myotubes indicating that tumor-derived factors can cause muscle-wasting by blocking myogenic differentiation [68]. Stable expression of a dominant negative inhibitor of NF-κB partially restored muscle differentiation in PC3 cells conditioned media-treated primary myoblasts [68]. Kuroda et al. [69] investigated the effect of dehydroxymethylepoxyquinomicin (DHMEQ), an inhibitor of NF-κB, on IL-6 production and cachexia using a mouse model of prostate cancer. Their study showed that intraperitoneal administration of DHMEQ to JCA-1 tumor-bearing mice significant ameliorated the cachexia phenotypes including the loss of skeletal muscle mass. They also reported that DHMEQ was effective in reducing the serum level of IL-6 in JCA-1 tumor-bearing mice or in cultured cells [69].
Direct evidence regarding the role of NF-κB in tumor-induced skeletal muscle-wasting came from a recent study of Cai et al. [10] wherein they employed transgenic approach to block the NF-κB activation in skeletal muscle of mice. The study suggested that the activation of NF-κB in skeletal muscle tissues was significantly increased after implantation of Lewis Lung Carcinoma (LLC) in the wild-type mice. The increase in NF-κB activity in the skeletal muscle was attributed mainly to the tumor-derived circulating factors because there was no evidence of tumor growth in the muscle tissue of these mice [10]. The study showed that muscle-specific inhibition of NF-κB activity by transgenic overexpression of a super-repressor mutant of IκBα protein (i.e. IκBαSR) significantly attenuated the loss of skeletal muscle mass and body weight of LLC-bearing mice [10].
In the recent years, several drugs (e.g. megestrol acetate, Dronabinol, Eicosapentaenoic acid-enriched oral supplements, Ghrelin, Pentoxiphylline, thalidomide, melatonin, nandrolone decanoate) have been identified which were effective in treatment of cachexia/anorexia to some extent in randomized clinical trails in cancer patients. Although the mechanisms of action of these drugs in prevention of cachexia in cancer patients remain to be determined, many of these drugs such as Dexanabinol [70], Eicosapentaenoic acid [71], Ghrelin [72], Pentoxiphylline [73], thalidomide [74], and melatonin [75] have been shown to inhibit NF-κB activity in different cell types.
4.3 Sepsis
Skeletal muscle-wasting is a significant clinical problem in sepsis resulting in severe muscle-weakness and fatigue which may delay ambulation and increase the risk for thromboembolic and pulmonary complications. Sepsis-induced muscle-wasting is associated with the activation of a number of nuclear transcription factors including NF-κB. In an experimental rat model of sepsis induced by cecal ligation and puncture (CLP), Penner et al. [76] demonstrated that the activity of NF-κB is significantly increased within 4h of induction of sepsis compared to the sham-operated controls. Because sepsis is an inflammatory disorder, increased levels of cytokines and chemokines might play a prominent role in skeletal muscle-wasting during sepsis [77]. Infiltration of macrophages and neutrophils has been strongly implicated in the pathogenesis of sepsis-induced diaphragmatic weakness [78]. The chemokines of the CC and CXC family, which are involved in the recruitment of macrophages and neutrophils, are highly expressed in the septic mouse diaphragm in vivo. Boyd et al. [79] have reported that lipopolysaccharide (LPS) causes a significant increase in the expression of chemokines such as monocyte chemoattractant protein-1 (MCP-1) and KC in skeletal muscle via the recruitment of toll like receptor 4 (TLR4). Stimulation of TLR4 with LPS augmented the activation of NF-κB in cultured myotubes [79]. Furthermore, the activation of NF-κB and the expression of MCP-1 and KC were significantly blocked by over-expression of a dominant negative mutant of IKKβ [79]. A similar study also demonstrated that NF-κB regulates the endotoxin-induced expression of several other proinflammatory molecules such as TNF-α, IL-1α, IL-1β, IL-6, macrophage inflammatory peptide-2, intercellular adhesion molecule-1, and iNOS in the diaphragm muscle [80]. Collectively, these evidences suggest that NF-κB might play a pivotal role in the expression of proinflammatory molecules and muscle loss during sepsis.
4.4 Chronic obstructive pulmonary disease (COPD)
Skeletal muscle-wasting is an important feature of chronic obstructive pulmonary disease (COPD) which contributes to symptoms and influences prognosis. Although the exact mechanisms leading to the loss of peripheral muscle in COPD remain poorly understood, elevated levels of proinflammatory cytokines have been consistently observed in circulation and muscle tissues of COPD patients [33, 81, 82]. Proinflammatory cytokines can inhibit myogenic differentiation and induce degradation of specific muscle proteins such as MyoD and myosin heavy chain via NF-κB-dependent mechanisms [9, 34, 42, 44]. Indeed, reduced tension and power output observed in the diaphragm muscle of MyoD−/− mice resemble the changes that occur during COPD further indicating that alterations in the levels of MyoD might contribute to skeletal muscle-wasting during COPD [83]. Langen et al. [33] demonstrated that the body and muscle weights of transgenic mice expressing TNF-α under the control of surfactant protein C (SP-C) promoter (a mouse model for COPD) was significantly lower compared to that of the normal mice. In addition, there was significant deficit in the muscle regeneration in SP-C/TNF-α transgenic mice in response to reloading of disuse-atrophied muscle suggesting that attenuated muscle regeneration might be responsible for muscle-wasting in COPD [33]. Agusti et al [84] compared the activation of NF-κB between COPD patients with normal body mass index (BMI) and patients with low BMI [84]. They found that the DNA-binding activity of NF-κB in quadriceps femoris muscle was significantly higher in COPD patients with low BMI compared to normal weight patients [84]. Furthermore, the expression level of inducible nitric oxide synthase (iNOS), a NF-κB-regulated gene, was found to be increased in skeletal muscle of low BMI COPD patients compared to those with normal BMI. In a recent study, Broekhuizen et al [85] have demonstrated that polyunsaturated fatty acids which also block NF-κB activity provide beneficial effects on exercise capacity in patients with COPD.
