Abstract
Background
Major depressive disorder (MDD) has been linked to inflammation, but this association may be due to common precursors to both depression and inflammation. Myeloperoxidase (MPO) is an inflammatory enzyme produced by activated leukocytes which predicts risk of coronary heart disease. We sought to examine whether MPO and other markers of inflammation are associated with MDD, and whether the association is confounded by genetic or other shared familial factors.
Methods
We examined 178 monozygotic and dizygotic middle-aged male twin pairs. We assessed MDD with the Structured Clinical Interview for Psychiatry Disorders. Blood markers of inflammation included MPO, interleukin-6, white blood cell count, C-reactive protein, tumor necrosis factor (TNF)-α, the TNF-α soluble receptor II, and fibrinogen. Analyses were conducted in the overall sample and among 67 twin pairs discordant for MDD using mixed effects regression.
Results
Twins with a history of MDD had 32% higher levels of MPO (p<0.0001); this difference persisted after adjusting for other risk factors. Among dizygotic MDD-discordant twin pairs, twins with MDD had 77% higher MPO than their brothers without MDD after adjusting for other factors (p<0.0001). In contrast, no significant association was found in monozygotic twins (p=0.13). Similar, but weaker, associations were found between MDD and other inflammatory biomarkers.
Conclusion
MPO is a useful biomarker of immune activation in MDD. However, the association between inflammation and MDD is largely due to common genetic liability. Our results are consistent with the hypothesis that genes promoting inflammation are involved in the pathogenesis of MDD.
Keywords: Biomarkers, depression, cardiovascular disease, genetic factors, inflammation, twins
Inflammation is a key pathway in the initiation and progression of coronary heart disease (CHD), and inflammatory biomarkers such as C-reactive protein (CRP) and interleukin-6 (IL-6) have shown consistent associations with incident CHD events (1). In recent years, myeloperoxydase (MPO) has drawn growing attention as a new inflammatory biomarker of CHD risk (2–4). MPO is an enzyme produced by activated leukocytes during the innate immune response which catalyzes the formation of reactive oxidant species. It is present in human atherosclerotic plaques and exhibits a variety of proatherogenic properties (5).
Increased inflammation is a key mechanism through which several risk factors increase CHD risk (6). Depression is a risk factor for CHD (7), and whether increased inflammation is involved has attracted considerable interest (8). A role of inflammation in depression was first proposed by Smith in 1991 (9). Since then, several studies have reported a link between major depressive disorder (MDD), or depressive symptoms, and a variety of inflammatory and immune biomarkers (10–15). However, others have found no independent association (16) or mixed results (17–19), and one study even found lower levels of inflammatory biomarkers in depressed cardiac outpatients (20).
It is increasingly recognized that the relationship between depression and inflammation is more complex than initially conceived (21). Depression may cause inflammation through altered neuroendocrine function and central adiposity (22). However, depression may also be a consequence of inflammation, since a pathogenic role of inflammatory cytokines in the etiology of depression has been described (23). Although given less consideration, a third possibility is that depression is a marker of some other underlying dimension that is separately linked to depression and inflammation. Recently, it has been proposed that such underlying factor could be a specific genetic makeup (24; 25). Evidence for a common genetic substrate for depression and inflammation would be of substantial scientific and clinical interest, because it would suggest that a common biological pathway links these two conditions.
In a sample of middle-aged male twins we sought to determine, first, whether plasma MPO and other markers of inflammation are associated with MDD, and, second, whether the association is confounded by shared genetic or environmental (familial) factors. Twins provide unique opportunities to study associations while controlling for shared environmental or genetic factors, because twin siblings share genes (50%, on average, if dizygotic, and all if monozygotic), maternal factors, and early familial environment.
Methods
Subjects
The Twins Heart Study (THS) is an investigation of psychological, behavioral and biological risk factors for subclinical cardiovascular disease using twins. Twins were selected from the Vietnam Era Twin (VET) Registry (26), which includes 7,369 middle-aged male-male twin pairs both of whom served in the United States military during the time of the Vietnam War.
THS included 93 monozygotic and 87 dizygotic twin pairs who were born between 1946 and 1956. Zygosity information was determined by DNA typing in all but 11 twin pairs. The zygosity of these 11 pairs was assessed using questionnaires supplemented with blood group typing data abstracted from military records, which in our sample had an accuracy of 94%. The twins were free of symptomatic cardiovascular disease based on survey data collected in 1990 (27). Two groups of twin pairs were randomly sampled from the VET Registry: one group included depression-discordant twins, where one member of the pair had a lifetime history of MDD previously assessed with the Diagnostic Interview Schedule (28) and the other did not; the second group of twins included pairs where neither had a history of MDD. Twin pairs were examined together at the Emory University General Clinical Research Center between March 2002 and March 2006, and maintained an identical schedule during examination. This protocol was approved by the Institutional Review Board at Emory University and informed consent was obtained from all subjects.
Assessment of Depression and Other Psychiatric Diagnoses
Lifetime history of MDD was assessed using the Structured Clinical Interview for DSM IV (SCID), which yields a clinical diagnosis of major depression based on a lifetime history of major depressive episodes (29). The SCID also provided a diagnosis of other psychiatric disorders, including a lifetime history of posttraumatic stress disorder (PTSD), and a lifetime history of alcohol and of drug abuse or dependence. Current substance abuse and other psychiatric diagnoses were infrequent. We also collected information on current level of depressive symptoms using the Beck Depression Inventory-II (BDI-II) (30), a standardized scale providing a continuous measure of depressive symptoms. This self-report instrument includes 21 symptom items and the twins rated the severity of each symptom from 0 to 3. The BDI is used extensively in community samples and has satisfactory test-retest reliability and internal consistency (31).
