SUMMARY
Holoprosencephaly (HPE), the most common forebrain malformation, is characterized by an incomplete separation of the cerebral hemispheres. Mutations in the homeobox gene SIX3 account for 1.3% of all cases of human HPE. Using zebrafish-based assays, we have now determined that HPE-associated Six3 mutant proteins function as hypomorphs. Haploinsufficiency of Six3 caused by deleting one allele of Six3 or by replacing wild-type Six3 with HPE-associated Six3 mutant alleles was sufficient to recapitulate in mouse models most of the phenotypic features of human HPE. We demonstrate that Shh is a direct target of Six3 in the rostral diencephalon ventral midline (RDVM). Reduced amounts of functional Six3 protein fail to activate Shh expression in the mutant RDVM and ultimately lead to HPE. These results identify Six3 as a direct regulator of Shh expression and reveal a cross-regulatory loop between Shh and Six3 in the ventral forebrain.
Keywords: holoprosencephaly, Six3, Shh, mouse, telencephalon, zebrafish
INTRODUCTION
Holoprosencephaly (HPE), the incomplete separation of the cerebral hemispheres, is the most common human forebrain malformation and is classified into three subtypes (alobar, semilobar, and lobar) based on the severity of the defects (DeMyer, 1977).
The cause of HPE in humans is heterogeneous and pathogenetically variable. Mutations in nine genes have been identified in HPE patients (Cohen, 2006). Three genes, SHH, PTCH1, and GLI2, belong to the Sonic Hedgehog (Shh) signaling pathway (Roessler and Muenke, 2003). Mutations in SHH account for about 17% of familial cases and about 3.7% of all cases of HPE (Cohen, 2006). In mice, Shh expression in the prechordal plate (PrCP) causes the separation of the two eye fields and the proper development of the ventral forebrain (Chiang et al., 1996). Consequently, the lack of Shh function results in cyclopia, the most severe form of HPE (Chiang et al., 1996). However, not much is yet known about the identity of upstream regulators of Shh expression in the ventral forebrain. Mutations in the homeobox gene SIX3 account for 1.3% of all cases of HPE (Cohen, 2006). Like other HPE genes, SIX3 mutations cause a wide spectrum of defects ranging from cyclopia to microforms (e.g., single median maxillary central incisor) (Muenke and Cohen, 2000). Moreover, a single SIX3 mutation can cause HPE with variable penetrance and expressivity such that a normal parent of a patient with HPE also harbors the mutation. This finding suggests that additional genetic or environmental factors are required to cause HPE (Muenke and Cohen, 2000).
SIX3 is the only HPE gene that has not been directly or indirectly linked to the Shh signaling pathway (Cohen, 2006), and the molecular mechanisms by which mutations in this gene lead to HPE remain unknown. During mouse development, Six3 is initially expressed throughout the anterior neural ectoderm (ANE). However, later it becomes restricted to the ventral forebrain and eye field (Oliver et al., 1995). Functional studies in mice revealed that Six3 protects the ANE from the posteriorizing activity of Wnt1 via direct repression of Wnt1 transcription (Lagutin et al., 2003). Consequently, the anterior expansion of canonical Wnt signaling in Six3-null embryos results in the absence of the telencephalon and rostral diencephalon (Lagutin et al., 2003).
Here, we used zebrafish-based functional assays to determine that HPE-associated Six3 mutations result in decreased activity of Six3. Detailed characterization of mouse models of Six3-promoted HPE demonstrated that Six3 directly activates Shh expression in the rostral diencephalon ventral midline (RDVM) and that in turn Shh maintains Six3 expression in the RDVM; deletion of one allele of Six3 or replacement of wild-type Six3 by HPE-associated mutant Six3 disrupt this positive regulatory loop and leads to HPE.
RESULTS
HPE-associated Six3-mutant proteins behave as hypomorphs
Eighteen SIX3 mutations have been identified in HPE-affected individuals. Eleven mutations are located in the Six domain; six, in the homeodomain; and one, in the C-terminal region (Wallis et al., 1999; Nanni et al., 2000; Pasquier et al., 2000; Dubourg et al., 2004; Pasquier 2005; Ribeiro et al., 2006). Of those 18 mutations, we selected five that were identified in cases of familial HPE for further analysis (Figure S1A). Six3V92G and Six3H173P have a single amino acid mutation in the Six domain and Six3GGins contains a GG insertion in the Six domain that is predicted to cause premature termination of translation in the homeodomain (Dubourg et al., 2004; Pasquier et al., 2005). Six3V250A and Six3R257P harbor a single amino acid substitution in the third helix of the homeodomain (Wallis et al., 1999).
To unravel the mechanisms by which these Six3 mutations lead to HPE in humans, we first performed zebrafish-based in vivo assays aimed to identify possible changes in the activities of these mutant proteins. To do this, these five HPE-associated SIX3 mutations were introduced into six3b, the zebrafish homolog of mammalian Six3 (Kobayashi et al., 1998), and their activity was compared to that of wild-type Six3b in three different assays. The first assay relied on our previous observation that injection of synthetic RNA encoding mouse Six3 rescued the eyeless phenotype of headless/tcf3 (hdl−/−) zebrafish mutant embryos in which wnt1 expression is anteriorly expanded (Lagutin et al., 2003; Kim et al., 2000). As shown in Figure 1A–D, similar phenotypic rescue was achieved by injections of very low doses of six3b RNA; RNAs encoding all five Six3b HPE-related mutant proteins also rescued the hdl eyeless phenotype, some almost as efficiently as wild-type Six3b (Six3V92G), others ones only when used at higher doses. In a second assay we tested the ability of these mutant forms to suppress the eyeless phenotype of zebrafish embryos deficient in two six3-related genes, six3b and six7 (Inbal et al., 2007). Similar to the results obtained in the first assay, some of the mutants suppressed the eyeless phenotype of six3b;six7-deficient embryos only when used at higher doses (Figures 1E–1I). However, some mutant forms were unable to suppress the eyeless phenotype even at high doses, thereby showing no activity in this assay (Figures 1E–1I). In a final assay we took advantage of the fact that injections of synthetic six3b RNA result in dorsalization of the zebrafish embryo (Figures S1B–S1Q), which is mostly likely due to the ability of Six3 to repress Wnt and BMP expression (Lagutin et al., 2003; Gestri et al., 2005). The mutant proteins showed a spectrum of hypomorphic activities that ranged from a slightly decreased ability to an inability to dorsalize embryos at the injected doses (Figures S1B–1Q).
Figure 1.
HPE-associated Six3 mutants function as hypomorphs. (A–D) The five generated six3b HPE-related mutant forms can rescue the headless phenotype in injected hdl/tcf3−/− mutant embryos. ddH2O and mGFP (250 pg) were injected as negative controls. Rescue was scored at 55 h post-fertilization (hpf) according to three phenotypic categories: no rescue (yellow) (A), partial rescue (light blue) (B), and full rescue (dark blue) (C). The results are graphically represented in D, which shows the samples and doses injected. (E–H) The six3b mutant forms show hypomorphic activity in suppressing the eyeless phenotype of six3b;six7 mutant morphants. The six3bvu87/vu87 mutants injected with six7 antisense morpholino oligonucleotide (MO) exhibit an eyeless phenotype. After coinjection of six3b mutants with six7 MO and six3b wild-type or mutant forms, embryos were graded according to the size of their eyes (0–6.5 point (pt) scale). (E–H) Examples are shown of absent eyes (0 pt), severely reduced eyes (arrowhead) (1.2 pt), moderately reduced eyes (3.0 pt), and full-size eyes (5.3 pt), respectively. (I) Graphical interpretation of these results. The E109Stop-null mutation found in six3bvu87 mutants was overexpressed as a negative control. Double asterisks indicate eye size significantly different from that of six3bvu87/vu87-six7 mutant-morphants (p≤0.01). The absence of asterisks on mutant form-injected samples indicates a p-value that exceeds 0.05 (statistically insignificant). (J) Summary of the three zebrafish-based functional assays. Five HPE-associated Six3 mutants can be separated into three groups based on their hypomorphic activity.
