Introduction
Symptomatic osteoarthritis (OA) causes substantial physical and psychosocial disability (1). In the early 1990’s, over 7 million Americans were limited in their ability to participate in their main daily activities, such as going to school or work or maintaining their independence — simply because of their arthritis (2). Interestingly, the risk for disability (defined as needing help walking or climbing stairs) attributable to knee OA is as great as that attributable to cardiovascular disease and greater than that due to any other medical condition in elderly persons (1). Like arthritis prevalence, the prevalence of arthritis-related disability is also expected to rise by the year 2020, when an estimated 11.6 million people will be affected (2).
Compounding this picture are the enormous financial costs that our nation bears for treating arthritis, its complications, and the disability that results from uncontrolled disease. The total annual cost in the United States is almost $65 billion— a figure equivalent to a moderate national recession (3). This amount includes an estimated medical bill of $15 billion each year for such expenses as 39 million physician visits and more than half a million hospitalizations (CDC, unpublished data). OA accounts for 90% of hip and knee replacements (4). The balance is largely due to indirect costs such as those from wage losses (3). Thus, arthritis has become one of our most pressing public health problems —a problem that is expected to worsen in the next millennium with the increasing prevalence of this disease.
This review delineates the characteristic symptoms and signs associated with OA and how they can be used to make the clinical diagnosis. The predominant symptom in most patients is pain. The remainder of the review focuses on what we know causes pain in OA and contributes to its severity. Much has been learnt over recent years however for the budding researcher much of this puzzle remains unexplored or inadequately understood.
What is OA?
OA can be viewed as the clinical and pathological outcome of a range of disorders that results in structural and functional failure of synovial joints (5). OA occurs when the dynamic equilibrium between the breakdown and repair of joint tissues is overwhelmed (6). This progressive joint failure may cause pain, physical disability, and psychological distress (1), although many persons with structural changes consistent with OA are asymptomatic (7). The reasons why there is this disconnect between disease severity and the level of reported pain and disability is unknown.
Typically OA presents as joint pain. During a one year period, 25% of people over 55 years have a persistent episode of knee pain, of whom about one in six consult their general practitioner about it (8). Approximately 50% of these persons have radiographic knee OA. The usefulness of x-rays relates more importantly to the exclusion of other diagnostic possibilities rather than confirmation of osteoarthritic disease (9). Factors differentiating symptomatic OA from asymptomatic radiographic disease are largely unknown. Symptomatic knee OA (pain on most days and radiographic features consistent with OA) occurs in approximately 12% of those aged over 55 (8).
While OA is common in the knee, it is even more prevalent in the hands, especially the distal (DIP) and proximal (PIP) interphalangeal joints and the base of the thumb (CMC). When symptomatic, especially so for the base of thumb joint, hand OA is associated with functional impairment (10;11). OA of the thumb carpo-metacarpal joint is a common condition that can lead to substantial pain, instability, deformity, and loss of motion (12). Over the age of 70 years, approximately 5% of women and 3% of men have symptomatic OA affecting this joint with impairment of hand function (10).
The prevalence of hip OA is about 9% in Caucasian populations (13). In contrast, studies in Asian, black, and East Indian populations indicate a very low prevalence of hip OA (14). The prevalence of symptomatic hip OA is approximately 4% (15).
What are the characteristic symptoms of OA?
The joint pain of OA is typically described as exacerbated by activity and relieved by rest. More advanced OA can cause rest and night pain leading to loss of sleep which further exacerbates pain. The cardinal symptoms that suggest a diagnosis of OA include:
pain (typically described as activity related or mechanical, may occur with rest in advanced disease; often deep, aching and not well localized; usually of insidious onset;),
reduced function
stiffness (of short duration, also termed “gelling” i.e. short-lived stiffness after inactivity),
joint instability, buckling or giving way
patients may also complain of reduced movement, deformity, swelling, crepitus, and increased age (OA is unusual before age 40) in the absence of systemic features (such as fever),
and when pain persists pain-relate psychological distress.
Tailoring the physical exam-what signs are associated with OA
Physical examination should include an assessment of body weight and body mass index, joint range of motion, the location of tenderness, muscle strength, and ligament stability. For lower limb joint involvement, this should include assessment of body mass and postural alignment in both standing and walking (16). A goniometer can be used to permit the examiner to visually bisect the thigh and lower leg along their lengths. The centers of both the patella and ankle should be located and marked with a pen. The center of the goniometer is placed on the center of the patella, and the arms of this goniometer are extended along the center of the thigh and along the axis of the lower leg to the center of the ankle.
The features on physical examination that suggest a diagnosis of OA include:
Tenderness, usually located over the joint line
Crepitus with movement of the joint
Bony enlargement of the joint, e.g., Heberden's and Bouchard's nodes, squaring of the first CMC, typically along the affected joint line in the knee.
Restricted joint range of motion
Pain on passive range of motion
Deformity, e.g., angulation of the DIP and PIP joints, varus (bowed legs) deformity of the knees
Instability of the joint
Altered gait
Muscle atrophy or weakness
Joint effusion
The Diagnosis of OA
Bearing in mind that radiographs are notoriously insensitive to the earliest pathological features of OA, the absence of positive radiographic findings should not be interpreted as confirming the complete absence of symptomatic disease. Conversely, the presence of positive radiographic findings does not guarantee that an osteoarthritic joint is also the active source of the patient’s current knee or hip symptoms where other sources of pain including periarticular sources such as pes anserine bursitis at the knee and trochanteric bursitis at the hip often contribute (7). According to the ACR criteria for classification of hand OA (unlike the hip and knee where radiographs enhance the sensitivity and specificity), x-rays are less sensitive and specific than physical examination in the diagnosis of symptomatic hand OA (17).
In clinical practice the diagnosis of OA should be made on the basis of your history and physical examination and the role of radiography is to confirm this clinical suspicion and rule out other conditions.
When disease is advanced, it is visible on plain radiographs, which show narrowing of joint space, osteophytes, and sometimes changes in the subchondral bone. MRI can be used in infrequent circumstances to facilitate the diagnosis of other causes of joint pain that can be confused with OA (osteochondritis dissecans, avascular necrosis). An unfortunate consequence of the frequent use of MRI in clinical practice is the frequent detection of meniscal tears. In the interests of preserving menisci an important cautionary note; meniscal tears are nearly universal in persons with knee OA and are not necessarily a cause of increased symptoms (18). The penchant to remove menisci is to be avoided, unless there are symptoms of locking or extension blockade (19).
Do not rely upon laboratory testing to establish the diagnosis of OA. Because OA is a non-inflammatory arthritis, laboratory findings are expected to be normal.
What are the diagnostic criteria for osteoarthritis?
When making the diagnosis of OA, consider using the criteria of the American College of Rheumatology for diagnostic purposes and classification of OA of the hip, knee, and hands in patients with pain in these joints (17;20). These are the criteria that are used in research studies and should be used to inform your diagnosis in individuals but not limiting your information gathering to these criteria and considering the wealth of other information that patients with OA may provide that can help to either confirm or refute an OA diagnosis.
In clinical practice the diagnosis of OA should be made on the basis of your history and physical examination and the role of radiography is to confirm this clinical suspicion and rule out other conditions.
In the process of taking a history it is important to ask how the pain has affected the persons function at home, work and in recreational activities. Also, ask about how the person is coping with pain and how well that is going. It is important to look for signs of psychological distress, e.g. signs of anxiety such as excessive pain avoidant posturing, sleep onset insomnia, or signs of depression such as early morning wakening, weight loss, irritability, or a marked in increase in memory/concentration problems.
