Abstract
Nek1, the first mammalian ortholog of the fungal protein kinase never in mitosis A, is involved early in the DNA damage sensing/repair pathway after ionizing radiation. Here we extend this finding by showing that Nek1 localizes to nuclear foci of DNA damage in response to many different types of damage in addition to IR. Untransformed cells established from kat2J/Nek1−/− mice fail to arrest properly at G1/S and M-phase checkpoints in response to DNA damage. G1-S-phase checkpoint control can be rescued by ectopically overexpressing wild-type Nek1. In Nek1−/− murine cells and in human cells with Nek1 expression silenced by siRNA, the checkpoint kinases Chk1 and Chk2 fail to be activated properly in response to ionizing or UV radiation. In cells without functional Nek1, DNA is not repaired properly, double-stranded DNA breaks persist long after low dose IR, and excessive numbers of chromosome breaks are observed. These data show that Nek1 is important for efficient DNA damage checkpoint control and for proper DNA damage repair.
Keywords: checkpoint control, radiation, S-phase, mitosis, chromosome breaks
Introduction
Nek1 (a.k.a. Nrk1)1 is a mammalian ortholog of NIMA (never in mitosis A) in Aspergillus nidulans. It was cloned after screening an expression library with anti-tyrosine antibodies and identified as the first mammalian NIMA-related kinase.2 Nek1 has dual serine-threonine and tyrosine kinase activity in vitro, but its important cellular substrates are unknown at present. Because Nek1 mRNA is abundantly expressed in mouse gonads and in specific neurons, authors have suggested that Nek1 may play a direct and unique role in meiosis or in regulating the cell division cycle.2,3 Germ line Nek1 mutations in either of two strains of mice, the so-called kidneys-anemia-testis (kat and kat2J) strains, result in pleiotropic and ultimately fatal defects including growth retardation, facial dysmorphism, chorioid plexus and neurologic abnormalities, male sterility, anemia and progressive polycystic kidney disease (PKD).4,5
Fungal NIMA has been better characterized than its mammalian orthologs, NIMA is increased in response to DNA damage, regulates G2-M phase progression, functions as a kinase for acidic proteins, and is important for orderly mitotic events including spindle organization and proper formation of the nuclear envelope.6–8 Indeed, Osmani et al., suggested in some of their early studies that NIMA is required during mitosis in a “limited capacity”, as part of a cell cycle checkpoint, either one involved in making sure that DNA is properly replicated, or in assuring that damaged DNA is repaired before it’s replicated.7
The functions of mammalian NIMA-related kinases (Neks or Nrks) are only beginning to be understood. Of the 11 known Neks, Nek2 is the best characterized to date. It has a role in controlling orderly mitosis and in preventing chromosome instability, in part through direct interactions with centrosome proteins.1,9,10 Nek6 and Nek7 have been implicated as well in regulating mitotic progression.11,12 Nek8, which like Nek1 has been linked genetically with a form of cystic kidney disease and which like Nek1 localizes to the primary cilium of each cell, also results in disordered mitosis when inactivated.13–15 A cell’s primary cilium at its base is directly attached to the centrosomes, which will form microtubular organizing centers or spindle poles during mitosis.16 Taken together, observations to date thus indicate that several Neks, like their lower eukaryotic ortholog, but each in unique ways, function to maintain orderly progression of the cell division cycle. When they’re missing or inactivated, the same cellular phenotype, aberrant mitosis, results.
We have previously uncovered a role for Nek1 in DNA damage responses by showing that Nek1-deficient fibroblasts are much more sensitive to the effects of IR-induced DNA damage than otherwise identical wild type fibroblasts.17 Nek1 kinase activity and expression are quickly upregulated in cells treated with ionizing radiation (IR). During the early time frame after IR, at the same time that kinase activity is upregulated, a portion of Nek1 consistently redistributes in cells from cytoplasm to discrete nuclear foci at sites of DNA damage. There it colocalizes with key proteins involved very early in the response to IR-induced DNA double strand breaks (DSBs), including γ-H2AX and MDC1/NFBD1. Nek1 has not yet been fit into known DNA damage response pathways, however.
DNA damage initiates a complex signaling network that rapidly senses the lesions, recruits damage response proteins into macromolecular nuclear foci at sites of damage, and activates a series of checkpoints that temporarily halt progression of the cell cycle and prevent the cell from duplicating its DNA or from undergoing mitosis until after DNA can be assessed and repaired. For prompt and accurate DNA repair, signals must be conveyed rapidly and precisely. If these signals fail or are delayed, or if cell cycle checkpoints are bypassed, then unrepaired or misrepaired DNA can be passed on to daughter cells. This, in turn, leads to defective chromatid pairing, chromosome breaks, mitotic missegregation, programmed cell death if the mitotic defects are severe, and ultimately to chromosome instability (CIN) in subsequent generations of the surviving daughter cells. Several syndromes associated with defective DNA damage proteins ultimately result in CIN, whether the defective proteins are involved directly in centrosome or kinetochore functions during mitosis or whether they instead function indirectly in maintaining orderly cell cycle checkpoints.18 Phenotypes associated with defects in proteins known to be involved in DNA damage signaling include premature aging, immunodeficiency, predisposition to tissue-specific cancers and extreme sensitivity to ionizing radiation and other forms of DNA damage.
To determine whether Nek1 is required for proper check-point activation and DNA repair, we characterized known DNA damage responses in Nek1 deficient and control cells to different types of DNA damage, as well as the consequences of Nek1 deficiency on checkpoint activation and DNA repair. We report here that Nek1 localizes to DNA damage sites initiated by different types of DNA damage agents. Nek1 deficient cells are defective in G1/S and M-phase checkpoints in response to DNA damage, fail to activate downstream mediators Chk1 and Chk2, fail to repair DNA breaks after relatively low dose DNA damage, and ultimately develop of chromatid breaks, the hallmarks of unstable chromosomes.