In summary, based on our results from the 3 assays, the five HPE-associated Six3 mutant proteins can be separated into three groups (Figure 1J). Group 1: Six3bV92G shows the strongest hypomorphic activity; Group 2: Six3bV250A and Six3bH173P exhibit a moderate level of hypomorphic activity; Group 3: Six3bGGins and Six3bR257P show only a residual activity, as they rescue the hdl phenotype at higher doses, and fail to suppress the six3b;six7-deficient phenotype or dorsalize wild-type embryos. Hence, although at different degrees, all five HPE-associated Six3 mutant proteins behave as hypomorphs in vivo.
Haploinsufficiency of Six3 causes HPE
We previously reported that Six3-heterozygous mice lack any obvious HPE-like phenotype in the used outbred background (Lagutin et al., 2003). However, recent results from other mouse models of HPE have shown strain-specific severity and that pronounced HPE phenotypes are observed in the inbred C57BL/6 mouse strain (Petryk et al., 2004; Zhang et al., 2006). Hence, to determine whether some of the Six3-mutant proteins also function as hypomorphs in mammals, we generated two strains of mutant mice. In one strain we replaced the endogenous wild-type Six3 with the HPE-associated Six3V250A mutation that exhibited strong hypomorphic activity in the zebrafish assays. Because our previously reported Six3+/− strain is maintained in an outbred background, we decided to generate an additional Six3 mutant strain by replacing Six3 with CreERT2 to overcome the time-consuming backcrossing process; this strain could eventually be used for lineage tracing and conditional deletion. Both strains are now maintained in the C57BL/6 inbred background. We reasoned that if, as suggested by the zebrafish experiments described above, the Six3V250A functions as a hypomorph, then Six3V250A-heterozygous and Six3-CreERT2-heterozygous mice (Six3+/−) should exhibit some features of the HPE-like phenotype. However, if Six3V250A functions as a Six3 antagonist, then only mice carrying this mutation should exhibit HPE.
To generate the Six3V250A conditional knock-in Six3+/cki strain, we introduced the single base pair substitution T749C into the first exon of Six3. In addition, we inserted a floxed cassette containing a transcription termination sequence (STOP) (Lakso et al., 1992) and a neomycin-resistance gene upstream of the first exon (Figures S2A–S2D). To remove the STOP and activate transcription of Six3V250A, we crossed Six3V250A (Six3+/cki)-heterozygous mice (in a 129/Sv and C57BL/6 mixed background) with an available Bf1-Cre strain, which in the 129/SvJ background exhibits Cre recombinase activity in the telencephalon from embryonic day (E) 8.5 onwards (Hebert and McConnell, 2000). No obvious phenotypic abnormalities were observed in the generated Six3+/cki;Bf1-Cre double-heterozygous embryos.
In a mixed background, Bf1-Cre mice reportedly exhibit variable levels of Cre activity outside the regions of endogenous Bf1 expression (Hebert and McConnell, 2000). Furthermore, we identified variable levels of Cre activity also in the germ line (unpublished observations). Therefore, we took advantage of this variable activity of Bf1-Cre in the mixed background to permanently delete the STOP and neomycin-resistance cassettes in the germ line and generate the Six3V250A knock-in mouse line Six3+/ki. Six3+/cki;Bf1-Cre double-heterozygous mice maintained in the mixed background were crossed with the Six3+/cki mice, and pups were genotyped by PCR (Figures S2A–S2D). Approximately 10% of the Six3+/ki embryos and pups generated using this strategy exhibited HPE-like phenotypes in the 129/Sv and C57BL/6 mixed background (Figures S3G and S3I). However, no phenotypic alterations were observed in an outbred background (data not shown). Furthermore, the anterior diencephalon (prethalamus and thalamus) that was absent in Six3−/− embryos (Lagutin et al., 2003; Lavado et al., 2008) was present in Six3ki/ki embryos (Figures S4A–S4L). This result supports the proposal that in mammals Six3V250A functions as a hypomorph.
The Six3-CreERT2 strain (Six3+/−) was generated by replacing in-frame the 22-nucleotide-long NcoI-BglII restriction fragment of Six3 exon 1 with CreERT2 cDNA (Figure S2E and S2F). Upon backcrossing this mouse strain into the C57BL/6 background, 16% of the F1 Six3+/− embryos exhibited HPE-like phenotypes (Figure S3J–S3L). When backcrossed further into the C57BL/6 background, more than 85% of the F3 Six3+/− embryos exhibited HPE-like phenotypes that were indistinguishable from those of Six3+/ki embryos in the 129/Sv and C57BL/6 mixed background. These results suggest that haploinsufficiency of Six3 causes HPE in a background-dependent manner.
Next, to further investigate Six3 dosage effects on the pathogenesis of HPE, we determined the amount of Six3 transcripts present in 8- to- 10-somite stage wild-type, Six3+/− and Six3+/ki.embryos using real-time PCR. The primers and probes used for this analysis were specific for either the Six3V250A transcript or for both, the wild-type and Six3V250A transcripts. As shown in Figure S4M, Six3+/− embryos contained approximately half of the amount of Six3 transcripts present in wild-type littermates; Six3+/ki and Six3+/− embryos contained similar amounts of wild-type Six3 and Six3+/ki embryos contained similar amounts of wild-type and Six3V250A transcripts. Therefore, as Six3V250A exhibits hypomorphic activity, the combined Six3 activity provided by wildtype and hypomorphic (Six3V250A) Six3 should be higher in Six3+/ki embryos than in Six3+/− embryos. This may explain why in comparison to Six3+/− embryos, fewer Six+/ki embryos exhibited the HPE-like phenotype.
To determine whether the hypomorphic activity of the Six3 mutant proteins was caused by reduced stability, we transfected NIH 3T3 cells with plasmids expressing various mutant forms of Six3 and analyzed the protein lysates 48 h later by Western blot. As shown in Figure S4N and with the exception of Six3GGins (premature termination of translation), the rest of the analyzed Six3 mutant proteins were expressed at comparable levels, a result indicating that their stability is not affected.
In conclusion, the 5 tested mutations affect Six3 function without affecting the stability of its mRNA or its protein. The amount of functional Six3 is crucial for patterning the telencephalon; consequently, Six3 haploinsufficiency results in HPE. To generate a mouse model that closely resembles human HPE and unless otherwise stated, all subsequent experiments were performed using the Six3+/ki strain.
Six3 cooperates with Shh in the genesis of HPE
The low percentage of Six3+/ki embryos exhibiting HPE-like phenotypes and strict background dependence are consistent with the general low penetrance of familial HPE, suggesting that other genetic or environmental modifiers are involved in the pathogenesis of HPE. All HPE-associated genes but Six3 have been shown to directly or indirectly affect Shh signaling, and Shh mutant embryos exhibit cyclopia. Therefore, we hypothesized that this pathway could function synergistically with SIX3 mutations in the pathogenesis of HPE. To test this possibility, we crossed Six3+/ki mice with phenotypically normal Shh-heterozygous mice (Shh+/−). From these crosses, approximately 75% of the isolated Six3+/ki;Shh+/− embryos exhibited obvious HPE-like phenotypes in the 129/Sv and C57BL/6 mixed background (Figure 2). This frequency increased to 100% when we used a C57BL/6 background (backcrossed for five generations). The HPE-like phenotype was observed in 100% of the Six3+/−;Shh+/− embryos when using the Sv129 and C57BL/6 mixed background. The phenotype observed in these HPE-like mutant embryos was similar to that of Six3+/ki or Six3+/− embryos described above (compare Figures 2I and 2K with Figures S3G–S3L and Figures S3M–S3O). These results demonstrated that Six3 and Shh synergistically contribute to the pathogenesis of HPE.