Factors that contribute to pain
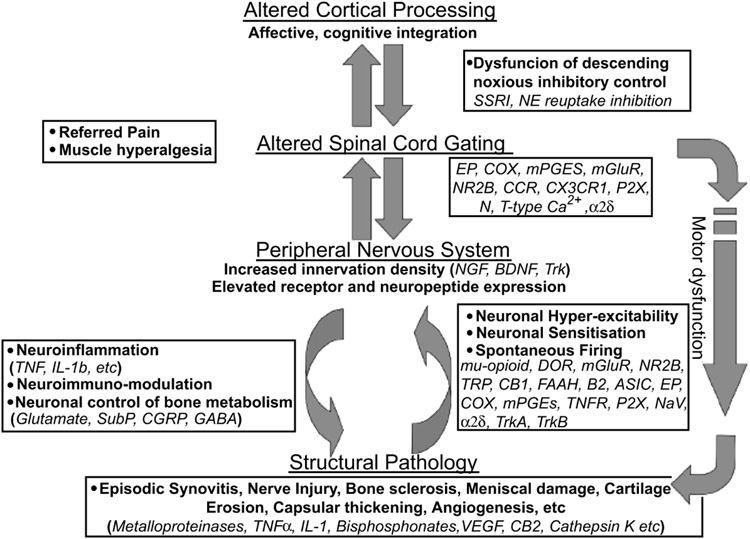
The source of pain is not particularly well understood and is best framed in a biopsychosocial framework (posits that biological, psychological and social factors all play a significant role in pain in OA) (21). Figure 1 depicts a schematic representing some of this complexity.
Figure 1.
Future Targets to Control Osteoarthritis Pain
(insert legend embedded in bottom of figure)
From Arthritis Research & Therapy 2007, 9:212 (doi:10.1186/ar2178)
From a biological perspective, neuronal activity in the pain pathway is responsible for the generation and ultimate exacerbation of the feeling of joint pain. During inflammation chemical mediators are released into the joint which sensitize primary afferent nerves such that normally innocuous joint movements (such as increased physical activity, high heeled shoes, weather changes) now elicit a painful response. This is the neurophysiological basis of allodynia i.e. the sensation of pain in response to a normally non-painful stimulus such as walking. Over time this increased neuronal activity from the periphery can cause plasticity changes in the central nervous system by a process termed "wind-up". In this instance, second order neurones in the spinal cord increase their firing rate such that the transmission of pain information to the somatosensory cortex is enhanced. This central sensitization phenomenon intensifies pain sensation and can even lead to pain responses from regions of the body remote from the inflamed joint i.e. referred pain.
Constitutional factors that can predispose to symptoms include self-efficacy, pain catastrophizing, and the social context of arthritis (social support, pain communication) are all important considerations in understanding the pain experience.
Local tissue pathology
The structural determinants of pain and mechanical dysfunction in OA are also not well understood, but are believed to involve multiple interactive pathways. Articular cartilage is both aneural and avascular. As such, cartilage is incapable of directly generating pain, inflammation, stiffness, or any of the symptoms that patients with OA typically describe (22). Given its relative unimportance to OA’s symptomatic presentation, it is ironic that articular cartilage has received so much attention while other common symptom sources in the joint are ignored.
In contrast the subchondral bone, periosteum, periarticular ligaments, periarticular muscle spasm, synovium and joint capsule are all richly innervated and are the source of nociception in OA.
In population studies there is a significant discordance between radiographically diagnosed OA and knee pain (7). Whilst radiographic evidence of joint damage predisposes to joint pain, it is clear that the severity of the joint damage on the radiograph bears little relation to the severity of the pain experienced.
However, utilising other imaging modalities such as magnetic resonance imaging (MRI) significant structural associations such as bone marrow lesions (23;24), sub-articular bone attrition (25), synovitis and effusion (26;27) have been related to knee pain. It remains unclear which of these local tissue factors predominate as until recently these analyses did not account for the fact that much of the structural change is collinear (a person who has more severe disease will have worse structural change in multiple tissues including the bone synovium, etc) and were not adjusting for other tissue changes. A recent analysis confirmed most beliefs that it is likely that changes in the subchondral bone and synovial activation/ effusion predominate (28).
Lesions in the bone marrow play an integral if not pivotal role in the symptoms that emanate from knee OA and its structural progression (23). Bone marrow lesions were found in 272 of 351 (77.5%) persons with painful knees compared with 15 of 50 (30%) persons with no knee pain (P < 0.001). Large lesions were present almost exclusively in persons with knee pain (35.9% vs. 2%; P < 0.001). After adjustment for severity of radiographic disease, effusion, age, and sex, lesions and large lesions remained associated with the occurrence of knee pain. More recently their relation to pain severity was also demonstrated (24). Other bone-related causes of pain include periostitis associated with osteophyte formation (29), subchondral microfractures (30), and bone angina due to decreased blood flow and elevated intraosseous pressure (31). The particular bone pathology most responsible for pain remains elusive however identifying this would be a major advance in delineating appropriate therapeutic targets. One likely source that remains underexplored is that of intra-osseous hypertension. The pathophysiology remains unclear, although phlebographic studies in OA indicate impaired vascular clearance from bone and raised intra-osseous pressure in the bone marrow near the painful joint (31–34). What may subsequently cause pain is as yet unknown. Increased trabecular bone pressure, ischemia and inflammation are all possible stimuli.
The synovial reaction in OA includes synovial hyperplasia, fibrosis, thickening of synovial capsule, activated synoviocytes and in some cases lymphocytic infiltrate (B- and T-cells as well as plasma cells) (35). The site of infiltration of the synovium is of obvious relevance as one of the most densely innervated structures of the joint is the white adipose tissue of the fat pad which also show evidence of inflammation and can act as a rich source of inflammatory adipokines (36). Synovial causes of pain include irritation of sensory nerve endings within the synovium from osteophytes and synovial inflammation that is due, at least in part, to the release of prostaglandins, leukotrienes, proteinases, neuropeptides and cytokines (37;38). Synovitis is frequently present in osteoarthritis and may predict other structural changes in osteoarthritis and correlate with pain and other clinical outcomes (26). Synovial thickening around the infra-patellar fat pad using non-contrast MRI has been shown on biopsy to represent mild chronic synovitis (39). A semi-quantitative measure of synovitis from the infrapatellar fat pad is associated with pain severity and similarly change in synovitis is associated with change in pain severity (27).
Another source of joint pain in OA may be from the nerves themselves. Following joint injury in which there is ligamentous rupture, the nerves which re-innervate the healing soft tissues contain an overabundance of algesic chemicals such as substance P and calcitonin gene-related peptide. An interesting observation of these new nerves was that their overall morphology was abnormal with fibres appearing punctate and disorganised (40;41). Since these phenomena are consistent with the innervation profiles described in nerve injury models, we speculate that injured joints may develop neuropathic pain post-trauma. Indeed, treatment of inflamed joints with the neuropathic pain analgesic gabapentin can also relieve arthritis pain (42).
Innervation in the Joint
The musculature, articular capsule, synovium, tendons, ligaments, and subchondral bone of the joint have a rich nerve supply, whereas the articular hyaline cartilage is aneural. In addition to post-ganglionic sympathetic efferents, joints are supplied by numerous sensory fibres whose subcategorization is based upon distinct anatomical features (43). Joint afferents which have a thick diameter and are myelinated are called Aβ (Group II) fibres, thin nerves with a myelin sheath which disappears in the terminal region to become a free nerve ending are termed Aδ (Group III) fibres, while the thin unmyelinated nerves are C (Group IV) fibres. Proprioceptive Aβ fibres of the joint terminate in the capsule, fat pad, ligaments, menisci and periosteum, whilst nociceptive Aδ and C fibres innervate the capsule, ligaments, menisci, periosteum and mineralised bone, (in particular in regions of high mechanical load) (37;43–45).