Results
Nek1 is involved in the DNA damage response to multiple agents
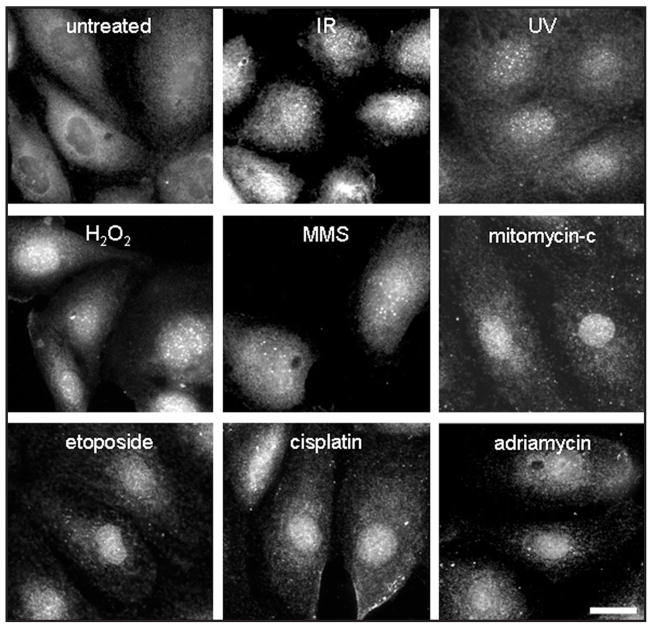
We have previously shown that Nek1 responds quickly to gamma irradiation by localization to DNA damage sites within minutes.17 To determine whether the localization of Nek1 to sites of DNA damage is a specific response to double strand breaks from IR, or a more generalized one, we used immunofluorescence staining to examine human kidney HK2 cells before and after exposure to other DNA damaging agents. We found that the relocalization of Nek1 to nuclear foci occurs in response to DNA damage from multiple agents including IR, UV radiation, H2O2/oxidative injury, and several different chemotherapeutic drugs that collectively activate both ATM- and ATR-dependent signaling pathways (Fig. 1). Nek1 kinase activity and expression also rapidly increase after IR, UV, and MMS treatment (not shown).
Figure 1.
Nek1 relocalizes to nuclear foci after treatment with multiple different DNA damaging agents. HK2 cells in exponential growth phase on coverslips were treated with γ-radiation (ionizing radiation or IR, 10 Gy); ultraviolet radiation (UV, 10 J/m2); H2O2 (0.001%), a free-radical generating agent that oxidizes DNA; methylmethane sulfonate (MMS, 0.01%), a DNA alkylating agent; mitomycin-c, a pyrimidine cross-linking agent (10 μg/ml); etoposide, a topoisomerase II inhibitor (0.2 μM); cisplatin (cis-DDP), another pyrimidine cross-linker (10 μg/ml); or adriamycin, an anthracycline DNA intercalating agent (0.2 μg/ml). One hour later, cells were fixed with 4% neutral buffered formalin and then incubated with rabbit polyclonal anti-Nek1 primary antibodies and fluorescence-tagged anti-rabbit IgG secondary antibodies.
Cell cycle checkpoints are defective in Nek1-deficient cells
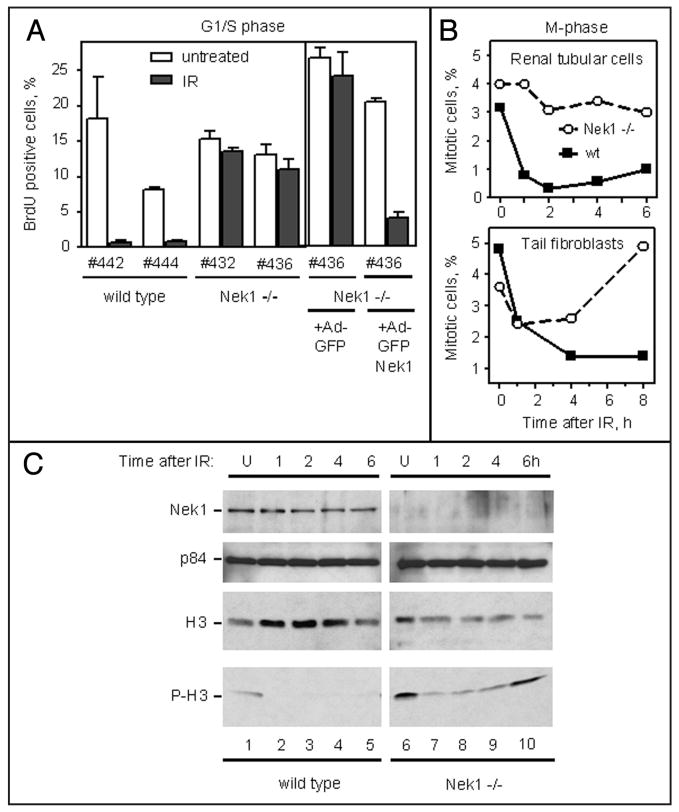
Cells suffering from DNA damage should arrest at G1/S, S-or G2/M-phase until the damage can be repaired. If key elements in the DNA damage response or repair pathway are defective, then cells will not arrest properly and will continue progression of the cell cycle, which will lead to cell death if DNA damage is severe. Since we observed hypersensitivity to the lethal effects of IR in Nek1−/− kat2J cells,17 we sought to determine whether this hypersensitivity might be due to defective checkpoint activation. We therefore examined two checkpoints directly in Nek1−/− and wild type cells treated with relatively low dose IR (4 Gy). Unsynchronized cells progressing into S-phase after this irradiation were scored 24 h later by BrdU incorporation. Cells without intact G1/S checkpoint control will continue entering S phase, where they will replicate their DNA and incorporate BrdU.19 Two different, untransformed, early passage, Nek1−/− cell lines failed to arrest at G1/S-phase (Fig. 2A). Cells cultured identically from wild type littermates did arrest as expected. To determine whether Nek1 is directly responsible for this G1/S checkpoint defect, one of the Nek1−/− cell lines was reconstituted with an adenovirus expressing tagged Nek1 or with the tag alone. Nek1 expression specifically restored the ability of irradiated Nek1−/− cells to arrest before S-phase (Fig. 2A).
Figure 2.