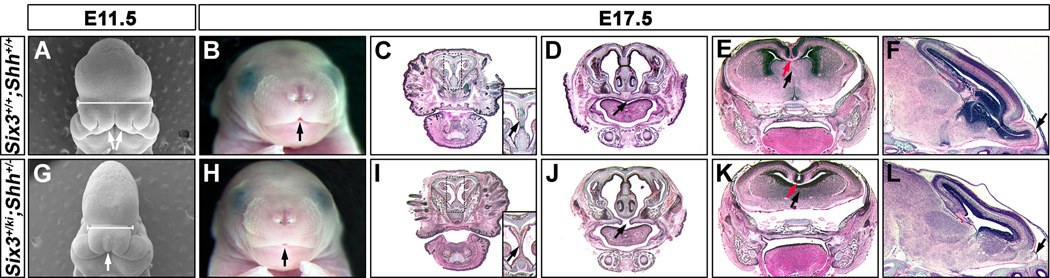
Figure 2.
Six3+/ki;Shh+/− embryos exhibit a semilobar HPE–like phenotype. (A) Electron micrograph of an E11.5 wild-type embryo shows the separation of the medial nasal prominences (MNPs, arrows). (G) The MNP is not separated in the Six3+/ki;Shh+/− embryo (arrow). The Six3+/ki;Shh+/− embryo also has microcephaly and ocular hypotelorism, as indicated by the shorter distance between the eyes (bars). (B and H) Frontal view of E17.5 embryos shows the agenesis of the philtrum in the Six3+/ki;Shh+/− embryo (arrows). Coronal sections of E17.5 wild-type (C–E) and Six3+/ki;Shh+/− (I–K) embryos stained with HE. The cartilage nasal septum (arrows in C and I insets), the septum (black arrows in E and K), and the corpus callosum (red arrows in E and K) are absent in the Six3+/ki;Shh+/− embryo, and the secondary palate did not fuse properly (arrows in D and J). (F, L) Sagittal sections of wild-type and Six3+/ki;Shh+/− embryos with HE staining shows the hypoplasia of the olfactory bulbs in the Six3+/ki;Shh+/− embryo (arrows).
Next, we performed a detailed morphological characterization of the HPE-like phenotype in Six3+/ki;Shh+/− embryos at various developmental stages. As shown in Figures 2A and 2G, defects in the separation of the medial nasal prominence (MNP), ocular hypotelorism (i.e., shorter distance between the eyes), and microcephaly (small brain) were obvious in E11.5 Six3+/ki;Shh+/− embryos assessed via scanning electron microscopy. At E17.5, the most obvious craniofacial phenotype was the absence of the philtrum (Figures 2B and 2H) caused by the lack of separation of the MNP. Coronal sections revealed that the mutant head also lacked the cartilage nasal septum (Figures 2C and 2I), the septum (Figures 2E and 2K), and the corpus callosum (Figures 2E and 2K). In addition, the mutant head had fusion defects of the secondary palates (Figures 2D and 2J) and hypoplasia of the olfactory bulbs (Figures 2F and 2L). No obvious defects were identified in the posterior separation of the two hemispheres by the thalamus (data not shown). No phenotype was observed in Shh+/− embryos maintained in a C57BL/6 background (Figures S3D–S3F). The phenotypes observed in the Six3+/ki;Shh+/− embryos were similar to those described in individuals with semilobar HPE (DeMyer, 1977).
HPE is thought to be associated with dorsoventral patterning defects of the telencephalon (Muenke and Beachy, 2000; Monuki, 2007). Accordingly, detailed molecular analysis of the telencephalon of E12.5 Six3+/ki;Shh+/− embryos identified putative dorsoventral patterning defects. As shown in Figure 3, the major dorsoventral patterning defects decreased in severity from rostral to caudal. At the most rostral level (Figures 3A–3H), the cerebral hemispheres of Six3+/ki;Shh+/− embryos were fused together, and the expression of the dorsal telencephalic markers Ngn2 and Pax6 (Schuurmans and Guillemot, 2002) was expanded ventrally such that it entirely surrounded the ventricle (Figures 3A–3D). The expression of the ventral telencephalic markers Mash1 and Dlx2 (Schuurmans and Guillemot, 2002) was not detected in the E12.5 mutant telencephalon (Figures 3E–3H). Toward the mid-caudal region, similar to the wild-type embryos, a distinct interhemispheric fissure was present in the mutant brain due to the invagination of the telencephalic dorsal midline (Figures 3I and 3J). Ngn2 expression was properly restricted to the dorsal telencephalon of the mutant embryos; however, its ectopic expression in the rostral dorsal midline (Figures 3I and 3J) indicated that this structure failed to properly develop. As indicated by Dlx2 expression, a single ganglionic eminence (GE) was present in the ventral telencephalon of the mutant embryos (Figure 3L). This GE expressed the lateral GE marker Ebf1 (Fuccillo et al., 2006; Figure 3N) but not the medial GE marker Nkx2.1 (Fuccillo et al., 2006; Figure 3P). Posteriorly, the cerebral hemispheres of Six3+/ki;Shh+/− embryos were normally separated by the diencephalon, and the dorsal midline structures (cortical hem, hippocampus, and choroid plexus) were normally located (Kimura et al., 2005; Figures S5A–S5H). Unlike the E12.5 Six3+/ki;Shh+/− telencephalon, that of Shh−/− embryos is composed of a very small single vesicle with very reduced levels of Dlx2 along the ventral midline, ventral expansion of Ngn2 throughout, and a lack of Nkx2.1 or Ebf1 expression (data not shown; Rallu et al., 2002).
Figure 3.
Dorsoventral patterning defects are observed in the Six3+/ki;Shh+/− telencephalon. Coronal sections of the telencephalon of E12.5 wild-type and Six3+/ki;Shh+/− embryos. (A and C) At the most anterior level, two well-separated cerebral hemispheres are present in the wild-type telencephalon (arrows). Ngn2 and Pax6 expression are restricted to the dorsal telencephalon (arrowheads). (E and G) Mash1 and Dlx2 are expressed in the wild-type ventral telencephalon. (B and D). In the Six3+/ki;Shh+/− telencephalon, the two cerebral hemispheres are fused together, and Ngn2 and Pax6 expression have expanded ventrally to encircle the single ventricle (arrowhead). The white dotted lines in B and D mark the ventricle. (F and H) Mash1 and Dlx2 expression are lost in the Six3+/ki;Shh+/− ventral telencephalon. (I) At the middle level, the two cerebral hemispheres are separated by the invagination of the dorsal midline (arrow). Ngn2 expression is restricted to the wild-type dorsal telencephalon, while it is excluded from the dorsal midline (arrowhead). (K, M, and O) The lateral and medial ganglionic eminences (LGE and MGE, respectively) are formed ventrally in the wild-type telencephalon, and (K) Dlx2 is expressed in both the MGE and LGE. (M) Ebf1 is expressed in the LGE, and (O) Nkx2.1 expression labels the MGE. (J) In the Six3+/ki;Shh+/− telencephalon, Ngn2 is restricted to the dorsal side, and the dorsal midline is invaginated (arrow). However, the dorsal midline is Ngn2-positive (arrowhead). (L and N) Only one ganglionic eminence (GE) is present ventrally in the Six3+/ki;Shh+/− telencephalon, and it is positive for Dlx2 and Ebf1. (P) No Nkx2.1 expression was detected in the Six3+/ki;Shh+/− telencephalon.