Joint nociceptors are typically localised within specific articular structures and their receptive field is normally restricted to the joint. During inflammation, however, this receptive field can expand into adjacent areas such that mechanical stimuli in non-articular tissues such as the surrounding muscle can suddenly become activated. Therefore, a typical neurone in the spinal cord with a receptive field in the joint may now respond to physical stimulation of extra-articular muscle for example (46;47).
Under disease conditions, the innervation territories of the various nerve fibers are highly plastic. An example of such plasticity, is the innervation of normally aneural tissues such as cartilage with substance P and calcitonin-gene-related peptide (CGRP) positive nerves in patients with OA (48). Therefore, the "normally" mechanically insensitive cartilage becomes potentially a candidate for tibiofemoral pain in OA although this has never been shown electrophysiologically. Furthermore, these peptide-containing nerves may also accelerate disease progression via localized neurogenic inflammatory mechanisms.
Tissue injury activates the nociceptive system, which generates the subjective pain experience. Spontaneous pain and mechanical hypersensitivity can develop as a consequence of sensitization of primary afferents directly by locally released inflammatory mediators, as well as following sensitization of neuronal processes in the spinal cord (central sensitization) or indeed higher centres (46).
In arthritis, inflammatory mediators such as bradykinin, histamine, prostaglandins, lactic acid, substance P, vasoactive intestinal peptide and calcitonin gene related peptide (CGRP) are released into the joint (38). These mediators reduce the firing threshold of joint nociceptors, making them more likely to respond to both non-noxious and noxious painful stimuli. As the disease progresses, more and more of these mediators accumulate in the joint, thereby triggering a self-perpetuating cycle of pain generation. The first study to explore which chemical mediators are responsible for OA pain in an animal model focused on the neuropeptide vasoactive intestinal peptide (VIP). VIP is a 28 amino acid peptide which was originally identified in the porcine intestine where it controls vascular tone and enzyme secretion (49). Over 20 years ago, VIP was localised in the synovial fluid and serum of arthritis patients (50) and then the peptide was forgotten by the rheumatology field. Recently it was shown that local administration of VIP to rat knees causes synovial hyperaemia (51) and sensitization of joint afferents leading to pain (52;53). Interestingly, treatment of OA knees with a VIP antagonist significantly attenuated peripheral sensitization and alleviated pain behaviour in this animal model of degenerative joint disease. Thus, VIP inhibition may be a useful means of controlling OA pain.
In addition to sensitizing mediators being released into OA joints to elicit pain, evidence is beginning to emerge which suggests that naturally produced desensitizing agents may also contribute to pain modulation in the joint. For example, the endogenous opioid endomorphin is present in high concentration in arthritic knees (54;55) where it can reduce afferent firing rate in response to joint movement (56). Similarly, endocannabinoid activity has been reported in OA knees and activation of the articular cannabinoid system can dramatically offset the hyperactivity of joint nociceptors (57). Even though these endogenous analgesic agents are present in significant amounts in articular tissues, the question still remains as to why the body's natural pain killers are unable to provide any appreciable relief from the debilitating effects of joint pain.
Silent Nociceptors
Polymodal Aδ and C fibers that innervate the joint increase their firing rate in response to noxious mechanical stimuli as well as in the presence of various chemical agents such as those released during inflammation. In addition to these classic nociceptors, there are also a number of fibers in the joint that are not normally activated by noxious stimulation but become responsive when damage or inflammation occurs in the joint. These fibers, called silent nociceptors, can make a major contribution to the pain sensation (46).
The neuroanatomy of mineralized bone, bone marrow and periosteum is well defined (45). A-β, A-δ, C-fibers and sympathetic fibers distribute densely throughout the periosteum, entering bone in close association with blood vessels (58). Of these tissues, the periosteum has the greatest density of sensory and sympathetic innervation, which may be further enhanced during joint inflammation. Electrophysiological studies of the mechano-sensitivity of joint innervation, indicate that generally A-β fibres are activated by non-noxious normal working range joint movement whilst approximately 50% of A-δ and 70% of C-fibers are classified as high threshold units (59). During inflammation, A-δ and C-fibers show increased mechano-sensitivity. Low threshold populations exhibit exaggerated responses, whilst high threshold populations and units that were initially mechano-insensitive are sensitized and now respond to movements in the normal working ranges of the joint (60). It is this increased activity of low threshold units and the awakening of the silent nociceptors which conspire to intensify joint pain sensation in arthritis.
Central Mechanisms
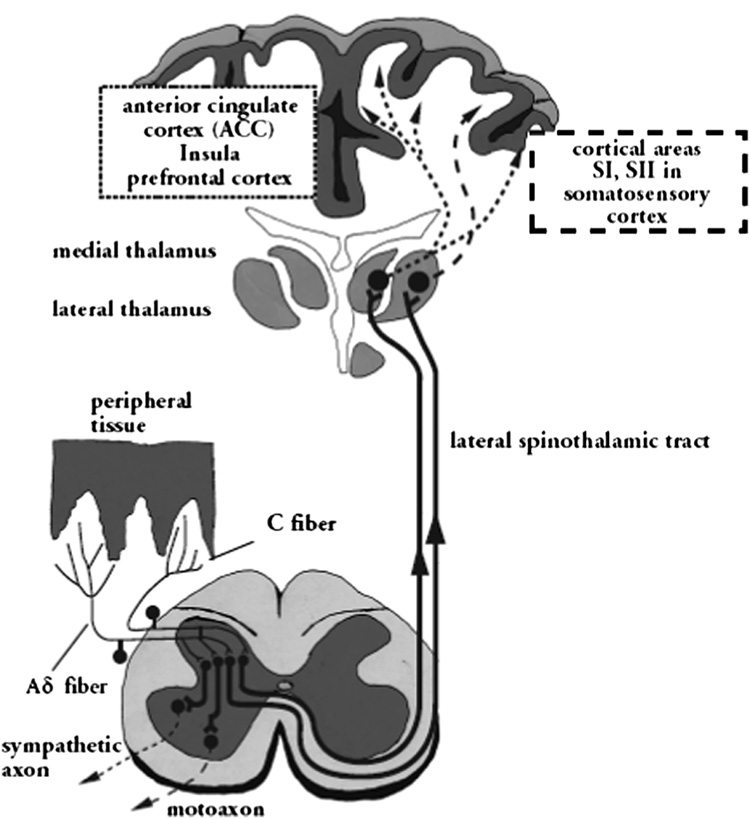
The A-δ fibers transmit impulses centrally through the peripheral nerve up through the dorsal root and into the dorsal horn of the spinal cord. The C fibers conduct impulses relatively slowly through the same route to the central nervous system (CNS) (61) (See Figure 2). The A-δ fibers terminate in laminae I and V of the dorsal horn, and the C fibers terminate predominantly in lamina II. From the dorsal horn, the signals are carried along the ascending pain pathways to the brain stem, hypothalamus, thalamus, and cerebral cortex.
Figure 2.
Pain transmission. Reproduced with permission from Schaible H-G et al. (44)
Descending pathways originating in supraspinal centers (somatosensory and limbic cortices) project through the periaqueductal gray area to the dorsal horn and modulate activity in the dorsal horn by controlling spinal pain transmission (62).
