Abstract
Members of the Fanconi anemia (FA) protein family are involved in repair of genetic damage caused by DNA cross-linkers. It is not clear whether the FA proteins function in oxidative DNA damage and oncogenic stress response. Here we report that deficiency in the Fanca gene in mice elicits a p53-dependent growth arrest and DNA damage response to oxidative DNA damage and oncogenic stress. Using a Fanca-/- Trp53-/- double knockout model and a functionally switchable p53 retrovirus, we define the kinetics, dependence, and persistence of p53-mediated response to oxidative and oncogenic stresses in Fanca-/- cells. Notably, oxidative stress induces persistent p53 response in Fanca-/- cells, likely due to accumulation of unrepaired DNA damage. On the other hand, whereas WT cells exhibit prolonged response to oncogene activation, the p53-activating signals induced by oncogenic ras are short-lived in Fanca-/- cells, suggesting that Fanca may be required for the cell to engage p53 during constitutive ras activation. We propose that the FA proteins protect cells from stress-induced proliferative arrest and tumor evolution by acting as a modulator of the signaling pathways that link FA to p53.
Introduction
Research on Fanconi anemia (FA) has recently generated great interest because the disease serves as an excellent model for hematopoietic failure and leukemic evolution. FA is a genetic disorder associated with bone marrow failure and cancers particularly leukemia (1-3). FA is genetically heterogeneous, with 13 complementation groups identified thus far. The genes encoding the groups A (FANCA), B (FANCB), C (FANCC), D1 (FANCD1/BRCA2), D2 (FANCD2), E (FANCE), F (FANCF), G (FANCG), -I (FANCI/KIAA1794), J (FANCJ/BRIP1), L (FANCL), M (FANCM), and N (FANCN/PALB2) have been cloned (4-18). The biological function of FA proteins has been the subject of intense investigation in recent years.
Because cells deficient for FA genes are hypersensitive to DNA cross-linking agents such as mitomycin C (MMC) and diepoxybutane (DEB), it has been proposed that the FA proteins may be involved in the sensing and/or repair of DNA inter-strand cross-links. This model is supported by evidence that shows that an intact FA nuclear complex is required for biochemical modification in the form of monoubiquitination of FANCD2 and FANCI, as mutations in any one of the FA proteins that disrupt the complex prevent the activation of FANCD2 and FANCI, leading to cross-linker hypersensitivity and the characteristic broken and radial chromosome formation in FA cells (3, 18). The common damage to DNA in vivo is oxidative stress and ample evidence has suggested that FA cells are in an in vivo pro-oxidant state (19) and that the FA proteins may play important roles in cellular responses to oxidative stress. For example, the FANCC protein has been found to interact with NADPH cytochrome P450 reductase and glutathione S-transferase P1-1 (20, 21), two enzymes involved in either triggering or detoxifying reactive intermediates including reactive oxygen species. In addition, mice with combined deficiencies of the anti-oxidative enzyme, Cu/Zn superoxide dismutase and the Fancc genes demonstrated a defective hematopoiesis (22). Another FA protein, FANCG, interacts with cytochrome P450 2E1 (23) and mitochondrial peroxiredoxin-3 (24), suggesting a possible role of FANCG in protection against oxidative DNA damage. Recently, Saadatzadeh et al. (25) showed that oxidant hypersensitivity of Fancc-/- cells was due to an altered redox regulation and hyperactivation of the serine-threonine kinase apoptosis signal-regulating kinase 1 (ASK1), an important kinase involved in oxidant-induced apoptosis. Moreover, oxidative stress induces complex formation by two major FA proteins, FANCA and FANCG (26). These observations corroborate a critical role for oxidative stress in FA phenotype and disease progression.
Cells from patients with FA gene mutations have high predisposition to leukemia and other cancers. However, little is known about whether these mutant cells have high susceptibility to oncogenic transformation. In respond to oncogenic activation, normal cells induce genetically encoded programs that prevent deregulated proliferation and thus protect multicellular organisms from cancer progression. Two such programs induced by oncogenic activation are apoptosis and senescence that are normally triggered by DNA damage or other stresses. Studies have demonstrated that overexpression of anti-apoptotic proteins such as Bcl-2 or deletion of apoptosis- and senescence-associated proteins such as p53 accelerates oncogene-induced tumorigenesis (27, 28). The theory that has arisen from these findings is that oncogene-driven proliferation must be associated with inhibition of apoptosis and senescence to allow malignant outgrowth.
The tumor suppressor p53 is a key transcription factor that activates vital damage containment procedures to restrict aberrant cell growth in response to DNA damage, oncogene activation and loss of normal cell contacts (29, 30). p53 restricts cellular growth by inducing senescence, cell cycle arrest or apoptosis (31). Thus, p53 plays a major role in the prevention of cancer. Consistent with this, emerging evidence suggests that p53 deficiency may increase cancer development in patients with FA and FA mice. For example, studies have indicated a higher proportion of human papillomavirus–positive squamous cell carcinomas in patients with FA than in healthy controls, indicating that loss of functional p53 facilitates the tumor development (32, 33) Mice deficient for Fancd1 or Fancd2 have been shown to have accelerated tumor development in Trp53-deficient background (34, 35). In addition, Fancc deficiency accelerates the development of certain blood and solid tumors in mice heterozygous at Trp53 (36). Moreover, these studies found that the FA proteins and p53 cooperate in apoptosis and cell-cycle checkpoint control following DNA damage (35, 37, 38). In this study, we show that p53 is engaged differentially in response to oxidative and oncogenic stresses in FA cells in vitro and in vivo.
Materials and Methods
Mice
WT, Fanca -/- mice and double knock out mice (p53-/-; Fanca -/-) were generated by interbreeding the heterozygous Fanca +/- and p53+/- mice. The genetic background of the mice was C57BL/6 mice which were used at ∼ 6-10 weeks of age. All experimental procedures conducted in this study were approved by the institutional Animal Care and Use Committee of Cincinnati Children's Hospital Medical Center.
Isolation of bone marrow lineage-depleted cells and differentiation assay
The femora and tibiae were harvested from the mice immediately after their sacrifice with CO2. Bone marrow cells were flushed from bones into Iscoves modified Dulbeco medium (IMDM; Invitrogen, Frederick, MD) containing 10% fetal calf serum (FCS) using a 21-guage needle and syringe. Low density BM mononuclear cells (BMMNCs) were separated by Ficoll Hypaque density gradient (Sigma, St Louis, MO) and washed with IMDM. BMMNC were depleted of lineage-committed cells using a lineage cell depletion kit (Miltenyi Biotec Inc.) in accordance with Manufacturer's instruction. For myeloid and lymphoid differentiation, BM Lin- cells were cultured with various cytokine combinations known to support myeloid [stem cell factor (SCF), 100 ng/ml; granulocyte–monocyte colony-stimulating factor (GM-CSF), 10 ng/ml] or lymphoid (SCF, 100 ng/ml; IL-3 and IL-7, 10 ng/ml each) differentiation. After 5 days, cells were stained with antibodies against myeloid markers Gr-1 and Mac-1 or lymphoid markers B220 and CD3e, followed by flow cytometric analysis using a FACSCalibur (Becton Dickinson, San Jose, CA).