The finding that Six3 becomes haploinsufficient upon additional inactivation of one allele of Shh suggested that Six3 and Shh function synergistically in the pathogenesis of HPE. Unlike the alobar HPE features present in Shh−/− embryos or in embryos with defective expression of Shh in the PrCP (Chiang et al., 1996; Anderson et al., 2002; Lowe et al., 2001), the phenotype of the Six3+/ki;Shh+/− double heterozygous telencephalon closely resembles the clinical features of semilobar HPE (DeMyer, 1977).
Haploinsufficiency of Six3 fails to activate Shh expression in the ventral forebrain
Having established a reproducible mouse model of HPE, we began investigating the molecular mechanisms of this pathogenesis. Given the cooperative effect of Six3 and Shh, we first compared the expression profiles of the two genes’ products during early development.
Normally, starting at around E7.5 to E8.0 (0–4 somite stage), Shh expression is detected throughout the axial mesoderm (Figure 4A; Shimamura et al., 1995), including the PrCP anteriorly (Figure 4A inset) and the notochord posteriorly. At this stage, Six3 expression is high in the ANE, which is located right above the Shh-expressing PrCP (Figure 4B). Weaker Six3 expression was detected in the PrCP (Figure 4B inset). At the 5-somite stage (E8.0), Six3 expression was maintained in the ANE while Shh was detected in the ventral midline of the midbrain besides the PrCP (data not shown; Shimamura et al., 1995). Around the 8-somite stage (E8.0), Shh expression in the neuroectoderm extended anteriorly into the RDVM and posteriorly into the hindbrain (Figure 4C; Shimamura et al., 1995). At this stage, Six3 expression was already restricted to the ventral forebrain and eye field (Figure 4D). At the 12-somite stage (E8.5), Shh expression was strong in the RDVM (Figure 4E; Shimamura et al., 1995), and Six3 was maintained in the ventral forebrain (Figure 4F). Shh and Six3 expression in the RDVM overlapped at this stage (Figures 4I–4L), and continued at least until the 18-somite stage (E9.0, data not shown). Later, Shh expression was excluded from the RDVM, except in the preoptic area (Figure 4G; Shimamura et al., 1995); its expression also appeared in the lateral tuberal hypothalamus (Figure 4G; Shimamura et al., 1995). In the preoptic area, Six3 and Shh expression continued to colocalize at least until E10.5 (Figures 4M–4P and data not shown). Around E10.0, Shh expression was detected in the ventral midline of the telencephalon, while Six3 was broadly expressed in the ventral telencephalon (data not shown; Shimamura et al., 1995).
Figure 4.
Shh and Six3 are coexpressed in the RDVM of 12-somite stage embryos. (A) Ventral view of a 1-somite staged embryo with the anterior side at the top. Shh is expressed in the axial mesoderm (arrowheads). The transverse section shows the expression of Shh in the prechordal plate (PrCP) and the rostral axial mesoendoderm (black arrow in inset). The level of the section is indicated by the black dotted line. (B) Dorsal view of a 1-somite staged embryo with the anterior side to the left. Six3 is expressed strongly in the anterior neuroectoderm (blue arrow in the inset). Trace levels of Six3 are also detected in the PrCP (black arrow in inset). The level of the section is indicated by the white dotted line. (C–H) Lateral views with the anterior side to the left. (C) At the 8-somite stage, Shh expression in the neuroectoderm starts to extend from the ventral midline of the midbrain anteriorly into the forebrain (arrow) and posteriorly into the hindbrain (arrowhead). (D) Six3 expression is restricted to the ventral forebrain (arrow in inset) and the eye field (arrowheads in inset) at this stage. (E) At the 12-somite stage, Shh is strongly expressed in the RDVM (arrow). (F) Six3 expression remains in the ventral forebrain and the eye field. (G) At the 24-somite stage, Shh expression is downregulated in the RDVM (red arrow), except in the preoptic area (black arrow). Instead, Shh is expressed in the lateral tuberal hypothalamus (arrowhead). The level of the coronal section shown in the inset is indicated by the red dotted line. (H) At this stage, Six3 remains in the ventral forebrain and the eye field. (I-P) Double immunostaining reveals the colocalization of Six3 (green) and Shh (red) in the RDVM at the 12-somite stage (I–L) and in the preoptic area at the 24-somite stage (M–P). The levels of the sections are indicated by the black dotted lines in E and G, respectively. The images in (K and O) and (L and P) are merged pictures at lower and higher magnification, respectively.
Based on these expression profiles, we envisaged two explanations for the HPElike phenotypes identified in Six3+/ki;Shh+/− embryos: First, Shh signaling activity in the PrCP is required for proper patterning of the forebrain (Muenke and Cohen, 2000), and although Six3 is only weakly expressed in the PrCP during a very brief period (data not shown), it may regulate Shh expression in this region. Alternatively, previous studies have demonstrated that Shh signaling from the PrCP activates its own expression and that of other downstream target genes such as the homeobox gene Nkx2.1 in the RDVM (Shimamura and Rubenstein, 1997). Because Six3 is also expressed in the RDVM, it might regulate Shh expression in this territory or provide competence to the ANE to respond to Shh signaling from the PrCP. To distinguish between these explanations we compared the patterns of Shh expression in wild-type and Six3+/ki;Shh+/− littermates. Shh expression was detected in the PrCP and RDVM of 10- to 11-somite stage (E8.5) wild-type (Figure 5A), Six3+/ki (Figure 5B), and Shh+/− embryos (data not shown). Although Shh expression was normal in the PrCP of Six3+/ki;Shh+/− littermates, it was absent from the RDVM (Figure 5C). Interestingly, Nkx2.1 expression was comparable between control and Six3+/ki;Shh+/− littermates in this same forebrain region (Figure 5D–F). Nkx2.1 is a target of PrCP-derived Shh signaling (Shimamura and Rubenstein, 1997); thus, the competence of the Six3+/ki;Shh+/− ANE to respond to Shh signaling was unaffected.
Figure 5.
Shh expression is lost in the RDVM of Six3+/ki;Shh+/− embryos. (A, B) In 10- to 11-somite staged wild-type or Six3+/ki;Shh+/+ embryos, Shh is expressed in the PrCP (arrowhead) and the RDVM (arrow); (D, E) Nkx2.1 colocalizes with Shh in the RDVM; and (G, H) Fgf8 demarcates the commissural plate (arrow). In Six3+/ki;Shh+/− embryos, Shh (C) is expressed normally in the PrCP (arrowhead); however, it fails to be activated in the RDVM (arrow). (F) Nkx2.1 and (I) Fgf8 expression in the mutant embryos is comparable to that of control embryos. (J–S) In 15-somite staged wild-type or Six3+/ki;Shh+/+ embryos (J, K), Shh is strongly expressed in the RDVM (arrow). (M, N) Gli1 is expressed in the ventral neural tube, and its expression extends to the commissural plate at the rostral end of the neural tube (arrow). (P, Q) Fgf8 continues to be expressed in the commissural plate (arrow). In Six3+/ki;Shh+/− embryos, (L) Shh expression is absent from the RDVM (arrow); (O) Gli1 expression ends posteriorly to the optic vesicles (arrow), and (S) Fgf8 is dramatically downregulated in the commissural plate (arrow).
These results demonstrate that Six3 dosage is crucial for the activation of Shh expression in the ventral forebrain and that Six3 in the RDVM and Shh signaling from the PrCP synergistically regulate Shh expression in the RDVM.
