Summary
Background
Large ventral lateral clock neurons (lLNv) exhibit higher daytime light-driven spontaneous action potential firing rates in Drosophila, coinciding with wakefulness and locomotor activity behaviour. To determine whether the lLNv are involved in arousal and sleep/wake behaviour we examined the effects of altered electrical excitation of the LNv.
Results
LNv-hyperexcited flies reverse the normal day/night firing pattern, showing higher lLNv firing rates at night and pigment dispersing factor-mediated enhancement of nocturnal locomotor activity behaviour and reduced quantity and quality of sleep. lLNv hyperexcitation impairs sensory arousal, as shown by physiological and behavioural assays. lLNv hyperexcited flies lacking sLNv neurons exhibit robust hyperexcitation-induced increases in nocturnal behaviour, suggesting that the sLNv are not essential for mediation of arousal.
Conclusions
Light-activated lLNv neurons modulate behavioural arousal and sleep in Drosophila.
Introduction
In mammals, multiple interlinked neuronal circuits shape sleep/wake behaviour including distinct arousal, circadian and sleep circuits [1]. The recent application of direct patch clamp neurophysiological measurements of Drosophila clock neurons [2-4], in combination with novel molecular genetic strategies designed to selectively dampen or enhance electrical excitability of these neurons [2, 5-9] has contributed to our functional understanding of the Drosophila circadian circuit. While progress has been made in describing circadian [10, 11] and sleep circuits [12, 13] in Drosophila, circuits that mediate arousal are less understood.
The small and large LNv (henceforth sLNv and lLNv) were among the first cells identified as crucial for normal light entrainment of circadian behaviour [14-16]. Several studies suggest that the sLNv are responsible for sustained circadian locomotor activity in constant darkness [9, 14], while the lLNv are less characterized. Recently we showed using whole-cell patch clamp electrophysiology that the lLNv acutely increase their firing rate in response to light in a CRYPTOCHROME-dependent fashion [3]. As light is a well-known sensory cue for arousal, as well as circadian entrainment, we tested whether altered electrical activity of the lLNv influences locomotor activity behaviour, sleep and arousal.
Results
Electrical hyperexcitation of lLNv neurons enhances nocturnal behavioural activity
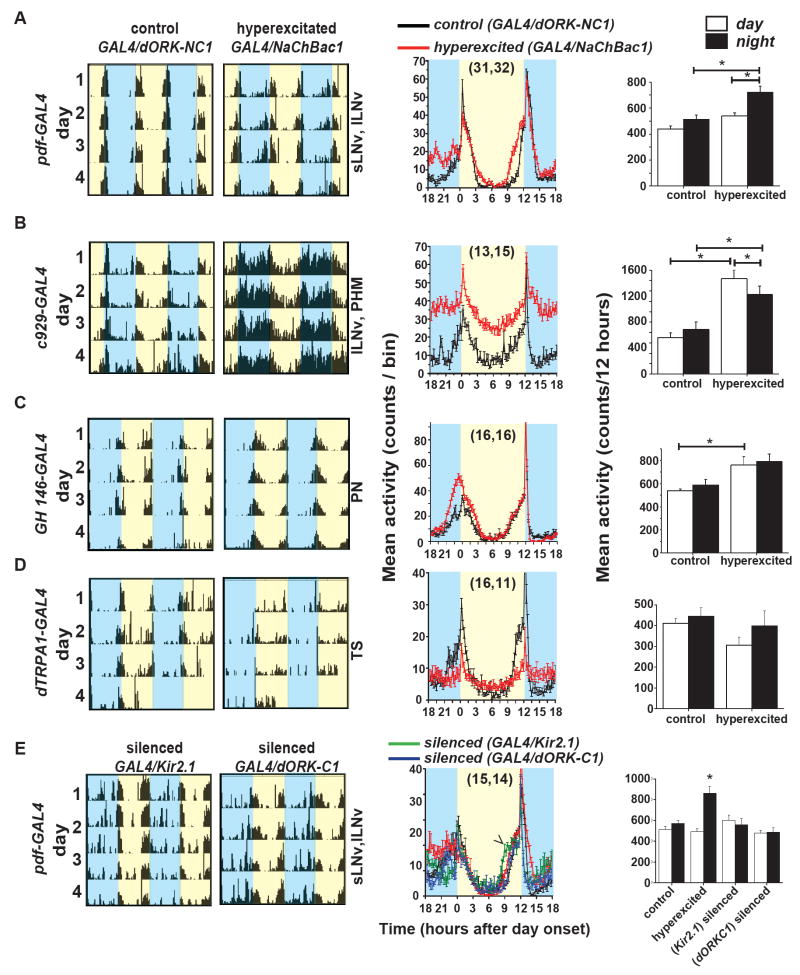
Drosophila pacemaker neurons can be physiologically assessed directly by patch clamp [2-4, 9]. Additionally, using molecular genetic manipulation, the electrical activity of these neurons can be hyperexcited (defined as net increased membrane depolarisation) [2, 4, 5, 8] or hypoexcited (defined as net decreased membrane depolarisation) [2, 6, 17]. We screened the effects of electrical hyperexcitation of neuronal circuits that include or exclude the lLNv by assaying locomotor activity behaviour of adult males under 12:12 h light/dark (LD) cycles. LNv hyperexcited (pdfGAL4/NaChBac1) flies show increased nocturnal activity (722.4 ± 46 counts/12h: mean ± SEM) relative to control (pdfGAL4/dORK-NC1) flies (513.2 ± 34 counts/12h; Mann-Whitney U test, p < 0.001; Fig. 1A). Although daytime (counts/12 h) as well as total (counts/24 h) activity of LNv hyperexcited flies are greater than controls, this increase in activity becomes most pronounced approximately 5 h after night onset and remains high almost until the end of night (ZT 18-23). Driver control pdfGAL4/+ flies show significantly lower nocturnal activity than hyperexcited flies and do not differ from pdfGAL4/dORK-NC1 flies either in absolute daytime and night time activity level or in their relative distribution of activity during the day/night cycle (Supplementary Fig. S1). Four out of the five NaChBac transgenic lines screened show enhanced nocturnal activity along with relatively little change in daytime activity except for blunting of the morning peak (activity profile in and UAS background controls in Supplementary Fig. S1C-F). We find similar increases in nocturnal activity along with a redistribution of daytime activity when we used other GAL4 driver lines (Mz520 and Mai179) to hyperexcite the LNv neurons [18], confirming that LNv hyperexcitation increases nocturnal preference for locomotor activity (Data not shown). When we hyperexcite a large and diverse group of peptidergic neurons, including the lLNv, but excluding all other known clock neurons using c929GAL4 driver [18, 19], the enhancement of both nocturnal and daytime activity was much greater than achieved by either pdfGAL4 (compare Fig. 1 A and B) or Mz520GAL4 drivers. It is difficult to assign which neurons are responsible for driving locomotor activity using the c929GAL4 driver as it is known to target 100-300 peptidergic (PHM) neurons in the brain along with the lLNv while excluding sLNv [19, 20]. To determine the relative contribution of lLNv to these behavioural effects we used pdfGAL80 in combination with c929GAL4 thereby restricting NaChBac mediated hyperexcitation to non-lLNv PHM neurons. In this genotype non-lLNv neurons also contribute to increased day and night locomotor activity (Supplementary Fig. S2A, B). These results suggest that hyperexcitation of LNv is sufficient to increase nocturnal locomotor activity but that hyperexcitation of peptidergic neurons outside the known circadian circuit can also modulate day and night locomotor activity. This result is not surprising as up to 17 different neuropeptides are expressed in c929GAL4 neurons [20].