Defective G1/S and M-phase checkpoints in Nek1−/− cells. (A) G1/S phase checkpoint. Four primary RTE lines in log phase growth, two each with the genotypes wild type and Nek1−/−, were treated with moderate dose IR (4 Gy) and then incubated 24 h later with BrdU. One of the Nek1−/− RTE lines was also reconstituted with adenoviruses expressing either green fluorescence protein (GFP) alone or GFP-tagged, wild type, human Nek1. 48 h later, these reconstituted cells were treated with IR in a manner identical to that used for the other four parental RTE lines. The percentages of cells that stained for incorporated BrdU, out of >200 individual cells per line, were determined. Histograms represent means ± SEM from three experiments. (B) M phase checkpoint. The same RTE cells, and tail fibroblasts originally from the same mice, were treated at time zero with IR, as above, and then fixed at hourly intervals thereafter and stained with DAPI. Mitotic cells were scored as a percentage of >1000 cells per line in duplicate experiments. (C) Histone H3 phosphorylation during M phase persists after IR in Nek1−/− cells. Within 1 h after γ-radiation (IR, 10 Gy), wild type cells arrest in mitosis and no longer phosphorylate H3 on Ser10 (P-H3). In Nek1−/− cells, H3 phosphorylation diminishes 1–4 h after IR, but is still evident and is back to the baseline amount by 6 h. Upper three panels (Nek1, p84 as loading control, and total histone H3) are Western blots using 20% of the amount of the same lysates used in the lowest panel (P-H3).
To assess the G2/M phase checkpoint, we examined wild type and Nek1−/− cells for mitotic figures at hourly intervals after IR. Whereas wild type renal tubular epithelial cells (RTEs) and tail fibroblasts arrested and formed no further mitotic figures within 1 h, Nek1−/− cells continued to progress into and through M-phase, either immediately (for RTEs) or within 2 hours (for fibroblasts) (Fig. 2B). Western blotting for M-phase-specific, phosphorylation of histone H3 on Ser1020 confirmed progression of Nek1−/− cells into M-phase after IR (Fig. 2C). These results show that lack of Nek1 is associated with failure of cells to arrest at G1/S- and G2/M-phase checkpoints properly after DNA damage.
Nek1 is required for Chk1 and Chk2 activation
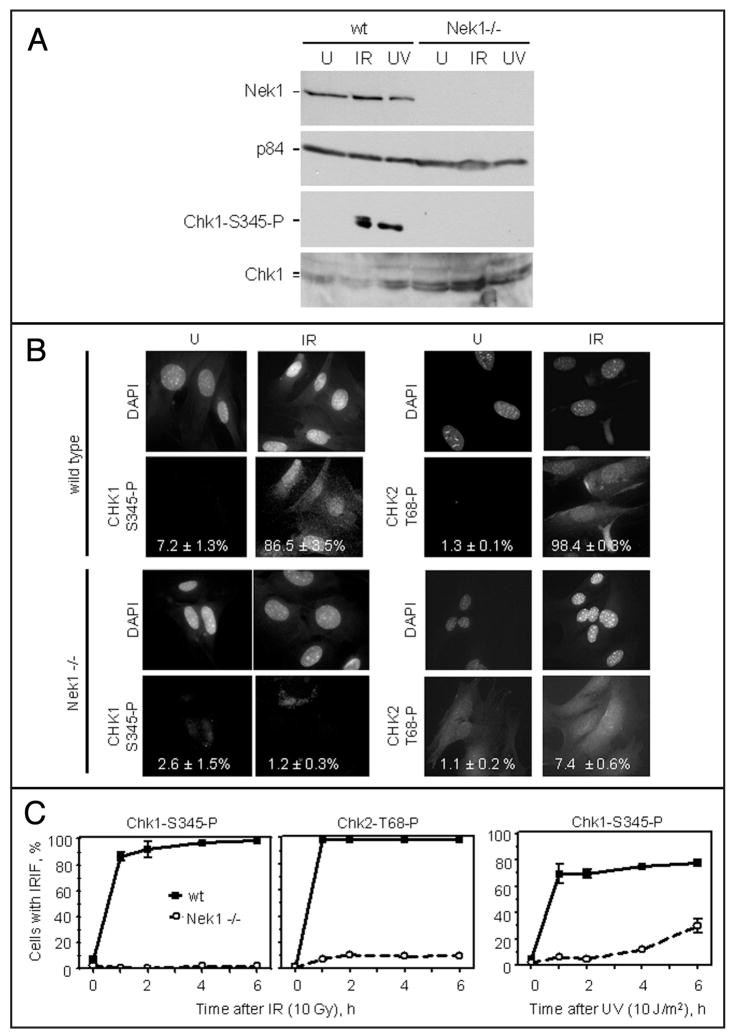
To explore further how Nek1 might be required for checkpoint activation, we examined crucial phosphorylations of the cell cycle checkpoint kinases Chk1 and Chk2,21–27 after defined DNA damage in wild type and Nek1−/− murine cells. Chk1 activation by phosphorylation on Ser345, assessed both by Western blotting (Fig. 3A, lower two blots) and by examination of individual cells for IR-induced immunofluorescent foci (IRIF) 10 min after γ-radiation (Fig. 3B, left), is conspicuously absent or markedly impaired in Nek1−/− cells. Using immunocytochemistry, we also found that Chk2, like Chk1, failed to be activated properly by phosphorylation on Thr68, and failed to localize to nuclear foci in Nek1−/− cells exposed to IR (Fig. 3B, right). The lack of proper Chk1 and Chk2 activation in Nek1−/− cells is not merely a delay, since very few Chk1 or Chk2 IRIF were seen in Nek1−/− deficient cells, even up 6 h after induction of DNA damage by IR (Fig. 3C). Neither was the lack of Chk1 activation in Nek1−/− cells specific for IR; UV irradiation also failed to result in proper localization of phospho-S345 Chk1 to IRIF up to 4–6 h later (Fig. 3C, rightmost).
Figure 3.