Loss of Shh expression in the RDVM of Six3+/ki;Shh+/− embryos affects the formation and function of key telencephalic signaling centers
As originally described by Ohkubo et al. (2002), at least three signaling centers that regulate each other work synergistically to control regional specification and morphogenesis of the telencephalon (Crossley et al., 2001; Monuki, 2007; Monuki and Walsh, 2001; Ohkubo et al., 2002; Sur and Rubenstein, 2005). Malfunctions of these patterning centers have been linked to HPE. The rostral-patterning center (commissural plate) expresses Fgf genes and controls the size and ventral patterning of the telencephalon (Crossley et al., 2001; Storm et al., 2003; Storm et al., 2006). HPE-like phenotype was observed in Fgf8 hypomorphic and conditional knock-out embryos (Storm et al., 2006). The dorsal-patterning center expresses different Bmp and Wnt genes and controls the development of dorsocaudal structures (cortical hem, hippocampus, and choroid plexus) (Monuki, 2007; Sur and Rubenstein, 2005). An excessive amount of Bmp causes HPE via downregulation of Fgf8 and Shh (Ohkubo et al., 2002), and in Shh-null embryos, Fgf8 expression is lost, and Bmp signaling–responsive genes are upregulated (Ohkubo et al., 2002). Finally, the ventral-patterning center (ventral midline of the telencephalon) expresses Shh whose function in that region has not yet been directly established (Monuki, 2007). Therefore, we next investigated whether the observed absence of Shh expression in the RDVM of early-somite stage Six3+/ki;Shh+/− embryos affected the formation and function of any of these signaling centers.
Consistent with previous studies showing that the initiation of Fgf8 expression is independent of Shh signaling (Ohkubo et al., 2002), Fgf8 expression was normal in the commisural plate of 10- to 11-somite stage Six3+/ki;Shh+/− embryos (Figure 5I). Later (15-somite stage, E9.0) and similar to what was observed at earlier stages (Figure 5C), we detected no Shh expression in the RDVM of those embryos (Figure 5L). A small number of Six3+/ki embryos also failed to express Shh in the RDVM (data not shown). As described before, 10% of Six3+/ki embryos exhibit HPE-like phenotype. Therefore, we hypothesized that the Six3+/ki embryos that do not express Shh in the RDVM will subsequently exhibit the HPE-like phenotype.
In control embryos, Gli1, a downstream target and a component of the Shh signaling pathway, was normally expressed in the ventral neural tube, along the entire anteroposterior axis (Hui et al., 1994; Lee et al., 1997; Figure 5M). However, consistent with the lack of Shh signaling anteriorly (Figure 5L), the most anterior domain of Gli1 expression in the ventral neural tube was missing in Six3+/ki;Shh+/− embryos (Figure 5O). At this later somite stage, Fgf8 expression was also dramatically downregulated in the commissural plate of Six3+/ki;Shh+/− embryos (Figure 4S), suggesting that maintenance of Fgf8 expression in the commissural plate requires RDVM-derived Shh activity. Expression analysis performed at E9.5 revealed that Fgf8 remained absent from the commissural plate of Six3+/ki;Shh+/− embryos (Figures 6A and 6B).
Figure 6.
Dorsal-ventral patterning centers and cell death are affected in the E9.5 Six3+/ki;Shh+/− telencephalon. In E9.5 wild-type embryos Fgf8 (A) is expressed in the commissural plate (arrow), Bmp4 (C) covers the dorsal midline of the telencephalon (arrows) and the anterior diencephalon (arrowhead), Nkx2.1 (E) demarcates both the hypothalamus (arrowhead) and the ventral telencephalon (arrow), and Shh (G) expression in the RDVM is downregulated, except in the preoptic area (arrow), and it is expressed in the lateral tuberal hypothalamus (arrowhead). In E9.5 Six3+/ki;Shh+/− embryos, Fgf8 (B) remains absent from the commissural plate (arrow), Bmp4 (D) fails to be expressed in the dorsal midline of the telencephalon (arrows), while it is expressed normally in the diencephalon (arrowhead), Nkx2.1 (F) is expressed normally in the hypothalamus (arrowhead); however, it fails to be activated in the ventral telencephalon (arrow), and Shh (H) expression in the preoptic area is lost (arrow), and that in the lateral tuberal hypothalamus is dramatically downregulated (arrowhead). (I-P) Coronal sections of E9.5 embryos processed for TUNEL (I–L) or anti-PH3 antibody (M–P). The levels of the sections are labeled by dotted lines in A and B. (I and K) TUNEL-positive cells are predominantly located in the dorsal and the ventral midline of the wild-type telencephalon (white arrowheads) and in the optic stalks (yellow arrowheads). (J and L) Comparable amounts of TUNEL-positive cells are detected in the dorsal midline of the Six3+/ki;Shh+/ telencephalon (white arrowheads). However, the ventral midline (white arrowhead) and optic stalk cells (yellow arrowheads) are TUNEL-negative. Instead, large numbers of TUNEL-positive cells are seen in the lateral dorsal telencephalon (arrows). (M and O) PH3-positive cells are detected in the ventricular zone of the wild-type telencephalon. (M and N) At the anterior level, fewer PH3-positive cells are detected in the Six3+/ki;Shh+/− telencephalon. (O and P) More posteriorly, the number of PH3-positive cells in the Six3+/ki;Shh+/− telencephalon is comparable to that in the wild-type.
At E9.5, Bmp4 is expressed in the dorsal midline of the telencephalon and the anterior diencephalon of wild-type embryos (Figure 6C). In Six3+/ki;Shh+/− littermates, Bmp4 expression was absent from the dorsal midline of the mutant telencephalon but remained in the dorsal midline of the anterior diencephalon (Figure 6D). By contrast, no alterations were detected in the expression of Wnt8b (Storm et al., 2006) in the dorsocaudal midline of the mutant telencephalon (data not shown). Previous studies have shown that Nkx2.1 expression in the ventral telencephalon is necessary for the subsequent activation of Shh in this region at around E10.0 (Jeong et al., 2006; Sussel et al., 1999). We found that Nkx2.1 expression was not activated in the ventral telencephalon of Six3+/ki;Shh+/− embryos at this later stage (Figure 6F), although it was normal in the hypothalamus, which is derived from the RDVM (Figure 6F). Finally, Shh expression remained absent from the preoptic area (anterior hypothalamus) of Six3+/ki;Shh+/− embryos at E9.5 and it was dramatically downregulated in the lateral tuberal hypothalamus (Figures 6G and 6H).
The E10.5 Six3+/ki;Shh+/− telencephalon was smaller than that of control littermates (Figures S5I and S5J). In E10.5 Six3+/ki;Shh+/− embryos, Shh expression remained absent in the preoptic area and most likely because of the lack of Nkx2.1 expression, it was also not activated in the ventral telencephalon (Figure S5J). However, Shh expression in the lateral tuberal hypothalamus was similar to that of control littermates at this stage (Figure S5J), indicating that in the mutant embryos, Shh expression in this region was delayed but not lost. Fgf8 and Bmp4 expression remained missing from the midline of the mutant telencephalon (Figures S5K–S5N), whereas the pattern of Wnt8b expression was similar to that of wild-type littermates (Figures S5O and S5P).
In conclusion, the loss of Shh expression in the RDVM of Six3+/ki;Shh+/− embryos was correlated with and likely affected the expression of Fgf8, Bmp4, and Shh along the midline of the telencephalon. The absence of these signaling molecules promoted the dorsoventral and midline patterning defects identified in the Six3+/ki;Shh+/− telencephalon.