Figure 1. Behavioural screen of hyperexcitation in different subsets of the circadian pacemaker circuit shows hyperexcitation of lLNv is sufficient for induction of higher nocturnal locomotor activity under 12:12 LD cycles.
(A-D) Representative double-plotted actograms of control (dORK-NC1 channel) and LNv hyperexcited (expressing NaChBac1 channel) flies under the control of different GAL4 drivers. Blue shaded areas denote night while yellow shaded areas denote day. The subsets of brain neurons targeted by each of the GAL4 driver lines are indicated next to the actograms. Panels in the middle are the corresponding average profiles of mean activity levels binned across 15 mins. Black lines represent mean activity (± S.E.M) of controls and red lines are hyperexcited flies. Numbers of flies whose activity was used for analysis are in parentheses (GAL4/dORK-NC1, GAL4/Nachbac1). Panels on the right are mean (± S.E.M) day (white) and night (black) activity (counts/12h) for each genotype. Non-parametric analyses using the Kruskal-Wallis ANOVA by ranks followed by multiple comparisons, or one-way ANOVA followed by Tukey’s HSD test were used to determine significant differences, p < 0.05 are indicated by horizontal lines and asterisk. (A) pdf-GAL4 driver targets sLNv and lLNv. (B) c929-GAL4 targets lLNv in addition to nearly 300 peptidergic neurons. (C) GH146-GAL4 does not drive expression in any known circadian neurons and targets projection neurons (PN) in antennal lobe. (D) Temperature sensing (TS neurons) are targeted by dTRPA-GAL4 driver. (E) Flies expressing Kir2.1 (green) and dORK-C1 (blue) ion channels that cause electrical silencing or hypoexcitation of LNv when driven using pdfGAL4 do not show any change in nocturnal activity levels compared to controls but have significantly lower activity than NaChBac1 flies. Kir2.1 expression in the LNv (green line) causes phase advance in the evening peak (arrowhead) and disrupts anticipation in the morning activity peak.
The lLNv are light-sensing neurons [3]. To determine whether increased nocturnal activity is due to non-specific effect of NaChBac on other sensory non-clock neurons, we used the GH146GAL4 driver [21] to hyperexcite olfactory sensory projection neurons. No significant night specific enhancement of activity is seen with this driver (Fig. 1C). dTRPAGAL4 [22] driven hyperexcitation in temperature sensing neurons also does not elicit nocturnal activity (Fig. 1D). The above results suggest that hyperexcitation of different subsets of circadian pacemaker circuit that includes the LNv (and in particular, the lLNv) is sufficient to increase night time locomotor activity in flies. We also tested the effects of electrical hypoexcitation: LNv expression of either Kir2.1 or dORK-C1 does not cause any change in nocturnal activity level (Fig. 1E). However, Kir2.1 expression in the LNv (green line) causes phase advance in the evening peak (arrowhead) and disrupts anticipation in the morning activity peak (Fig. 1E, [6]). We compared daytime and night time activities in flies expressing NaChBac1, Kir2.1, dORK-C1 or control non-conducting channel dORK-NC1. While daytime activity is not different among the four genotypes, night time activity of NaChBac1 flies was significantly higher than all other genotypes (Kruskal-Wallis ANOVA by ranks, H3,62 = 20.13, p = 0.0002; multiple comparisons, p < 0.03). There was no difference in night time activity between pdfGAL4 driven dORK-NC1 controls and either Kir2.1 or dORK-C1 (Kruskal-Wallis test, H2,47 = 2.02, p = 0.36) and there is no significant contribution of genetic background to these effects (Supplementary Fig. S2C).
Reversal of the day/night pattern of peak firing rate in hyperexcited lLNv
LNv hyperexcitation evokes spontaneous giant sustained action potential firing [2, 4]. Whole cell current clamp recordings on lLNv expressing control dORK-NC1 (Fig. 2 upper panels) or NaChBac1 (Fig. 2 middle and bottom panels) compare day (left panels, ZT 1-6, Table 1) versus night firing (right panels, ZT 18-22, Table 1). Control lLNv show high proportions of high rate burst firing during the day (Fig. 2 upper left) as previously seen [4] while at night they exhibit low rate tonic or no spontaneous firing (Fig. 2, upper right panel). While tonic firing controls show on average 0.024 ± 0.002 sec action potential duration, LNv hyperexcited flies show much larger sustained depolarisations that last for 1.45 ± 0.4 sec (Students t test, t = -3.5; p = 0.007). Thus, while NaChBac induced lLNv nocturnal firing frequency is 10-fold less than controls, the depolarisation duration is 60-fold greater. LNv hyperexcited flies show significant differences in resting membrane potential and higher firing frequency at night compared to our reported day measurements [4] prepared from identical genotype flies (-103 ± 7 mV and 0.03 ± 0.005 Hz; Kolmogorov-Smirnov Test). Thus LNv hyperexcitation reverses the day/night pattern of firing frequency relative to normal controls (Table 1). lLNv net depolarisation is high during the day and low at night in wild-type flies, while in LNv hyperexcited flies, lLNv net depolarisation is higher at night than during the day. C929GAL4 driven NaChBac expression results in a qualitatively distinct firing pattern with a greater probability of the cell being in a depolarised state compared to controls (Fig. 2 bottom). Consistent with the behavioural results, there is no difference in day versus night firing frequency or resting membrane potential in C929GAL4/NaChBac.
Figure 2. NaChBac expression in LNv causes hyperexcitation and reversal of day/night pattern of net excitation in lLNv.