4.5. Chronic heart failure (CHF)
Heart failure is a syndrome characterized by decreased exercise capacity and is accompanied by severe muscle-wasting also known as cardiac cachexia. Many different mechanisms have been proposed to explain muscle-wasting in patients with heart failure; however, the pathogenesis remains largely obscure. Higher levels of TNF-α and IL-1β have been observed in the circulation and in the skeletal muscle of patients with CHF which might be responsible, at least in part, for the observed muscle wasting in CHF patients [86]. Whilst direct TNFD and IL-1β antagonism has therapeutic appeal, manipulation of the downstream pathways such as NF-κB might be useful in the treatment of cachexia during CHF. Adams et al. [87] reported significantly increased levels of IL-1β and inducible nitric oxide (iNOS) in the muscle biopsies of CHF patients as compared to healthy controls. The IL-1β-mediated iNOS induction was completely suppressed on inhibition of NF-κB in cultured muscle cells [87]. A linear correlation between NF-κB activation and iNOS expression has also been reported in muscle biopsies of CHF patients suggesting that NF-κB-mediated increase in iNOS expression in skeletal muscle may contribute to exercise intolerance and early fatigue in CHF patients [88]. Furthermore, increased activation of NF-κB has also been reported in peripheral blood leukocytes, activation of which generally promotes muscle-wasting in chronic diseases including CHF [89, 90]. Although clinical trails for treatment for CHF cachexia are still lacking, there is a possibility that polyunsaturated fatty acids and other drugs which ameliorate muscle-wasting in cancer and COPD, will also provide beneficial effects in treatment of cardiac cachexia.
4.6 Diabetes
Skeletal muscular atrophy is a well-documented complication of diabetes and has been attributed to the direct effect of low serum insulin on the motor end plates and on the synthesis of contractile proteins. Deficiency of insulin in animals leads to accelerated muscle atrophy and protein degradation [91]. Recent studies have suggested that insulin resistance and type II diabetes are associated with muscle-wasting in adults [92, 93]. NF-κB and oxidative stress are well known for their contribution to the pathogenesis of type I and type II diabetes [16]. Interestingly, both NF-κB and oxidative stress are also the potent stimuli for the protein degradation in skeletal muscle [94]. Although the direct evidence regarding the role of NF-κB in muscle-wasting in diabetes is still lacking, NF-κB seems to have no role in the development of insulin resistance in muscle. Using transgenic mice expressing either constitutive active IKKβ mutant (MIKK) or a super-repressor mutant of IκBα (MISR) in skeletal muscle, Cai et al. [10] investigated whether the activation of IKKβ/NF-κB causes skeletal muscle-wasting by modulating insulin sensitivity. They found that insulin sensitivity was not altered in either MIKK or MISR mice suggesting that IKKβ and NF-κB mediate muscle-wasting without being directly involved in the development of insulin resistance in this tissue [10].
Mastrocola et al. [95] investigated the contribution of oxidative stress in skeletal muscle-wasting in diabetic rats. They showed that the level of activation of IKKβ and the amount of NF-κB p65 protein in the nuclear extract from the gastrocnemius of STZ-treated rats were significantly higher than in the control rats. Administration of dehydroepiandrosterone (DHEA), a multifunctional steroid with multi-targeted antioxidant properties, prevented the loss of gastrocnemius muscle mass and partially restored the normal levels of IKKβ and NF-κB activity in diabetic rats [95].
4.7. Unloading
The majority of muscle atrophy in the general population results from disuse. Reduction in skeletal muscle activity as a result of hindlimb unloading, immobilization, microgravity, or denervation leads to significant loss of skeletal muscle mass and strength. Hunter et al. [19] demonstrated that hindlimb unloading leads to a drastic increase in the NF-κB DNA-binding and reporter gene activities in rat soleus muscle. Compared to weight-bearing rats, there was significant increase in the nuclear level of NF-κB/IκB family proteins p50, c-Rel and Bcl3 in unloaded soleus muscle [19]. Analysis of the NF-κB/DNA complex showed that it mainly contained p50, c-Rel, and Bcl-3 but not other members of the NF-κB/IκB family [19]. The interesting observation of this study was that there was no evidence of the activation of classical p50-p65 NF-κB dimers, which are generally activated in response to proinflammatory cytokines and implicated in regulation of skeletal muscle wasting in chronic diseases.