Chk1 and Chk2 are not activated by phosphorylation after DNA damage in Nek1−/− cells. (A) After either IR or UV irradiation, Chk1 fails to be activated by phosphorylation on Ser345 in Nek1−/− cells. Western blotting confirmed the presence or absence of Nek1 and total Chk1 in the cells. p84 was included to control for loading. (B) Within 1 h after IR in wild type cells, Chk1 is activated by phosphorylation on S345 and localizes in discrete IRIF at sites of damaged DNA. Likewise, Chk2 is activated by phosphorylation on Tyr68 and localizes to IRIF in wild type cells. In contrast, Chk1 and Chk2 are not activated, or activated to a very minor degree, in identically treated Nek1−/− cells. (C) Graphic comparisons of time courses 0–6 h after IR (for activated Chk1-S345-P and activated Chk2-T68-P) and UV radiation (for activated Chk1-S345-P). More than 400 cells for each of two cell lines were scored for each time point.
Downregulation of Nek1 expression by RNA interference in normal cells results in phosphorylation of proximal ATM and ATR substrate but not Chk2
To make sure that our observations in murine cells were not consequences only of a specific, hypomorphic Nek1 mutation in the kat2J mouse strain, we also examined key DNA damage responses in human RTE (HK2) cells with Nek1 expression silenced by RNA interference (short inhibitory RNA). Silencing of Nek1 expression by an adenovirus-mediated shRNA was specific and efficient (Fig. 4A and B). Although infection of cells with recombinant adenoviral vectors non-specifically activated the DNA damage response to a minor degree, even in cells that weren’t further treated with IR (Fig. 4B and C),28 IR caused further phosphorylation and activation of an early, ATM-dependent, DNA damage response protein, γ-H2AX (Fig. 4C). Chk2, however, was not similarly or normally activated by phosphorylation on Thr68 in the same conditions (Fig. 4C). These results after silencing Nek1 expression in human cells were entirely consistent to those we observed in murine Nek1−/− cells. Therefore, our observations about the role of Nek1 in DNA damage response and in checkpoint control are not unique only to the specific, Nek1/kat2J mutation. Rather, they are the direct consequences of Nek1 inactivation.
Figure 4.
Chk2 is not activated properly after IR in cells with Nek1 expression silenced. (A) Silencing of Nek1 expression by RNA interference in human RTEs. HK2 cells were either left untreated (U), infected with an adenovirus vector carrying shRNA specific for silencing the firefly luciferase gene (Luci), or transfected with the same backbone vector carrying shRNA specific for human Nek1 (Nek1i). 72 h after transfection, cells were lysed. Protein lysates were separated by SDS gel electrophoresis, transferred to membranes, and Western blotted with anti-Nek1 and anti-p84 antibodies, the latter to control for loading. (B) The same HK2 cells, on coverslips and pretreated with Luci or Nek1i 72 h earlier, were fixed and stained before (untreated, U) or 1 h after DNA damage by IR (4 Gy). DAPI staining was used to identify cell nuclei and indirect immunocytochemistry to localize Nek1. (C) Quantification of nuclear foci (IRIF) of γ-phosphoryated H2AX, 1 h after mock treatment or IR (4 Gy). 2-way ANOVA p < 0.05 when comparing percentages of cells with IRIF before and after irradiation. Chk2 activation by phosphorylation on T68 and decoration of nuclei as IRIF, although intact in HK2 cells untreated or pretreated with the irrelevant Luci, are absent in HK2 cells pretreated with Nek1i. More than 400 cells were scored in duplicate experiments for each condition. Means ± SEM are shown in the histograms. 2-way ANOVA p < 0.01 before and after IR for untreated cells, when comparing untreated cells and cells pretreated with Luci. (D) Time course of Chk2 activation by phosphorylation on T68 and expression in nuclear IRIF, 0–6 h after γ-radiation (IR). Cells were either left untreated or pretreated 72 h earlier with adenoviruses carrying siRNA constructs (Luci or Nek1i). More than 400 cells were scored in 3–4 experiments for each condition. Means ± SEM are shown for each time point. 2-way ANOVA p < 0.001 by for all time points other than time zero, when comparing cells pretreated with Nek1 siRNA vs. untreated cells or cells treated with luciferase siRNA.
Nek1 mutation results in unrepaired DNA damage and chromosome breaks
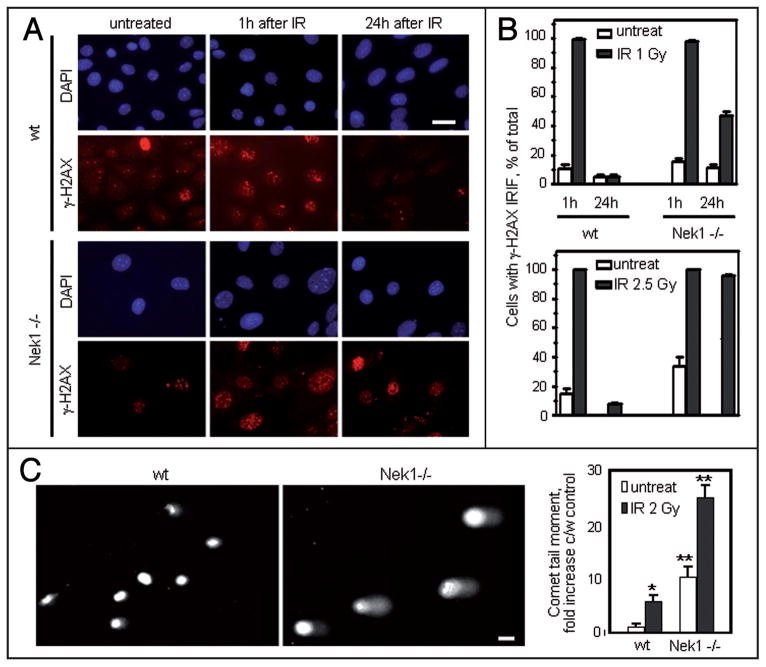
To determine whether the abnormalities in DNA damage responses and checkpoint control lead directly to deficient DNA repair, we examined loss of γ-H2AX nuclear foci as an indicator for repair of DSBs at intervals after low dose IR in wild type and Nek1−/− mouse cells. One hour after IR, γ-H2AX localized to nuclear foci of DNA damage in nearly 100% of wild type and Nek1−/− cells (Fig. 5A and B). By 24 h after IR, γ-H2AX foci were gone from wild type cells, indicating that these cells had repaired the DNA breaks. In contrast, 50% of Nek1−/− cells still had γ-H2AX nuclear foci 24 h after very low dose IR (1 Gy). After a slightly higher dose (2.5 Gy), nearly 100% of Nek1−/− still had γ-H2AX foci after 24 h, indicating that these cells had difficulty in repairing the damaged DNA. To confirm our finding, we also performed comet assays of individual cell nuclei 24 h after low dose IR (2 Gy). Nek1−/− mouse cells had significantly longer and denser comet moments, i.e., further evidence of excessive, unrepaired DSBs in their DNA (Fig. 5C and D). These results demonstrate that non-lethal DNA damage persists long after IR when cells have no functional Nek1.