Assessment of Inflammatory Biomarkers
MPO was measured in serum with a commercially available ELISA (CardioMPO Test, Prognostix, Cleveland, Ohio). The inter-and intra-assay variabilities were 2% and 6%. In addition to MPO, we measured a battery of other biomarkers that were previously associated with either MDD or CHD or both. These included interleukin-6 (IL-6), white blood cell count (WBC), C-reactive protein (CRP), tumor necrosis factor α (TNF- α), the TNF-α soluble receptor II (sTNF RII), and fibrinogen. IL-6, TNF-α, and sTNF RII were assessed using commercially available ELISA kits from R and D Systems. Interand intra-assay variability for these assays is <10%. CRP was measured with the Beckman Coulter High Sensitivity C-Reactive Protein assay on the Synchron LX-20 analyzer. WBC count was measured with the Beckman Coulter LH 750 hematology analyzer (Brea, CA). Fibrinogen was measured by using the Dade Behring BCS coagulation analyzer. All biochemical assays for each twin pair were processed in the same analytical run.
Other Measurements
A medical history and a physical exam were obtained from all twins. Weight and height were used to calculate the body mass index. Systolic and diastolic blood pressure was measured by standard procedures; the average of two measurements 5-minutes apart was used in the analysis. Venous blood samples were drawn for the measurements of glucose and lipid profile after an overnight fast. Total triglycerides were determined by enzymatic methods (Beckman Coulter Diagnostics, Fullerton, CA). Direct high-density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterol were measured with homogeneous assays (Equal Diagnostics, Exton, PA). Glucose was measured on the Beckman CX7 chemistry autoanalyzer. Physical activity was assessed with a modified version of the Baecke Questionnaire of Habitual Physical Activity used in the Atherosclerosis Risk in Communities (ARIC) Study (32); this is a 16-question instrument documenting level of physical activity at work, during sports and non-sports activities. The global physical activity score was used in the analysis. Cigarette smoking was classified into current versus never or past smoker. A history of CHD was defined as a previous diagnosis of myocardial infarction or angina pectoris, or previous coronary revascularization procedures. Diabetes mellitus was defined as having a fasting glucose level > 126 mg/dl or being treated with anti-diabetic medications. Information on current use of other medications was also collected.
Statistical Analysis
Initial descriptive analyses compared means and percents of study factors according to MDD status. P values were corrected for the correlation between co-twins using generalized estimating equations for categorical variables and mixed-effects models for continuous variables with a random intercept for each pair.
The association between MDD and inflammatory markers was first examined in the entire sample of twins, treating twins as separate individuals in mixed-effects models. Analyses were done before and after adjusting for factors that were significantly different according to MDD status, or that were a priori considered potentially important confounders. These included marital status, body mass index, habitual physical activity (Baecke score), antidepressant medications, previous history of CHD, and zygosity; we also adjusted for the Framingham risk score, which incorporates age, low-density-lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, blood pressure, diabetes mellitus, and current smoking (33). Because smoking status is potentially a major confounder in our analysis, we repeated our models by including current smoking as a stand-alone variable in place of the FRS, in addition to the other variables. In order to asses if current level of depressive symptoms explained the association between MDD and inflammation, we also ran models which also adjusted for the BDI score.
The association between MDD and inflammatory biomarkers, controlling for shared genetic and environmental factors, was examined in a co-twin control analysis of the MDD-discordant twin pairs using mixed-effects models. This was a matched-pair analysis in which healthy co-twins (without MDD) were used as controls for their brothers with MDD. Twins share maternal factors, familial and childhood/adolescent environment if they are raised together as in our sample. The matched nature of the co-twin control design minimizes confounding by these factors. If the paired effects are smaller than the effects seen when twins are analyzed as separate individuals, this is evidence that there is confounding by factors shared by co-twins.
In a co-twin control study, confounding by genetic factors can be inferred by stratifying the analysis according to zygosity and testing the interaction with zygosity. Monozygotic twins share 100% of their segregating genes; therefore genetic factors are fully controlled for when monozygotic twins are compared with each other. Dizygotic twins, however, only share on average 50% of genes, and therefore genetic factors are only partially accounted for when comparing dizygotic twins within a pair. Thus, if the paired effect is smaller in monozygotic than in dizygotic twins, this is evidence that the association is confounded by genetic factors. All analyses were conducted using SAS software version 9.1 (SAS Institute). Significance level was set at 0.05, two-sided.
Results
Of the 180 THS twin pairs we excluded one pair because of missing MPO data, and another pair because of implausible TNF-α values. Of the remaining 178 pairs, 67 pairs were discordant for lifetime history of MDD and 111 were not. The majority of twins with MDD were in remission, with only 8 subjects meeting DMS-IV criteria for a current major depressive episode and 77% having had the last depressive episode >1 year before examination.
Both in the entire population of twins and within the MDD-discordant twin pairs, twins with MDD were less likely to be married, more likely to smoke and to have lower levels of physical activity (Table 1). They were also more likely to have PTSD, although the number of twins with PTSD was small (N=23), and to have a higher level of depressive symptoms. There were no substantial differences in other characteristics according to MDD status, including medication use except for antidepressants. There were no significant differences in study variables between monozygotic and dizygotic twins, except that dizygotic twins were slightly older. (Supplemental Table 1)
Table 1.