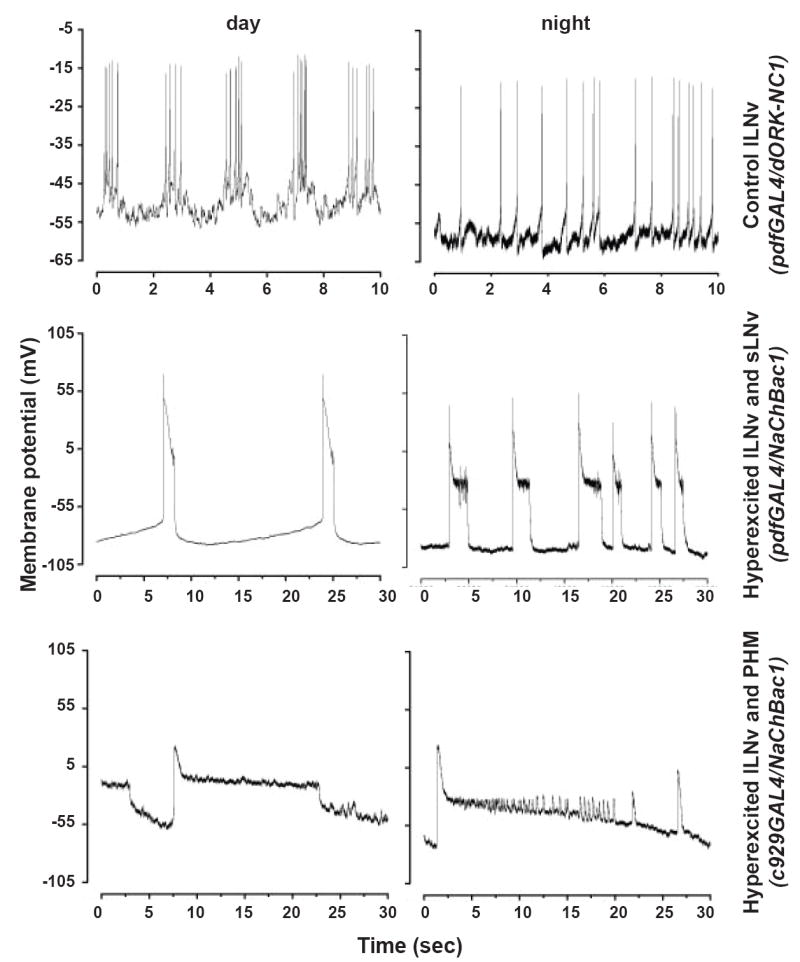
Representative traces of whole cell current clamp recordings from control (uppermost panels, expressing dORK-NC1) or hyperexcited lLNv (middle and lowermost panel, expressing NaChBac1) recorded from brains dissected during the day (left panels, ZT 1-6) or night (right panels, ZT 18-22). At night four out of six control lLNv show tonic firing with mean frequency 1.7 ± 0.5 Hz (mean ± SEM) and resting membrane potential −45 ± 3 mV (the remaining two silent lLNv showed membrane potential within the range of the other four lLNv). Hyperexcited lLNv show large spontaneous depolarisations with mean firing frequency of 0.17 ± 0.04 Hz starting from hyperpolarized resting potentials of −77 ± 14 mV (n = 5) when no other cells in the brain express NaChBac. When NaChBac is expressed using the c929GAL4 driver both day and night phases show higher probability of depolarisation with mean resting membrane potential of −78 ± 5 mV (n = 4 for day and night). (Note the differences in scales of both x and y-axes between uppermost control panels and experimental panels due to extremely large depolarisations in hyperexcited lLNv.
Table 1.
Whole cell current clamp recording of lLNv expressing control dORK-NC1 or NaChBac1 under the control of pdfGAL4 or c929GAL4 drivers made during day (ZT 1-6) and night (ZT 18-22). Resting membrane potential (RMP), firing frequency (FF) and duration of action potential (AP duration) are shown.
| DAY | NIGHT | ||
|---|---|---|---|
|
Control lLNv
(pdfGAL4/dORK-NC1) |
RMP (mV) | -41.5 ± 1.1 | -45 ± 3 |
| FF (Hz) | 3.1 ± 0.4 | 1.7 ± 0.5 | |
| AP duration (s) | 0.023 ± 0.003 | 0.024 ± 0.002 | |
|
Hyperexcited lLNv and sLNv
(pdfGAL4/NaChBac1) |
RMP (mV) | -103 ± 7 | -77 ± 14 |
| FF (Hz) | 0.03 ± 0.005 | 0.17 ± 0.04 | |
| AP duration (s) | 0.94 ± 0.2 | 1.45 ± 0.4 | |
|
Hyperexcited Llnv and PHM
(c929GAL4/NaChBac1) |
RMP (mV) | -78 ± 5 | -78 ± 5 |
| FF (Hz) | 0.3 ± 0.1 | 0.05 ± 0.01 | |
| AP duration (s) | 7.4 ± 4 | 11.2 ± 3 |
LNv hyperexcitation disrupts nocturnal sleep
The observation that LNv hyperexcitation increases nocturnal activity led us to examine whether there is a concomitant disruption in the quantity and quality of sleep. We analysed activity/sleep parameters (using 5 min binning interval) of male control and LNv hyperexcited or hypoexcited flies subjected to 12:12 LD (70-90 lux during the day phase) for ages day 5, 7, 9 and 11. To account for potential genetic background effects we also assayed respective control flies without the pdfGAL4 driver (dORK-NC1/+ and NaChBac1/+) and the driver line itself (pdfGAL4/+). The average amount of sleep per hour of LNv hyperexcited flies (pdfGAL4/NaChBac1, red line Fig. 3A) is significantly reduced throughout the night compared to controls (pdfGAL4/dORK-NC1, black line) and driver control (pdfGAL4/+, grey line). LNv hyperexcited flies sleep significantly less at night compared to all other genotypes (Fig. 3B, One way ANOVA, F4, 74 = 14.17, p < 0.001; and post-hoc multiple comparisons with Tukey’s HSD, p < 0.001). Correspondingly, mean nocturnal activity (counts/12h ± SEM) of LNv hyperexcited flies is significantly greater than all other genotypes (data not shown, One way ANOVA, F4, 71 = 12.4, p < 0.001; and post-hoc multiple comparisons with Tukey’s HSD, p < 0.001). Daytime sleep does not differ between LNv hyperexcited and control flies (data not shown). To examine whether LNv hyperexcitation induces general behavioural hyperactivity we compared the activity counts per waking minute and find no significant difference in activity levels either during the night (Fig. 3C) or day (data not shown) between control and LNv hyperexcited flies. Therefore increased night time activity counts in LNv hyperexcited flies appear to be a consequence of reduced sleep at night. LNv hyperexcited flies have significantly shorter nocturnal sleep bout duration compared to controls, indicating sleep fragmentation (Fig. 3D, Kruskal-Wallis Test: H4,77 = 24.3, p = 0.001; multiple comparisons, p < 0.02), while the number of nocturnal sleep bouts in LNv hyperexcited flies does not differ from controls (Kruskal-Wallis Test: H4,77 = 1.45; p = 0.83, data not shown). The latency of sleep onset after dusk in LNv hyperexcited flies (99.14 ± 10 min, Fig. 3E) is also significantly longer than all other genotypes tested (one-way ANOVA F4,75 = 14.5, p <0.001; Tukey’s HSD, p < 0.05). Correspondingly the amount of night time waking is significantly higher in LNv hyperexcited flies (Supplementary Fig. S3A). Thus, LNv hyperexcitation causes night time arousal and specifically modulates the quality and duration of nocturnal sleep rather than the overall level of behavioural activity in adult flies. As expected from the examination of locomotor activity, hyperexcitation of larger number of neurons using c929-GAL4 driver causes reduction in both daytime and night time sleep (Supplementary Fig. S3B). No change in sleep levels is seen either during day or night when olfactory projection neurons are hyperexcited (Supplementary Fig. S3C). Under prolonged 12:12 LD we find that pdfGAL4 driven hyperexcitation induced changes in sleep parameters are robust for up to 15 days of age after which rest levels tend to approach that of controls (Supplementary Fig. S3D-F). We also examined whether the effects of NaChBac expression is attenuated by constant light (LL) and find that hyperexcited flies continue to show decreased rest compared to controls even under LL conditions (Supplementary Fig. S3G). For comparison with results in [4], the LL result depicted in Supplementary Fig. S3G is also found when the data is analysed in 15 min bins.