The role of p50, Bcl-3, and c-Rel proteins in unloading-induced muscle atrophy was later evaluated using knockout mice [11, 96]. Genetic ablation of either Nfkb1 or Bcl-3 gene in mice prevented the unloading-induced NF-κB reporter gene activity in the soleus muscle [11]. Furthermore, unloading-induced soleus muscle atrophy, as evaluated by measuring the fiber cross-sectional area and transition from slow to fast fiber type were also significantly inhibited in Nfkb1- or Bcl3-gene knockout mice. Further analysis revealed that the inhibition of atrophy in Nfkb1-knockout mice was due to the lack of atrophy in the fast fibers, while in Bcl3-knockout mice, the atrophy was inhibited in both the fast and the slow fiber type [11]. The same group of investigators also reported that c-Rel subunit was not involved in unloading-induced muscle atrophy because there was no difference in the NF-κB reporter gene activity and the fiber cross-section area between c-Rel-knockout mice where compared to the corresponding wild-type mice [96]. Collectively, these reports suggest that p50 and Bcl3 are the major components of the NF-κB signaling system which contribute to the unloading-induced skeletal muscle atrophy and other associated muscle phenotypic transitions.
4.8 Denervation
Muscle and motor neurons have great influences on each other; hence, denervation of skeletal muscle causes a wide range of molecular and cellular alterations: the most important one is atrophy. Denervation-induced atrophy generally results in rounded atrophied fibers with reduced cross-sectional areas. The role of NF-κB in denervation induced skeletal muscle was recently elucidated by two independent studies using transgenic approaches [10, 12]. Mourkioti et al [12] demonstrated that skeletal muscle-restricted NF-κB inhibition in mice through targeted deletion of IKK2 subunit of the IKK complex causes an increase in soleus muscle intermediate type fibers, total cross-sectional area, and specific twitch and titanic forces [12]. Moreover, compared to controls, activation of NF-κB and the loss of skeletal muscle mass and function in response to sciatic nerve injury were significantly blocked in muscle-restricted IKK2-deficient mice. Muscle-specific deletion of IKK2 also enhanced the skeletal muscle regeneration after cardiotoxin injury and the increase in muscle regeneration was attributed to the reduced inflammatory response in IKK2-depleted skeletal muscle [12].
Cai et al [10] studied the role of NF-κB in denervation-induced skeletal muscle atrophy through transgenic overexpression of a super-repressor mutant of NF-κB inhibitory protein IκBα (IκBαSR) in the skeletal muscle of mice. NF-κB activity increased 9-fold in the hindlimb muscles of the wild-type mice 14 days after sciatic nerve injury. On the other hand, there was only a 1.5-fold increase in the NF-κB activity in the denervated muscle of IκBαSR-expressing transgenic mice. Furthermore, transgenic expression of IκBαSR protein in the skeletal muscle significantly blunted the denervation-induced loss of skeletal muscle mass and fiber size suggesting that NF-κB is involved in the denervation-induced skeletal muscle atrophy [10].
4.9 Aging
Loss of skeletal muscle mass, strength, and quality observed in elderly humans is often referred to as sarcopenia. Several biochemical mechanisms have been suggested for age-related muscle loss. These include reduced proliferation/activation of satellite cells, decreased muscle protein synthesis, increased oxidative stress, and impaired glucose metabolism [97]. In addition, catabolic cytokines (e.g. IL-6, IL1β, and TNF-α) may also play a role in age-related muscle loss. Indeed, the levels of NF-κB activating inflammatory cytokines have been found to be elevated in elderly people with significant sarcopenia [98].
Studies performed in animal models of sarcopenia have suggested that increased activity of NF-κB may contribute to age-related muscle loss. Bar-Shai et al. [99] studied the kinetics of activation of NF-κB in 24-months old rats in response to four weeks of immobilization by external fixation. Compared to controls, the activity of classical p65/p50 dimer of NF-κB in quadriceps muscles was down-regulated during the first two weeks of limb immobilization [99]. However, in the third week the activity of p65/p50 returned to the basal levels of the control muscles, while after the fourth week, there was a significant increase in p65/p50 activity [99]. In another study, Cuthbertson et al. [100] reported decreased anabolic response in skeletal muscle of healthy older men compared to young after ingesting different amounts of essential amino acids. They also observed that the level of NF-κB was increased by almost four-folds in myofibers of older men compared to young men [100]. Although the physiological significance of activation of NF-κB in skeletal muscle of older men remains unclear, increased activation of NF-κB coupled with reduced anabolism may lead to skeletal muscle-wasting.