Abnormal apoptosis and cell proliferation in Six3+/ki;Shh+/− embryos
Proper cell proliferation and apoptosis shape the developing telencephalon (Monuki and Walsh, 2001). As changes in the activity of Fgf8 or Bmp4 in the telencephalon affect cell proliferation and apoptosis (Crossley et al., 2001; Ohkubo et al., 2002; Storm et al., 2003; Storm et al., 2006), we investigated these cellular responses in the E9.5 Six3+/ki;Shh+/− telencephalon. TUNEL-positive cells are normally abundant in the dorsal and ventral midlines of the telencephalon and in the optic stalks at this stage (Figures 6I and 6K). We detected similar amounts of TUNEL-positive cells in the dorsal midline of Six3+/ki;Shh+/− embryos; however, none or very few apoptotic cells were detected in the ventral midline and optic stalks of the mutant embryos (Figures 6J and 6L). This lack of cell death in the ventral midline of the Six3+/ki;Shh+/− telencephalon may lead to the fusion of the lateral GE at later stages. Interestingly, a large number of TUNEL-positive cells were present in the Six3+/ki;Shh+/− lateral dorsal telencephalon (Figures 6J and 6L); this alteration may cause the microcephaly phenotype in the mutant embryos. We also assayed for the M-phase cell cycle marker phosphohistone-3 (PH3) to examine changes in cell proliferation. A small reduction in the number of PH3-positive cells was detected in the Six3+/ki;Shh+/− lateral telencephalon at this stage (Figures 6M and 6N, Figures S5Q and S5R). In conclusion, local changes in cell proliferation and apoptosis in the Six3+/ki;Shh+/− telencephalon may contribute to the morphological defects observed in the Six3+/ki;Shh+/− brain.
Six3 and Shh regulate each other in the ventral forebrain
We have demonstrated that Six3 in the RDVM and Shh signaling from the PrCP synergistically regulate Shh expression in the RDVM. To validate these results and confirm that Six3 is an upstream regulator of Shh and its activity is required for Shh expression in the ventral forebrain, we analyzed the expression of Shh in Six3-null embryos (Lagutin et al., 2003). At the 10-somite stage, Shh was expressed in the Six3-null PrCP; however, its expression failed to extend anteriorly into the RDVM (Figure 7B). Confirmation that at least part of the RDVM remained in the Six3-null brain was provided by the normal expression of Nkx2.1 and the anterior diencephalic marker Lhx5 (Sheng et al., 1997; Figures 7C–7F). These results demonstrate that Six3 activity is necessary for Shh expression in the RDVM.
Figure 7.
Six3 and Shh regulate each other in the RDVM. (A–F) 10- to 11-somite staged embryos. (A) Shh is expressed in the wild-type RDVM (arrow) and prechordal plate (PrCP) (arrowhead). (B) In the Six3-null embryo, Shh is expressed in the PrCP (arrowhead) but not in the RDVM (arrow). (C) Nkx2.1 expression covers the RDVM in the wild-type embryo. (D) In the Six3-null embryo, a group of cells in the ventral midline are Nkx2-positive. (E) Lhx5 is expressed in the entire wild-type anterior diencephalon. (F) Lhx5-positive cells are also present in Six3-null embryos. (G) 15-somite Six3+/−;Shh-LacZ embryo showing X-gal staining in the RDVM (arrow). (H) X-gal staining in the RDVM is lost in the Six3+/−;Shh LacZ littermate. (I) ChIP and real-time PCR analysis. Fold enrichment is the quantitative ratio between the samples precipitated with or without anti-Six3 antibody. For SBE2, the fold enrichment of the head (Six3-positive) samples is around two-fold higher than that of the posterior trunk (Six3-negative). However, for a control DNA sequence (6.5 kb downstream to SBE2), no difference in the fold enrichment was detected. (J–Q) Six3 expression is gradually lost in the ventral forebrain of Six3+/ki;Shh+/− embryos; I–L, frontal views and M–P, lateral views (anterior to the left). (J and K) At the 8-somite stage, Six3 expression in the ventral forebrain of the Six3+/ki;Shh+/− embryo is comparable to that in the wild-type embryo (arrow). (L and M) At the 12-somite stage, Six3 expression in the ventral forebrain of the mutant embryo is slightly less that in the wild-type embryo (arrow). (N and O) At the 15-somite stage, Six3 expression in the ventral forebrain of the mutant embryo is severely downregulated (arrow); however, Six3 expression in the eye field appears normal (arrowhead). (P and Q) By the 24-somite stage, Six3 expression remains dramatically downregulated in the ventral forebrain of the mutant embryo (arrow), while that in the optic vesicles and optic stalks is normal (arrowhead). Insets in P and Q are frontal views of the wild-type and mutant embryos, respectively. (S) Proposed model of Six3 action during telencephalon development.
Next, we used a Shh-LacZ reporter line that recapitulates the endogenous pattern of Shh expression in the ventral forebrain (Jeong et al., 2006) to determine whether lack of Six3 will affect expression of the reporter gene. F2 Six3+/− mice were crossed with the Shh-LacZ reporter strain and as expected, X-gal staining was detected in the RDVM territory of control E9.0 (15 somites) Six3+/+;Shh-LacZ embryos (Figure 7G); however, this staining was absent in Six3+/−;Shh-LacZ littermates (Figure 7H). In a related paper, Jeong et al (personal communication) reported that this Shh regulatory element contains a Six3-binding site (SBE2: Shh Brain Enhancer-2), and this site was mutated in an individual with semilobar HPE. Introduction of this mutation into the Shh-LacZ reporter strain caused severe downregulation of the reporter gene expression in the RDVM (Jeong et al., personal communication). Therefore, to determine whether Six3 binds directly to this regulatory element and activates Shh expression in vivo, we performed chromatin immunoprecipitation (ChIP) using chromatin from the Six3-expressing head or the Six3-negative posterior trunk of E9.0 (13–18 somites) mouse embryos. Using Taqman-based real-time PCR we detected around two-fold enrichment of SBE2 in the head chromatin compared to that in the posterior trunk (Figure 7I). However, equal amounts of a control fragment located 6.5kb downstream of SBE2 were detected in the two samples (Figure 7I). Hence, in the RDVM Six3 acts as a direct upstream activator of Shh expression.
Finally, we also analyzed Six3 expression in Six3+/ki;Shh+/− embryos at different stages. At the 8-somite stage, Six3 expression was normal in the ventral forebrain of the mutant embryos (Figures 7J and 7K). Eight hours later (12-somite stage) and in agreement with the loss of Shh expression in the RDVM, Six3 expression was slightly weaker in the mutant ventral forebrain (Figures 7L and 7M). Fourteen hours later (15-somite stage), Six3 expression was severely downregulated in the mutant ventral forebrain; however, its expression around the developing eyes appeared normal (Figures 7N and 7O). By E9.5, Six3 expression in the mutant ventral forebrain remained dramatically downregulated, but it was normal in the optic vesicles and optic stalks (Figures 7P and 7Q). These results indicate that a positive regulatory loop between Six3 and Shh operates during the patterning of the ventral forebrain. Therefore, we concluded that Six3 and Shh regulate each other in the RDVM.
DISCUSSION
Using zebrafish-based in vivo assays, we determined that although at different degrees, HPE-associated Six3-mutant proteins function as hypomorphs. Analysis of generated mouse models revealed that haploinsufficiency of Six3 causes HPE and that Six3 and Shh cooperate in the pathogenesis of HPE, i.e., the loss of one copy of Shh in the presence of one hypomorphic Six3 mutation dramatically increased the percentage of embryos with HPE features. These findings provide experimental evidence in support of the multi-hit hypothesis proposed for the pathogenesis of HPE (Ming and Muenke, 2002), whereby hypomorphic mutations in SIX3 become haploinsufficient when combined with mutations in different genes that impair SHH signaling.
Importantly, we have determined that Six3 is a direct upstream activator of Shh and that its activity is necessary for Shh expression in the ventral forebrain. Shh signaling plays multiple roles during forebrain development, including the establishment of the ventral midline, separation of the eye field, and growth and patterning of the telencephalon (Chiang et al., 1996; Shimamura and Rubenstein, 1997). Previous studies have suggested that temporal perturbation in Shh signaling is associated with the severity of the resulting HPE phenotype (Cordero et al., 2004). In Six3+/ki;Shh+/− embryos, early Shh expression in the PrCP remained unaffected and was sufficient to establish the ventral midline and to separate the eye fields. However, the lack of Shh expression in the RDVM at later stages resulted in the semilobar HPE phenotype. This phenotype was similar to that described for Cdo−/− embryos in which Shh expression in the RDVM is lost (Zhang et al., 2006). These results confirm that the severity of the HPE phenotype is closely related to the stages at which perturbations in the Shh signaling pathway occur, i.e., the earlier the alteration, the more severe the resulting HPE phenotype.