Distribution of demographic, behavioral and coronary risk factors in the entire sample (n=356) and in 67 twin pairs discordant for MDD.
| All Twins |
MDD-Discordant Pairs |
|||||
|---|---|---|---|---|---|---|
| No MDD n=275 | MDD n=81 | P Value* | No MDD n=67 | MDD n=67 | P Value* | |
| Age, years, mean ± SD | 54.6 ± 2.9 | 53.6 ± 2.9 | 0.11 | 53.9 ± 2.8 | 53.9 ± 2.8 | -- |
| Greater than high school education, % | 31.3 | 22.2 | 0.15 | 31.3 | 25.4 | 0.35 |
| Married, % | 83.3 | 64.2 | 0.001 | 85.1 | 64.2 | 0.003 |
| Systolic blood pressure, mm Hg, mean ± SD | 130 ± 16.0 | 127 ± 15.0 | 0.16 | 127 ± 17.1 | 126 ± 14.0 | 0.51 |
| Diastolic blood pressure, mm Hg, mean ± SD | 81.2 ± 10.8 | 79.3 ± 10.0 | 0.18 | 79.7 ± 10.2 | 78.5 ± 9.0 | 0.38 |
| LDL-cholesterol, mg/dL, mean ± SD | 122 ± 33.6 | 124 ± 35.2 | 0.62 | 122 ± 36.2 | 124 ± 35.8 | 0.72 |
| HDL-cholesterol, mg/dL, mean ± SD | 38.4 ± 8.9 | 39.1 ± 12.0 | 0.87 | 37.6 ± 8.4 | 36.8 ± 9.5 | 0.49 |
| Diabetes, % | 7.7 | 14.8 | 0.12 | 12.1 | 13.4 | 0.72 |
| Body mass index, mean ± SD | 29.2 ± 4.8 | 29.2 ± 5.2 | 0.69 | 29.5±5.3 | 29.0 ± 4.7 | 0.47 |
| Current smoker, % | 16.7 | 32.1 | 0.001 | 16.4 | 34.3 | 0.01 |
| Framingham Risk Score | 5.7 ± 2.2 | 5.6 ± 2.1 | 0.95 | 5.4 ± 2.4 | 5.5 ± 2.1 | 0.64 |
| Physical activity (Baecke) score, mean ± SD | 7.4 ± 1.6 | 6.9 ± 2.2 | 0.02 | 7.5 ± 1.8 | 7.0 ± 2.2 | 0.12 |
| Number of alcoholic beverages in typical week, mean ± SD | 4.5 ± 7.2 | 6.3 ± 13.7 | 0.14 | 4.1 ± 5.6 | 5.1 ± 9.1 | 0.41 |
| Prior coronary heart disease, % | 9.4 | 11.1 | 0.89 | 13.4 | 10.5 | 0.52 |
| Lifetime history of PTSD, % | 4.4 | 13.6 | 0.002 | 4.5 | 14.9 | 0.03 |
| Lifetime history of alcohol abuse or dependence, % | 41.1 | 55.5 | 0.02 | 41.8 | 55.2 | 0.80 |
| Lifetime history of drug abuse or dependence, % | 16.0 | 14.8 | 0.89 | 14.9 | 16.4 | 0.11 |
| BDI score | 3.8±4.9 | 8.8±9.6 | <0.0001 | 3.9±4.7 | 8.4±9.7 | <0.0001 |
| Taking antidepressants, % | 9.1 | 32.1 | <0.0001 | 8.9 | 28.3 | 0.01 |
| Taking ace-inhibitors, % | 13.4 | 16.0 | 0.73 | 19.4 | 13.4 | 0.35 |
| Taking statins, % | 24.4 | 24.7 | 0.80 | 34.3 | 28.3 | 0.39 |
| Taking beta-blocker medications, % | 7.3 | 12.3 | 0.25 | 10.4 | 13.4 | 0.56 |
| Taking aspirin, % | 26.5 | 22.2 | 0.44 | 23.9 | 20.9 | 0.65 |
BDI: Beck Depression Inventory; MDD: Major Depression Disorder. SD: Standard Deviation.
P values are obtained from mixed models for continuous variables or generalized estimating equations (GEE) for categorical variables.
Most of the inflammatory biomarkers were mildly correlated with each other, both in the entire population and in the MDD-discordant co-twins: 90% of the correlation coefficients were <0.40 (Supplemental Table 2). MPO and TNF-α were the least correlated with other markers.
In the entire sample of twins (twins analyzed as separate individuals), MPO showed a highly significant association with MDD (Table 2), with values that were 32% higher in twins with MDD than those without MDD (p<0.0001). IL-6, WBC count, fibrinogen and sTNF RII also showed significant (or borderline significant) associations, although the magnitude of the difference was smaller than for MPO. All the biomarkers considered, except TNF-α, had numerically higher values in twins with MDD. Multivariable analysis did not materially alter the association for MPO while it attenuated the association for most of the other biomarkers.
Table 2.
Unadjusted and adjusted differences in inflammatory biomarkers in the entire sample (n=356), considering twins as separate individuals.
| Unadjusted | ||||||||
|---|---|---|---|---|---|---|---|---|
| MPO (pmol/L) | IL-6 (pg/mL) | WBC Count (1000/µL) | CRP (mg/L) | TNFα (pg/mL) | sTNF R-II (pg/mL) | Fibrinogen (mg/dL) | ||
| No MDD, mean* | 603 | 1.80 | 6.08 | 1.21 | 1.29 | 2.00 | 327 | |
| (95% CI) | (556, 654) | (1.65, 1.97) | (5.86, 6.31) | (1.03, 1.42) | (1.20, 1.39) | (1.93, 2.07) | (319, 336) | |
| MDD, mean* | 794 | 2.16 | 6.70 | 1.42 | 1.30 | 2.10 | 346 | |
| (95% CI) | (701, 899) | (1.85, 2.52) | (6.31, 7.11) | (1.10, 1.83) | (1.16, 1.46) | (1.99, 2.22) | (330, 362) | |
| % Difference | 31.7 | 20.0 | 10.2 | 17.4 | 1.0 | 5.0 | 5.8 | |
| P | <0.0001 | 0.04 | 0.002 | 0.22 | 0.93 | 0.07 | 0.03 | |
| Adjusted† | ||||||||
| MPO (pmol/L) | IL-6 (pg/mL) | WBC Count (1000/µL) | CRP (mg/L) | TNFα (pg/mL) | sTNF R-II (pg/mL) | Fibrinogen (mg/dL) | ||
| No MDD, mean* | 606 | 1.84 | 6.09 | 1.25 | 1.28 | 2.01 | 329 | |
| (95% CI) | (559, 656) | (1.69, 2.00) | (5.88, 6.30) | (1.07, 1.44) | (1.19, 1.38) | (1.94, 2.08) | (321, 338) | |
| MDD, mean* | 791 | 2.04 | 6.65 | 1.35 | 1.29 | 2.04 | 343 | |
| (95% CI) | (696, 899) | (1.74, 2.38) | (6.27, 7.06) | (1.05, 1.73) | (1.14, 1.45) | (1.93, 2.16) | (327, 359) | |
| % Difference | 30.6 | 10.6 | 9.3 | 8.3 | 1.0 | 1.6 | 4.2 | |
| P | 0.0001 | 0.26 | 0.008 | 0.56 | 0.92 | 0.57 | 0.12 | |
MPO: myeloperoxidase. IL-6: interleukin-6. WBC: white blood cell. CRP: C-reactive protein. TNFα: tumor-necrosis factor α. sTNF R-II: soluble TNFα receptor II. MDD: major depressive disorder. SE: standard error.