5. CONCLUDING REMARKS
From the above description, it is clear that NF-κB can induce muscle loss by multiple mechanisms and inhibition of NF-κB activity has enormous potential for the prevention and treatment of muscle-wasting not only in chronic diseases but also in many conditions such as unloading, denervation, space flight, aging etc. Like in many other NF-κB-related disorders, both molecular and pharmacological approaches can be used to inhibit the activation of NF-κB in response to various muscle-wasting stimuli/conditions. Because NF-κB appears to be an important molecular target in muscle-wasting, more studies need to be performed in experimental animals to test the efficacy of specific molecular and pharmacological inhibitors of NF-κB in muscle-wasting. Such studies will provide strong basis for future clinical trials in patients with skeletal muscle atrophy. However, it is also important to recognize that while offering great clinical potential for muscle-wasting and several other inflammatory diseases, inhibition of NF-κB in vivo can also be detrimental because NF-κB controls multiple functions in homeostasis including development and function of immune system, cell proliferation and survival. The balance between therapeutic benefit and potential changes in normal cellular function and response during drug induced NF-κB inhibition will be one of the most important challenges in future clinical studies. Furthermore, it is also important to identify the genes NF-κB regulates in atrophying skeletal muscle. Modulating the expression and/or activity of specific NF-κB target genes can also be used as a potential alternative therapeutic approach for the management of NF-κB-related muscle-wasting. For example, the expression of MuRF-1 ubiquitin ligase is directly regulated by NF-κB in atrophying conditions. Therefore, instead of inhibiting the activity of NF-κB, modulation of the activity of MuRF1 could be a better approach because it is specifically expressed in skeletal muscle and primarily involved in muscle protein breakdown. In addition, it is important to take into consideration that many other cell types especially the cells of immune system (a major source for inflammatory/catabolic cytokines) contribute significantly to skeletal muscle atrophy. Specific modulation of NF-κB activity in these cells along with skeletal muscle might produce better results for the treatment of skeletal muscle atrophy. The availability of conditional knockout mice of various NF-κB family proteins will be helpful in identifying the NF-κB target genes expressed during different muscle-wasting conditions and to understanding the effects of cell/tissue-specific modulation of NF-κB activity on skeletal muscle atrophy.
Acknowledgements
We would like to apologize to the many researchers whose contributions were not cited due to our oversight or space limitation. This work was supported by a RO1 grant AG029623 from National Institute of Health (to AK).
Abbreviations
- AP-1
activator protein-1
- CHF
chronic heart failure
- COPD
chronic obstructive pulmonary disorder
- DMD
Duchenne muscular dystrophy
- MAPK
mitogen-activated protein kinase
- IFN
interferon
- IGF
insulin growth factor
- IkB
I kappa B
- IKK
IkB kinase
- LGMD2A
Limb-girdle muscular dystrophy type 2A
- LLC
Lewis lung carcinoma
- MMP
matrix metalloproteinase
- MuRF1
muscle RING finger protein 1
- NBD
NEMO-bidning domain
- NSAID
Nonsteroidal anti-inflammatory drug
- NIK
NF-κB-inducing kinase
- iNOS
inducible nitric oxide synthase
- PDTC
pyrrolidine dithiocarbamate
- PIF
proteolysis inducing factor
- PI3K
phosphatidyl inosital 3-kinase
- TWEAK
TNF-related weak inducer of apoptosis
REFERENCES
- 1.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287:C834–843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 2.Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve. 2006;33:155–165. doi: 10.1002/mus.20442. [DOI] [PubMed] [Google Scholar]
- 3.Boonyarom O, Inui K. Atrophy and hypertrophy of skeletal muscles: structural and functional aspects. Acta Physiol (Oxf) 2006;188:77–89. doi: 10.1111/j.1748-1716.2006.01613.x. [DOI] [PubMed] [Google Scholar]
- 4.Tisdale MJ. Cancer cachexia. Langenbecks Arch Surg. 2004;389:299–305. doi: 10.1007/s00423-004-0486-7. [DOI] [PubMed] [Google Scholar]
- 5.Ventadour S, Attaix D. Mechanisms of skeletal muscle atrophy. Curr Opin Rheumatol. 2006;18:631–635. doi: 10.1097/01.bor.0000245731.25383.de. [DOI] [PubMed] [Google Scholar]
- 6.Eley HL, Tisdale MJ. Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J Biol Chem. 2007;282:7087–7097. doi: 10.1074/jbc.M610378200. [DOI] [PubMed] [Google Scholar]
- 7.Kandarian SC, Stevenson EJ. Molecular events in skeletal muscle during disuse atrophy. Exerc Sport Sci Rev. 2002;30:111–116. doi: 10.1097/00003677-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 8.McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell. 2004;119:907–910. doi: 10.1016/j.cell.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Guttridge DC. Signaling pathways weigh in on decisions to make or break skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:443–450. doi: 10.1097/01.mco.0000134364.61406.26. [DOI] [PubMed] [Google Scholar]