Based on the original model proposed by Ohkubo et al. (2002), we propose a modified model that incorporates Six3 function in the dorsoventral patterning of the telencephalon and in promoting HPE when mutated (Figure 7S). At the 8- to 10-somite stage, Six3 directly activates Shh expression in the RDVM. Upon activation, Shh activity from the RDVM positively feeds back to maintain Six3 expression in the ventral forebrain and promotes Fgf8 expression in the commissural plate via a yet unknown mechanism. Fgf8 activity from the commissural plate positively regulates Six3 expression in the ventral forebrain and is required for the proper expression of Bmp4 in the dorsal midline. Fgf8 signaling also activates the expression of Nkx2.1 in the ventral telencephalon. Subsequently, Nkx2.1 activates Shh expression in the ventral telencephalon. Thus, three signaling centers are established along the midline, and they function synergistically to ensure the proper dorsoventral patterning and morphogenesis of the telencephalon.
In Six3-haploinsufficient embryos, the amount of Six3 activity is not enough to activate Shh expression in the RDVM and consequently, this leads to the loss of all of the above-mentioned signaling molecules in the telencephalon. The fact that Nkx2.1 failed to be activated in the Six3-haplosufficient embryos suggests that Six3 is required for induction of telencephalic Nkx2.1 expression, which in turn is required for the induction of Shh expression. However, this cascade is not essential for preventing ventral holoprosencephaly, as Nkx2.1 mutant embryos do not exhibit HPE (Sussel et al., 1999). Rather, the maintenance of Fgf8 expression by Six3 appears more relevant for HPE pathogenesis as Fgf8 severe hypomorphs exhibit this phenotype (Storm et al., 2006). Conversely, the fact that Fgf8 promotes Six3 expression (Storm et al., 2006) suggests that reduced Six3 expression likely contributes to the HPE phenotype described in Fgf8 hypomorphs.
Consistent with our model, Marcucio et al., have shown that blocking Shh signaling specifically from the RDVM in 10-somite stage chicken embryos causes loss of Shh expression in the ventral telencephalon, reduction in expression of the ventral markers Nkx2.1 and Dlx2, and ventral expansion of the dorsal marker Pax6 (Marcucio et al., 2005). Furthermore, as suggested by the analysis of six3b−/−;smo−/− mutant zebrafish embryos (Inbal et al., personal communication), the lack of Six3 expression observed in the ventral forebrain of Six3+/ki;Shh+/− embryos could also contribute to the dorsal-ventral patterning defects observed in these mutant embryos in a Shh- independent manner.
In summary, we have identified Six3 as a direct upstream regulator of Shh expression and shown that Six3 and Shh signaling from the PrCP synergistically activate Shh expression in the RDVM. However, it is not yet clear how Shh regulates the Six3 transcriptional activity. SIX3 mutations in human are associated with HPE phenotypes ranging from mild (microform) to severe (cyclopia). However, in Six3+/−, Six3+/−;Shh+/−, Six3+/ki, and Six3+/ki;Shh+/− embryos, semilobar HPE was the predominant phenotype observed in the C57BL/6 background; more or less severe forms of HPE were rarely observed in embryos with mixed genetic backgrounds (Figure S6). These phenotypic variations were probably caused by additional genetic modifiers.
EXPERIMENTAL PROCEDURES
Mice
NMRI and C57BL/6 mice were purchased from the Jackson Laboratory. Shh+/− mice were provided by Dr. Chin Chiang (Vanderbilt University, Nashville, TN), Bf1-Cre mice were provided by Dr. Susan McConnell (Stanford University, Stanford, CA), and Six3+/− and Shh-LacZ (447L17βlacZ) mice have been previously reported (Lagutin et al., 2003; Jeong et al., 2006). Generation of the different mouse strains is described in Supplementary Materials. All of the mouse experiments were approved by the St. Jude Children’s Research Hospital Animal Care and Use committee.
Zebrafish
Mutant six3b synthetic RNA
For the generation of the HPE-related mutant forms, wild-type six3b zebrafish cDNA (Kobayashi et al., 1998) was used as a template for site-specific mutagenesis using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Mutant DNAs were linearized and synthetic RNAs were transcribed with the mMESSAGE mMACHINE High Yield Capped RNA Transcription Kit (SP6 polymerase) (Ambion, Austin, TX).
RNA injection
The hdl/tcf3−/− mutant rescue assay. Homozygous hdl -mutant embryos at the one-cell stage (Kim et al., 2000) were injected with either wild-type six3b or the mutant RNAs. Embryos were then evaluated at 55 hpf and categorized according to the degree of rescue of the eyeless hdl phenotype.
The six3bvu87/vu87 mutant-six7 morphant suppression assay. An antisense morpholino oligonucleotide (MO) against the 5’ UTR of six7 (2 ng) was injected into six3bvu87/vu87 mutant embryos (Inbal et al., 2007). The six7 MO was coinjected with wild-type or mutant six3b RNA constructs at the one-cell stage. Embryos were scored at 55 hpf; 10 representative embryos were selected from each injected sample and photographed. The eye size of the embryos was digitally measured using Adobe Photoshop (Adobe Systems, San Jose, CA) on a 0- to 6.5 point scale, with 5.0 to 6.5 points being equivalent to full-sized eyes. Results were then quantified for statistical significance using the t-test in comparison to six3bvu87/vu87-six7 -mutant morphants. Experimental details on the dorsalization assay are described in Supplementary Materials.
Chromatin immunoprecipitation (ChIP)
ChIP was performed as previously described (Cawley et al. 2004) with slight modifications described in Supplementary Materials.
In situ hybridization, immunohistochemistry and X-gal staining
For in situ hybridization, embryos were fixed in 4% PFA at 4 °C overnight and processed as described (Lagutin et al. 2003). For immunohistochemistry, embryos were fixed in 4% PFA on ice for 30 min and processed for cryosection. Immunohistochemistry was performed on frozen sections (10 µm) as described (Liu et al., 2006). The primary antibodies were rabbit anti-Six3 and mouse anti-Shh (Hybridoma Bank), and the secondary antibodies were Alexa 488–conjugated donkey anti-rabbit (Molecular Probes) and Cy3-conjugated goat anti-mouse (Jackson Laboratories). DAB immunostaining was performed using rabbit anti-Pax6 (Covance) as the primary antibody, donkey anti-rabbit biotin-conjugated secondary antibody (Jackson Laboratories) and diaminobenzidine as substrate (ABC kit, Vector). X-gal staining was performed as described (Lagutin et al. 2003).