Figure 3. LNv hyperexcitation disrupts nocturnal sleep.
(A) Average amount of sleep plotted for days 5, 7, 9 and 11 under 12:12 LD. Error bars are S.E.M. LNv hyperexcited flies (red line) experience significantly lower levels of sleep throughout the night phase compared to the pdfGAL4 driver (grey line) and controls expressing non-conducting dORK-NC1 channel (black line). (B) Mean night time sleep (± SEM) of LNv hyperexcited flies is significantly lower than all other genotypes (One way ANOVA, F4,74 = 14.17, p < 0.001; and post-hoc multiple comparisons with Tukey’s HSD, p < 0.001). (C) Box plot of night time intensity of activity showing LNv hyperexcited flies are not hyperactive relative to the both genetic background controls and dORK-NC1 expressing flies (Kruskal-Wallis Test: H4,80 = 4.7, p = 0.31 (D) Box plot of night time sleep bout duration showing LNv hyperexcited flies experience significantly lower duration sleep episodes relative to the both genetic background controls and dORK-NC1 expressing flies (Kruskal-Wallis Test: H4,77 = 24.3, p <0.001; multiple comparisons, p < 0.01). Median, 25-75 percentile and range are indicated. (E) Sleep latency after night onset is greater in LNv hyperexcited flies compared to all other genotypes (one-way ANOVA F4,75 = 14.5, p < 0.001; Tukey’s HSD, p < 0.05).
NaChBac in LNv phase advances the waveform of PER oscillation under LD throughout the circadian pacemaker circuit
Does LNv hyperexcitation merely phenocopy the effects of light-activation? We measured PERIOD (PER) protein levels in the sLNv, lLNv, and 5th sLNv and the four dorsal downstream neuronal groups of the circadian circuit (LNd, DN1, DN2, and DN3) in male control (pdfGAL4/dORK-NC1) and LNv hyperexcited flies (pdfGAL4/NaChBac1) at two-hour intervals through a 24-hour period under three different photoperiods (Supplementary Fig. S4). Under a wide range of LD cycles, hyperexcitation of LNv causes phase advance in PER oscillation in a majority of downstream dorsally located circadian pacemaker neurons, but in no case is clock cycling stopped. PER oscillation, while phase shifted by LNv hyperexcitation, clearly persists in all pacemaker neuronal groups in contrast to light driven synaptic inputs that induce PER degradation [23].
PDF is necessary for increased nocturnal behaviour in LNv hyperexcited flies
Since the neurotransmitter Pigment Dispersing Factor (PDF) is a key regulator of normal entrainment to LD cycles [16] and contributes to synchronization of the downstream dorsal clock neuron oscillators to changes in LNv activity [4, 8, 24-26], we determined whether PDF is required for LNv hyperexcitation induced elevated nocturnal activity counts. LNv hyperexcitation was tested both in wild type and pdf01 backgrounds (Fig. 4A) using the NaChBac4 line that enabled the creation of homozygous pdf01 mutant background [4]. Average activity profiles show that in the absence of PDF, LNv hyperexcitation does not elicit increased nocturnal behaviour (Fig. 4B left panel, green line, NaChBac4/pdfGAL4; pdf01 Kruskal-Wallis ANOVA H3,60 =24.5, p = 0.001; multiple comparisons at p < 0.05). In contrast, nocturnal activity counts are robustly enhanced when NaChBac4 is driven by pdfGAL4 in a wild-type background (Fig. 4B left, red line, NaChBac4/pdfGAL4/+). The activity profile of the driver and NaChBac4 lines in a pdf01 genetic background shows impaired anticipation to light onset as well as phase-advanced evening anticipation, both characteristic features of pdf01 flies (Fig. 4B right, dark blue line- pdfGAL4/pdf01 and grey line- NaChBac4;pdf01). Comparing activity counts of LNv hyperexcited flies in the presence and absence of PDF suggests that while daytime activity counts do not differ significantly (Fig. 4B left, red and green lines respectively, Mann-Whitney U test, p = 0.11), nocturnal activity counts of flies lacking PDF is significantly lower (612.8 ± 50 versus 251.8 ± 22 counts per night; Mann-Whitney U test, p < 0.001). However these flies still retain the PDF-null characteristic phase-advanced evening peak (Fig. 4B). Furthermore, in flies lacking PDF, the overall level of locomotor activity is lower and the amplitude of the morning activity peak is dampened (Fig. 4B), suggesting attenuation of acute lLNv light response. Thus LNv hyperexcitation drives higher locomotor activity at night via a PDF-dependent mechanism.
Figure 4. Enhanced nocturnal activity behaviour in LNv hyperexcited flies is PDF dependent.