- 10.Cai D, Frantz JD, Tawa NE, Jr., Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest. 2004;114:1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mourkioti F, Kratsios P, Luedde T, Song YH, Delafontaine P, Adami R, Parente V, Bottinelli R, Pasparakis M, Rosenthal N. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J Clin Invest. 2006;116:2945–2954. doi: 10.1172/JCI28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sahenk Z, Weinstein M, Gardner KL, Rafael-Fortney JA, Karin M, Tidball JG, Baldwin AS, Guttridge DC. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 15.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 19.Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. FASEB J. 2002;16:529–538. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- 20.Cao PR, Kim HJ, Lecker SH. Ubiquitin-protein ligases in muscle wasting. Int J Biochem Cell Biol. 2005;37:2088–2097. doi: 10.1016/j.biocel.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 23.Whitehouse AS, Tisdale MJ. Increased expression of the ubiquitin-proteasome pathway in murine myotubes by proteolysis-inducing factor (PIF) is associated with activation of the transcription factor NF-kappaB. Br J Cancer. 2003;89:1116–1122. doi: 10.1038/sj.bjc.6601132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spate U, Schulze PC. Proinflammatory cytokines and skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:265–269. doi: 10.1097/00075197-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Debigare R, Cote CH, Maltais F. Peripheral muscle wasting in chronic obstructive pulmonary disease. Clinical relevance and mechanisms. Am J Respir Crit Care Med. 2001;164:1712–1717. doi: 10.1164/ajrccm.164.9.2104035. [DOI] [PubMed] [Google Scholar]
- 26.Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003;6:295–299. doi: 10.1097/01.mco.0000068965.34812.62. [DOI] [PubMed] [Google Scholar]
- 27.Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Celemin L, Pasko N, Blomart V, Thissen JP. Inhibition of muscle insulin-like growth factor I expression by tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab. 2002;283:E1279–1290. doi: 10.1152/ajpendo.00054.2002. [DOI] [PubMed] [Google Scholar]
- 29.Foulstone EJ, Meadows KA, Holly JM, Stewart CE. Insulin-like growth factors (IGF-I and IGF-II) inhibit C2 skeletal myoblast differentiation and enhance TNF alpha-induced apoptosis. J Cell Physiol. 2001;189:207–215. doi: 10.1002/jcp.10017. [DOI] [PubMed] [Google Scholar]
- 30.Demoule A, Divangahi M, Danialou G, Gvozdic D, Larkin G, Bao W, Petrof BJ. Expression and regulation of CC class chemokines in the dystrophic (mdx) diaphragm. Am J Respir Cell Mol Biol. 2005;33:178–185. doi: 10.1165/rcmb.2004-0347OC. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava AK, Qin X, Wedhas N, Arnush M, Linkhart TA, Chadwick RB, Kumar A. Tumor necrosis factor-alpha augments matrix metalloproteinase-9 production in skeletal muscle cells through the activation of transforming growth factor-beta-activated kinase 1 (TAK1)-dependent signaling pathway. J Biol Chem. 2007;282:35113–35124. doi: 10.1074/jbc.M705329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 33.Langen RC, Schols AM, Kelders MC, van der Velden JL, Wouters EF, Janssen-Heininger YM. Muscle wasting and impaired muscle regeneration in a murine model of chronic pulmonary inflammation. Am J Respir Cell Mol Biol. 2006;35:689–696. doi: 10.1165/rcmb.2006-0103OC. [DOI] [PubMed] [Google Scholar]
- 34.Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J. 2001;15:1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- 35.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitin N, Kudla AJ, Konieczny SF, Taparowsky EJ. Differential effects of Ras signaling through NFkappaB on skeletal myogenesis. Oncogene. 2001;20:1276–1286. doi: 10.1038/sj.onc.1204223. [DOI] [PubMed] [Google Scholar]
- 37.Lehtinen SK, Rahkila P, Helenius M, Korhonen P, Salminen A. Down-regulation of transcription factors AP-1, Sp-1, and NF-kappa B precedes myocyte differentiation. Biochem Biophys Res Commun. 1996;229:36–43. doi: 10.1006/bbrc.1996.1754. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Hertlein E, Bakkar N, Sun H, Acharyya S, Wang J, Carathers M, Davuluri R, Guttridge DC. NF-kappaB regulation of YY1 inhibits skeletal myogenesis through transcriptional silencing of myofibrillar genes. Mol Cell Biol. 2007;27:4374–4387. doi: 10.1128/MCB.02020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaloor D, Miller KJ, Gephart J, Mitchell PO, Pavlath GK. Systemic administration of the NF-kappaB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am J Physiol. 1999;277:C320–329. doi: 10.1152/ajpcell.1999.277.2.C320. [DOI] [PubMed] [Google Scholar]