Figure 5.
Excessive and persistent DNA damage in Nek1−/− cells. (A) Wild type and Nek1−/− cells were either left untreated, or treated identically with low dose γ-radiation (IR, 1 Gy). They were then fixed 1 h or 24 h later and immunostained with primary antibodies recognizing γ-H2AX (Alexa 594 red-tagged secondary antibody). Representative microscopic fields are shown. Bar = 20 μm. (B) Histograms show percentages of nuclei with >5 nuclear foci (IRIF) before and at different time points after IR (1 Gy or 2.5 Gy). Histograms represent means ± SEM from 2 separate experiments with >200 cells per time point in each experiment. (C) Representative examples of single cells gel electrophoresed under alkaline conditions and stained with SYBR green to demonstrate fast-migrating, damaged DNA in the “comet” tails (CometAssay™). Bar, 10 μm. Histograms in the rightmost panel show quantification of relative comet tail length, density, and fraction of total DNA in the tail, measured as “comet tail moment” and normalized to the control (untreated wt cells). * 2-way ANOVA p < 0.002 when comparing untreated wt cells and wt cells treated with IR (2 Gy); ** p < 0.0001 when comparing untreated wt cells with untreated Nek1−/− cells or Nek1−/− cells before and after treatment with IR (2 Gy).
Finally, we looked at the mitotic consequences of unrepaired DNA damage in Nek1−/− cells. Unrepaired DNA DSBs will lead to breaks in the chromosomes during mitosis.29,30 To explore this phenotype, we examined chromosome spreads and looked for chromosome breaks in wild type and Nek1−/− cells cultured in identical conditions for the same number of passages. Giemsa stained mitotic chromosomes were less compact and more variable in length in Nek1−/− cells, and individual chromosomes showed many more gross breaks per spread in Nek1−/− cells than in wild type cells (Fig. 6A and B). These gross chromosomal abnormalities suggest that the impaired DNA damage checkpoint control and the inability to repair the DNA breaks in Nek1−/− cells can lead to faulty chromatid pairing during mitosis, and ultimately to chromosome instability.
Figure 6.
Chromsome breaks in Nek1−/− cells. (A) Examples of chromosome breaks (arrows) in Giemsa-stained mitotic spreads from Nek1−/− RTEs. Bar, 30 μm. (B) Number of chromosome breaks per cell. Number of chromosome spreads examined: N = 62 for wt, N = 43 for Nek1−/− cells.
Discussion
We have now built upon our earlier report indicating that Nek1 is involved early in the DNA damage response.17 Our new data demonstrate Nek1 is important for proper checkpoint activation and DNA repair. Nek1-deficient cells fail to arrest at the G1/S or M-phase checkpoints in response to DNA damage. This defective checkpoint control is associated with failure to activate properly the crucial kinases, Chk1 and Chk2. Nek1-deficent cells also fail to repair damaged DNA after low dose ionizing radiation, and develop excessive numbers of DNA breaks.
Comparisons between known functions of fungal NIMA and our data regarding the role of Nek1 in DNA damage checkpoint control and in maintaining the fidelity of mitosis are informative. Aspergillus nimA mutants never enter mitosis; they instead arrest in late G2 phase with duplicated spindle pole bodies, the fungal equivalents of mammalian cell centrosomes.31 Overexpression of NIMA in Aspergillus, yeast, and even human cells, causes premature condensation of chromosomes independent of Cdc2, the proximal cyclin dependent kinase controlling access to M phase.8,32–34 If nimA5 Aspergillus mutants are forced to bypass G2/M phase arrest by acquisition of an additional mutation in bimE7 (the gene encoding an anaphase-promoting complex subunit APC1, an E3 ubiquitin ligase required for degradation of several mitotic proteins),8 however, they develop grossly aberrant mitotic phenotypes. These include multiple spindle pole bodies, spindles with disparate sizes and shapes, disorganized microtubules moving in multiple directions other than orthogonal ones, and defects in nuclear envelope structure and in nucleokinesis.7 We have observed grossly disordered mitoses in multiply-passed, murine Nek1−/− cells (manuscript in preparation) When accounting for the differences between Aspergillus versus mammalian mitosis, these aberrations are very similar to those observed in nimA5 + bimA7 mutants. Indeed, Nek1, like NIMA and other Nrks, may not to be essential for entry into or progression through mitosis, but instead may be important for regulating the sequence and fidelity of chromosomal segregation. Proper checkpoint control is not an all-or-none phenomenon. Rather, it must be precisely timed and coordinated after DNA damage, especially in higher eukaryotes.
DNA damage responses work through a series of feed-forward transducers to amplify signals after initial injury.35 Nek1, the kinase activity and expression of which is rapidly upregulated in both cytoplasm and at nuclear foci after oxidative injury that includes DNA damage (ref. 36 and manuscript in preparation), may be required to facilitate or amplify some of the same downstream transducers and effectors as ATM or ATR, such that a full or robust DNA damage response is not possible after stress without functional Nek1.