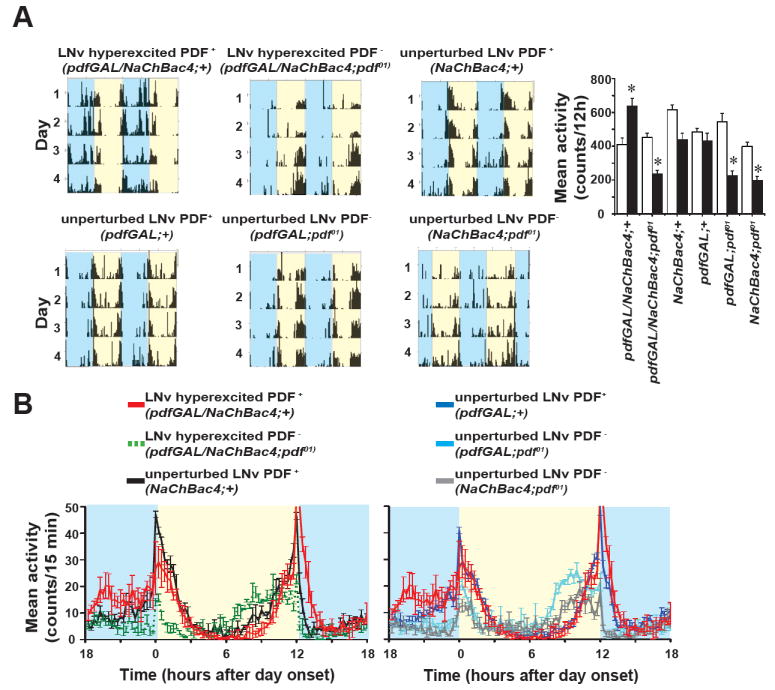
(A) Left panel shows representative actograms of LNv hyperexcitation in wild type and pdf01 backgrounds and the control driver line in pdf01 background showing LNv hyperexcitation induced enhanced nocturnal activity only in presence of PDF. Right panel gives mean (± SEM) of activity (counts/12h) during the day (white bar) and night (black bar). * indicates significantly higher night activity count of NaChBac expressing flies in presence of PDF compared to all other genotypes. * also indicates significantly lower activity of pdf01 background flies compared to wild type genetic background. (B, Left panel) Average activity profiles show that hyperexcitation of LNv by NaChBac expression cannot elicit nocturnal behaviour in the absence of PDF (green line). Black line shows wild type activity profile of the NaChBac4 line in the absence of the driver. Red line shows enhanced nocturnal activity when LNv are hyperexcited (pdfGAL4/NaChBac4) in a wild type genetic background. (B, Right panel) Background control activity profiles show profile of the pdfGAL4 driver in a pdf01 genetic background (cyan) with no anticipation of day onset and phase advanced anticipation to night onset, both characteristic features of pdf01 flies. Normal activity profile is seen in the driver line in PDF+ background (dark blue). Both overall activity level and the morning peak is reduced in the absence of PDF (grey and cyan lines) thus PDF in LNv is essential for the overall activity level and the acute response to day onset.
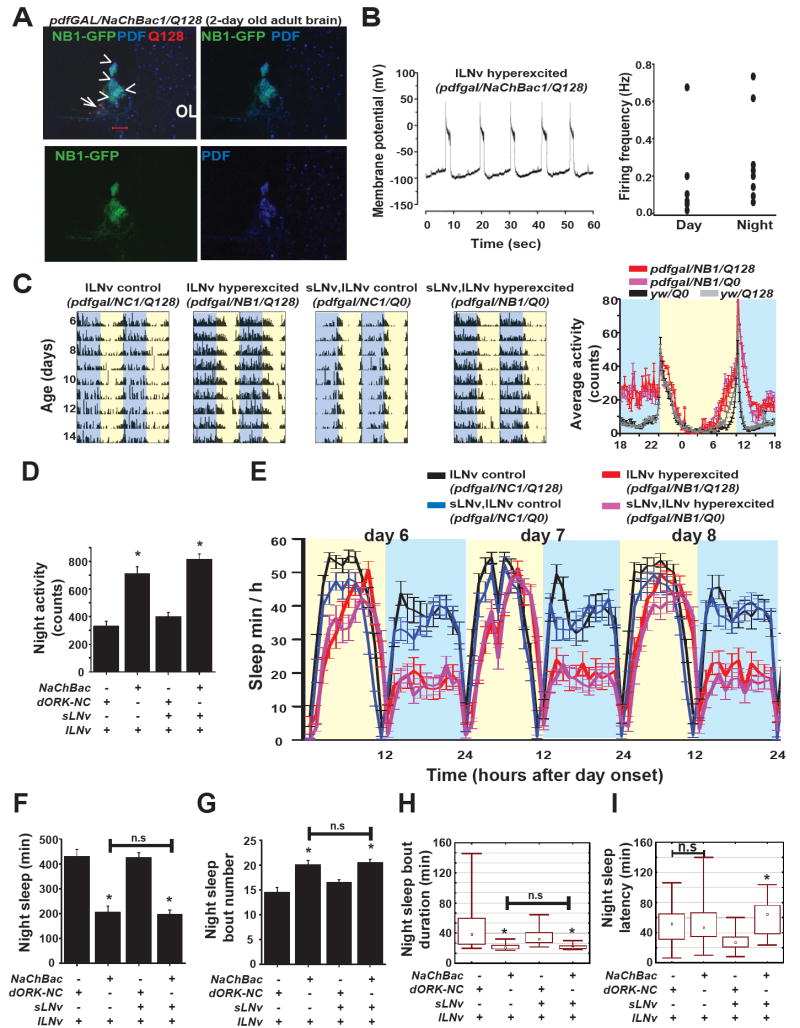
Large LNv hyperexcitation reduced nocturnal sleep in the absence of functional sLNv
The results above provoke questions regarding the relative contribution of the lLNv and sLNv subsets for LNv hyperexcitation-induced modulation of wakefulness, locomotor activity, and sleep. To narrow the effects of hyperexcitation to lLNv while simultaneously removing sLNv from the circuit, we co-expressed human Huntingtin protein with a 128-repeat poly-glutamine neurotoxic insert [27] (HTT-Q128) along with NaChBac which functionally ablated sLNv while hyperexciting lLNv (UAS-HTT-Q128/UAS-NaChBac-GFP/pdfGAL4, henceforth pdfGAL/NaChBac1/Q128). Male pdfGAL/NaChBac1/Q128 flies were dissected at 1-2 days of age post-eclosion and stained with antibodies against HTT and PDF to visualize LNv (Fig. 5A). NaChBac expression is seen as clear homogenous green fluorescence on average in 3 lLNv (n = 13 brain hemispheres). All lLNv cells in 1-2 day old pdfGAL/NaChBac1/Q128 flies also express clear PDF staining and faint diffuse HTT-Q128, and show normal gross morphology of ipsilateral and contralateral projections. In pdfGAL/NaChBac1/Q128 flies by day 1-2 post-eclosion, sLNv are completely lost with no PDF or GFP signal in the expected location in the accessory medulla where dense HTT-immunopositive aggregates are seen, suggesting the presence of cellular debris from the former sLNv. None of the sLNv-like HTT-immunopositive aggregates are associated with NaChBac-GFP. Furthermore, the functional selectivity of the Q128-induced sLNv lesion is confirmed by behavioural arrhythmicity in DD 1-2 day old flies when Q128 alone is expressed (data not shown). Whole cell current clamp recordings of lLNv of pdfGAL/NaChBac1/Q128 flies (2-7 days post eclosion) made at night (ZT 18-22) and day (ZT 1-6) confirms both NaChBac expression by the presence of characteristic large sustained depolarisation events seen in all spontaneously firing cells, and lLNv functionality. At night, the large sustained NaChBac-induced depolarisation events (present in all cells recorded) last on average for 0.7 ± 0.14 secs for each depolarisation and fire at a frequency of 0.29 ± 0.09 Hz (n= 8, Fig. 5B). This is higher than daytime firing rate (0.19 ± 0.10 Hz, n = 6). Although the increase in firing frequency at night was not statistically significant Fig. 5B right panel shows that there is a tendency for lLNv to fire at higher rates at night. Thus, in pdfGAL/NaChBac1/Q128 flies lacking functional sLNv, the lLNv subset are functional and specifically hyperexcited, showing the same general pattern of large action potential firing seen when both subsets of LNv express NaChBac [4]. PolyQ protein expression per se does not cause elevation in nocturnal activity, as shown