Abstract
Lipopolysaccharide (LPS) stimulates cytoplasmic accumulation of pro-interleukin (IL)-1β. Activation of P2X7 receptors stimulates conversion of pro-IL-1β into mature IL-1β, which is then secreted. Because both LPS (in vivo) and IL-1β (in vitro) decrease vascular reactivity to contractile agents, we hypothesized that: 1) P2X7 receptor activation contributes to LPS-induced vascular hyporeactivity; and 2) IL-1β mediates this change. Thoracic aortas were obtained from 12-week-old male C57BL/6 mice. The aortic rings were incubated 24 hours in Dulbecco’s Modified Eagle’s Medium (DMEM), LPS, benzoylbenzoyl-ATP (BzATP, P2X7 receptor agonist), LPS plus BzATP, oxidized ATP (oATP, P2X7 receptor antagonist) or oATP plus LPS plus BzATP. Following the treatment, the rings were either mounted in a myograph for evaluation of contractile activity, or homogenized for IL-1β and inducible nitric oxide synthase (iNOS) protein measurement. In endothelium-intact aortic rings, phenylephrine (PE)-induced contractions were not altered by incubation with LPS or BzATP, but significantly decreased in aortic rings incubated with LPS plus BzATP. Treatment with oATP or IL-1ra (IL-1β receptor antagonist) reversed LPS plus BzATP-induced hyporeactivity to PE. In the presence of NG-nitro-l-arginine methyl ester (l-NAME) or N-([3-(aminomethyl)phenyl]methyl)ethanimidamide (1400W, selective iNOS inhibitor), the vascular hyporeactivity induced by LPS plus BzATP on PE responses was not observed. BzATP augmented LPS-induced IL-1β release and iNOS protein expression, and these effects were also inhibited by oATP. Moreover, incubation of endothelium-intact aortic rings with IL-1β induced iNOS protein expression. Thus, activation of P2X7 receptor amplifies LPS-induced hyporeactivity in mouse endothelium-intact aorta, which is associated with IL-1β-mediated release of nitric oxide by iNOS.
Introduction
Invasion of the bloodstream by bacteria, viruses or parasites induces activation of the immune system and triggers systemic defense mechanisms (Pinsky, 1996). Extreme triggering of defense mechanisms leads to severe inflammation [sepsis/septic shock (Morrison and Ulevitch, 1978; McCabe, et al., 1983)]. In 1988, Cerami and Beutler showed that endotoxic shock is not caused directly by toxins secreted from microorganisms, but it is the result of a strong, incontrollable immune response in the host (Cerami and Beutler, 1988). Lipopolysaccharide (LPS), a major component of Gram-negative bacteria, once released into bloodstream induces multiple and excessive endogenous pro-inflammatory mediators, including cytokines [such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1,-6,-8], leukotrienes and thromboxane A2, all playing a role in the responses associated with sepsis/septic shock. For example, intravenous injection of IL-1 induces lowering of blood pressure (Dinarello and Wolff, 1993). Prolonged exposure to IL-1β results in decreased vascular tone (Beasley, et al., 1989; Takizawa, et al., 1999), and it also increases nitric oxide synthase (NOS) protein expression and activity (French, et al., 1991; Gui, et al., 2000). Many studies have indicated that a subtype of purinergic receptors, the P2X7 receptor, plays a key role in IL-1 processing and release in inflammatory cells. The P2 family of purinergic receptors includes, as of now, 15 members that have been cloned and classified into subfamilies: P2Y receptors and P2X receptors, which are G protein-coupled and cation-selective channels, respectively (Burnstock and Knight, 2004). The P2X7 receptor, a member of the P2X purinergic superfamily of receptors, functions as an ATP-gated ion channel (Di, 1995; Falzoni, et al., 1995). Activation of toll like receptors by LPS in monocytes, macrophages, and microglial cells induces large quantity synthesis of pro-IL-1β (molecular mass, 31 kDa, a form of biologically inactive pro-cytokine), which accumulates and aggregates with the inflammasome component in the cell cytoplasm. Activation of P2X7 receptors by ATP triggers potassium efflux, procaspase-1 cleavage, conversion of pro-IL-1β into mature IL-1β (molecular mass, 17 kDa, a biologically active form of cytokine) and mature IL-1β release to the extracellular environment (Perregaux and Gabel, 1994; Sanz and Di, 2000; Ferrari, et al., 2006). Experiments performed in vitro and in vivo in P2X7 receptor knockout mice conclusively identified the receptor responsible for ATP-dependent IL-1β release, upon LPS challenge, as the P2X7 receptor (Ferrari, et al., 1997; Solle, et al., 2001; Labasi, et al., 2002).