With the present data we don’t know precisely how lack of Nek1 prevents activating the crucial kinases Chk1 and Chk2. Since cells and animals with inactivating mutations in Nek1 are viable, Nek1 must play a regulatory rather than an essential role in the cell division cycle. It may be required for ATM or ATR activation of Chk1 and Chk2. Since γ-H2AX is activated and localized to DNA damage sites soon after IR in Nek1 deficient cells, it is not likely that Nek1 is required for ATM or ATR activation. More likely, Nek1 may be required for efficient cytoplasmic to nuclear transport of Chk1 and/or Chk2. If newly synthesized Chk1 or Chk2 were not able to enter the nucleus, it could not be activated by ATM or ATR, and thus could not function properly in checkpoint control. A requirement for Nek1 to activate Chk1 and Chk2 could be direct if Nek1 phosphorylates a precursor site before ATM or ATR phosphorylates other activating sites, or indirect through Nek1’s effects on other kinases or phosphatases required for Chk1 and Chk2 to interact with ATM or ATR. Both Chk1 and Chk2 have consensus phosphorylation sites for Nek1. Nek1 could even be a redundant kinase more important stochiometrically in response to oxidative injury, such that cells without Nek1 would develop problems only if stressed.
Nek1 may also play a synergistic role in proper activation of other ATM/ATR substrates and participants in the DNA damage signaling response. Because Nek1 is a kinase, it’s likely to have several substrates, which it could phosphorylate in a hit-and-run manner, perhaps differentially depending on the stimulus and the phase of the cell cycle. Our preliminary, unpublished results and those from other labs indicate that Nek1 interacts with several proteins involved in DNA damage response signaling, some upstream of Chk1 and Chk2, and at least one involved in the effector arm of DNA damage repair.
The most proximal or upstream sensor in the canonical DNA damage response is thought to be the Mre11-Rad50-Nbs1 (MRN) complex, which may be a sensor.37 This complex, comprised of the endonuclease Mre11, the structural maintenance of chromosome protein Rad50, and a protein associated when mutated with Nijmegen breakage syndrome, Nbs1, is recruited rapidly to sites of DNA breaks. There it tethers and processes the broken DNA ends.38 Mre11 is thus also an effector of DNA damage repair. Interestingly, it has been identified in a yeast 2-hydrid screen as a potential direct target of Nek1.39 53BP1, a transducer of amplified DNA damage signals, one which lies both upstream and downstream of ATM/ATR in the feed-forward loop,40 but upstream of Chk1 and Chk2, has likewise been identified as a potential target of Nek1. We are currently determining whether these potential Nek1-interacting proteins are actual kinase substrates, and whether interaction with or phosphorylation by Nek1 directly affects their activities. The present report gives impetus to clarifying these important details, for Nek1 might be a unique protein in the DNA damage response, a protein with important functions distinct from but intertwined with the known functions of the canonical, upstream, signaling kinases ATM and ATR.
Materials and Methods
Cell culture
Human HK2 human proximal renal tubular epithelial cells were obtained from American Type Tissue Collection (Rockville, MD) and cultured in 50/50 Ham’s F-12/Delbecco’s modified Eagle medium containing 10% fetal bovine serum and antibiotics. Primary fibroblasts and renal tubular epithelial cells were obtained from Nek1/kat2J mice and their wild type littermates, as previously described,17 and cultured in the same Ham’s F-12/DMEM.
Ionizing and ultraviolet radiation
Cells were γ-irradiated using cesium 40 at the rate of 116 cGy/min. For UV irradiation, cells were first washed with PBS twice and then placed inside a UV cross-linker (Stratagene, LaJolla, CA). The dose of UV irradiation was monitored with a UV meter.
Chemicals
H2O2 and the chemotherapeutic drugs (methylmethane sulfonate, mitomycin-c, etoposide, cis-platin and adriamycin) were purchased from Sigma-Aldrich (St. Louis, MO) and reconstituted according to the manufacturer’s instructions immediately before addition to cultured cells.
Antibodies
Rabbit polyclonal anti-Nek1 antibodies have been described and characterized.15,17 Anti-p84 mAb 5E10,41 was purchased from GeneTex (San Antonio, TX). Anti-γ-H2AX, anti-Chk1-phospho-S345, anti-Chk2-phospho-T68, anti-histone H3 and anti-phospho-H3 were purchased from Cell Signaling Technology (Danvers, MA).
Kinase assays
1 ×106 cells were lysed and immunoprecipitated with anti-Nek1 antibodies, as previously described.17 After extensive washing with lysis buffer, the immune complexes were washed twice with TBS (10 mM NaCl, 10 mM Tris-HC1, pH 7.5), twice with ddH2O, and finally twice with kinase buffer (100 mM Tris-HCl, pH 7.5, 5 mM MnCl2, 2 mM DTT, 1 mM ATP). Immune complexes were stored in 100 μl of kinase buffer at −80°C. The kinase reaction was carried out in a total volume of 30 μl, with 20 μl of immune complexes and 5 μl ofβ-casein in the presence of 3 μCi of γ-32P-ATP. After incubation for 30 min at 37°C, equal volumes of SDS sample buffer with EDTA were added to final concentration of 2 mM for the kinase reactions.
Immunoblot analysis
Cells resuspended in Lysis 250 buffer were subjected to three freeze/thaw cycles (liquid N2/37°C), then centrifuged at 14,000 rpm for 2 min at room temperature, as previously described.9
Scoring for immunofluorescent nuclear foci at sites of DNA damage
A cell was scored positive for immunoflourescent nuclear foci (IRIF) at sites if at least 5 discrete, fluorescing dots were evident per nucleus. Percentages of nuclei positive for IRIF were calculated by dividing number of nuclei with ≥5 IRIF by the total number of nuclei stained with DAPI in the same microscopic fields. The procedure has previously been validated and described in detail.42
Recombinant, GFP-tagged adenoviruses and RNA interference
Adenoviruses were generated using an AdEasy Vector System (Stratagene, LaJolla, CA). Briefly, wild type Nek1 was fused first in-frame to GFP in the pEGFP-Cl vector. The resulting GFP fusion fragment was then subcloned into transfer vector (pShuttle-CMV). After linearizing with PmeI, pShuttle-CMV-GFP-Nekl was mixed with pAdEasy-1 and electroporated into competent E. coli BJ5183 cells. A recombinant Ad-GFP-Nek1 plasmid was obtained, purified, and linearized with PacI to transfect into 293 cells. Recombinant GFP-Nek1 adenovirus was then generated, amplified, and purified for further infections. Recombinant adenoviruses expressing DNA-based, GFP-tagged, short inhibitory RNA (shRNA) sequences specific for human Nek1 (GGAGAGAAGUUGCA GUAUUG) and for an irrelevant control gene (firefly Luciferase, AAGAUUCAAAGUGCGCUGCUG) were generated in a similar manner. Multiplicities of infection of approximately 30 viral particles per cell were used to obtain efficient gene transduction in all cases using the recombinant adenoviruses, and resulted in >99% of the cells expressing GFP.