by co-expression with control dORK-NC1 (henceforth pdfGAL/dORK-NC1/Q128, Fig. 5C,D). Moreover the elevation in nocturnal locomotor activity is not different from when non-toxic (HTT-Q0) is co-expressed with NaChBac (pdfGAL/NaChBac1/Q0, both LNv subsets are intact and hyperexcited, Fig. 5C,D). Genetic background Q0 and Q128 do not contribute to the observed changes in nocturnal activity (Fig. 5C last panel). Correspondingly, reduced nocturnal sleep persists when lLNv are hyperexcited in the absence of sLNv (Fig. 5E red line, 5F) and is indistinguishable from that observed when both sLNv and lLNv subsets are hyperexcited (Fig. 5E pink line, 5F). Thus, hyperexcitation of lLNv specifically drives enhanced nocturnal locomotor activity and reduction in sleep (Fig. 5 D-F). No contribution of sLNv hyperexcitation is required for enhancement of nocturnal locomotor activity and the presence of sLNv is not necessary for circuit conveyance of these effects of lLNv hyperexcitation. Further, nocturnal sleep in lLNv hyperexcited pdfGAL/NaChBac1/Q128 flies is more fragmented as shown by increased number of sleep bouts per night (Fig. 5G) and decreased bout duration (Fig. 5H). Comparison of night time sleep latency however shows that when lLNv alone are hyperexcited there is no increase compared to controls whereas when both LNv subsets are hyperexcited latency is significantly increased (Fig. 5I), suggesting that sLNv modulate the time of sleep onset after nightfall.
Figure 5. Large LNv drives arousal and modulate sleep independent of functional sLNv.
(A) GFP tagged NaChBac1 (Green), anti-PDF (Blue) and anti-HTT (red) staining of 2 day old adult brains of flies co-expressing NaChBac1 and HTT-Q128 (pdfgal/NaChBac/Q128). pdfGAL4 driven NaChBac-GFP and PDF are clearly seen in four lLNv while there is no NaChBac-GFP or PDF in the region where Q128 ablated sLNv are expected to be located. Scale bar = 20 mm. Arrowheads indicate lLNv and arrow points to expected location of sLNv in the accessory medulla. OL = Optic lobe. (B Left panel) Whole cell current clamp recordings during night (ZT 18-22) of lLNv co-expressing NaChBac1 and HTT-Q128 shows typical NaChBac features. (B Right panel) Scatter plot of day and night firing frequencies. (C) Representative actograms showing increased activity during the night in NaChBac expressing flies even when Q128 HTT ablates sLNv. (D) Mean night time locomotor activity is significantly higher when lLNv are hyperexcited both in absence or presence of hyperexcited sLNv (One-way ANOVA F3,121 =33.8, p < 0.001; multiple comparisons p < 0.001 for comparisons between genotypes indicated by * with all others except among themselves). (E) Profile of day and night time sleep under 12:12 LD of flies with hyperexcited lLNv alone (red line, NaChBac1/Q128, n = 30) and both-LNv hyperexcited (pink, NaChBac1/Q0, n = 32) showing decreased nocturnal sleep relative to respective controls where dORK-NC1 is co expressed with HTT-Q128 or Q0 (black, blue; n = 32 for both genotypes). (F) Mean night time sleep is significantly lower when lLNv alone (NaChBac/Q128) or when both sLNv and lLNv (NaChBac/Q0) are hyperexcited compared to controls (dORK-NC1) when either lLNv alone or both LNv are present (One-way ANOVA F3,121 =65.8, p < 0.001; multiple comparisons p < 0.001 for comparisons between genotypes indicated by * with all others except among themselves). (G) The number of night time sleep bouts is significantly higher when lLNv alone or both LNv are hyperexcited (one-way ANOVA F3,120 = 13.2, p < 0.001; Tukey’s HSD, p < 0.02 for comparisons between genotypes indicated by * with all others except among themselves). (H) Duration of the bouts of night time sleep is also significantly shorter when lLNv alone or both LNv are hyperexcited, thus quality of sleep is also significantly affected by selectively hyperexciting lLNv (Kruskal-Wallis Test: H3,124 = 66.3, p < 0.001; multiple comparisons, p < 0.001 for comparisons between genotypes indicated by * with all others except among themselves). (I) The latency of night time sleep of lLNv hyperexcited flies is not significantly greater than control flies with unperturbed lLNv alone while hyperexcitation of both LNv significantly increases sleep latency (Kruskal-Wallis Test: H3,125 = 33.1, p < 0.001; multiple comparisons, p < 0.001).