Mouse embryonic fibroblast (MEF) isolation and culture
MEF were prepared from day 13.5 embryo derived from crosses of p53+/- with Fanca+/- mice. The head and the red organs were removed, and torso was minced and dispersed with 0.25% trypsin-EDTA by using 20-gauge needle. Single cell suspension obtained were cultured in DMEM containing 10% fetal bovine serum, glutamine, MEM non-essential amino acid, penicillin/streptomycin and gentamycin under 5% CO2 for three days till the genotypes were confirmed. Cells were then trypsinized and expanded for experimental use. MEFs were further maintained on the basis of 3T3 protocol.
Construction of retroviral expression vectors
The full length human FANCA cDNA was subcloned into the NotI site of retroviral vector MIEG3 to create MIEG3-FANCA. The WT mouse p53 (mp53wt), a temperature-sensitive mutant p53 (tsp53 V143A), and H-ras V12 cloned in MIEG3 were kindly provided by Dr. Yi zheng (Cincinnati Children's Medical Center). The plasmids (10μg each) were used to produce retroviral supernatant.
Retroviral mediated gene transfer
Helper-virus-free Phoenix packaging cells (kindly provided by Dr. Gary Nolan, Stanford University, Stanford, CA) were plated at the density of 3×106 in 10cm tissue culture dish and incubated for 24 hrs. Cells were then transfected by calcium phosphate precipitation with 10 μg of retroviral plasmids (15 hrs at 37°C). Retroviral supernatant was collected at different time points (24, 36, and 48 hrs) respectively. After transfection, the virus containing medium was filtered (0.45 μm filter, Millipore) and aliquoted at -80°C. WT, Fanca -/-, p53-/-;Fanca+/+ and p53-/-;Fanca-/- MEFs were plated at the density of 8×105 cells per 10cm dish and incubated for overnight at 37°C. For infections, culture medium was replaced by appropriate retroviral supernatant in presence of 4 μg/ml polybrene (Sigma) for 12 hrs. Infection process was repeated two times. 24 hrs after infection, EGFP-positive cells were used for different assays as indicated in results.
Colony and cell proliferation assay
Low density BM cells were plated in a 35 mm tissue culture dish in 4 ml of semisolid medium containing 3 ml of MethoCult M 3134 (Stem Cell Technologies) and the following growth factors: 100 ng/mL SCF, 10 ng/mL IL-3, 100 ng/mL granulocyte colony-stimulating factor (G-CSF), and 4 U/mL erythropoietin (Peprotech). On day 10 after plating, erythroid and myeloid colonies were enumerated. Hematopoietic clonal growth results were expressed as means (of triplicate plates) ± SD of three experiments. For proliferation, cells were cultured for 24 h in normal growth medium supplemented with 10 μM BrdU (Sigma), harvested and fixed in 70% ethanol. BrdU-labeled cells fixed in 70% ethanol were treated with 2 N HCl (20 min at room temperature followed by addition of 2 volumes of 0.1 M sodium borate (pH 8.5). The cells were incubated with an anti-BrdU mouse monoclonal antibody, washed, and incubated with fluorescein isothiocyanate-conjugated anti-mouse antibody. Cells were counterstained overnight with 5 μg of propidium iodide per ml containing 40 μg of RNase per ml. The stained cells were analyzed by flow cytometry.
Apoptosis assay and cell cycle analysis
Cells were stained with annexin V and 7AAD using BD ApoAlert Annexin V kit (BD Pharmingen) in accordance with the manufacturer's instruction. Apoptosis were analyzed by quantification of annexin V positive cell population by flow cytometry. For cell cycle analysis, cells were fixed with 0.25% formaldehyde in PBS and permiabilized with 0.3% Nonidet P-40. Cells were then stained with propidium iodide containing 1mg/ml RNase A, followed by FACS analysis for G0/G1, S, and G2/M populations using a FACS caliber.
Immunocytochemistry
Cells were plated on 18-mm diameter glass cover slips and were allowed to recover for 24 hrs before treatment with H2O2. Treated cells were fixed with 4% formaldehyde in PBS for 20 min at room temperature. Cells were then washed with PBS and permeabilized with 0.2% triton-X-100 in 3% bovine serum albumin (PBS/BSA) for 10 min at room temperature. The cells were then washed with PBS and blocked with 10% BSA/PBS solution for 1 hr at room temperature. Cells were then incubated with primary antibodies against p53, p53ser 20, or p21WAF1 (Cell signaling Technology) in 10% BSA/PBS solution at room temperature for 2 hrs. After extensive washes, cells were incubated with Rhodamine Red-X -conjugated Goat Anti-Rabbit IgG or Rhodamine Red-X -conjugated Goat Anti-mouse IgG (Jackson Immuno Research). DNA was then labeled with DAPI (4, 6 diamino-2-phenylindole, Sigma). Slides were finally mounted in mounting medium (Vector, Burlingame, CA), and were visualized under Carl Zeiss invert Axiovert 200M microscope (Cottingen Germany) with OpenLab 4.0.3 software (Improvision, Lexington, MA).
Temperature shifts and cell proliferation analysis
For the temperature shift experiment, 1×105 tsp53 transduced p53-/-;Fanca+/+ and p53-/-;Fanca-/- MEF cells were cultured in triplicate in 6-well tissue culture plates. Plates were incubated at 37°C or 32°C. Every third day cells were trypsinized, counted with a hemacytometer, and same number of cells was re-plated in 6-well tissue culture plates.
Senescence-Associated β-Galactosidase
SA-β-galactosidase (SA-β-gal) activity was determined using a SA-β-gal staining kit (Cell Signaling Technology) according to the manufacturer's instructions. Briefly cells were washed once with PBS, and fixed with 0.5% glutaraldehyde. Cells were further washed with PBS and stained with X-gal solution (1mg/ml X-gal) for overnight at 37°C. The percentage of positive stained cells was quantified under a phase-contrast microscope.
Immunohistochemistry
Spleen, thymus and bone were fixed with formalin, and embedded in paraffin for sectioning. After deparaffinization and hydration, sections were blocked with 10% serum in PBS for 30 min and then incubated with 1:50 diluted anti-p53, or 1:50 diluted anti-p21WAF1 (all from Santa Cruz Technologies) primary antibodies overnight at 4°C followed by three washing with PBS at 5 min interval. Detection of p53 and p21WAF1 staining was performed using the biotin peroxidase complex (ABC) method (Vectastain ABC kits, Vector Laboratories, Burlingame, CA). Color was developed with diaminobenzidie tetrahydrochloride, and nuclei were stained with hematoxylin.