G1/S checkpoint
Untransformed, early passage RTEs were treated with γ-irradiation (4 Gy). This moderate, sublethal dose was chosen so that damaged DNA would be repaired after normal the cells would transiently arrest at cell cycle checkpoints. 24 h post irradiation, cells were incubated with BrdU (20 μM) for 1 h. After washing with PBS, cells were fixed with 4% formaldehyde and probed with anti-BrdU antibodies using a proliferation kit [GE Healthcare Bio-Sciences (Piscataway, NJ)]. Cells incorporating BrdU were scored as percentages of total numbers of cells.
Mitotic checkpoint
Primary RTEs were γ-irradiated (4 Gy), then fixed and stained with DAPI at different time points thereafter. Cells were photographed using a fluorescence microscope and cells with mitotic figures were counted as percentages of total cell numbers. Only cells with clearly condensed chromosomes were scored as mitotic.
Chromosome spreads and breaks
Cycling primary RTEs were treated with colchicine (1 μg/ml, from Sigma) for 30 min at 37°C. All cells, including those in the supernatants, were then collected by trypsinization and swollen in 75 mM KCl for 15 min at 37°C. Disbursed cells were then fixed with freshly prepared methanol: acetic acid (3:1). Free chromosomes were dropped onto slides and stained with Giemsa. For chromosome break analysis, cells were incubated with hydroxyurea (0.1 μM, from Sigma) for 24 h before colchicine treatment.
Analyses of ability of individual cells to repair DNA
Cells were cultured in normal media but changed to serum-free media 24 h before exposure to low dose gamma radiation. After low dose IR (1 to 2.5 Gy), the cells were continued in culture with serum-free media for an additional 24 h before they were washed with PBS and fixed with formalin for examination of γ-H2AX IRIF by indirect immunofluorescence microscopy. The same cells, cultured using identical conditions, were also examined by single cell gel electrophoresis (SCGE) under alkaline conditions, using a CometAssay™ kit from Trevigen, Inc., (Gaithersburg, MD). Nuclei stained with SYBR green were analyzed using a Ziess fluorescence microscope and by photographing multiple, random fields with the associated digital camera. For >90 individual nuclei per condition, “comet” tail length, intensity, percentage of DNA in the tail versus the head, and the area of the tail were measured, and were used to calculate tail moments and olives using CometScore™ 1.5 software (http://autocomet.com/products_cometscore.php). Comet tail moments were normalized to control (i.e., mean tail moment of wild type cells without any IR), and expressed in histograms as fold increase compared with control.
Acknowledgments
This work was supported by grants from the PKD foundation, the American Society of Nephrology, the National Kidney Foundation, and the NIH (R01-DK067339) to Y.C.; a George M. O’Brien Kidney Research Center grant from the NIH to Y.C. (P50-DK061597), Hanna E. Abboud, Program Director); and a grant from the NIH to D.J.R. (R01-DK61626). We thank Huai-Chin Chiang and Sergio Garcia for technical support, and a Veterans Administration Renal Research Excellence Award to the South Texas Veterans Health Care System, Audie L. Murphy Division, for help with core services, equipment, and institutional infrastructure.
References
- 1.O’Connell MJ, Krien MJ, Hunter T. Never say never. The NIMA-related protein kinases in mitotic control Trends. Cell Biol. 2003;13:221–8. doi: 10.1016/s0962-8924(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 2.Letwin K, Mizzen L, Motro B, Ben-David Y, Bernstein A, Pawson TA. The mammalian dual specificity protein kinase, Nek1, is related to the NIMA cell cycle regulator and highly expressed in meiotic germ cells. EMBO J. 1992;11:3521–31. doi: 10.1002/j.1460-2075.1992.tb05435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arama E, Yanai A, Kilfin G, Bernstein A, Motro B. Murine NIMA-related kinases are expressed in patterns suggesting distinct functions in gametogenesis and a role in the nervous system. Oncogene. 1998;16:1813–23. doi: 10.1038/sj.onc.1201710. [DOI] [PubMed] [Google Scholar]
- 4.Upadhya P, Birkenmeier EH, Birkenmeier CS, Barker JE. Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc Natl Acad Sci USA. 2000;97:217–21. doi: 10.1073/pnas.97.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogler C, Homan S, Pung A, Thorpe C, Barker J, Birkenmeier EH, Uphadhya P. Clinical and pathologic findings in two new allelic murine models of polycystic kidney disease. J Am Soc Nephrol. 1999;10:2534–39. doi: 10.1681/ASN.V10122534. [DOI] [PubMed] [Google Scholar]
- 6.Osmani AH, McGuire SL, Osmani SA. Parallel activation of the NIMA and p34cdc2 cell cycle-regulated protein kinases is required to initiate mitosis in A. nidulans. Cell. 1991;67:283–91. doi: 10.1016/0092-8674(91)90180-7. [DOI] [PubMed] [Google Scholar]
- 7.Osmani AH, O’Donnell K, Pu RT, Osmani SA. Activation of the nimA protein kinase plays a unique role during mitosis that cannot be bypassed by absence of the bimE checkpoint. EMBO J. 1991;10:2669–79. doi: 10.1002/j.1460-2075.1991.tb07810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osmani SA, Engle DB, Doonan JH, Morris NR. Spindle formation and chromatin condensation in cells blocked at interphase by mutation of a negative cell cycle control gene. Cell. 1988;52:241–51. doi: 10.1016/0092-8674(88)90513-2. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Riley DJ, Zheng L, Chen PL, Lee WH. Phosphorylation of the mitotic regulator protein Hec1 by Nek2 kinase is essential for faithful chromosome segregation. J Biol Chem. 2002;277:49408–16. doi: 10.1074/jbc.M207069200. [DOI] [PubMed] [Google Scholar]