Large LNv modulate behavioural arousal
To determine whether lLNv are physiologically light sensitive when hyperexcited we conducted whole cell current clamp recordings of lLNv in flies co-expressing Q128 and NaChBac1. Unlike controls [3] which show immediate increase in firing frequency and depolarisation of membrane potential in response to 7-10 klux intensity light, NaChBac expressing lLNv do not show any detectable change in either firing frequency or resting membrane potential (Fig. 6A,B). In contrast, in sLNv ablated flies that are not hyperexcited, the lLNv are still physiologically light responsive (pdfGAL4/dORK-NC1/Q128, data not shown). These results suggest that NaChBac expression impairs the normal light response by inhibiting light driven inputs to lLNv. To test whether there is a general loss in arousal sensitivity to sensory stimuli due to such alteration in membrane properties of lLNv we subjected flies to a series of mechanical stimuli with increasing intensity during the night (5 levels, arbitrary units 1-10). As expected, control flies show a gradient in increase in response as measured by the percentage of flies woken from sleep after stimulation. NaChBac flies show a higher threshold for response to mechanical stimulation as seen by a significantly lower fraction of flies being woken up by the intermediate stimulus strength while there is no significant difference in response to lower and higher intensity of stimuli (Fig. 6C). We find that NaChBac expression alters the firing pattern of the large LNv to such an extent that it impairs multiple sensory inputs while at the same time drives output that increases arousal behaviour at night. Since the lLNv hyperexcited flies are more likely to be spontaneously awake than controls, their reduced responsiveness to stimulation is actually greater than shown. These results suggest that the membrane properties of lLNv subgroup of neurons modulate behavioural arousal in the fly brain.
Figure 6. Membrane hyperexcitation impairs lLNv mediated multisensory input.
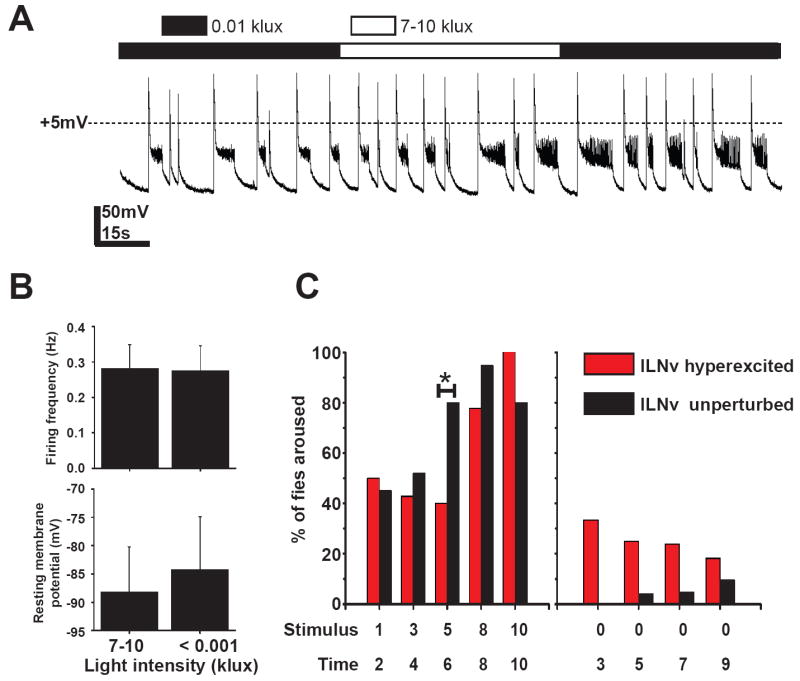
(A) Representative trace of whole cell current clamp recordings from hyperexcited lLNv in the absence of sLNv, recorded from brains dissected during the night showing no response to a 1000-fold change in light intensity. (B) Bar graphs show no change in firing frequency or resting membrane potential between the two recording conditions (mean ± SEM). (C) Hyperexcitation of lLNv reduces the sensitivity of flies to mechanical stimulation. X- axis top line denotes increasing strength of vertical mechanical stimulation (arbitrary units). The lower line values denote the time of application of stimulus in hours after onset of night. The threshold for arousal in lLNv hyperexcited flies is greater than controls as shown by the left panel where significantly lower fraction of flies can be woken up from sleep at the intermediate stimulus intensity. Right panel shows that LNv hyperexcited flies have an intrinsically higher probability of being awake during the time of the assay compared to controls.
Discussion
The temporal pattern of arousal tends to be strongly species-specific with a few notable exceptions [28]. Our studies show that alteration of the balance of day/night neuronal firing by hyperexcitation of lLNv clock neurons in D. melanogaster directs behavioural activity preference towards increased nocturnality and modulates the quantity and quality of nocturnal sleep. Furthermore, other peptidergic neurons encompassed within the c929 expression pattern modulate both day and night wakefulness and sleep. The results herein, in combination with our earlier detailed electrophysiological analysis [3] suggest that lLNv neurons constitute a light-activated arousal circuit. Further support for this is shown by decreased behavioural responsiveness to day onset in PDF-lacking flies and flies with electrically altered LNv. It has been shown previously that plasticity in temporal day/night behavioural preference in mammals and other animals can result from changes in environmental sensory time cues or manipulations that alter sensory input pathways to circadian clocks [28]. In Drosophila, mutants with partial or complete loss of photoreceptors sometimes show greater activity at night [29]. Mutations in a widely expressed putative cation channel DMα1U (narrow abdomen, na) also results in a switch from diurnal to nocturnal activity [30]. Rescue of diurnal activity was achieved by expression of wild-type channel in parts of the circuit that included lLNv [31].
In the case of NaChBac induced hyperexcitation of lLNv, the normal pattern of light driven activity during the day is reversed to a novel pattern of firing rate that favours higher activity in the night. The lLNv are not likely to be “nocturnal” neurons. Rather, they appear to drive locomotor activity according to their relative day/night pattern of excitation. Considering that the wild-type electrophysiological firing properties of lLNv are so dramatically different from NaChBac evoked firing and sustained hyperexcitation, it is reasonable to ask how NaChBac expression in LNv, and specifically lLNv subset yields such a coherent pattern of behavioural activity. The results above show that lLNv neurons modulate arousal and sleep and altering the relative pattern of day/night excitability is sufficient to evoke a temporal change in behavioural output. Based on these observations we hypothesize that the precise firing pattern or timing of lLNv electrical activity is probably not important, making NaChBac expression an appropriate tool to study these neurons and likely other modulatory circuits for which general changes in the gain of activity rather than the precise pattern of activity dictates functional output.
Our results reveal that the PDF-expressing peptidergic lLNv modulate arousal and wakeful behaviour as well as sleep stability. Considering the functional studies herein, lLNv appear to act as an arousal circuit that is physiologically activated by light and borders with, but is distinct from, the circadian pacemaker and downstream sleep circuits. A number of other recently described modulatory systems in Drosophila influence behavioural locomotion and sleep, including aminergic [32-34] and GABAergic neurons [35] (See schematic in Supplementary Fig 5). As noted, many of the overall features of morning and evening peaks in locomotor activity are retained when lLNv and other peptidergic neuronal subsets are hyperexcited. Considering the naturalistic implications, temporal niche switching has been observed in a few animals, including the social ant species Camponotus compressus. Among the worker class of these ants, some individuals are diurnal and others nocturnal, and these plastic behavioural differences are associated with differences in their underlying free-running circadian period [36]. Based on our results, we propose that it is possible that activity changes in a relatively small number of arousal neurons could influence both short-term temporal niche switching or long term evolutionary commitment to a given temporal niche.