Preparation of cell extracts and immunoblotting
To prepare cell protein, single cell suspension was prepared from BMMNCs, spleenocytes or trypsinized MEFs, washed with ice cold PBS, and resuspended in ice cold lysis buffer containing 50mM Tris-HCL (pH 7.4), 0.1% NP40, and 1M NaCl supplemented with protease and phosphatase inhibitors (10μg/ml aprotinin, 25μg/ml leupeptin, 10μg/ml pepstatin A, 2mM PMSF, 0.1M NaP2O4, 25mM NaF and 2mM sodium orthovandate) for 30 min on ice. Cell debris was removed from the lysates by centrifuging them at 14,000 rpm for 30 min. Protein concentration was quantified by using Biorad reagent. 100μg of cell lysates were resolved on (12%) SDS- PAGE and transferred onto nitrocellulose membranes. Immunoblots were then incubated with primary antibodies specific for p53, p53ser20 and p21WAF1 (Santa Cruz Biotechnologies), γ H2AX (Upstate Biotechnology), and phosphorylated pRB, phosphorylated p38, phosphorylated ERK1/2, and the pan kinases p38 and ERK1/2 (all from Cell Signaling) or β actin (Sigma) for 12-16 hr at 4°C. Signals were revealed after incubation with anti-mouse or anti-rabbit secondary antibodies.
Statistics
Data was analyzed statistically using a Student's t test. The level of statistical significance stated in the text was based on the p values. p < 0.05 was considered statistically significant.
Results
Hematopoietic cells and MEFs from Fanca-/- mice are hypersensitive to oxidants
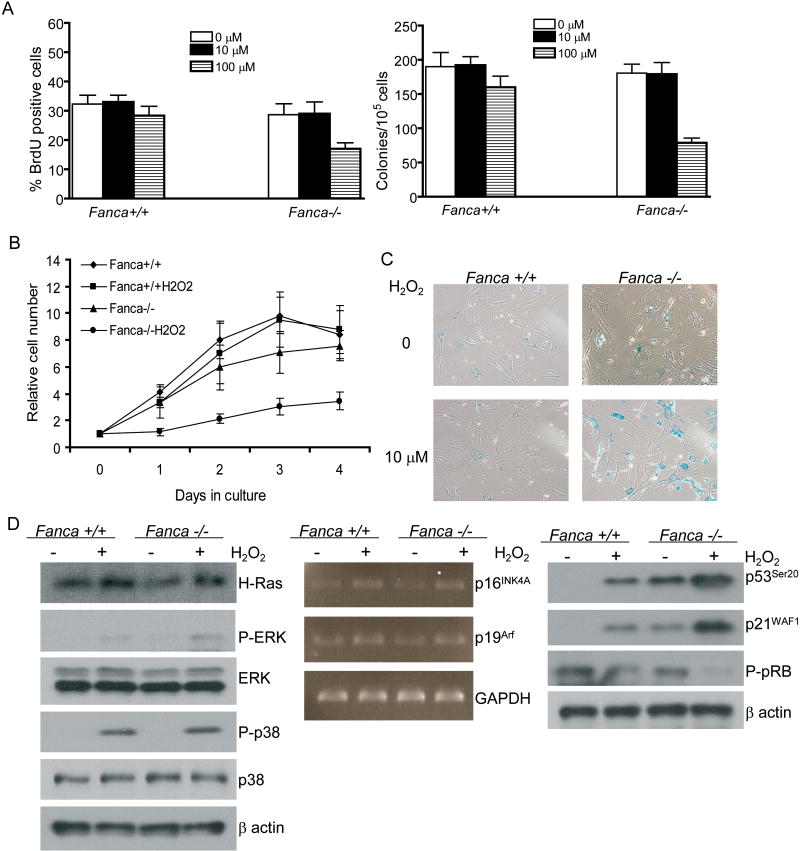
Since FA patients have severe defects in hematopoietic function and since it is not clear whether the FA proteins function in oxidative stress response, we used mice deficient for the FA complementation group A (Fanca) gene to examine the sensitivity of primary FA cells to oxidative stress. We first treated bone marrow mononuclear cells (BMMNCs) from Fanca-/- or their WT littermates with increasing concentrations of the oxidant hydrogen peroxide (H2O2) and analyzed cell proliferation using the BrdU incorporation assay. We observed that treated Fanca-/- cells proliferated much slower than their WT counterparts (Fig. 1A, left panel). Because FA hematopoietic failure is direct consequence of the defect in the hematopoietic stem cell/progenitor (HSC/P) compartment, we tested whether the FA HSC/P cells were hypersensitive to oxidative damage. Indeed, the number of colonies formed by Fanca-/- HSC/P cells was markedly decreased with 100 μM of H2O2, while WT HSC/P cells showed little effect H2O2 treatment (Fig. 1A, right panel).
Figure 1.
Fanca-/- cells are hypersensitive to oxidants. A, left panel, 2×105 BMMNCs from Fanca-/- mice and their WT littermates were treated with increasing concentration of H2O2 and cultured in triplicate wells at 37 °C for 45 min. Cells were then washed and cultured for 24 h in normal growth medium supplemented with 10 μM BrdU, and level of BrdU incorporation was determined by flow cytometry. Data represent mean ± SD of three experiments. Right panel, 1×105 BMMNCs from Fanca-/- mice and their WT littermates were cultured in semi-solid medium containing increasing concentration of H2O2 and progenitor proliferation was analyzed by clonogenic assay. Data represents the mean ± SD of three experiments. B, Equal density (1×105) of MEFs isolated from Fanca-/- mice or their WT littermates were treated with or without H2O2 (50 μM) and cultured in triplicate wells at 37 °C for 4 days, when viable cells were counted each day. Experiments were performed on three MEF cultures derived from individually isolated embryos for each genotype. C, WT, Fanca-/- MEFs at passage 4 were incubated with or without H2O2 (10 μM) for 5 days, with medium and H2O2 changed each day. Cells were then stained for SA-β-gal. D, WT, Fanca-/- MEFs at passage 4 were incubated with or without H2O2 (10 μM) for 5 days, with medium and H2O2 changed each day. Whole cell extracts or total RNAs were subjected to Western blot (left and right panels) or RT-PCR (middle panel) analysis, respectively.
We also established mouse embryonic fibroblasts (MEFs) from Fanca-/- or WT mice and utilized this well-established cell type as a cellular model system for the evaluation of cellular and molecular alterations in oxidative response in FA mutant cells. Similar to BMMNCs, the growth of Fanca-/- MEFs was severely inhibited even at low concentrations of H2O2, which had little effect on the proliferation of WT cells (Fig. 1B). In addition, a sublethal dose (10μM) of H2O2 induced premature senescence, as tested by senescence-associated β-galactosidase (SA-β-gal) staining, in Fanca-/- MEFs (Fig. 1C) Together, these results indicate that loss of FA function results in cellular hypersensitivity to oxidants.