In the present study, we thus addressed the hypothesis that P2X7 receptor activation contributes to LPS-induced changes in vascular reactivity through IL-1β release. To test our hypothesis, we determined, in mouse thoracic aortas, the effects of an agonist of P2X7 receptors, BzATP, on vascular responses and IL-1β release induced by LPS. In addition, we elucidated the downstream mediators of this signaling pathway.
Methods
Materials
Bacterial lipopolysaccharide (LPS, E. coli serotype 0.127:B8), phenylephrine (PE), NG-nitro-l-arginine methyl ester (l-NAME), N-([3-(aminomethyl)phenyl]methyl)ethanimidamide (1400W), benzoylbenzoyl-ATP (BzATP), and oxidized ATP (oATP) were all obtained from Sigma Chemical Co., St. Louis, MO, USA. Recombinant mouse IL-1β receptor antagonist (IL-1ra) was obtained from R&D Systems, Inc., Minneapolis, MN, USA. Dulbecco’s Modified Eagle’s Medium (DMEM) was obtained from Invitrogen Corporation (Gibco), Carlsbad, CA, USA. Fetal bovine serum was obtained from HyClone, Logan, UT, USA.
Isolated artery preparation and vascular incubation protocol
Thoracic aortas were obtained from 12-week-old male C57BL/6 mice. The thoracic aortas were cleaned of adhering periadventitial fat, cut into 3 mm length rings, and then incubated 24 hours with DMEM (supplemented with 10% fetal bovine serum, 0.5% penicillin/streptomycin and 1% L-glutamine), LPS (100 μg/ml), BzATP (P2X7 receptor agonist, 150 μM), LPS plus BzATP, oATP (P2X7 receptor antagonist, 50 μM), oATP plus LPS plus BzATP (oATP 50 μM incubated one hour before LPS and BzATP) or IL-1β (20 ng/ml). In some experiments, the endothelium was mechanically removed from the aortic rings by rubbing the intimal surface with a stainless steel wire.
Vascular function studies
Following the incubation, the rings were mounted in a myograph (Danish Myo Technology A/S) containing warmed (37 °C), oxygenated (95 % O2 /5 % CO2) physiological salt solution consisting of (mM): NaCl 130, KCl 4.7, KH2PO4 1.18, MgSO4·7H2O 1.18, CaCl2·2H2O 1.56, NaHCO3 14.9, glucose 5.6 and EDTA 0.03. The preparations were equilibrated for at least 60 min under a passive tension of 5 mN. After the equilibration period, the aortic rings were stimulated with PE (0.1 μM), followed by relaxation with acetylcholine (1 μM), which was used as evidence of an intact endothelium. Cumulative concentration-response curves to PE (10-10 M-10-5 M) were performed. Functional experiments were also performed in the presence of IL-1ra (IL-1β receptor antagonist, 100 ng/ml; 4 hours), l-NAME (NOS inhibitor, 100 μM; 40 minutes), or 1400W [selective inducible NOS (iNOS) inhibitor, 1 μM; 40 minutes). The contractile response to 120 mM KCl was also tested at the end of each time functional study to rule out the possibility of vascular damage due to the 24 hours incubation with the different reagents.
Measurement of vascular IL-1β by ELISA
Following 24 hours of incubation (as described above), aortas were homogenized in a T-PER tissue protein extraction reagent (Pierce Biotechnology, IL, USA) and proteinase inhibitor mixture (Sigma Chemical Co., St. Louis, MO, USA). After a 30-min centrifugation at 14,000 g, the supernatant was aliquoted and used for protein assay (with BCA protein assay reagent A and B; Pierce Biotechnology, IL, USA), IL-1β measurement with an enzyme-linked immunoadsorbent assay (ELISA) kit (Pierce Biotechnology, IL, USA), or western blots analysis of iNOS expression.
iNOS protein expression in aorta by western blot
Forty μg of protein were eluted from the supernatant and loaded directly into the sodium dodecyl sulfate (SDS) sample buffer for 10 % SDS-polyacrylamide gel electrophoresis. Following transfer onto 0.45 μm pure nitrocellulose membrane (Trans-Blot Transfer Medium; Bio-Rad Lab., Hercules, CA, USA), the membranes were blocked with 5 % defatted milk in Tris buffer solution (TBS) containing 0.1 % Tween-20 for 1 h and then incubated with 1:100 dilution of specific polyclonal antibody against iNOS (BD Transduction Laboratories, Lexington, KY) in TBS containing 0.1 % Tween-20 for 24 h at 4°C. The membranes were washed and finally incubated with a 1:1000 dilution of sheep anti-mouse IgG-horseradish peroxidase (HRP) antibody (GE healthcare, UK) for 1 h at room temperature. After successive washes, the immunocomplexes were developed using an enhanced peroxidase/luminol chemiluminescence reaction (ECL Western blotting detection reagents; Pierce Biotechnology, IL, USA) and exposed to X-ray film (Eastman Kodak Company, Rochester, NY, USA).