- 40.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci. 2005;1056:206–217. doi: 10.1196/annals.1352.010. DOI 1056/1/206 [pii] 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 41.Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J. 2007;21:1857–1869. doi: 10.1096/fj.06-7537com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dogra C, Changotra H, Mohan S, Kumar A. Tumor necrosis factor-like weak inducer of apoptosis inhibits skeletal myogenesis through sustained activation of nuclear factor-kappaB and degradation of MyoD protein. J Biol Chem. 2006;281:10327–10336. doi: 10.1074/jbc.M511131200. [DOI] [PubMed] [Google Scholar]
- 43.Berkes CA, Tapscott SJ. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol. 2005;16:585–595. doi: 10.1016/j.semcdb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 45.Sitcheran R, Cogswell PC, Baldwin AS., Jr. NF-kappaB mediates inhibition of mesenchymal cell differentiation through a posttranscriptional gene silencing mechanism. Genes Dev. 2003;17:2368–2373. doi: 10.1101/gad.1114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Marco S, Mazroui R, Dallaire P, Chittur S, Tenenbaum SA, Radzioch D, Marette A, Gallouzi IE. NF-kappa B-mediated MyoD decay during muscle wasting requires nitric oxide synthase mRNA stabilization, HuR protein, and nitric oxide release. Mol Cell Biol. 2005;25:6533–6545. doi: 10.1128/MCB.25.15.6533-6545.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaliman P, Canicio J, Testar X, Palacin M, Zorzano A. Insulin-like growth factor-II, phosphatidylinositol 3-kinase, nuclear factor-kappaB and inducible nitric-oxide synthase define a common myogenic signaling pathway. J Biol Chem. 1999;274:17437–17444. doi: 10.1074/jbc.274.25.17437. [DOI] [PubMed] [Google Scholar]
- 48.Canicio J, Ruiz-Lozano P, Carrasco M, Palacin M, Chien K, Zorzano A, Kaliman P. Nuclear factor kappa B-inducing kinase and Ikappa B kinase-alpha signal skeletal muscle cell differentiation. J Biol Chem. 2001;276:20228–20233. doi: 10.1074/jbc.M100718200. [DOI] [PubMed] [Google Scholar]
- 49.Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M, Acharyya S, Rudnicki MA, Hollenbach AD, Guttridge DC. IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol. 2008;180:787–802. doi: 10.1083/jcb.200707179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalkilic I, Kunkel LM. Muscular dystrophies: genes to pathogenesis. Curr Opin Genet Dev. 2003;13:231–238. doi: 10.1016/s0959-437x(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 51.Hoffman EP, Brown RH, L.M. M. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 52.Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol. 2001;98:235–243. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- 53.Chen YW, Zhao P, Borup R, Hoffman EP. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J Cell Biol. 2000;151:1321–1336. doi: 10.1083/jcb.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tidball JG, Wehling-Henricks M. Evolving therapeutic strategies for Duchenne muscular dystrophy: targeting downstream events. Pediatr Res. 2004;56:831–841. doi: 10.1203/01.PDR.0000145578.01985.D0. [DOI] [PubMed] [Google Scholar]
- 55.Kumar A, Boriek AM. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J. 2003;17:386–396. doi: 10.1096/fj.02-0542com. [DOI] [PubMed] [Google Scholar]
- 56.Monici MC, Aguennouz M, Mazzeo A, Messina C, Vita G. Activation of nuclear factor-kappaB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology. 2003;60:993–997. doi: 10.1212/01.wnl.0000049913.27181.51. [DOI] [PubMed] [Google Scholar]
- 57.Carlson CG, Samadi A, Siegel A. Chronic treatment with agents that stabilize cytosolic IkappaB-alpha enhances survival and improves resting membrane potential in MDX muscle fibers subjected to chronic passive stretch. Neurobiol Dis. 2005;20:719–730. doi: 10.1016/j.nbd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Messina S, Bitto A, Aguennouz M, Minutoli L, Monici MC, Altavilla D, Squadrito F, Vita G. Nuclear factor kappa-B blockade reduces skeletal muscle degeneration and enhances muscle function in Mdx mice. Exp Neurol. 2006;198:234–241. doi: 10.1016/j.expneurol.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 59.Messina S, Altavilla D, Aguennouz M, Seminara P, Minutoli L, Monici MC, Bitto A, Mazzeo A, Marini H, Squadrito F, Vita G. Lipid peroxidation inhibition blunts nuclear factor-kappaB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am J Pathol. 2006;168:918–926. doi: 10.2353/ajpath.2006.050673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 61.Richard I, Roudaut C, Marchand S, Baghdiguian S, Herasse M, Stockholm D, Ono Y, Suel L, Bourg N, Sorimachi H, Lefranc G, Fardeau M, Sebille A, Beckmann JS. Loss of calpain 3 proteolytic activity leads to muscular dystrophy and to apoptosis-associated IkappaBalpha/nuclear factor kappaB pathway perturbation in mice. J Cell Biol. 2000;151:1583–1590. doi: 10.1083/jcb.151.7.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baghdiguian S, Martin M, Richard I, Pons F, Astier C, Bourg N, Hay RT, Chemaly R, Halaby G, Loiselet J, Anderson LV, Lopez de Munain A, Fardeau M, Mangeat P, Beckmann JS, Lefranc G. Calpain 3 deficiency is associated with myonuclear apoptosis and profound perturbation of the IkappaB alpha/NF-kappaB pathway in limb-girdle muscular dystrophy type 2A. Nat Med. 1999;5:503–511. doi: 10.1038/8385. [DOI] [PubMed] [Google Scholar]