Geometric means calculated from means of log-transformed values.
Adjusted for marital status, body mass index, habitual physical activity (Baecke score), antidepressant medications, the Framingham risk score, zygosity and previous history of coronary heart disease.
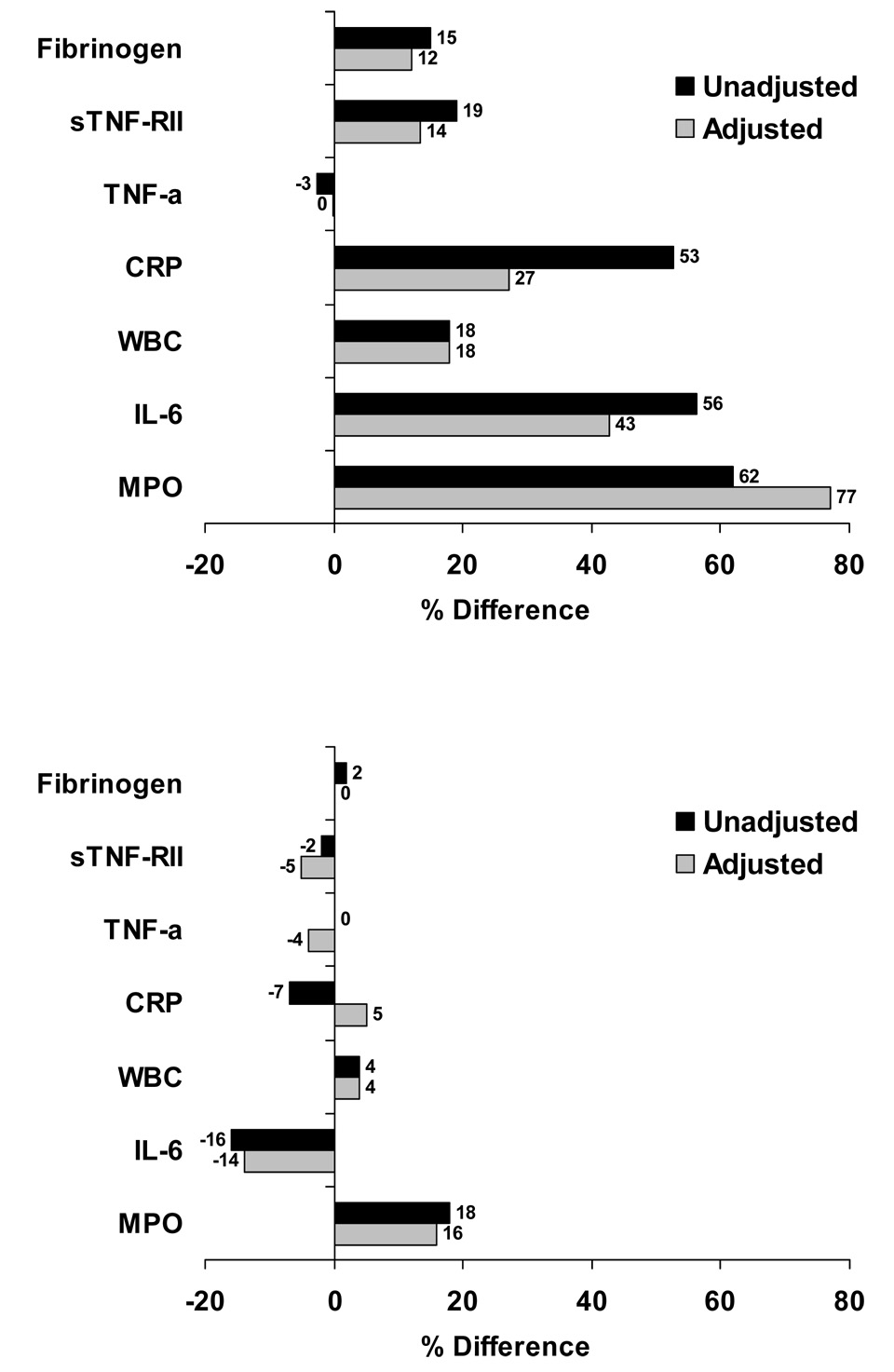
Table 3 and Figure 1 show the results of the association between MDD and inflammation from the co-twin control analysis among twin pairs discordant for MDD, stratified by zygosity. This analysis controlled by design for all factors shared by the twins. Consistent with the hypothesis of genetic confounding, larger associations between MDD and inflammatory markers were found among dizygotic twins than among monozygotic twins. Among dizygotic twins, those with MDD had significantly higher MPO, IL-6, WBC count, sTNF RII and fibrinogen levels than their brothers without MDD. Again, the difference was most marked for MPO, with 62% higher levels in MDD twins (p<0.0001). As in the analysis of the entire sample, adjustment for risk factors did not weaken the association between MPO and MDD, with 77% higher MPO levels in MDD twins in multivariable analysis. However, adjustment did attenuate the relationship of the other biomarkers, with only WBC count and (borderline) sTNF RII remaining significantly associated with MDD in multivariable analysis. In contrast, there was no evidence for an association of MDD and any of the inflammatory biomarkers in monozygotic twins. The interaction between MDD and zygosity was significant for MPO, IL-6, and sTNF RII. Although not statistically significant, the interaction p value approached significance for all the remaining biomarkers except TNF-α. These results confirm a confounding effect due to genetic factors. On the contrary, shared environment did not confound in the association between MDD and inflammation, since the paired differences in biomarker levels between dizygotic twin brothers were no less, and actually numerically larger, than in the overall analysis of twins as separate individuals.