Oxidative stress induces premature senescence by activating several major signaling pathways, including the p16INK4A/pRB and ARF/p53 pathways (39). Oncogene Ras has also been shown to induce cell growth arrest after oxidative injury by activating the MAPK pathway (40). We therefore examined the expression and activities of proteins and enzymes in the Ras, p53 and pRB pathways. We did not find significantly elevated Ha-Ras expression or the downstream activation of the MAPK kinases ERK and p38 in senescent Fanca-/- cells treated with 10μM H2O2 (Fig 1D, left panel), suggesting that the Ras pathway was not responsible for the 10μM H2O2-induced premature senescence in Fanca-/- cells. There was only a marginal increase in the expression of p16INK4A and p19Arf, which was correlated with a decrease in phosphorylated pRB, in response to 10μM H2O2 in both WT and Fanca-/- cells as determined by RT-PCR or Western blotting, respectively (Fig. 1D, middle and right panels). However, the activity of p53, as reflected by its phosphorylation at Ser20 (p53Ser20, which is a specific indicator of oxidative DNA damage; 41) and p21WAF1 expression, was significantly increased in senescent Fanca-/- cells treated with 10μM H2O2 (Fig. 1D, right panel). These results suggest that the p53 pathway may play a predominant role in H2O2-induced premature senescence in Fanca-/- cells.
H2O2 induces G2/M cell-cycle arrest and DNA double-strand breaks in Fanca-/- BM cells
One hallmark of FA cells in response to genotoxic stress is increased retardation of the cells at the G2/M phase of the cell cycle (1-3). We thus examined whether H2O2 induced abnormal G2/M cell-cycle arrest in primary FA BM cells. Indeed, H2O2-treated Fanca-/- BM cells displayed a significant increase in the percentage of cells in G2/M as compared to H2O2-treated WT cells (Fig. 2A). Since G2 cell-cycle arrest is a major cellular response to DNA damage preceding the decision to repair or enter the mitosis and since FA cells are known to be defective in the repair of DNA double-strand breaks (DSB; 1-3), we determined whether H2O2-treated Fanca-/- BM cells accumulated high levels of DNA damage by examining the well-established DSB marker, phosphorylated histone H2AX (γH2AX; 42). As shown in Figure 2B, there was no significant accumulation of DSB in WT BM cells, indicating that either the H2O2 treatments have little effect on WT BM cells or DNA damage induced by H2O2 had been mostly repaired in these WT BM cells. Strikingly, H2O2 induced significant more DSB in Fanca-/- BM cells than in untreated Fanca-/- or H2O2-treated WT BM cells (Fig. 2B). Thus like MMC, H2O2 induces abnormal G2/M cell-cycle arrest in primary FA BM cells, possibly in a DNA damage-dependent manner.
Figure 2.
H2O2 induces G2/M cell-cycle arrest and DNA-strand breaks in Fanca-/- BM cells. A, BM cells from Fanca-/- mice or their WT littermates were treated with or without H2O2 (100 μM) for 16 hrs and stained with propidium iodide (PI) followed by analysis for cell cycle distribution. Shown are representative flow cytometric presentations of three independent experiments. Numbers in plots indicate percent of cells in G2/M phases. B, BM cells from Fanca-/- mice or their WT littermates were treated with or without H2O2 (100 μM) for 4 hrs and whole cell extracts were prepared and analyzed for the expression of γH2AX.
H2O2 overactivates DNA damage response in Fanca-/- cells in vitro and in vivo
Reasoning that the abnormal G2/M accumulation induced by H2O2 in Fanca-/- BM cells might have been resulted from the prolonged activation of the oxidative DNA damage and DNA double-strand break checkpoints, we examined the expression of several oxidative damage response molecules p53, p53Ser20 and p21WAF1. We found increased expression of both total and activated form (p53Ser20) of p53 in H2O2-treated Fanca-/- cells compared to WT cells (Fig. 3A). Consistent with its activation, p21WAF1, the target of p53, was upregulated in H2O2-treated Fanca-/- cells.
Figure 3.
H2O2 overactivates DNA damage response in Fanca-/- cells in vitro and in vivo. A, WT or Fanca-/- mice were injected i.v. with H2O2 (250 μmol/kg body weight) or with equal volume (100 μl) of PBS. The mice were then sacrificed 4 hrs later and BM cells were isolated and stained for the p53Ser20, p53, or p21WAF1 proteins. B, BM cells and splenocytes of PBS-treated or H2O2-treated WT and Fanca-/- mice as described in (A) were analyzed for the expression of the p53Ser20, p53, or p21WAF1 proteins by western blotting. Total protein levels were normalized using β-actin as a control. C, Paraffin-embedded bone marrow, spleen, or thymus sections from untreated or H2O2-treated mice were stained with the antibody against p53, or p21WAF1.
Next, we examined oxidative DNA damage response in vivo. For this purpose we injected (i.v.) H2O2 into WT and Fanca-/- mice and examined the expression of p53, p53Ser20 and p21WAF1. Western blot analysis showed that all these DNA damage response molecules were elevated in response to H2O2 treatment in BM and spleen harvested from Fanca-/- mice (Fig. 3B). Similar results were noticed in the paraffin embedded tissue sections of bone marrow, spleen and thymus prepared from H2O2-treated mice (Fig. 3C). These results suggested that loss of FA function causes strong oxidative DNA damage response, possibly through upregulation of the p53 pathway.
Kinetics of H2O2-induced DNA damage response in Fanca-/- mice
The observation that H2O2 induced strong DNA damage response in Fanca-/- cells and tissues prompted us to study the kinetics of H2O2-mediated p53 signaling in Fanca-/- mice. We treated (through tail vein injection) WT and Fanca-/- mice with a single dose (250 μmol/kg) of H2O2, and sacrificed the injected animals 2, 4, 6, 12 and 24 hrs after H2O2 injection. The kinetics of DNA damage response in the bone marrow and spleen of the treated mice was analyzed for the expression of p53Ser20 and γH2AX. As shown in Figure 4, p53 activation (in the form of p53Ser20) was short-lived in both marrow (Fig 4A) and spleen (Fig 4B) of WT mice, which peaked at 4 hrs but was decayed rapidly to background level by 6 hrs after H2O2 treatment. In Fanca-/- mice, in contrast, high level of p53 activation was evident at 2 hrs and persisted for 24 hrs post-injection. Although the expression kinetics of γH2AX, a well-established DSB marker (42), varied somewhat among the mouse tissues, Fanca-/- mice showed prolonged expression of the DNA damage marker compared to WT mice, in which γH2AX expression was undetectable by 12-24 hrs after injection. Therefore, DNA damage response initiated by oxidative stress is persistent in Fanca-/- mouse hematopoietic tissues in vivo.
Figure 4.