- 10.Hayward DG, Fry AM. Nek2 kinase in chromosome instability and cancer. Cancer Lett. 2006;237:155–66. doi: 10.1016/j.canlet.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Roig J, Mikhailov A, Belham C, Avruch J. Nercc1, a mammalian NIMA-family kinase, binds the Ran GTPase and regulates mitotic progression. Genes Dev. 2002;16:1640–58. doi: 10.1101/gad.972202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yissachar N, Salem H, Tennenbaum T, Motro B. Nek7 kinase is enriched at the centrosome, and is required for proper spindle assembly and mitotic progression. FEBS Lett. 2006;580:6489–95. doi: 10.1016/j.febslet.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 13.Bowers AJ, Boylan JF. Nek8, a NIMA family kinase member, is overexpressed in primary human breast tumors. Gene. 2004;328:135–42. doi: 10.1016/j.gene.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–46. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- 15.Mahjoub MR, Trapp ML, Quarmby LM. NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J Am Soc Nephrol. 2005;16:3485–9. doi: 10.1681/ASN.2005080824. [DOI] [PubMed] [Google Scholar]
- 16.Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129:1255–7. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Polci R, Peng A, Chen PL, Riley DJ, Chen Y. NIMA-related protein kinase 1 is involved early in the ionizing radiation-induced DNA damage response. Cancer Res. 2004;64:8800–3. doi: 10.1158/0008-5472.CAN-04-2243. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–41. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 19.Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Volgelstein B, Fornace AJ., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–97. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 20.Hendzel MJ, Wei Y, Mancini MA, Van Hoooser A, Ranaldi T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–60. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- 21.Brown AL, Lee CH, Schwarz JK, Mitiku N, Piwnica-Worms H, Chung JH. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc Natl Acad Sci USA. 1999;96:3745–50. doi: 10.1073/pnas.96.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaturvedi P, Eng WK, Zhu Y, Mattern MR, Mishra R, Hurle MR, Zhang X, Annan RS, Lu Q, Faucette LF, Scott GF, Li X, Carr SA, Johnson RK, Winkler JD, Zhou BB. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–54. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA, Elledge SJ. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–59. [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–97. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA. 2000;97:10389–94. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–39. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 28.Sinclair A, Yarranton S, Schelcher C. DNA-damage response pathways triggered by viral replication. Expert Rev Mol Med. 2006;8:1–11. doi: 10.1017/S1462399406010544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Llorente B, Smith CE, Symington LS. Break-induced replication: what is it and what is it for? Cell Cycle. 2008;7:859–64. doi: 10.4161/cc.7.7.5613. [DOI] [PubMed] [Google Scholar]
- 30.Morgan WF, Corcoran J, Hartmann A, Kaplan MI, Limoli CL, Ponnaiya B. DNA double-strand breaks, chromosomal rearrangements and genomic instability. Mutat Res. 1998;404:125–8. doi: 10.1016/s0027-5107(98)00104-3. [DOI] [PubMed] [Google Scholar]
- 31.Oakley BR, Morris NR. A mutation in Aspergillus nidulans that blocks the transition from interphase to prophase. J Cell Biol. 1983;96:1155–8. doi: 10.1083/jcb.96.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu KP, Hunter T. Evidence for a NIMA-like mitotic pathway in vertebrate cells. Cell. 1995;81:413–24. doi: 10.1016/0092-8674(95)90394-1. [DOI] [PubMed] [Google Scholar]
- 33.O’Connell MJ, Norbury C, Nurse P. Premature chromatin condensation upon accumulation of NIMA. EMBO J. 1994;13:4926–37. doi: 10.1002/j.1460-2075.1994.tb06820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pu RT, Osmani SA. Mitotic destruction of the cell cycle regulated NIMA protein kinase of Aspergillus nidulans is required for mitotic exit. EMBO J. 1995;14:995–1003. doi: 10.1002/j.1460-2075.1995.tb07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–68. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 36.Riley DJ, Achinger SG, Chen Y. Expression of Nek1 protein kinase during kidney development and after ischemic tubular injury (Abstract) J Am Soc Nephrol. 2003;14:366. [Google Scholar]
- 37.Shiloh Y. The ATM-mediated DNA-damage response: taking shape. Trends Biochem Sci. 2006;31:402–10. doi: 10.1016/j.tibs.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Stracker TH, Theunissen JW, Morales M, Petrini JH. The Mre11 complex and the metabolism of chromosome breaks: the importance of communicating and holding things together. DNA Repair (Amst) 2004;3:845–54. doi: 10.1016/j.dnarep.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Surpili MJ, Delben TM, Kobarg J. Identification of proteins that interact with the central coiled-coil region of the human protein kinase NEK1. Biochemistry. 2003;42:15369–76. doi: 10.1021/bi034575v. [DOI] [PubMed] [Google Scholar]
- 40.Mochan TA, Venere M, DiTullio RA, Jr, Halazonetis TD. 53BP1, an activator of ATM in response to DNA damage. DNA Repair (Amst) 2004;3:945–52. doi: 10.1016/j.dnarep.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Durfee T, Mancini MA, Jones D, Elledge SJ, Lee WH. The amino-terminal region of the retinoblastoma gene product binds a novel nuclear matrix protein that co-localizes to centers for RNA processing. J Cell Biol. 1994;127:609–22. doi: 10.1083/jcb.127.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng A, Chen PL. NFBD1, like 53BP1, is an early and redundant transducer mediating Chk2 phosphorylation in response to DNA damage. J Biol Chem. 2003;278:8873–6. doi: 10.1074/jbc.C300001200. [DOI] [PubMed] [Google Scholar]