Table 3.
Unadjusted and adjusted differences in inflammatory biomarkers in 67 twin pairs discordant for MDD. Matched co-twin control analysis.
| Unadjusted |
Adjusted |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dizygotic (32 Twin Pairs) |
Dizygotic (32 Twin Pairs) |
|||||||||||||
| MPO (pmol/L) | IL-6 (pg/mL) | WBC Count (1000/µL) | CRP (mg/L) | TNFα (pg/mL) | sTNF R-II (pg/mL) | Fibrinogen (mg/dL) | MPO (pmol/L) | IL-6 (pg/mL) | WBC Count (1000/µL) | CRP (mg/L) | TNFα (pg/mL) | sTNF R-II (pg/mL) | Fibrinogen (mg/dL) | |
| No MDD, mean* (95% CI) |
534 (439, 650) |
1.71 (1.25, 2.33) |
5.57 (5.04, 6.16) |
1.26 (0.53, 1.51) |
1.16 (0.94, 1.42) |
1.80 (1.65, 1.98) |
317 (289, 348) |
518 (427, 628) |
1.80 (1.31, 2.47) |
5.60 (5.08, 6.17) |
1.00 (0.60, 1.65) |
1.14 (0.93, 1.41) |
1.85 (1.68, 2.03) |
323 (294, 354) |
| MDD, mean* (95% CI) |
866 (712, 1053) |
2.67 (1.95, 3.64) |
6.57 (5.95, 7.27) |
1.37 (0.81, 2.30) |
1.13 (0.92, 1.39) |
2.15 (1.96, 2.35) |
365 (333, 400) |
917 (753, 1115) |
2.57 (1.86, 3.54) |
6.58 (5.95, 7.26) |
1.27 (0.76, 2.12) |
1.13 (0.92, 1.39) |
2.10 (1.91, 2.31) |
360 (328, 396) |
| P | 0.0004 | 0.03 | 0.006 | 0.12 | 0.79 | 0.005 | 0.002 | <0.0001 | 0.13 | 0.01 | 0.43 | 0.90 | 0.051 | 0.11 |
|
Monozygotic (35 Twin Pairs) |
Monozygotic (35 Twin Pairs) |
|||||||||||||
| MPO (pmol/L) | IL-6 (pg/mL) | WBC Count (1000/µL) | CRP (mg/L) | TNFα (pg/mL) | sTNF R-II (pg/mL) | Fibrinog en (mg/dL) | MPO (pmol/L) | IL-6 (pg/mL) | WBC Count (1000/µL) | CRP (mg/L) | TNFα (pg/mL) | sTNF R-II (pg/mL) | Fibrinog en(mg/dL) | |
| No MDD, mean* (95% CI) |
623 (499, 780) |
2.04 (1.62, 2.57) |
6.79 (6.17, 7.48) |
1.32 (0.87, 1.99) |
1.49 (1.27, 1.75) |
2.15 (1.96, 2.37) |
320 (301, 341) |
628 (508, 776) |
2.01 (1.65, 2.50) |
6.78 (6.16, 7.46) |
1.24 (0.84, 1.83) |
1.52 (1.29, 1.79) |
2.19 (2.00, 2.41) |
325 (305, 346) |
| MDD, mean* (95% CI) |
732 (586, 916) |
1.72 (1.36, 2.16) |
7.08 (6.42, 7.80) |
1.23 (0.81, 1.85) |
1.49 (1.27, 1.75) |
2.11 (1.92, 2.32) |
327 (306, 349) |
727 (588, 899) |
1.74 (1.40, 2.16) |
7.07 (6.42, 7.80) |
1.30 (0.89, 1.92) |
1.46 (1.24, 1.72) |
2.08 (1.89, 2.28) |
322 (301, 344) |
| P | 0.13 | 0.21 | 0.38 | 0.65 | 0.99 | 0.57 | 0.64 | 0.18 | 0.28 | 0.37 | 0.75 | 0.64 | 0.14 | 0.85 |
| P for MDD-zygosity interaction | 0.046 | 0.01 | 0.09 | 0.11 | 0.84 | 0.005 | 0.10 | 0.02 | 0.02 | 0.14 | 0.10 | 0.57 | 0.009 | 0.10 |
MPO: myeloperoxidase. IL-6: interleukin-6. WBC: white blood cell. CRP: C-reactive protein. TNFα: tumor-necrosis factor α. sTNF R-II: soluble TNFα receptor II. MDD: major depressive disorder. SE: standard error.
Geometric means calculated from means of log-transformed values.
Adjusted for marital status, body mass index, habitual physical activity (Baecke score), antidepressant medications, the Framingham risk score, and previous history of coronary heart disease.
Figure 1.
Unadjusted and adjusted percent differences in inflammatory markers in dizygotic (upper panel) and monozygotic (lower panel) twin pairs discordant for MDD, comparing twins with MDD to their co-twins without MDD. Based on data in Table 5 (see this table for p values).