H2O2-induced DNA damage response is prolonged in Fanca-/- mice. A, WT and Fanca-/- littermates were injected with a single dose (250 μmol/kg) of H2O2, and sacrificed 2, 4, 6, 12 and 24 hrs after H2O2 injection. Whole cell extracts prepared from bone marrow cells were analyzed for the expression of p53Ser20 and γH2AX. B, WT and Fanca-/- littermates were injected with a single dose (250 μmol/kg) of H2O2, and sacrificed 2, 4, 6, 12 and 24 hrs after H2O2 injection. Whole cell extracts prepared from spleen were analyzed for the expression of p53Ser20 and γH2AX. C, BM Lin- cells from WT and Fanca-/- littermates were cultured with cytokine combinations known to support myeloid (SCF, 100 ng/ml; GM-CSF, 10 ng/ml) or lymphoid (SCF, 100 ng/ml; IL-3, 10 ng/ml; IL-7, 10 ng/ml) differentiation for 5 days. Cells were then stained with antibodies for lineage-specific markers (myeloid, Gr-1 and Mac-1; lymphoid, B220 and CD3e) followed by flow cytometry. Horizontal axes, fluorescence intensity; vertical axes, relative cell number; solid line, staining with test antibody; dashed line, staining with an isotype-matched control antibody. D, H2O2-induced DNA double-strand breaks in Fanca-/- BM myeloid and lymphoid cells. Fanca-/- BM myeloid and lymphoid cells described in (C) were treated with H2O2 (100 μM) for 0, 4 or 8 hrs, and protein extracts were prepared and analyzed by immunoblotting with anti-γH2AX and anti-actin antibodies.
The observation that H2O2-induced expression of the DSB marker γH2AX appeared to be more pronounced and sustained in the BM than in spleen raised the question as to whether there was a lineage-specific effect on oxidative DNA damage in Fanca-/- mice. To address this, we determined H2O2-induced DSBs in lymphoid and myeloid cells (Fig 4C) from the BM of Fanca-/- mice. While increased and prolonged expression of γH2AX was seen in Fanca-/- cells compared to WT cells, no significant difference in H2O2-induced DSBs was evident between lymphoid and myeloid cells (Fig 4D).
The dependency and persistence of p53-mediated response to oxidative and oncogenic stresses in Fanca-/- cells
To explore the requirement for p53 activity and potential FA-p53 interaction in oxidative DNA damage response, we generated Fanca-/- mice with deletion of the Trp53 gene, isolated primary MEFs from Fanca+/+;Trp53-/- and Fanca-/-;Trp53-/- mice, and expressed in these cells the temperature-sensitive p53 mutant tsp53V143A, which can be reverted to functional state at the permissive temperature of 32°C (43). The functional reversibility of the mutant tsp53V143A was verified by Western analysis demonstrating that H2O2 induced increased p21WAF1 expression in all the tsp53V143A-expressing cells cultured at 32°C (Fig. 5A). A prolonged time-course analysis of cell growth indicated that at non-permissive temperature (37°C) loss of p53 function almost completely abolished growth response to H2O2 in cells with or without the Fanca protein (Fig. 5B), suggesting that H2O2-induced damage response requires a functional p53. Indeed, when the function of p53 was restored at 32°C, H2O2-induced growth inhibition was clearly evident in both Fanca+/+ and Fanca-/- cells (Fig. 5C). However, we observed differential responses to H2O2-induced stress in cells with different Fanca status. In Fanca+/+ cells, the p53 response to oxidative stress appeared to be relatively short (3-6 days) after the insult; whereas Fanca-/- cells underwent prolonged growth inhibition (Fig. 5C). Interestingly, TUNEL assay indicated that p53 deficiency increased H2O2-induced apoptosis in Fanca-/- cells, which could be inhibited by functional tsp53V143A (Fig. 5D). Thus, although p53 caused prolonged proliferative suppression in Fanca-/- cells, a functional p53 appeared to be required for the survival of the mutant cells under oxidative stress.
Figure 5.
Differential responses to oxidative stress in Fanca+/+;Trp53-/- and Fanca-/-;Trp53-/- cells expressing a switchable p53. A, The functional reversibility of the tsp53V143A at permissive temperature (32°C). Fanca+/+;Trp53-/- and Fanca-/-;Trp53-/- MEFs were infected at passage 2 with MIEG3 empty vector or MIEG3- tsp53V143A retrovirus. Transduced cells were sorted by FACS, cultured at 37 °C for three passages, and shifted to 32 °C for functional tsp53V143A expression. 16 hrs later, cells were incubated with H2O2 (100 μM) for 4 hrs. Western blot analysis was then performed for the expression of p53 and p21WAF1. 100 μg of total proteins was loaded in each lane, and equal loading was ensured by probing the blot with anti-β-actin. B, A prolonged time course analysis of cell growth at non-permissive temperature (37 °C). Equal density (2×104) of MEFs described in (A) were cultured in the presence of 50 μM H2O2 in triplicate wells at 37 °C for 15 days. Viable cells were counted at the indicated time points. C, A prolonged time course analysis of cell growth at permissive temperature (32 °C). Equal density (2×104) of MEFs described in (A) were cultured in the presence of 50 μM H2O2 in triplicate wells at 32 °C for 15 days. Viable cells were counted at the indicated time points. D, MEFs described in (A) were cultured in the presence of 100 μM H2O2 at 37 °C or 32 °C for 16 hrs, and analyzed by TUNEL staining. Apoptosis was quantified by counting three random fields of 500 cells from each of three cultures.
We next used the same cell system to explore the requirement for p53 function in response to oncogenic stress, specifically activated ras that is a well-studied p53-antagonizing oncogene (44). We infected tsp53V143A-expressing Fanca+/+;Trp53-/- and Fanca-/-;Trp53-/- MEFs with an H-Ras V12 retrovirus and monitored cell proliferation at 32°C or 37°C for a period of 15 days. Growth kinetics showed that at 37°C where p53 was inactivated, all cell lines proliferated well regardless of the Fanca genotype (Fig. 6A). When the cells were cultured at 32°C where p53 was functional, Fanca+/+ cells exhibited persistent response to activated ras-induced growth arrest (Fig. 6A). Interestingly, Fanca-/- cells initially responded to activated ras-triggered growth arrest then started to proliferate like p53-null cells after day 6. These arrested cells expressed senescence-associated (SA)-β-galactosidase and appeared morphologically senescent (Fig. 6B).
Figure 6.