Processing the Perception of Pain
Nociception is processed throughout the nervous system, but it reaches conscious levels and is interpreted through connections between the thalamus and cortex. There are 2 main systems in the brain that are responsible for the perception of pain: the lateral system and the medial system of the lateral spinothalamic tract (63). The lateral system involves the activation of thalamic nuclei in the ventral lateral thalamus and the relay of information to the somatosensory cortex, where the noxious stimulus is analyzed for location, duration, intensity, and quality.
The medial system involves the relay of information by other (midline and intralaminar) thalamic nuclei to different parts of the brain such as the amygdala. The medial system comprises large areas of the brain that are responsible for pain perception as well as for functions in other contexts, such as affective responses, attention, and learning. This may explain the discrepancy between the degree of joint damage and the severity of pain. Because of the importance of the medial system in OA pain, a non-pharmacologic approach to management may be just as important as a pharmacologic strategy.
Finally, the perception of pain is modified by the patient’s affective status (e.g. level of depression, anxiety, or anger) and cognitive state (e.g. pain beliefs, expectations, memories of pain). Age, gender, socioeconomic status, racial and cultural background, pain communication skills, and previous pain experiences can contribute to the way a patient perceives pain.
Central Sensitization
The characteristic feature of most chronic pains is that hitherto non-noxious stimuli, such as walking or standing, are perceived as painful. It is now clear that pain pathways, far from being static or hardwired, exhibit marked plasticity and that sensitization at peripheral, spinal and cortical levels accounts for many of the clinical features associated with chronic pain. Consistent with this, the three chronic pain categories currently recognised, including neuropathic pain, neuroplastic or inflammatory pain and idiopathic pain, all exhibit features of an underlying central sensitization state (38).
Like peripheral sensitization previously described, central nociceptive transmission in the dorsal horn also can be sensitized. Increased input from peripheral nociceptors modulates spinal cord pain-transmitting neurons and leads to increased synaptic excitability and decreased firing thresholds that outlast the initiating input, amplifying responses to both noxious and innocuous inputs.
Thus, neuronal responses to noxious input is exaggerated (hyperalgesia), or normally innocuous input is now perceived as painful (allodynia), and sensitivity is expanded, with pain experienced beyond the original site of tissue damage (secondary hyperalgesia) (64).
Central sensitization involves activation and modulation, as well as modification. Modification of dorsal horn neurons leads to changes in receptors and transmitters in addition to structural reorganization (or physical rearrangement of the neurons) and disinhibition of dorsal horn nociceptors. According to one theory, disinhibition of dorsal horn nociceptors results from the death of local inhibitory interneurons, which potentially are replaced by excitatory A-δ̣ fibers that “sprout” from the dorsal horn. Peripheral and central sensitization represent the “plasticity,” or modifiability, of the nervous system, which can mold itself to new functions in response to changing inputs (38;64).
Hyperexcitability of Spinal Cord Neurons
Spinal cord hyperexcitability can originate from either nociceptive or neuropathic types of pain, though the mechanisms through which this occurs may be different (65). When a noxious stimulus is used to induce active inflammation, the sensitized area expands and additional neurons become activated. This process lowers the pain threshold and increases the sensitivity of adjacent neurons to stimulation (65). Central sensitization occurs as a consequence of tissue damage and peripheral sensitization and also as a consequence of abnormal discharges from damaged nerve fibers. A spinal cord neuron that has been sensitized often has an expanded receptive field. In addition, as a result of the process of central sensitization, more neurons in a spinal segment respond to noxious stimuli. Central sensitization has been seen mainly in the wake of tissue damage. In some forms of neuropathy, eg, after sectioning of peripheral nerves, many spinal cord neurons are silent and have no receptive field. Only a few neurons are active and show abnormal discharges. Other parts of the CNS also have the capacity for plasticity: After denervation, cortical maps may be changed, and this cortical process may be responsible for the chronicity of pain. It is this plastic quality of the central nervous system which should enable us to reverse chronic pain in long term diseases such as OA. By inhibiting the nociceptive input from the joint to the central nervous system it should be possible to rewire the brain gradually such that the sensation of chronic joint pain can be unlearned. Peripherally restricted pharmacological agents perhaps in combination with a physical therapy approach may help us ultimately to dismantle the neurophysiological processes which were constructed during OA pain development.
Modulatory Mediators
Glutamate is the primary excitatory neurotransmitter in the CNS. It is the neurotransmitter in A-β, A-δ, and C fibers. During repetitive noxious stimulation, glutamate activates N-methyl- D-aspartate (NMDA) in the spinal cord, and neuropeptide receptors are activated by neuropeptides that are co-released with glutamate from synaptic endings (65). Additionally, many modulatory mediators are present, including substance P, CGRP, opioids, neurotrophins, and prostaglandins, all of which also act in the CNS. Substance P, which is released in the superficial part of the dorsal horn into the gray matter, increases the pain response to noxious inputs from spinal cord neurons (66).
Prostaglandins are also important, both in the periphery and in the spinal cord. They have a major impact on the sensitivity of neighboring spinal cord neurons (67).
The Concept of Wind Up
When action potentials reach the nerve terminal, the presynaptic membrane is depolarized. This opens calcium channels, and calcium flows into the presynaptic ending, where it triggers the release of transmitters. The definition of wind up is quite specific: In a classic situation, a peripheral nerve is stimulated repeatedly at C-fiber strength. This produces a response in a spinal neuron that grows from stimulus to stimulus; this is termed wind up. Wind up is short-lived, surviving stimulation for only a very short time (seconds to minutes). Wind up intensifies pain during repetitive noxious stimulation. It is probably not produced by increased transmitter release but rather by postsynaptic changes such as NMDA receptor activation and, possibly, by calcium influx into the postsynaptic neuron. Wind up also occurs when the skin is stimulated repeatedly with short heat pulses (65).
The Sympathetic Response
When a noxious stimulus is received, the sympathetic nervous system releases norepinephrine into the peripheral tissues, which decreases the firing threshold of peripheral nerve cells and makes them more sensitive to stimulation. During noxious painful movement, sympathetic postganglionic nerve activity increases leading to a rise in mean arterial pressure and heart rate (68). Since sympathetic nerve stimulation leads to synovial vasoconstriction (69), then it is possible that the resulting hypoxemia could contribute to joint pain. These findings indicate that activation of joint mechanonociceptors causes reflex sympathetic discharges which could further augment joint pain sensitivity.
So far this review has focused on peripheral sensory input and central mechanisms although clearly modulation through cognitive, genetic, affective and environmental influences forms the net pain experience. The remainder of the review will focus on constitutional and environmental factors that may modulate the pain experience.
Constitutional factors
Pain has long been recognized as a complex sensory and emotional experience (70). Each individual has a unique experience of pain influenced by their life experience and genotypic profile. An individual’s stable psychological characteristics (trait) and the immediate psychological context in which pain is experienced (state) both influence perception of pain.
A full understanding of pain requires consideration of psychological and social environmental processes mediating a patient’s response to their disease (71). The biopsychosocial model is a very useful approach to understanding and assessing the experience of pain in persons with OA (72). Numerous studies have supported the importance of psychological factors in understanding OA pain (72). Two of the most important factors are self-efficacy and pain catastrophizing. Self-efficacy has been defined as an individuals confidence in their abilities to accomplish a desired task (e.g. control arthritis pain). Keefe, Lefebvre, et al. (73) found that OA patients who reported higher self-efficacy for pain control had higher thresholds and tolerance for thermal pain stimuli. Furthermore, increases in self-efficacy occurring over the course of a pain coping skills training protocol for OA patients was found to be one of the most important predictors of short- and long-term treatment outcome (74;75). In fact, Lorig et al, (76) found that increases in self-efficacy that occured following participation in an arthritis self-help intervention were related to improvements in pain and psychological functioning at 4 years follow-up. Pain catastrophizing refers to the tendency to focus upon, ruminate upon, and feel helpless in the face of pain. OA patients who catastrophize report higher levels of pain, psychological distress, and physical disability and also exhibit more pain behavior (77). Interestingly, pain catastrophizing has also been shown to relate to abnormal processing of pain signals in imaging studies suggesting it may influence pain perception in a fundamental fashion (78).