TUNEL and proliferation assays
TUNEL was performed on frozen sections (10 µm) using the ApoTag Plus Peroxidase Kit (Chemicon) according to the manufacturer’s instructions. PH3 immunohistochemistry was performed mouse anti-PH3 (Upstate) and Cy3-conjugated goat anti-mouse (Jackson Laboratories) as secondary antibody.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. C. Chiang and S.K. McConnell for the Shh+/− and Bf1-Cre mouse strains respectively. For plasmids, we thank Drs. S. Aizawa, R. Grosschedl, J. L. Rubenstein, Q. Ma, A. McMahon, P Gruss, P. McKinnon and B.L. Hogan. We also thank L. Zhang for confocal imaging, L. Boykins for scanning electron microscopy, Y. Lee for technical support with real-time PCR, R. S. Srinivasan and A. Lavado for critical reading of this manuscript, and A. McArthur for editing the manuscript. This project was supported in part by NIH grants R01 NS052386 (to G.O. and LSK), Cancer Center Support CA-21765 (G.O), the American Lebanese Syrian Associated Charities (ALSAC), and Vanderbilt University Zebrafish Initiative AVCF
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr Holoprosencephaly: clinical, anatomic, and molecular dimensions. Birth Defects Res. A. Clin. Mol. Teratol. 2006;76:658–673. doi: 10.1002/bdra.20295. [DOI] [PubMed] [Google Scholar]
- Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA. Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J. Clin. Invest. 2004;114:485–494. doi: 10.1172/JCI19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108:183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- DeMyer W. Holoprosencephaly (cyclopia-arhinencephaly) Amsterdam: North-Holland Publishing Co.; 1977. [Google Scholar]
- Dubourg C, Lazaro L, Pasquier L, Bendavid C, Blayau M, Le Duff F, Durou MR, Odent S, David V. Molecular screening of SHH, ZIC2, SIX3, and TGIF genes in patients with features of holoprosencephaly spectrum: Mutation review and genotype-phenotype correlations. Hum. Mutat. 2004;24:43–51. doi: 10.1002/humu.20056. [DOI] [PubMed] [Google Scholar]
- Fuccillo M, Rutlin M, Fishell G. Removal of Pax6 partially rescues the loss of ventral structures in Shh null mice. Cereb. Cortex. 2006;16 Suppl 1:i96–i102. doi: 10.1093/cercor/bhk023. [DOI] [PubMed] [Google Scholar]
- Gestri G, Carl M, Appolloni I, Wilson SW, Barsacchi G, Andreazzoli M. Six3 functions in anterior neural plate specification by promoting cell proliferation and inhibiting Bmp4 expression. Development. 2005;132:2401–2413. doi: 10.1242/dev.01814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev. Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev. Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- Inbal A, Kim SH, Shin J, Solnica-Krezel L. Six3 represses nodal activity to establish early brain asymmetry in zebrafish. Neuron. 2007;55:407–415. doi: 10.1016/j.neuron.2007.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y, El-Jaick K, Roessler E, Muenke M, Epstein DJ. A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers. Development. 2006;133:761–772. doi: 10.1242/dev.02239. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J, Suda Y, Kurokawa D, Hossain ZM, Nakamura M, Takahashi M, Hara A, Aizawa S. Emx2 and Pax6 function in cooperation with Otx2 and Otx1 to develop caudal forebrain primordium that includes future archipallium. J. Neurosci. 2005;25:5097–5108. doi: 10.1523/JNEUROSCI.0239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Toyama R, Takeda H, Dawid IB, Kawakami K. Overexpression of the forebrain-specific homeobox gene six3 induces rostral forebrain enlargement in zebrafish. Development. 1998;125:2973–2982. doi: 10.1242/dev.125.15.2973. [DOI] [PubMed] [Google Scholar]
- Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HR, McKinnon PJ, Solnica-Krezel L, Oliver G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–379. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Sauer B, Mosinger B, Jr, Lee EJ, Manning RW, Yu SH, Mulder KL, Westphal H. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc. Natl. Acad. Sci. U S A. 1992;89:6232–6236. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Lagutin OV, Oliver G. Six3 inactivation causes progressive caudalization and aberrant patterning of the mammalian diencephalon. Development. 2008;135:441–450. doi: 10.1242/dev.010082. [DOI] [PubMed] [Google Scholar]
- Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2552. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Yamada S, Kuehn MR. Genetic dissection of nodal function in patterning the mouse embryo. Development. 2001;128:1831–1843. doi: 10.1242/dev.128.10.1831. [DOI] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Dev. Biol. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Ming JE, Muenke M. Multiple hits during early embryonic development: digenic diseases and holoprosencephaly. Am. J. Hum. Genet. 2002;71:1017–1032. doi: 10.1086/344412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monuki ES. The morphogen signaling network in forebrain development and holoprosencephaly. J. Neuropathol. Exp. Neurol. 2007;66:566–575. doi: 10.1097/nen.0b013e3180986e1b. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Walsh CA. Mechanisms of cerebral cortical patterning in mice and humans. Nat. Neurosci. 2001 4 Suppl:1199–1206. doi: 10.1038/nn752. [DOI] [PubMed] [Google Scholar]
- Muenke M, Beachy PA. Genetics of ventral forebrain development and holoprosencephaly. Curr. Opin. Genet. Dev. 2000;10:262–269. doi: 10.1016/s0959-437x(00)00084-8. [DOI] [PubMed] [Google Scholar]
- Muenke M, Cohen MM., Jr Genetic approaches to understanding brain development: holoprosencephaly as a model. Ment. Retard. Dev. Disabil. Res. Rev. 2000;6:15–21. doi: 10.1002/(SICI)1098-2779(2000)6:1<15::AID-MRDD3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Nanni L, Croen LA, Lammer EJ, Muenke M. Holoprosencephaly: molecular study of a California population. Am. J. Med. Genet. 2000;90:315–319. doi: 10.1002/(sici)1096-8628(20000214)90:4<315::aid-ajmg10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Chiang C, Rubenstein JL. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Pasquier L, Dubourg C, Blayau M, Lazaro L, Le Marec B, David V, Odent S. A new mutation in the six-domain of SIX3 gene causes holoprosencephaly. Eur. J. Hum. Genet. 2000;8:797–800. doi: 10.1038/sj.ejhg.5200540. [DOI] [PubMed] [Google Scholar]
- Pasquier L, Dubourg C, Gonzales M, Lazaro L, David V, Odent S, Encha-Razavi F. First occurrence of aprosencephaly/atelencephaly and holoprosencephaly in a family with a SIX3 gene mutation and phenotype/genotype correlation in our series of SIX3 mutations. J. Med. Genet. 2005;42:e4. doi: 10.1136/jmg.2004.023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryk A, Anderson RM, Jarcho MP, Leaf I, Carlson CS, Klingensmith J, Shawlot W, O'Connor MB. The mammalian twisted gastrulation gene functions in foregut and craniofacial development. Dev. Biol. 2004;267:374–386. doi: 10.1016/j.ydbio.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Rallu M, Machold R, Gaiano N, Corbin JG, McMahon AP, Fishell G. Dorsalventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development. 2002;129:4963–4974. doi: 10.1242/dev.129.21.4963. [DOI] [PubMed] [Google Scholar]
- Ribeiro LA, El-Jaick KB, Muenke M, Richieri-Costa A. SIX3 mutations with holoprosencephaly. Am. J. Med. Genet. A. 2006;140:2577–2583. doi: 10.1002/ajmg.a.31377. [DOI] [PubMed] [Google Scholar]
- Roessler E, Muenke M. How a Hedgehog might see holoprosencephaly. Hum. Mol. Genet. 2003;12:15–25. doi: 10.1093/hmg/ddg058. [DOI] [PubMed] [Google Scholar]
- Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr. Opin Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Bertuzzi S, Chiang C, Shawlot W, Taira M, Dawid I, Westphal H. Expression of murine Lhx5 suggests a role in specifying the forebrain. Dev. Dyn. 1997;208:266–277. doi: 10.1002/(SICI)1097-0177(199702)208:2<266::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JL. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Storm EE, Rubenstein JL, Martin GR. Dosage of Fgf8 determines whether cell survival is positively or negatively regulated in the developing forebrain. Proc. Natl. Acd. Sci. U. S. A. 2003;100:1757–1762. doi: 10.1073/pnas.0337736100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Wallis DE, Roessler E, Hehr U, Nanni L, Wiltshire T, Richieri-Costa A, Gillessen-Kaesbach G, Zackai EH, Rommens J, Muenke M. Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly. Nat. Genet. 1999;22:196–198. doi: 10.1038/9718. [DOI] [PubMed] [Google Scholar]
- Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS. Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev. Cell. 2006;10:657–665. doi: 10.1016/j.devcel.2006.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.