Differential responses to oncogenic stress in Fanca+/+;Trp53-/- and Fanca-/-;Trp53-/- cells expressing a switchable p53. A, The tsp53V143A-expressing Fanca+/+;Trp53-/- and Fanca-/-;Trp53-/- MEFs described in Figure 5 (A) were infected with an H-Ras V12 retrovirus and cultured at 32°C or 37°C for 12 days. Viable cells were counted every two days. B, The tsp53V143A-expressing Fanca+/+;Trp53-/- and Fanca-/-;Trp53-/- MEFs infected with an H-Ras V12 retrovirus and cultured at 32°C or 37°C. Cells were examined for senescence on day 6 by SA-β-gal stain. C, The tsp53V143A-expressing Fanca+/+;Trp53-/- and Fanca-/-;Trp53-/- MEFs infected with an H-Ras V12 retrovirus and cultured at 37°C for either 3 or 12 days and then restored p53 function by shifting the cultures to 32°C. After 24 hrs, cell proliferation was analyzed by BrdU incorporation. D, The tsp53V143A-expressing Fanca+/+;Trp53-/- and Fanca-/-;Trp53-/- MEFs infected with an H-Ras V12 retrovirus and cultured at 37°C for either 3 or 12 days and then restored p53 function by shifting the cultures to 32°C. After 24 hrs, cell extracts were prepared for Western blot analysis of p53 and p21WAF1.
The interesting observation that Fanca-deficient cells failed to respond to ras-induced growth arrest after 6 days of culture at permissive temperature (32°C) suggested that Fanca-/- cells might have lost their ability to engage p53 during constitutive ras activation. To test this hypothesis, we cultured H-Ras V12-transduced tsp53V143A-expressing cells at 37°C for either 3 or 12 days and then restored p53 function by shifting the cultures to 32°C. Cell proliferation and induction of p21WAF1 were analyzed by BrdU incorporation and Western blot, respectively. Both cell lines shifting to 32°C at day 3 underwent approximately same level of growth arrest with few cells in the S-phase population (Fig. 6C), which was accompanied by strong induction of p21WAF1 (Fig. 6D). Whereas p53 restoration at day 12 could still triggered immediate growth arrest in Fanca+/+;Trp53-/- cells; Fanca-/-;Trp53-/- cells showed lack of activated ras-induced growth arrest and p21WAF1 expression (Fig. 6C,D). Therefore, loss of Fanca impairs p53 function during prolonged ras activation.
Discussion
Cellular stresses, most significantly oxidative DNA damage and oncogene insults, have been shown to activate p53 (45). Given that p53 activation can lead to cell growth arrest or apoptosis, cells, cancer cells in particular, must have developed strategies to regulate the p53 activity. In addition to the p53 negative regulator Mdm2, several p53-interacting proteins have been shown to regulate p53 activity. The interaction of p53 with other cellular proteins is known to influence the activity of the tumor suppressor (46). Our finding that the Fanca protein coordinates with p53 to regulate oxidative and oncogenic stress responses supports the notion that a fine control of p53 activity is fundamentally important for cellular homeostasis and cancer prevention.
Patients with Fanconi anemia (FA) have an increased susceptibility to cancer and cells from FA patients are deficient in repair of DNA damage induced by certain genotoxic agents. This suggests that the FA proteins may interplay with other tumor suppressor pathways that are involved in DNA damage repair and oncogenic signaling. Encouraged by recent reports that p53 deficiency increases cancer development in patients with FA and FA knockout mice (32-36), we formally tested the hypothesis that the FA proteins may functionally interact with the p53-activating signals in response to oxidative and oncogenic stresses. Our results suggest that the major FA protein, Fanca, may coordinate with p53 in regulation of oxidative and oncogenic stress responses. This notion is supported by (i) hypersensitive response to oxidative stress in tissues in vivo and cells in vitro derived from Fanca-/- mice is correlated with a persistent overactivation of the tumor suppressor p53; (ii) loss of Fanca function causes prolonged oxidative DNA damage response through upregulation of the p53 pathway; and (iii) the functional status of p53 dictates the kinetics and persistence of response to oxidative and oncogenic stresses in these FA cells.
The mechanistic link between p53 signaling and FA has not been well defined. The involvement of p53 in FA pathophysiology has been highlighted by recent studies that show accelerated tumor development in Fancd1, Fancd2 or Fancc mice also deficient for the Trp53 gene (34-36). Furthermore, developmental defects and increased apoptosis in Fancd2-deficient zebrafish could be corrected by knockdown of p53, suggesting that p53-dependent apoptosis may be an underlying mechanism for developmental defect in the Fancd2-/- fish (38). The current study establishes the requirement for p53 signaling in oxidative and oncogenic stress responses in Fanca-deficient cells. We have used primary MEFs from Fanca+/+;Trp53-/- and Fanca-/-;Trp53-/- mice combined with a functionally switchable p53 mutant to define the kinetics, dependence, and persistence of p53-mediated response to oxidative and oncogenic stresses in FA cells. One important finding of our study is that we observed different pattern of p53-dependent response to H2O2- and deregulated oncogene-induced stress in cells with different Fanca status. For instance, restoration of p53 function elicited a transient response to oxidative stress in Fanca+/+ cells; whereas Fanca-/- cells underwent prolonged p53-dependent growth inhibition (Fig 5). This persistent p53 engagement may be due to the fact that Fanca-deficient cells accumulated high levels of oxidative DNA damage as a consequence of impairment in DNA damage repair. We previously reported that bone marrow progenitor cells from Fancc-/- mice contained high levels of oxidative DNA damage and exhibited persistent DNA damage response (47). Thus, the prolonged p53 response in Fanca-/- cells might have been induced by the unrepaired DNA damage, leading to persistent DNA damage response.
Another interesting finding in the current study is that, although activation of p53 by oxidative stress causes increased proliferative suppression in Fanca-/- cells compared to WT cells (Fig. 1), studies with the Fanca-/-;Trp53-/- double-knockout cells indicated that p53 deficiency increased H2O2-induced apoptosis in Fanca-/- cells, which could be inhibited by functional tsp53V143A (Fig 5). Thus, a functional p53 appears to be required for the survival of the mutant cells under oxidative stress. Circumstantial evidence indicates that the selectivity of p53 to regulate the pro-survival or pro-apoptotic signaling is affected by growth environment, the type of stress used, the cellular context, and differential expression of the target genes (48, 49). In response to stress, p53 induces cell growth arrest thus preventing the damaged cells from further replication (45, 50). More importantly, p53 can activate cell-cycle checkpoint in cells with damaged DNA, which allows cells to repair the damage before re-entering cell cycle. Indeed, it has been reported that p53-null cells are more sensitive to mitotic catastrophe induced by genotoxic drug (51, 52). Since H2O2-induced arrest in Fanca-/- cells with a functional p53 occurs at the G2M cell cycle checkpoint, inactivation of p53 would lead to the impairment of the checkpoint. Consequently, failure of the damaged Fanca-/- cells to arrest at G2 would result in entry into mitosis and potential death through mitotic catastrophe as well as apoptosis.