OA pain occurs in a social context and factors such as social support can play an important role in determining how patients adjust to arthritis pain (79). Patients and their partners, however, may vary with respect to their abilities to communicate about and manage OA pain as a couple. In a recent study, (80), we examined key aspects of pain communication (self-efficacy for pain communication and holding back from discussing pain and arthritis-related concerns) in patients with osteoarthritis (OA) and their partners. Results indicated that patients who reported higher levels of self-efficacy for pain communication experienced much lower levels of pain, physical and psychological disability, and their partners reported much lower levels of negative affect. Patients who reported holding back on discussions about pain and related arthritis concerns experienced much higher levels of psychological disability. Interestingly, when partners reported they held back on discussions of pain and related arthritis concerns, they reported higher levels of caregiver strain and their patient-partners were more likely to report high levels of psychological disability. Taken together, these findings suggest that patients' and partners' self-efficacy for pain communication and tendency to hold back on pain communication may be important in understanding patient and partner adjustment to OA pain. These findings also underscore the importance of involving spouses of OA patients in pain management efforts, something that has been shown to improve the outcomes of pain coping skills training (74;75).
Further, central nervous system processing associated with pain perception is closely integrated with hypothalamic-pituitary axis (HPA) and autonomic nervous system (ANS) activity. Variations in pain perception within populations may reflect genetic polymorphisms in all three systems, with current attention being focused on serotonin transporter re-uptake protein (SERT-P), Alpha-2 receptor and catechol-O-methyltransferase (COMT) although a number of other candidate genes are under review (45).
Environmental stimuli
In the presence of OA local stimuli that typically would not be noxious can precipitate alteration in the severity of pain through either micro-structural damage of the joint or by decreasing the pain threshold level. There is evidence that patients with OA do experience fluctuations in pain severity or exacerbations of pain (26;81). A brief consideration of some of the factors that could predispose to fluctuations in pain severity are discussed here.
I. Physical activity
Numerous studies have assessed the relation of physical activity to the risk of radiographic knee OA with little or no attention paid to the relation of physical activity and OA symptoms. These include studies of runners (82–84), heavy physical activity in daily life (85), and occupational activities including prolonged standing and knee bending activities (86–89) however few if any of these studies have investigated the relation of these activities to symptom severity. In fact there is a paucity of epidemiological data to explain which particular activities are painful or more injurious than others however we know from clinical practice that different activities predispose to exacerbation of pain where in a normal joint they typically would not. Identification of these factors that exacerbate pain is important as these are potentially modifiable.
II. Foot wear
Appropriate supportive footwear is recommended in guidelines for treating symptomatic OA although there is little data to support this recommendation (90). There are a number of ways in which footwear can potentially modify impact loading through the lower limb and thus reduce impact that potentially may lead to pain in subjects with OA. Impact force during locomotion increases with increasing age as a function of diminishing foot position awareness (91); this impact force could be reduced through the addition of supportive shoes (92).
Another link between footwear and knee loads comes from gait analysis studies demonstrating that high- heeled shoes increase compressive forces across the patellofemoral and medial tibiofemoral joints (93). Women’s shoes, even with only moderately high heels (1.5 in) were found to increase the forces that strain both the tibiofemoral and patellofemoral joints during walking (94). Given the increased predilection for women experiencing symptomatic knee OA (female to male ratio is typically reported as 2:1) clarifying the impact high heeled shoes have on symptoms could have public health import.
III. Injury and Trauma
Among both genders, a past history of injury to the stabilizing or load bearing structures of the knee renders the joint highly vulnerable to radiographic OA in subsequent years (95). Persons with OA have quadriceps weakness (96) and impaired proprioception (97) that makes them more susceptible to falls (98) and injury risk. In contrast to the knowledge about the development of radiographic OA following injury, the relationship of pain exacerbation in subjects with pre-existing OA to joint injury/falls/ trauma remains unknown and warrants further exploration.
IV. Weather
Many people believe that weather conditions can influence joint pain, but science offers little proof (99;100). If the phenomenon were real, cause-and-effect mechanisms might provide clues that would aid treatment of joint pain. Some theorize that alterations in barometric pressure and humidity can alter the synovial fluid (volume and content) in the joint and predispose to alteration in symptoms. The factors that have been considered include ambient temperature, barometric pressure, relative humidity, sunshine, wind speed and precipitation; however the literature on the subject is sparse, conflicting, and vulnerable to bias (101;102). However, for patients who believe that weather can influence their pain, the biological mechanisms may not be fully understood, but the effect seems to be real.
Conclusion
The pathophysiology of pain in OA is complex and similarly the symptomatic presentation in OA diverse and heterogeneous. Attention to the many modulating factors that alter the experience of pain may improve the way we treat this disease.
Acknowledgements
Preparation of this article for Francis Keefe was supported in part by NIH grants: AG026010, AR47218, AR049059, AR050245, and AR05462.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. American Journal of Public Health. 1994;84(3):351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthritis prevalence and activity limitations--United States, 1990. MMWR - Morbidity & Mortality Weekly Report. 1994;43(24):433–438. [PubMed] [Google Scholar]
- 3.Yelin E, Callahan LF. The economic cost and social and psychological impact of musculoskeletal conditions. National Arthritis Data Work Groups. Arthritis & Rheumatism. 1995;38(10):1351–1362. doi: 10.1002/art.1780381002. [see comments] [Review] [68 refs]. [DOI] [PubMed] [Google Scholar]
- 4.Segal L, Day SE, Chapman AB, Osborne RH. Can we reduce disease burden from osteoarthritis? Medical Journal of Australia. 2004;180(5 Suppl):S11–S17. doi: 10.5694/j.1326-5377.2004.tb05907.x. [see comment]. [DOI] [PubMed] [Google Scholar]
- 5.Nuki G. Osteoarthritis: a problem of joint failure. Zeitschrift fur Rheumatologie. 1999;58(3):142–147. doi: 10.1007/s003930050164. [Review] [55 refs]. [DOI] [PubMed] [Google Scholar]