- 63.Benayoun B, Baghdiguian S, Lajmanovich A, Bartoli M, Daniele N, Gicquel E, Bourg N, Raynaud F, Pasquier MA, Suel L, Lochmuller H, Lefranc G, Richard I. NF-{kappa}B-dependent expression of the antiapoptotic factor c-FLIP is regulated by calpain 3, the protein involved in limb-girdle muscular dystrophy type 2A. FASEB J. 2007;22:1521–1529. doi: 10.1096/fj.07-8701com. [DOI] [PubMed] [Google Scholar]
- 64.Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care. 2005;8:265–269. doi: 10.1097/01.mco.0000165004.93707.88. [DOI] [PubMed] [Google Scholar]
- 65.Wyke SM, Russell ST, Tisdale MJ. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-kappaB activation. Br J Cancer. 2004;91:1742–1750. doi: 10.1038/sj.bjc.6602165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore-Carrasco R, Busquets S, Almendro V, Palanki M, Lopez-Soriano FJ, Argiles JM. The AP-1/NF-kappaB double inhibitor SP100030 can revert muscle wasting during experimental cancer cachexia. Int J Oncol. 2007;30:1239–1245. [PubMed] [Google Scholar]
- 67.Wang Z, Corey E, Hass GM, Higano CS, True LD, Wallace D, Jr., Tisdale MJ, Vessella RL. Expression of the human cachexia-associated protein (HCAP) in prostate cancer and in a prostate cancer animal model of cachexia. Int J Cancer. 2003;105:123–129. doi: 10.1002/ijc.11035. [DOI] [PubMed] [Google Scholar]
- 68.Jiang Z, Clemens PR. Cellular caspase-8-like inhibitory protein (cFLIP) prevents inhibition of muscle cell differentiation induced by cancer cells. FASEB J. 2006;20:2570–2572. doi: 10.1096/fj.06-6347fje. [DOI] [PubMed] [Google Scholar]
- 69.Kuroda K, Horiguchi Y, Nakashima J, Kikuchi E, Kanao K, Miyajima A, Ohigashi T, Umezawa K, Murai M. Prevention of cancer cachexia by a novel nuclear factor {kappa}B inhibitor in prostate cancer. Clin Cancer Res. 2005;11:5590–5594. doi: 10.1158/1078-0432.CCR-04-2561. [DOI] [PubMed] [Google Scholar]
- 70.Juttler E, Potrovita I, Tarabin V, Prinz S, Dong-Si T, Fink G, Schwaninger M. The cannabinoid dexanabinol is an inhibitor of the nuclear factor-kappa B (NF-kappa B). Neuropharmacology. 2004;47:580–592. doi: 10.1016/j.neuropharm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 71.Mishra A, Chaudhary A, Sethi S. Oxidized omega-3 fatty acids inhibit NF-kappaB activation via a PPARalpha-dependent pathway. Arterioscler Thromb Vasc Biol. 2004;24:1621–1627. doi: 10.1161/01.ATV.0000137191.02577.86. [DOI] [PubMed] [Google Scholar]
- 72.Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C, Weintraub NL. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–2226. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 73.Futakuchi M, Ogawa K, Tamano S, Takahashi S, Shirai T. Suppression of metastasis by nuclear factor kappaB inhibitors in an in vivo lung metastasis model of chemically induced hepatocellular carcinoma. Cancer Sci. 2004;95:18–24. doi: 10.1111/j.1349-7006.2004.tb03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Majumdar S, Lamothe B, Aggarwal BB. Thalidomide suppresses NF-kappa B activation induced by TNF and H2O2, but not that activated by ceramide, lipopolysaccharides, or phorbol ester. J Immunol. 2002;168:2644–2651. doi: 10.4049/jimmunol.168.6.2644. [DOI] [PubMed] [Google Scholar]
- 75.Gilad E, Wong HR, Zingarelli B, Virag L, O'Connor M, Salzman AL, Szabo C. Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: role of inhibition of NFkappaB activation. FASEB J. 1998;12:685–693. doi: 10.1096/fasebj.12.9.685. [DOI] [PubMed] [Google Scholar]
- 76.Penner CG, Gang G, Wray C, Fischer JE, Hasselgren PO. The transcription factors NF-kappab and AP-1 are differentially regulated in skeletal muscle during sepsis. Biochem Biophys Res Commun. 2001;281:1331–1336. doi: 10.1006/bbrc.2001.4497. [DOI] [PubMed] [Google Scholar]
- 77.Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr Opin Clin Nutr Metab Care. 2005;8:255–263. doi: 10.1097/01.mco.0000165003.16578.2d. [DOI] [PubMed] [Google Scholar]
- 78.Callahan LA, Nethery D, Stofan D, DiMarco A, Supinski G. Free radical-induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol. 2001;24:210–217. doi: 10.1165/ajrcmb.24.2.4075. [DOI] [PubMed] [Google Scholar]
- 79.Boyd JH, Divangahi M, Yahiaoui L, Gvozdic D, Qureshi S, Petrof BJ. Toll-like receptors differentially regulate CC and CXC chemokines in skeletal muscle via NF-kappaB and calcineurin. Infect Immun. 2006;74:6829–6838. doi: 10.1128/IAI.00286-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Demoule A, Divangahi M, Yahiaoui L, Danialou G, Gvozdic D, Labbe K, Bao W, Petrof BJ. Endotoxin triggers nuclear factor-kappaB-dependent up-regulation of multiple proinflammatory genes in the diaphragm. Am J Respir Crit Care Med. 2006;174:646–653. doi: 10.1164/rccm.200509-1511OC. [DOI] [PubMed] [Google Scholar]