Patients with FA have high predisposition to leukemia and other cancers. Whether FA cells have high susceptibility to oncogenic transformation is not known. We have used an in vitro system in which a functional p53 could be restored in Fanca-/-;Trp53-/- cells to investigate the dynamic interplay between Fanca and p53 in response to oncogenic stress. While WT cells exhibit prolonged response to oncogene activation, the p53-activating signals induced by oncogenic ras were short-lived in Fanca-/- cells. Indeed, when p53 function was restored 12 days after ras activation, Fanca-/-;Trp53-/- cells showed lack of activated ras-induced growth arrest and p21WAF1 expression (Fig 6). This suggests that a functional Fanca may be required for the cell to engage p53 during constitutive ras activation. In contrast to oxidative DNA damage, which triggers p53 activation only transiently, activated Ras elicits persistent p53-activating signals in WT cells. Furthermore, our results demonstrate that the Fanca function is required to maintain p53 response to Ras-induced growth arrest, as restoration of p53 function after 12 days of constitutive ras activation failed to suppress proliferation of cells deficient for Fanca.
In conclusion, our results derived from both in vivo and in vitro studies using the restorable p53 model shed new light on the potential interplay between p53 and the FA pathway during cellular response to oxidative and oncogenic stresses. Our studies also define the important kinetic difference between WT and FA cells during prolonged response to oxidative DNA damage and activated oncogene signals. Additional studies into functional interaction between the p53 and FA pathways in DNA damage and oncogenic stress response may aid us in better understanding how cells can bypass the normal checkpoints and continue to proliferate in the presence of damaged DNA and oncogenic activation. In the context of FA, new insights on the role of the FA proteins in oxidative DNA damage response/repair and oncogene activation can suggest new pathways and proteins to target for therapeutic prevention of cancer progression of the disease.
Acknowledgments
We thank Dr. Madeleine Carreau (Laval University, Canada) for the Fanca+/- mice, Dr. Yi zheng (Cincinnati Children's Hospital Medical Center) for the tsp53 V143A and H-Ras V12 retroviral vectors, Dr. Xiaoling Zhang for technical assistance and discussion, and the Vector Core of the Cincinnati Children's Research Foundation (Cincinnati Children's Hospital Medical Center) for the preparation of retroviruses.
This work was supported in part by NIH grants R01 CA109641 and R01 HL076712. Q.P. is supported by a Leukemia and Lymphoma Scholar award.
Nonstandard abbreviations used
- BMMNCs
bone marrow (BM) mononuclear cells
- DEB
diepoxybutane
- FANCA
Fanconi anemia (FA) complementation group A
- MEF
Mouse embryonic fibroblast
- MMC
mitomycin C
- SA-β-gal
Senescence-Associated β-Galactosidase
References
- 1.Bagby GC. Genetic basis of Fanconi anemia. Curr Opin Hematol. 2003;10:68–76. doi: 10.1097/00062752-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Collins N, Kupfer GM. Molecular pathogenesis of Fanconi anemia. Int J Hematol. 2005;82:176–83. doi: 10.1532/IJH97.05108. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy RD, D'Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005;19:2925–40. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- 4.Timmers C, Taniguchi T, Hejna J, et al. Positional cloning of a novel Fanconi anemia gene, FANCD2. Mol Cell. 2001;7:241–8. doi: 10.1016/s1097-2765(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 5.Strathdee CA, Gavish H, Shannon WR, Buchwald M. Cloning of cDNAs for Fanconi's anaemia by functional complementation. Nature. 1992;356:763–7. doi: 10.1038/356763a0. [DOI] [PubMed] [Google Scholar]
- 6.Lo Ten Foe JR, Rooimans MA, Bosnoyan-Collins L, et al. Expression cloning of a cDNA for the major Fanconi anaemia gene, FAA. Nature Genet. 1996;14:320–3. doi: 10.1038/ng1196-320. [DOI] [PubMed] [Google Scholar]
- 7.de Winter JP, Waisfisz Q, Rooimans MA, et al. The Fanconi anaemia group G gene FANCG is identical with XRCC9. Nat Genet. 1998;20:281–3. doi: 10.1038/3093. [DOI] [PubMed] [Google Scholar]
- 8.de Winter JP, Rooimans MA, van Der WL, et al. The Fanconi anaemia gene FANCF encodes a novel protein with homology to ROM. Nat Genet. 2000;24:15–6. doi: 10.1038/71626. [DOI] [PubMed] [Google Scholar]
- 9.de Winter JP, Leveille F, van Berkel CG, et al. Isolation of a cDNA Representing the Fanconi Anemia Complementation Group E gene. Am J Hum Genet. 2000;67:1306–8. doi: 10.1016/s0002-9297(07)62959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howlett NG, Taniguchi T, Olson S, et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–9. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]
- 11.Meetei AR, de Winter JP, Medhurst AL, et al. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet. 2003;35:165–70. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- 12.Meetei AR, Levitus M, Xue Y, et al. X-linked inheritance of Fanconi anemia complementation group B. Nat Genet. 2004;36:1219–24. doi: 10.1038/ng1458. [DOI] [PubMed] [Google Scholar]
- 13.Meetei AR, Medhurst AL, Ling C, et al. A human ortholog of archael DNA repair protein HEF is defective in Fanconi anemia complementation group M. Nat Genet. 2005;37:958–63. doi: 10.1038/ng1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levran O, Attwooll C, Henry RT, et al. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat Genet. 2005;37:931–3. doi: 10.1038/ng1624. [DOI] [PubMed] [Google Scholar]
- 15.Levitus M, Waisfisz Q, Godthelp BC, et al. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat Genet. 2005;37:934–5. doi: 10.1038/ng1625. [DOI] [PubMed] [Google Scholar]
- 16.Xia B, Dorsman JC, Ameziane N, et al. Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nat Genet. 2006;39:159–61. doi: 10.1038/ng1942. [DOI] [PubMed] [Google Scholar]
- 17.Reid S, Schindler D, Hanenberg H, et al. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet. 2006;39:162–4. doi: 10.1038/ng1947. [DOI] [PubMed] [Google Scholar]
- 18.Smogorzewska A, Matsuoka S, Vinciguerra P, et al. Identification of the FANCI Protein, a Monoubiquitinated FANCD2 Paralog Required for DNA Repair. Cell. 2007;129:1–13. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagano G, Degan P, d'Ischia M, et al. Oxidative stress as a multiple effector in Fanconi anaemia clinical phenotype. Eur J Haematol. 2005;75:93–100. doi: 10.1111/j.1600-0609.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 20.Kruyt FA, Hoshino T, Liu JM, Joseph P, Jaiswal AK, Youssoufian H. Abnormal microsomal detoxification implicated in Fanconi anemia group C by interaction of the FAC protein with NADPH cytochrome P450 reductase. Blood. 1998;92:3050–6. [PubMed] [Google Scholar]