- 6.Eyre DR. Collagens and cartilage matrix homeostasis. Clinical Orthopaedics & Related Research. 2004;(427 Suppl):S118–S122. doi: 10.1097/01.blo.0000144855.48640.b9. [Review] [37 refs]. [DOI] [PubMed] [Google Scholar]
- 7.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. Journal of Rheumatology. 2000;27(6):1513–1517. [PubMed] [Google Scholar]
- 8.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Annals of the Rheumatic Diseases. 2001;60(2):91–97. doi: 10.1136/ard.60.2.91. [see comments.]. [Review] [45 refs]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cibere J. Do we need radiographs to diagnose osteoarthritis? Best Practice & Research in Clinical Rheumatology. 2006;20(1):27–38. doi: 10.1016/j.berh.2005.08.001. [Review] [60 refs]. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Niu J, Kelly-Hayes M, Chaisson CE, Aliabadi P, Felson DT. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: The Framingham Study. American Journal of Epidemiology. 2002;156(11):1021–1027. doi: 10.1093/aje/kwf141. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham LS, Kelsey JL. Epidemiology of musculoskeletal impairments and associated disability. American Journal of Public Health. 1984;74(6):574–579. doi: 10.2105/ajph.74.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. Journal of Hand Surgery - British Volume. 1994;19(3):340–341. doi: 10.1016/0266-7681(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 13.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis & Rheumatism. 1998;41(8):1343–1355. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [Review] [116 refs]. [DOI] [PubMed] [Google Scholar]
- 14.Nevitt MC, Xu L, Zhang Y, Lui LY, Yu W, Lane NE, et al. Very low prevalence of hip osteoarthritis among Chinese elderly in Beijing, China, compared with whites in the United States: the Beijing osteoarthritis study. Arthritis & Rheumatism. 2002;46(7):1773–1779. doi: 10.1002/art.10332. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis & Rheumatism. 1998;41(5):778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [see comments.]. [DOI] [PubMed] [Google Scholar]
- 16.Kraus VB, Vail TP, Worrell T, McDaniel G, Kraus VB, Vail TP, et al. A comparative assessment of alignment angle of the knee by radiographic and physical examination methods. Arthritis & Rheumatism. 2005;52(6):1730–1735. doi: 10.1002/art.21100. [DOI] [PubMed] [Google Scholar]
- 17.Altman RD. Classification of disease: osteoarthritis. Seminars in Arthritis & Rheumatism. 1991;20 6 Suppl 2:40–47. doi: 10.1016/0049-0172(91)90026-v. [Review] [38 refs]. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. Journal of Bone & Joint Surgery - American Volume. 2003;85-A(1):4–9. doi: 10.2106/00004623-200301000-00002. [comment]. [DOI] [PubMed] [Google Scholar]
- 19.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis & Rheumatism. 2004;50(9):2811–2819. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 20.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis & Rheumatism. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 21.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965–973. doi: 10.1016/S0140-6736(05)71086-2. [Review] [100 refs]. [DOI] [PubMed] [Google Scholar]
- 22.Felson D. The sources of pain in knee osteoarthritis. Current Opinion in Rheumatology. 2005;17(5):624–628. doi: 10.1097/01.bor.0000172800.49120.97. [Review] [34 refs]. [DOI] [PubMed] [Google Scholar]
- 23.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Annals of Internal Medicine. 2001;134(7):541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [see comments.]. [DOI] [PubMed] [Google Scholar]
- 24.Hunter D, Gale D, Grainger G, Lo G, Conaghan P. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Annals of the Rheumatic Diseases. 2008;67(2):206–211. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 25.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis & Cartilage. 2006;14(10):1033–1040. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. Journalof Rheumatology. 2001;28(6):1330–1337. [PubMed] [Google Scholar]
- 27.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Changes in synovitis are associated with changes in pain in knee osteoarthritis. Arthritis & Rheumatism. 2005;52(9) supplement:S71. Ref Type: Abstract. [Google Scholar]
- 28.Lo G, McAlindon T, Niu J, Zhang Y, Beals C, Dabrowski C, et al. Strong Association of Bone Marrow Lesions and Effusion with Pain in Osteoarthritis. Arthritis & Rheumatism. 2008;56(9):S790. Ref Type: Abstract. [Google Scholar]
- 29.Cicuttini FM, Baker J, Hart DJ, Spector TD. Association of pain with radiological changes in different compartments and views of the knee joint. Osteoarthritis & Cartilage. 1996;4(2):143–147. doi: 10.1016/s1063-4584(05)80323-1. [DOI] [PubMed] [Google Scholar]
- 30.Burr DB. The importance of subchondral bone in the progression of osteoarthritis. Journal of Rheumatology - supplement. 2004;70:77–80. [Review] [13 refs]. [PubMed] [Google Scholar]
- 31.Simkin P. Bone pain and pressure in osteoarthritic joints. Novartis Foundation Symposium. 2004;260:179–186. [Review] [34 refs]. [PubMed] [Google Scholar]
- 32.Arnoldi CC, Lemperg K, Linderholm H. Intraosseous hypertension and pain in the knee. Journal of Bone & Joint Surgery - British Volume. 1975;57(3):360–363. [PubMed] [Google Scholar]
- 33.Arnoldi CC, Djurhuus JC, Heerfordt J, Karle A. Intraosseous phlebography, intraosseous pressure measurements and 99mTC-polyphosphate scintigraphy in patients with various painful conditions in the hip and knee. Acta Orthopaedica Scandinavica. 1980;51(1):19–28. doi: 10.3109/17453678008990764. [DOI] [PubMed] [Google Scholar]
- 34.Arnoldi CC. Vascular aspects of degenerative joint disorders. A synthesis. Acta Orthopaedica Scandinavica. 1994;261 Supplementum:1–82. [Review] [270 refs]. [PubMed] [Google Scholar]
- 35.Roach HI, Aigner T, Soder S, Haag J, Welkerling H, Roach HI, et al. Pathobiology of osteoarthritis: pathomechanisms and potential therapeutic targets. Current Drug Targets. 2007;8(2):271–282. doi: 10.2174/138945007779940160. [Review] [138 refs]. [DOI] [PubMed] [Google Scholar]
- 36.Ushiyama T, Chano T, Inoue K, Matsusue Y, Ushiyama T, Chano T, et al. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Annals of the Rheumatic Diseases. 2003;62(2):108–112. doi: 10.1136/ard.62.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDougall J. Arthritis and pain. Neurogenic origin of joint pain. Arthritis Research & Therapy. 2006;8(6):220. doi: 10.1186/ar2069. [Review] [138 refs]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altman R. Management of Osteoarthritis Knee Pain: The State of the Science. Littleton, CO: Medical Education Resources; 2006. Ref Type: Report. [Google Scholar]
- 39.Fernandez-Madrid F, Karvonen RL, Teitge RA, Miller PR, An T, Negendank WG. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magnetic Resonance Imaging. 1995;13(2):177–183. doi: 10.1016/0730-725x(94)00119-n. [DOI] [PubMed] [Google Scholar]
- 40.McDougall JJ, Bray RC, Sharkey KA. Morphological and immunohistochemical examination of nerves in normal and injured collateral ligaments of rat, rabbit, and human knee joints. Anatomical Record. 1997;248(1):29–39. doi: 10.1002/(SICI)1097-0185(199705)248:1<29::AID-AR4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 41.McDougall JJ, Yeung G, Leonard CA, Bray RC. A role for calcitonin gene-related peptide in rabbit knee joint ligament healing. Canadian Journal of Physiology & Pharmacology. 2000;78(7):535–540. [PubMed] [Google Scholar]