Supplementary Material
Materials and Methods and Supplementary figure S1-S5 and legends.
Acknowledgments
We thank Benjamin White, Michael Nitabach, Jay Park, Paul Taghert, Francois Rouyer, Troy Littleton, Gillian Bates, Diane O`Dowd, Ralf Stanewsky and Jeff Hall for sharing fly lines and antibodies. We thank Diane O’Dowd and the reviewers for constructive comments on the manuscript, Paul Shaw for macros to analyse sleep parameters, Huaiyu Gu for advice on electrophysiology experiments, and Nicole Lam for assistance with behavioural experiments. While preparing this manuscript, we learned that Leslie Griffith, Michael Rosbash and colleagues had conducted similar experiments and reached similar conclusions, including that NaChBac hyperexcitation of the lLNv increases nocturnal locomotor behaviour. This work was supported by NSF grant IBN-0757242, and NIH grant NS046750 to TCH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nature Reviews. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- 2.Holmes TC, Sheeba V, Mizrak D, Rubovszky B, Dahdal D. Circuit-breaking and behavioral analysis by molecular genetic manipulation of neural activity in Drosophila. In: North G, Greenspan R, editors. Invertebrate Neurobiology. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2007. pp. 19–52. [Google Scholar]
- 3.Sheeba V, Gu H, Sharma VK, O’Dowd DK, Holmes TC. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. Journal of Neurophysiology. 2008;99:976–988. doi: 10.1152/jn.00930.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheeba V, Sharma VK, Gu H, Chou YT, O’Dowd DK, Holmes TC. Pigment dispersing factor-dependent and -independent circadian locomotor behavioral rhythms. J Neurosci. 2008;28:217–227. doi: 10.1523/JNEUROSCI.4087-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luan H, Lemon WC, Peabody NC, Pohl JB, Zelensky PK, Wang D, Nitabach MN, Holmes TC, White BH. Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila. J Neurosci. 2006;26:573–584. doi: 10.1523/JNEUROSCI.3916-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 7.Nitabach MN, Holmes TC, Blau J. Membranes, ions, and clocks: testing the njus-sulzman-hastings model of the circadian oscillator. Methods in Enzymology. 2005;393:682–693. doi: 10.1016/S0076-6879(05)93036-X. [DOI] [PubMed] [Google Scholar]
- 8.Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheeba V, Kaneko M, Sharma VK, Holmes TC. The Drosophila circadian pacemaker circuit: Pas De Deux or Tarantella? Critical Reviews in Biochemistry and Molecular Biology. 2008;43:37–61. doi: 10.1080/10409230701829128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helfrich-Förster C, Shafer OT, Wulbeck C, Grieshaber E, Rieger D, Taghert P. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol. 2007;500:47–70. doi: 10.1002/cne.21146. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko M, Hall JC. Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol. 2000;422:66–94. doi: 10.1002/(sici)1096-9861(20000619)422:1<66::aid-cne5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 13.Pitman JL, McGill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–756. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- 14.Helfrich-Förster C. Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J Comp Physiol [A] 1998;182:435–453. doi: 10.1007/s003590050192. [DOI] [PubMed] [Google Scholar]
- 15.Helfrich-Forster C, Homberg U. Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp Neurol. 1993;337:177–190. doi: 10.1002/cne.903370202. [DOI] [PubMed] [Google Scholar]
- 16.Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 17.Nitabach MN, Sheeba V, Vera DA, Blau J, Holmes TC. Membrane electrical excitability is necessary for the free-running larval Drosophila circadian clock. Journal of Neurobiology. 2005;62:1–13. doi: 10.1002/neu.20053. [DOI] [PubMed] [Google Scholar]
- 18.Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 19.Taghert PH, Hewes RS, Park JH, O’Brien MA, Han M, Peck ME. Multiple amidated neuropeptides are required for normal circadian locomotor rhythms in Drosophila. J Neurosci. 2001;21:6673–6686. doi: 10.1523/JNEUROSCI.21-17-06673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park D, Veenstra JA, Park JH, Taghert PH. Mapping peptidergic cells in Drosophila: where DIMM fits in. PLoS ONE. 2008;3:e1896. doi: 10.1371/journal.pone.0001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. Journal of neurobiology. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes & Development. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helfrich-Forster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron. 2001;30:249–261. doi: 10.1016/s0896-6273(01)00277-x. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila free-running rhythms require intercellular communication. PLoS Biology. 2003;1:E13. doi: 10.1371/journal.pbio.0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- 27.Lee WC, Yoshihara M, Littleton JT. Cytoplasmic aggregates trap polyglutamine-containing proteins and block axonal transport in a Drosophila model of Huntington’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3224–3229. doi: 10.1073/pnas.0400243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mrosovsky N, Hattar S. Diurnal mice (Mus musculus) and other examples of temporal niche switching. Journal of Comparative Physiology. 2005;191:1011–1024. doi: 10.1007/s00359-005-0017-1. [DOI] [PubMed] [Google Scholar]
- 29.Rieger D, Stanewsky R, Helfrich-Forster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. Journal of Biological Rhythms. 2003;18:377–391. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- 30.Nash HA, Scott RL, Lear BC, Allada R. An unusual cation channel mediates photic control of locomotion in Drosophila. Curr Biol. 2002;12:2152–2158. doi: 10.1016/s0960-9822(02)01358-1. [DOI] [PubMed] [Google Scholar]
- 31.Lear BC, Lin JM, Keath JR, McGill JJ, Raman IM, Allada R. The ion channel narrow abdomen is critical for neural output of the Drosophila circadian pacemaker. Neuron. 2005;48:965–976. doi: 10.1016/j.neuron.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods in Enzymology. 2005;393:759–772. doi: 10.1016/S0076-6879(05)93040-1. [DOI] [PubMed] [Google Scholar]
- 33.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking experience affects sleep need in Drosophila. Science. 2006;313:1775–1781. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 34.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 35.Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of GABA(A) receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nature Neuroscience. 2008;11:354–359. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma VK, Lone SR, Mathew D, Goel A, Chandrashekaran MK. Possible evidence for shift work schedules in the media workers of the ant species Camponotus compressus. Chronobiology International. 2004;21:297–308. doi: 10.1081/cbi-120037817. [DOI] [PubMed] [Google Scholar]
- 37.Gu H, O’Dowd DK. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. J Neurosci. 2006;26:265–272. doi: 10.1523/JNEUROSCI.4109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods and Supplementary figure S1-S5 and legends.