- 21.Cumming RC, Lightfoot J, Beard K, Youssoufian H, O'Brien PJ, Buchwald M. Fanconi anemia group C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nat Med. 2001;7:814–20. doi: 10.1038/89937. [DOI] [PubMed] [Google Scholar]
- 22.Hadjur S, Ung K, Wadsworth L, et al. Defective hematopoiesis and hepatic steatosis in mice with combined deficiencies of the genes encoding Fancc and Cu/Zn superoxide dismutase. Blood. 2001;98:1003–11. doi: 10.1182/blood.v98.4.1003. [DOI] [PubMed] [Google Scholar]
- 23.Futaki M, Igarashi T, Watanabe S, et al. The FANCG Fanconi anemia protein interacts with CYP2E1: possible role in protection against oxidative DNA damage. Carcinogenesis. 2002;23:67–72. doi: 10.1093/carcin/23.1.67. [DOI] [PubMed] [Google Scholar]
- 24.Mukhopadhyay SS, Leung KS, Hicks MJ, Hastings PJ, Youssoufian H, Plon SE. Defective mitochondrial peroxiredoxin-3 results in sensitivity to oxidative stress in Fanconi anemia. J Cell Biol. 2006;175:225–35. doi: 10.1083/jcb.200607061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saadatzadeh MR, Bijangi-Vishehsaraei K, Hong P, Bergmann H, Haneline LS. Oxidant hypersensitivity of Fanconi anemia type C-deficient cells is dependent on a redox-regulated apoptotic pathway. J Biol Chem. 2004;279:16805–12. doi: 10.1074/jbc.M313721200. [DOI] [PubMed] [Google Scholar]
- 26.Park SJ, Ciccone SL, Beck BD, Hwang B, Freie B, Clapp DW, Lee SH. Oxidative stress/damage induces multimerization and interaction of Fanconi anemia proteins. J Biol Chem. 2004;279:30053–9. doi: 10.1074/jbc.M403527200. [DOI] [PubMed] [Google Scholar]
- 27.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–3. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 28.Elson A, Deng C, Campos-Torres J, Donehower LA, Leder P. The MMTV/c-myc transgene and p53 null alleles collaborate to induce T-cell lymphomas, but not mammary carcinomas in transgenic mice. Oncogene. 1995;11:181–90. [PubMed] [Google Scholar]
- 29.Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–83. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 30.Lohrum MA, Vousden KH. Regulation and activation of p53 and its family members. Cell Death Differ. 1999;6:1162–8. doi: 10.1038/sj.cdd.4400625. [DOI] [PubMed] [Google Scholar]
- 31.Jin S, Levine AJ. The p53 functional circuit. J Cell Sci. 2001;114:4139–40. doi: 10.1242/jcs.114.23.4139. [DOI] [PubMed] [Google Scholar]
- 32.Kutler DI, Wreesmann VB, Goberdhan A, et al. Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2003;95:1718–21. doi: 10.1093/jnci/djg091. [DOI] [PubMed] [Google Scholar]
- 33.Lowy DR, Gillison ML. A new link between Fanconi anemia and human papillomavirus-associated malignancies. J Natl Cancer Inst. 2003;95:1648–50. doi: 10.1093/jnci/djg125. [DOI] [PubMed] [Google Scholar]
- 34.Jonkers J, Meuwissen R, van der Gulden H, et al. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–25. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 35.Houghtaling S, Granville L, Akkari Y, et al. Heterozygosity for p53 (Trp53+/-) accelerates epithelial tumor formation in fanconi anemia complementation group D2 (Fancd2) knockout mice. Cancer Res. 2005;65:85–91. [PubMed] [Google Scholar]
- 36.Freie B, Li X, Ciccone SL, et al. Fanconi anemia type C and p53 cooperate in apoptosis and tumorigenesis. Blood. 2003;102:4146–52. doi: 10.1182/blood-2003-03-0971. [DOI] [PubMed] [Google Scholar]
- 37.Freie BW, Ciccone SL, Li X, et al. A role for the Fanconi anemia C protein in maintaining the DNA damage-induced G2 checkpoint. J Biol Chem. 2004;279:50986–93. doi: 10.1074/jbc.M407160200. [DOI] [PubMed] [Google Scholar]
- 38.Liu TX, Howlett NG, Deng M, Langenau DM, Hsu K, Rhodes J, Kanki JP, D'Andrea AD, Look AT. Knockdown of zebrafish Fancd2 causes developmental abnormalities via p53-dependent apoptosis. Dev Cell. 2003;5:903–14. doi: 10.1016/s1534-5807(03)00339-3. [DOI] [PubMed] [Google Scholar]
- 39.Satyanarayana A, Rudolph KL. p16 and ARF: activation of teenage proteins in old age. J Clin Invest. 2004;114:1237–40. doi: 10.1172/JCI23437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lander HM, Milbank AJ, Tauras JM, et al. Redox regulation of cell signaling. Nature. 1996;381:380–1. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 41.Banin S, Moyal L, Shieh S, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–7. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 42.Celeste A, Difilippantonio S, Difilippantonio MJ, et al. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell. 2003;114:371–83. doi: 10.1016/s0092-8674(03)00567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedlander P, Haupt Y, Prives C, Oren M. Mutant p53 that discriminates between p53-responsive genes cannot induce apoptosis. Mol Cell Biol. 1996;16:4961–71. doi: 10.1128/mcb.16.9.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferbeyre G, de Stanchina E, Lin AW, et al. Oncogenic ras and p53 cooperate to induce cellular senescence. Mol Cell Biol. 2002;22:3497–508. doi: 10.1128/MCB.22.10.3497-3508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 46.Scolnick DM, Chehab NH, Stavridi ES, et al. CREB-binding protein and p300/CBP-associated factor are transcriptional coactivators of the p53 tumor suppressor protein. Cancer Res. 1997;57:3693–6. [PubMed] [Google Scholar]
- 47.Zhang X, Sejas DP, Qiu Y, Williams DA, Pang Q. Inflammatory ROS promote and cooperate with Fanconi anemia mutation for hematopoietic senescence. J Cell Sci. 2007;120:1572–83. doi: 10.1242/jcs.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 49.Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–42. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 50.Levine AJ, Finlay CA, Hinds PW. P53 is a tumor suppressor gene. Cell. 2004;116:S67–70. doi: 10.1016/s0092-8674(04)00036-4. [DOI] [PubMed] [Google Scholar]
- 51.Ianzini F, Bertoldo A, Kosmacek EA, Phillips SL, Mackey MA. Lack of p53 function promotes radiation-induced mitotic catastrophe in mouse embryonic fibroblast cells. Cancer Cell Int. 2006;6:11–8. doi: 10.1186/1475-2867-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SS, Eom YW, Choi KS. Cdc2 and Cdk2 play critical roles in low dose doxorubicin-induced cell death through mitotic catastrophe but not in high dose doxorubicin-induced apoptosis. Biochem Biophys Res Commun. 2005;334:1014–21. doi: 10.1016/j.bbrc.2005.06.192. [DOI] [PubMed] [Google Scholar]