- 42.Hanesch U, Pawlak M, McDougall JJ, Hanesch U, Pawlak M, McDougall JJ. Gabapentin reduces the mechanosensitivity of fine afferent nerve fibres in normal and inflamed rat knee joints. Pain. 2003;104(1–2):363–366. doi: 10.1016/s0304-3959(03)00043-5. [DOI] [PubMed] [Google Scholar]
- 43.Freeman MA, Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. Journal of Anatomy. 1967;101(Pt 3):505–532. [PMC free article] [PubMed] [Google Scholar]
- 44.Schaible H, Richter F. Pathophysiology of pain. Langenbecks Archives of Surgery. 2004;389(4):237–243. doi: 10.1007/s00423-004-0468-9. [Review] [55 refs]. [DOI] [PubMed] [Google Scholar]
- 45.Dray A, Read SJ, Dray A, Read SJ. Arthritis and pain. Future targets to control osteoarthritis pain. Arthritis Research & Therapy. 2007;9(3):212. doi: 10.1186/ar2178. [Review] [176 refs]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaible H, Schmelz M, Tegeder I. Pathophysiology and treatment of pain in joint disease. Advanced Drug Delivery Reviews. 2006;58(2):323–342. doi: 10.1016/j.addr.2006.01.011. [Review] [226 refs]. [DOI] [PubMed] [Google Scholar]
- 47.Konttinen YT, Kemppinen P, Segerberg M, Hukkanen M, Rees R, Santavirta S, et al. Peripheral and spinal neural mechanisms in arthritis, with particular reference to treatment of inflammation and pain. Arthritis & Rheumatism. 1994;37(7):965–982. doi: 10.1002/art.1780370701. [Review] [55 refs]. [DOI] [PubMed] [Google Scholar]
- 48.Suri S, Gill SE, Massena dC, Wilson D, McWilliams DF, Walsh DA, et al. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Annals of the Rheumatic Diseases. 2007;66(11):1423–1428. doi: 10.1136/ard.2006.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Said S, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169(951):1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- 50.Lygren I, Ostensen M, Burhol PG, Husby G, Lygren I, Ostensen M, et al. Gastrointestinal peptides in serum and synovial fluid from patients with inflammatory joint disease. Annals of the Rheumatic Diseases. 1986;45(8):637–640. doi: 10.1136/ard.45.8.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDougall JJ, Barin AK. The role of joint nerves and mast cells in the alteration of vasoactive intestinal peptide (VIP) sensitivity during inflammation progression in rats. British Journal of Pharmacology. 2005;145(1):104–113. doi: 10.1038/sj.bjp.0706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuelert N, McDougall JJ, Schuelert N, McDougall JJ. Electrophysiological evidence that the vasoactive intestinal peptide receptor antagonist VIP6-28 reduces nociception in an animal model of osteoarthritis. Osteoarthritis & Cartilage. 2006;14(11):1155–1162. doi: 10.1016/j.joca.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 53.McDougall JJ, Watkins L, Li Z. Vasoactive intestinal peptide (VIP) is a modulator of joint pain in a rat model of osteoarthritis. Pain. 2006;123(1–2):98–105. doi: 10.1016/j.pain.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 54.McDougall JJ, Baker CL, Hermann PM. Attenuation of knee joint inflammation by peripherally administered endomorphin-1. Journal of Molecular Neuroscience. 2004;22(1–2):125–137. doi: 10.1385/JMN:22:1-2:125. [DOI] [PubMed] [Google Scholar]
- 55.McDougall JJ, Barin AK, McDougall CM. Loss of vasomotor responsiveness to the mu-opioid receptor ligand endomorphin-1 in adjuvant monoarthritic rat knee joints. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2004;286(4):R634–R641. doi: 10.1152/ajpregu.00464.2003. [DOI] [PubMed] [Google Scholar]
- 56.Li Z, Proud D, Zhang C, Wiehler S, McDougall JJ, Li Z, et al. Chronic arthritis down-regulates peripheral mu-opioid receptor expression with concomitant loss of endomorphin 1 antinociception. Arthritis & Rheumatism. 2005;52(10):3210–3219. doi: 10.1002/art.21359. [see comment]. [DOI] [PubMed] [Google Scholar]
- 57.Schuelert N, McDougall JJ, Schuelert N, McDougall JJ. Cannabinoid-mediated antinociception is enhanced in rat osteoarthritic knees. Arthritis & Rheumatism. 2008;58(1):145–153. doi: 10.1002/art.23156. [DOI] [PubMed] [Google Scholar]
- 58.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113(1):155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 59.Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain. 1993;55(1):5–54. doi: 10.1016/0304-3959(93)90183-P. [Review] [438 refs]. [DOI] [PubMed] [Google Scholar]
- 60.Schaible HG, Schmidt RF, Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. Journal of Neurophysiology. 1985;54(5):1109–1122. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 61.Schwartzman RJ, Grothusen J, Kiefer TR, Rohr P, Schwartzman RJ, Grothusen J, et al. Neuropathic central pain: epidemiology, etiology, and treatment options. Archives of Neurology. 2001;58(10):1547–1550. doi: 10.1001/archneur.58.10.1547. [Review] [38 refs]. [DOI] [PubMed] [Google Scholar]
- 62.Basbaum AI. Spinal mechanisms of acute and persistent pain. Regional Anesthesia & Pain Medicine. 1999;24(1):59–67. doi: 10.1016/s1098-7339(99)90167-0. [Review] [46 refs]. [DOI] [PubMed] [Google Scholar]
- 63.Treede RD, Kenshalo DR, Gracely RH, Jones AK, Treede RD, Kenshalo DR, et al. The cortical representation of pain. Pain. 1999;79(2–3):105–111. doi: 10.1016/s0304-3959(98)00184-5. [see comment]. [Review] [58 refs]. [DOI] [PubMed] [Google Scholar]
- 64.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [Review] [73 refs]. [DOI] [PubMed] [Google Scholar]
- 65.Ollat H, Cesaro P. Pharmacology of neuropathic pain. Clinical Neuropharmacology. 1995;18(5):391–404. doi: 10.1097/00002826-199510000-00002. [Review] [62 refs]. [DOI] [PubMed] [Google Scholar]
- 66.Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiology Clinics of North America. 2005;23(1):21–36. doi: 10.1016/j.atc.2004.11.013. [Review] [94 refs]. [DOI] [PubMed] [Google Scholar]
- 67.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410(6827):471–475. doi: 10.1038/35068566. [see comment]. [DOI] [PubMed] [Google Scholar]
- 68.Sato Y, Schaible HG. Discharge characteristics of sympathetic efferents to the knee joint of the cat. Journal of the Autonomic Nervous System. 1987;19(2):95–103. doi: 10.1016/0165-1838(87)90002-6. [DOI] [PubMed] [Google Scholar]
- 69.McDougall J, Karimian SM, Ferrell WR. Prolonged alteration of vasoconstrictor and vasodilator responses in rat knee joints by adjuvant monoarthritis. Experimental Physiology. 1995;80(3):349–357. doi: 10.1113/expphysiol.1995.sp003852. [DOI] [PubMed] [Google Scholar]
- 70.Kane RL, Bershadsky B, Lin WC, Rockwood T, Wood K, Kane RL, et al. Efforts to standardize the reporting of pain. Journal of Clinical Epidemiology. 2002;55(2):105–110. doi: 10.1016/s0895-4356(01)00429-2. [DOI] [PubMed] [Google Scholar]
- 71.Orbell S, Johnston M, Rowley D, Espley A, Davey P. Cognitive representations of illness and functional and affective adjustment following surgery for osteoarthritis. Social Science & Medicine. 1998;47(1):93–102. doi: 10.1016/s0277-9536(97)10132-0. [DOI] [PubMed] [Google Scholar]
- 72.Keefe FJ, Smith SJ, Buffington AL, Gibson J, Studts JL, Caldwell DS. Recent advances and future directions in the biopsychosocial assessment and treatment of arthritis. Journal of Consulting & Clinical Psychology. 2002;70(3):640–655. doi: 10.1037//0022-006x.70.3.640. [Review] [126 refs]. [DOI] [PubMed] [Google Scholar]