When the analyses were repeated by including current smoking as a stand-alone variable in place of the FRS, the results were virtually identical and are not reported. In addition, we examined whether current level of depressive symptoms, measured with the BDI, would explain the relationship between MDD and inflammatory biomarkers among the dizygotic twins. The BDI score was not significantly correlated with MPO (r=0.02, p=0.69), but it was significantly associated with IL-6, CRP, WBC count and sTNF RII. However, adjustment for BDI score did not substantially affect the relationship between MDD and any of the biomarkers, suggesting that the relationship was not due to current level of depressive symptoms.
Discussion
We found that MDD is associated with higher levels of inflammation, and that this association is particularly robust for MPO, an inflammatory biomarker that was never studied before in relation to depression. However, we also found evidence for genetic confounding in this association. Our results are consistent with the hypothesis that there is a common genetic substrate linking MDD and inflammation, suggesting that these two phenotypes share a common pathophysiological mechanism.
MPO, Other Inflammatory Markers, and Depression
MPO is an enzyme of the innate immune system which exhibits a wide array of pro-atherogenic features (5; 34). MPO is secreted upon leukocyte activation, contributing to innate host defenses. However, it also increases oxidative stress thereby contributing to tissue damage during inflammation and atherogenesis. MPO generates numerous reactive oxidants that cause lipid peroxidation, post-translational modifications to target proteins and decrease of nitric oxide bioavailability resulting into oxidation of LDL and apolipoprotein A1, protein carbamylation and endothelial dysfunction (5; 35; 36). Transgenic mice containing the human MPO gene show significantly larger atherosclerosis build up than the wild type (34; 37). In humans, individuals with total or subtotal MPO deficiency, a defect with a frequency of 1 in every 2000 to 4000 whites, are less likely to develop cardiovascular diseases, and those harboring a promoter polymorphism associated with a 2-fold reduction in MPO expression appear cardioprotected (5; 38–40). Consistent with these pro-atherogenic properties, MPO has received growing attention as a novel risk marker for future cardiovascular events (2–4).
Oxidative stress has also been linked to neuronal degeneration in the central nervous system (41; 42). MPO is both expressed and enzymatically active in the human brain (43; 44) and is associated with Alzheimer’s disease (44). Previous studies have described abnormalities of oxidant-antioxidant systems in MDD suggestive of higher oxidative stress. For example, elevated levels of antioxidant enzymes, particularly superoxide dismutase (SOD), and biomarkers of oxidation, such as malondialdehyde, were found in plasma, red blood cells or other peripheral tissues of acutely depressed MDD patients compared with controls (45–47). In some cases (46; 47), but not others (45), these abnormalities were reduced with antidepressant treatment. SOD coenzyme concentrations are also higher in postmortem brain tissue (prefrontal cortex) of MDD patients than in control brains (48).
Overall, twins with MDD had 32% higher levels of MPO than those without MDD, an association that was not explained by other risk factors. Other inflammatory biomarkers, except TNF-α, tended to be elevated in twins with MDD; in contrast to MPO, however, these associations became weaker and mostly non-significant after adjusting for behavioral and CHD risk factors. Depressive symptoms were not correlated with MPO, and adjustment for BDI score in the analysis did not alter the results for any of the biomarkers. It is possible that MPO is a more specific indicator of brain immune activation than other inflammatory biomarkers. MPO is a myeloid-specific enzyme produced by activated phagocytic cells, including brain microglia (43). In contrast, inflammatory cytokines are produced by a variety of cell types, while acute-phase proteins such as CRP and fibrinogen are mostly secondary products in response to cytokine signaling (49). Thus, MPO is potentially a more specific marker of microglial immune activation and therefore more relevant for MDD and other brain disorders.
Genetic Influences
We found that the paired association between MDD and MPO was significantly larger in the dizygotic than in the monozygotic MDD-discordant twin pairs, a finding that was paralleled by other inflammatory biomarkers. These results suggest that the link between inflammation and depression is, at least in part, due to shared genes, perhaps genes regulating immune function and inflammatory response. Our data indicate that inflammation and depression are both expression of a common pathophysiological process linked to immune dysregulation. Our work substantiates the suspicion that there may be shared etiological mechanisms across chronic conditions, and that a key link may be a shared genetic vulnerability to inflammation (24; 25). This might be true for other chronic conditions, such as CHD, diabetes, cancer and neurodegenerative diseases (50). Our results also provide a clue for the inconsistencies in previous literature concerning the association between MDD and inflammation (21), since results may vary depending on genetic admixture and population stratification.
Previous twin and family studies have demonstrated a role of genetic factors in the liability to depression (51) and to inflammation (52; 53); however, no study has examined whether a common genetic substrate underlies vulnerability to both. Potential candidates, of course, are gene variations influencing the encoding or expression or inflammatory cytokines (25). Given our results, genetic polymorphisms influencing MPO levels could be implicated, but these variants have never been studied in relation to depression. A functional G-463A polymorphism in the promoter region of the MPO gene, which increases MPO production (54), has been associated with a variety of diseases with inflammatory components, including CHD (39) and Alzheimer’s disease (43). Data, however, are not entirely consistent, and in some studies the A variation was the risk allele (55). Other common genes that may influence both depression and inflammation include those involved in the hypothalamus-pituitary-adrenal (HPA) axis, or in the sympathetic, parasympathetic and serotonin pathways, which have been related to stress and depression (51) and may be relevant to immune regulation secondary to the stress response (53; 56).
Limitations
Our study is cross-sectional, thus limited in the ability to discern the temporal order between MDD and inflammation. However, based on our results, the covariation of these two phenotypes may mostly be due to a common genetic precursor rather than a cause-effect relationship. Another limitation is that few twins met the criteria for a current major depressive episode, preventing us from examining the data in relation to current versus past depression. However, there was no relationship between MPO and current depressive symptoms measured with the BDI, and although other inflammatory markers were related to the BDI score, adjustment for BDI did not alter the results. These data suggest that the link between depression and inflammation is due to common genetic liability between MDD, a heritable trait, and inflammation, and that current depressive symptoms do not play a role. Furthermore, the finding that a relationship between MDD and inflammation is seen in our sample of persons mostly in remission indicates that such a relationship may be of significance for chronic consequences of MDD such as CHD. Lastly, because our twins were all middle-aged male military veterans, caution should be used in generalizing our results to women or older individuals. Our study provides the foundation for future investigations where our findings can be confirmed in diverse sociodemographic groups.