- 81.Casadevall C, Coronell C, Ramirez-Sarmiento AL, Martinez-Llorens J, Barreiro E, Orozco-Levi M, Gea J. Upregulation of pro-inflammatory cytokines in the intercostal muscles of COPD patients. Eur Respir J. 2007;30:701–707. doi: 10.1183/09031936.00152005. [DOI] [PubMed] [Google Scholar]
- 82.Gosker HR, Wouters EF, van der Vusse GJ, Schols AM. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectives. Am J Clin Nutr. 2000;71:1033–1047. doi: 10.1093/ajcn/71.5.1033. [DOI] [PubMed] [Google Scholar]
- 83.Staib JL, Swoap SJ, Powers SK. Diaphragm contractile dysfunction in MyoD gene-inactivated mice. Am J Physiol Regul Integr Comp Physiol. 2002;283:R583–590. doi: 10.1152/ajpregu.00080.2002. [DOI] [PubMed] [Google Scholar]
- 84.Agusti A, Morla M, Sauleda J, Saus C, Busquets X. NF-kappaB activation and iNOS upregulation in skeletal muscle of patients with COPD and low body weight. Thorax. 2004;59:483–487. doi: 10.1136/thx.2003.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Broekhuizen R, Wouters EF, Creutzberg EC, Weling-Scheepers CA, Schols AM. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax. 2005;60:376–382. doi: 10.1136/thx.2004.030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bolger AP, Anker SD. Tumour necrosis factor in chronic heart failure: a peripheral view on pathogenesis, clinical manifestations and therapeutic implications. Drugs. 2000;60:1245–1257. doi: 10.2165/00003495-200060060-00002. [DOI] [PubMed] [Google Scholar]
- 87.Adams V, Nehrhoff B, Spate U, Linke A, Schulze PC, Baur A, Gielen S, Hambrecht R, Schuler G. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation: an in vitro and in vivo study. Cardiovasc Res. 2002;54:95–104. doi: 10.1016/s0008-6363(02)00228-6. DOI S0008636302002286 [pii] [DOI] [PubMed] [Google Scholar]
- 88.Adams V, Spate U, Krankel N, Schulze PC, Linke A, Schuler G, Hambrecht R. Nuclear factor-kappa B activation in skeletal muscle of patients with chronic heart failure: correlation with the expression of inducible nitric oxide synthase. Eur J Cardiovasc Prev Rehabil. 2003;10:273–277. doi: 10.1097/00149831-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 89.Jankowska EA, von Haehling S, Czarny A, Zaczynska E, Kus A, Anker SD, Banasiak W, Ponikowski P. Activation of the NF-kappaB system in peripheral blood leukocytes from patients with chronic heart failure. Eur J Heart Fail. 2005;7:984–990. doi: 10.1016/j.ejheart.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 90.Siednienko J, Jankowska EA, Banasiak W, Gorczyca WA, Ponikowski P. Nuclear factor-kappaB activity in peripheral blood mononuclear cells in cachectic and non-cachectic patients with chronic heart failure. Int J Cardiol. 2007;122:111–116. doi: 10.1016/j.ijcard.2006.03.095. [DOI] [PubMed] [Google Scholar]
- 91.Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR. Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. Am J Physiol. 1999;276:C1132–1138. doi: 10.1152/ajpcell.1999.276.5.C1132. [DOI] [PubMed] [Google Scholar]
- 92.Guillet C, Boirie Y. Insulin resistance: a contributing factor to age-related muscle mass loss? Diabetes Metab. 2005:5S20–25S26. doi: 10.1016/s1262-3636(05)73648-x. 31 Spec No 2. [DOI] [PubMed] [Google Scholar]
- 93.Willey KA, Singh MA. Battling insulin resistance in elderly obese people with type 2 diabetes: bring on the heavy weights. Diabetes Care. 2003;26:1580–1588. doi: 10.2337/diacare.26.5.1580. [DOI] [PubMed] [Google Scholar]
- 94.Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol. 2003;285:C806–812. doi: 10.1152/ajpcell.00129.2003. [DOI] [PubMed] [Google Scholar]
- 95.Mastrocola R, Reffo P, Penna F, Tomasinelli CE, Boccuzzi G, Baccino FM, Aragno M, Costelli P. Muscle wasting in diabetic and in tumor-bearing rats: role of oxidative stress. Free Radic Biol Med. 2008;44:584–593. doi: 10.1016/j.freeradbiomed.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 96.Judge AR, Koncarevic A, Hunter RB, Liou HC, Jackman RW, Kandarian SC. Role for IkappaBalpha, but not c-Rel, in skeletal muscle atrophy. Am J Physiol Cell Physiol. 2007;292:C372–382. doi: 10.1152/ajpcell.00293.2006. [DOI] [PubMed] [Google Scholar]
- 97.Degens H, Alway SE. Control of muscle size during disuse, disease, and aging. Int J Sports Med. 2006;27:94–99. doi: 10.1055/s-2005-837571. [DOI] [PubMed] [Google Scholar]
- 98.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 99.Bar-Shai M, Carmeli E, Coleman R, Rozen N, Perek S, Fuchs D, Reznick AZ. The effect of hindlimb immobilization on acid phosphatase, metalloproteinases and nuclear factor-kappaB in muscles of young and old rats. Mech Ageing Dev. 2005;126:289–297. doi: 10.1016/j.mad.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 100.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]