- 73.Keefe FJ, Lefebvre JC, Maixner W, Salley AN, Jr, Caldwell DS, Keefe FJ, et al. Self-efficacy for arthritis pain: relationship to perception of thermal laboratory pain stimuli. Arthritis Care & Research. 1997;10(3):177–184. doi: 10.1002/art.1790100305. [DOI] [PubMed] [Google Scholar]
- 74.Keefe FJ, Caldwell DS, Baucom D, Salley A, Robinson E, Timmons K, et al. Spouse-assisted coping skills training in the management of osteoarthritic knee pain. Arthritis Care & Research. 1996;9(4):279–291. doi: 10.1002/1529-0131(199608)9:4<279::aid-anr1790090413>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 75.Keefe FJ, Caldwell DS, Baucom D, Salley A, Robinson E, Timmons K, et al. Spouse-assisted coping skills training in the management of knee pain in osteoarthritis: long-term followup results. Arthritis Care & Research. 1999;12(2):101–111. doi: 10.1002/1529-0131(199904)12:2<101::aid-art5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 76.Lorig KR, Mazonson PD, Holman HR, Lorig KR, Mazonson PD, Holman HR. Evidence suggesting that health education for self-management in patients with chronic arthritis has sustained health benefits while reducing health care costs. Arthritis & Rheumatism. 1993;36(4):439–446. doi: 10.1002/art.1780360403. [DOI] [PubMed] [Google Scholar]
- 77.Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87(3):325–334. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 78.Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120(3):297–306. doi: 10.1016/j.pain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 79.Penninx BW, van Tilburg T, Deeg DJ, Kriegsman DM, Boeke AJ, van Eijk JT, et al. Direct and buffer effects of social support and personal coping resources in individuals with arthritis. Social Science & Medicine. 1997;44(3):393–402. doi: 10.1016/s0277-9536(96)00156-6. [DOI] [PubMed] [Google Scholar]
- 80.Porter L, Keefe F, Wellington C, Williams A. Pain communication in the context of osteoarthritis: Patient and partner self-efficacy for pain communication and holding back from discussion of pain and arthritis-related concerns. Clinical Journal of Pain. doi: 10.1097/AJP.0b013e31816ed964. In press. [DOI] [PubMed] [Google Scholar]
- 81.McAlindon T, Formica M, LaValley M, Lehmer M, Kabbara K. Effectiveness of glucosamine for symptoms of knee osteoarthritis: results from an internet-based randomized double-blind controlled trial. American Journal of Medicine. 2004;117(9):643–649. doi: 10.1016/j.amjmed.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 82.Panush RS, Schmidt C, Caldwell JR, Edwards NL, Longley S, Yonker R, et al. Is running associated with degenerative joint disease? JAMA. 1986;255(9):1152–1154. [PubMed] [Google Scholar]
- 83.Panush R, Hanson C, Caldwell J, Longley S, Stork J, Thoburn R. Is running associated with osteoarthritis? An eight year follow-up study. J Clin Rheumatol. 1995;1:35–39. doi: 10.1097/00124743-199502000-00008. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- 84.Lane NE, Michel B, Bjorkengren A, Oehlert J, Shi H, Bloch DA, et al. The risk of osteoarthritis with running and aging: a 5-year longitudinal study. Journal of Rheumatology. 1993;20(3):461–468. [PubMed] [Google Scholar]
- 85.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. American Journal of Medicine. 1999;106(2):151–157. doi: 10.1016/s0002-9343(98)00413-6. [DOI] [PubMed] [Google Scholar]
- 86.Croft P, Cooper C, Wickham C, Coggon D. Osteoarthritis of the hip and occupational activity. Scandinavian Journal of Work, Environment & Health. 1992;18(1):59–63. doi: 10.5271/sjweh.1608. [DOI] [PubMed] [Google Scholar]
- 87.Maetzel A, Makela M, Hawker G, Bombardier C. Osteoarthritis of the hip and knee and mechanical occupational exposure--a systematic overview of the evidence. Journal of Rheumatology. 1997;24(8):1599–1607. [PubMed] [Google Scholar]
- 88.Felson DT. Do occupation-related physical factors contribute to arthritis? Baillieres Clinical Rheumatology. 1994;8(1):63–77. doi: 10.1016/s0950-3579(05)80225-0. [Review] [58 refs]. [DOI] [PubMed] [Google Scholar]
- 89.Vingard E, Alfredsson L, Goldie I, Hogstedt C. Occupation and osteoarthrosis of the hip and knee: a register-based cohort study. International Journal of Epidemiology. 1991;20(4):1025–1031. doi: 10.1093/ije/20.4.1025. [DOI] [PubMed] [Google Scholar]
- 90.Anonymous. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis & Rheumatism. 2000;43(9):1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 91.Robbins S, Waked E, Allard P, McClaran J, Krouglicof N. Foot position awareness in younger and older men: the influence of footwear sole properties. Journal of the American Geriatrics Society. 1997;45(1):61–66. doi: 10.1111/j.1532-5415.1997.tb00979.x. [DOI] [PubMed] [Google Scholar]
- 92.Robbins S, Waked E, Krouglicof N. Vertical impact increase in middle age may explain idiopathic weight-bearing joint osteoarthritis. Archives of Physical Medicine & Rehabilitation. 2001;82(12):1673–1677. doi: 10.1053/apmr.2001.26255. [DOI] [PubMed] [Google Scholar]
- 93.Kerrigan DC, Lelas JL, Karvosky ME. Women's shoes and knee osteoarthritis. Lancet. 2001;357(9262):1097–1098. doi: 10.1016/S0140-6736(00)04312-9. [DOI] [PubMed] [Google Scholar]
- 94.Kerrigan DC, Johansson JL, Bryant MG, Boxer JA, Croce UD, Riley PO, et al. Moderate-heeled shoes and knee joint torques relevant to the development and progression of knee osteoarthritis. Archives of Physical Medicine & Rehabilitation. 2005;86(5):871–875. doi: 10.1016/j.apmr.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 95.Davis MA, Ettinger WH, Neuhaus JM, Cho SA, Hauck WW. The association of knee injury and obesity with unilateral and bilateral osteoarthritis of the knee. American Journal of Epidemiology. 1989;130(2):278–288. doi: 10.1093/oxfordjournals.aje.a115334. [DOI] [PubMed] [Google Scholar]
- 96.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, et al. Quadriceps weakness and osteoarthritis of the knee. Annals of Internal Medicine. 1997;127(2):97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 97.Hurley MV, Scott DL, Rees J, Newham DJ. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Annals of the Rheumatic Diseases. 1997;56(11):641–648. doi: 10.1136/ard.56.11.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pandya NK, Draganich LF, Mauer A, Piotrowski GA, Pottenger L, Pandya NK, et al. Osteoarthritis of the knees increases the propensity to trip on an obstacle. Clinical Orthopaedics & Related Research. 2005;(431):150–156. doi: 10.1097/01.blo.0000150316.97009.f2. [DOI] [PubMed] [Google Scholar]
- 99.Quick DC. Joint pain and weather. A critical review of the literature. Minnesota Medicine. 1997;80(3):25–29. [Review] [24 refs]. [PubMed] [Google Scholar]
- 100.Wilder FV, Hall BJ, Barrett JP. Osteoarthritis pain and weather. Rheumatology. 2003;42(8):955–958. doi: 10.1093/rheumatology/keg264. [DOI] [PubMed] [Google Scholar]
- 101.Laborde JM, Dando WA, Powers MJ. Influence of weather on osteoarthritics. Social Science & Medicine. 1986;23(6):549–554. doi: 10.1016/0277-9536(86)90147-4. [DOI] [PubMed] [Google Scholar]
- 102.Strusberg I, Mendelberg RC, Serra HA, Strusberg AM. Influence of weather conditions on rheumatic pain. Journal of Rheumatology. 2002;29(2):335–338. [PubMed] [Google Scholar]