Conclusions
Inflammation is a correlate of MDD largely because of common genetic liability. Of several biomarkers, MPO, a specific marker of leukocyte activation, shows the strongest association with MDD. Inflammatory genes involved in MPO expression or in other aspects of immune/inflammatory regulation should be investigated as potential precursors of MDD. Overall, our results are consistent with the hypothesis that inflammation and depression are both expression of a common pathophysiological process linked to immune dysregulation.
Supplementary Material
Acknowledgments
The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance, including: VA Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; NIH; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University.
We gratefully acknowledge the continued cooperation and participation of the members of the Vietnam Era Twin Registry. Without their contribution this research would not have been possible.
Funding Sources
This study was supported by K24HL077506, R01 HL68630 and R01 AG026255, and P01 HL076491 from the National Institutes of Health; by the Emory University General Clinical Research Center MO1-RR00039 and by grant 0245115N from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
Dr. Hazen is named as co-inventor on patents filed by the Cleveland Clinic Foundation that relate the use of biomarkers, including myeloperoxidase, for inflammatory and cardiovascular diseases. Dr. Hazen is the scientific founder of PrognostiX Inc. Dr Hazen has received honoraria for educational presentations from Merck, BioSite, Abbott, Pfizer, Lilly, Wyeth, GlaxoSmithKline and AstraZeneca. He has served as a consultant for PrognostiX, Wyeth, Merck, Pfizer, Biophysical, and is on the advisory board of PrognostiX.
All the other authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 2.Brennan M-L, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 3.Zhang R, Brennan M-L, Fu X, Aviles RJ, Pearce GL, Penn MS, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 4.Meuwese MC, Stroes ES, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;50:159–165. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 6.Danesh J, Muir J, Wong YK, Ward M, Gallimore JR, Pepys MB. Risk factors for coronary heart disease and acute-phase proteins. A population-based study. Eur Heart J. 1999;20:954–959. doi: 10.1053/euhj.1998.1309. [DOI] [PubMed] [Google Scholar]
- 7.Wulsin LR. Is depression a major risk factor for coronary disease? A systematic review of the epidemiologic evidence. Harv Rev Psychiatry. 2004;12:79–93. doi: 10.1080/10673220490447191. [DOI] [PubMed] [Google Scholar]
- 8.Kop WJ, Gottdiener JS. The role of immune system parameters in the relationship between depression and coronary artery disease. Psychosom Med. 2005;67:S37–S41. doi: 10.1097/01.psy.0000162256.18710.4a. [DOI] [PubMed] [Google Scholar]
- 9.Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- 10.Empana JP, Sykes DH, Luc G, Juhan-Vague I, Arveiler D, Ferrieres J, et al. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: The Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2005;111:2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- 11.Kling MA, Alesci S, Csako G, Costello R, Luckenbaugh DA, Bonne O, et al. Sustained low-grade pro-inflammatory state in unmedicated, remitted women with major depressive disorder as evidenced by elevated serum levels of the acute phase proteins C-reactive protein and serum amyloid A. Biol Psychiatry. 2007;62:309–313. doi: 10.1016/j.biopsych.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danner M, Kasl SV, Abramson JL, Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom Med. 2003;65:347–356. doi: 10.1097/01.psy.0000041542.29808.01. [DOI] [PubMed] [Google Scholar]
- 13.Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol. 2002;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- 14.Dentino AN, Pieper CF, Rao KMK, Currie MS, Harris T, Blazer DG, et al. Association of interleukin-6 and other biological variables with depression in older people living in the community. J Am Geriatr Soc. 1999;47:6–11. doi: 10.1111/j.1532-5415.1999.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 15.Penninx BWJH, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, et al. Inflammatory markers and depressed mood in older persons: results from the health, aging and body composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 16.Steptoe A, Kunz-Ebrecht SR, Owen N. Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychol Med. 2003;33:667–674. doi: 10.1017/s0033291702007250. [DOI] [PubMed] [Google Scholar]
- 17.Tiemeier H, Hofman A, van Tuijl HR, Kiliaan AJ, Meijer J, Breteler MM. Inflammatory proteins and depression in the elderly. Epidemiology. 2003;14:103–107. doi: 10.1097/00001648-200301000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Lesperance F, Frasure-Smith N, Theroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and Creactive protein in patients With recent acute coronary syndromes. Am J Psychiatry. 2004;161:271–277. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- 19.Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, et al. Inflammation and coagulation factors in persons >65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89:419–424. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 20.Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314–320. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glassman AH, Miller GE. Where there is depression, there is inflammation sometimes…sometimes! Biol Psychiatry. 2007;62:280–281. doi: 10.1016/j.biopsych.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 22.Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53:897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 23.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Geus EJ. Genetic pleiotropy in depression and coronary artery disease. Psychosom Med. 2006;68:185–186. doi: 10.1097/01.psy.0000208628.90274.bc. [DOI] [PubMed] [Google Scholar]
- 25.McCaffery JM, Frasure-Smith N, Dube MP, Theroux P, Rouleau GA, Duan Q, et al. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med. 2006;68:187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Res. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 27.Scherrer JF, Xian H, Bucholz KK, Eisen SA, Lyons MJ, Goldberg J, et al. A Twin study of depression symptoms, hypertension, and heart disease in middle-aged men. Psychosom Med. 2003;65:548–557. doi: 10.1097/01.psy.0000077507.29863.cb. [DOI] [PubMed] [Google Scholar]
- 28.Robins LM, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 29.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM IV-Patient Edition (SCID-P) Washington, D.C.: American Psychiatric Press; 1995. [Google Scholar]
- 30.Beck AT, Steer RA, Brown GK. BDI-II. Beck Depression Inventory: Second Edition. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 31.Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL. Reliability and validity of the Beck depression inventory--II with adolescent psychiatric inpatients. Psychol Assess. 2004;16:120–132. doi: 10.1037/1040-3590.16.2.120. [DOI] [PubMed] [Google Scholar]
- 32.Richardson MT, Ainsworth BE, Wu H, Jacobs DR, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisuretime physical activity. Int J Epidemiol. 1995;24:685–693. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 33.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbeshatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 34.McMillen TS, Heinecke JW, LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 2005;111:2798–2804. doi: 10.1161/CIRCULATIONAHA.104.516278. [DOI] [PubMed] [Google Scholar]
- 35.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 37.Castellani LW, Chang JJ, Wang X, Lusis AJ, Reynolds WF. Transgenic mice express human MPO −463G/A alleles at atherosclerotic lesions, developing hyperlipidemia and obesity in −463G males. J Lipid Res. 2006;47:1366–1377. doi: 10.1194/jlr.M600005-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Asselbergs FW, Reynolds WF, Cohen-Tervaert JW, Jessurun GA, Tio RA. Myeloperoxidase polymorphism related to cardiovascular events in coronary artery disease. Am J Med. 2004;116:429–430. doi: 10.1016/j.amjmed.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 39.Nikpoor B, Turecki G, Fournier C, Theroux P, Rouleau GA. A functional myeloperoxidase polymorphic variant is associated with coronary artery disease in French-Canadians. Am Heart J. 2001;142:336–339. doi: 10.1067/mhj.2001.116769. [DOI] [PubMed] [Google Scholar]
- 40.Pecoits-Filho R, Stenvinkel P, Marchlewska A, Heimburger O, Barany P, Hoff CM, et al. A functional variant of the myeloperoxidase gene is associated with cardiovascular disease in end-stage renal disease patients. Kidney Int Suppl. 2003:S172–S176. doi: 10.1046/j.1523-1755.63.s84.32.x. [DOI] [PubMed] [Google Scholar]
- 41.Koutsilieri E, Scheller C, Grunblatt E, Nara K, Li J, Riederer P. Free radicals in Parkinson's disease. J Neurol. 2002;249 Suppl 2:II1–II5. doi: 10.1007/s00415-002-1201-7. [DOI] [PubMed] [Google Scholar]
- 42.Grunblatt E, Schlosser R, Fischer P, Fischer MO, Li J, Koutsilieri E, et al. Oxidative stress related markers in the "VITA" and the centenarian projects. Neurobiol Aging. 2005;26:429–438. doi: 10.1016/j.neurobiolaging.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds WF, Rhees J, Maciejewski D, Paladino T, Sieburg H, Maki RA, et al. Myeloperoxidase polymorphism is associated with gender specific risk for Alzheimer's disease. Exp Neurol. 1999;155:31–41. doi: 10.1006/exnr.1998.6977. [DOI] [PubMed] [Google Scholar]
- 44.Green PS, Mendez AJ, Jacob JS, Crowley JR, Growdon W, Hyman BT, et al. Neuronal expression of myeloperoxidase is increased in Alzheimer's disease. J Neurochem. 2004;90:724–733. doi: 10.1111/j.1471-4159.2004.02527.x. [DOI] [PubMed] [Google Scholar]
- 45.Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Human psychopharmacology. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 46.Lukash AI, Zaika VG, Kucherenko AO, Miliutina NP. Free radical processes and antioxidant system in depression and treatment efficiency. Zh Nevrol Psikhiatr Im S S Korsakova. 2002;102:41–44. [PubMed] [Google Scholar]
- 47.Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 48.Michel TM, Frangou S, Thiemeyer D, Camara S, Jecel J, Nara K, et al. Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder--a postmortem study. Psychiatry Res. 2007;151:145–150. doi: 10.1016/j.psychres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 50.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 51.Levinson DF. The genetics of depression: a review. Biol Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 52.de Maat MPM, Bladbjerg EM, von Bornemann Hjelmborg J, Bathum L, Jespersen J, Christensen K. Genetic influence on inflammation variables in the elderly. Arterioscler Thromb Vasc Biol. 2004;24:2168–2173. doi: 10.1161/01.ATV.0000143856.01669.e7. [DOI] [PubMed] [Google Scholar]
- 53.Wessel J, Moratorio G, Rao F, Mahata M, Zhang L, Greene W, et al. C-reactive protein, an 'intermediate phenotype' for inflammation: human twin studies reveal heritability, association with blood pressure and the metabolic syndrome, and the influence of common polymorphism at catecholaminergic/beta-adrenergic pathway loci. J Hypertens. 2007;25:329–343. doi: 10.1097/HJH.0b013e328011753e. [DOI] [PubMed] [Google Scholar]
- 54.Piedrafita FJ, Molander RB, Vansant G, Orlova EA, Pfahl M, Reynolds WF. An Alu element in the myeloperoxidase promoter contains a composite SP1-thyroid hormoneretinoic acid response element. J Biol Chem. 1996;271:14412–14420. doi: 10.1074/jbc.271.24.14412. [DOI] [PubMed] [Google Scholar]
- 55.Pope SK, Kritchevsky SB, Ambrosone C, Yaffe K, Tylavsky F, Simonsick EM, et al. Myeloperoxidase polymorphism and cognitive decline in older adults in the Health, Aging, and Body Composition Study. Am J Epidemiol. 2006;163:1084–1090. doi: 10.1093/aje/kwj146. [DOI] [PubMed] [Google Scholar]
- 56.Sanders VM, Straub RH. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav Immun. 2002;16:290–332. doi: 10.1006/brbi.2001.0639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.