Statistical analysis
All values in the figures and text are expressed as mean±S.E.M. of n observations, where n represents the number of animals studied. For measurement of IL-1β and iNOS, three aortas from the same group were pooled and each pool was considered as one number. Statistical evaluation was performed by using analysis of variance (ANOVA) followed by a Bonferroni’s multiple comparison test in the vascular functional studies. Differences in IL-1β production and iNOS expression were analyzed by a Dunnett’s multiple comparison test. A P value less than 0.05 was considered to be statistically significant.
Results
Effects of LPS and P2X7 receptor activation on PE vascular reactivity
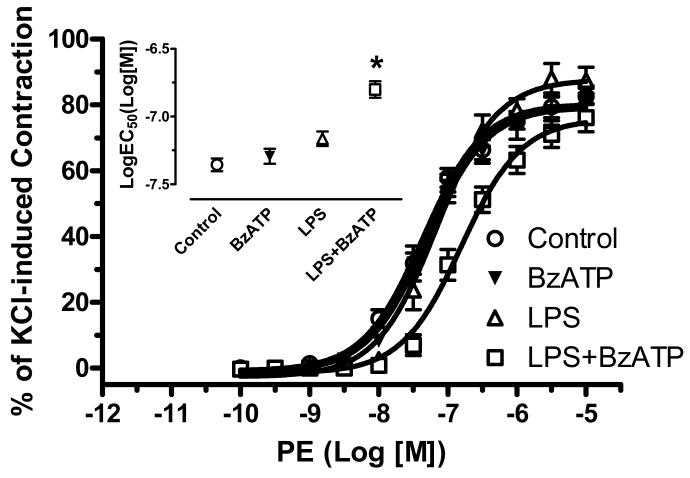
Incubation with LPS (100 μg/ml) plus BzATP (150 μM) for 24 hours, but not with BzATP or LPS alone, significantly attenuated PE-induced contractile responses in endothelium-intact aortic rings, compared with control (DMEM) (LogEC50 of Control: -7.36± 0.05; BzATP: -7.29± 0.06; LPS: -7.16± 0.05; LPS plus BzATP: -6.80± 0.06) (Figure 1). No changes in PE-induced contractile responses were observed in endothelium-denuded aortic rings incubated with DMEM and LPS plus BzATP. (LogEC50 of Control: -7.62± 0.06; LPS plus BzATP: -7.49± 0.06).
Figure 1. PE-induced vascular contractile responses are attenuated by LPS plus BzATP.
Endothelium-intact aortic rings were incubated with DMEM (Control; n=8), LPS (100 μg/ml; n=9), BzATP (150 μM; n=5), and LPS plus BzATP (LPS 100 μg/ml plus BzATP 150 μM; LPS+BzATP; n=7). Data are plotted as the percentage of maximum contractile response to 120 mM KCl. Log EC50 to PE are represented. Data are expressed as mean±SEM of n animals. *P<0.001 vs. Control.
Effect of IL-1ra and NOS inhibitors on LPS plus BzATP-induced hyporeactivity to PE
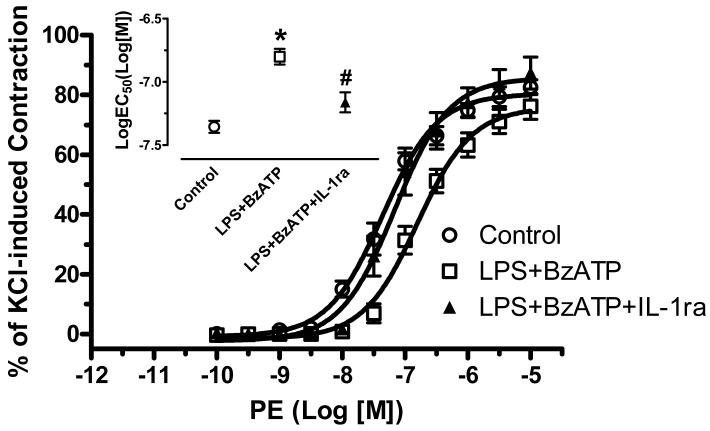
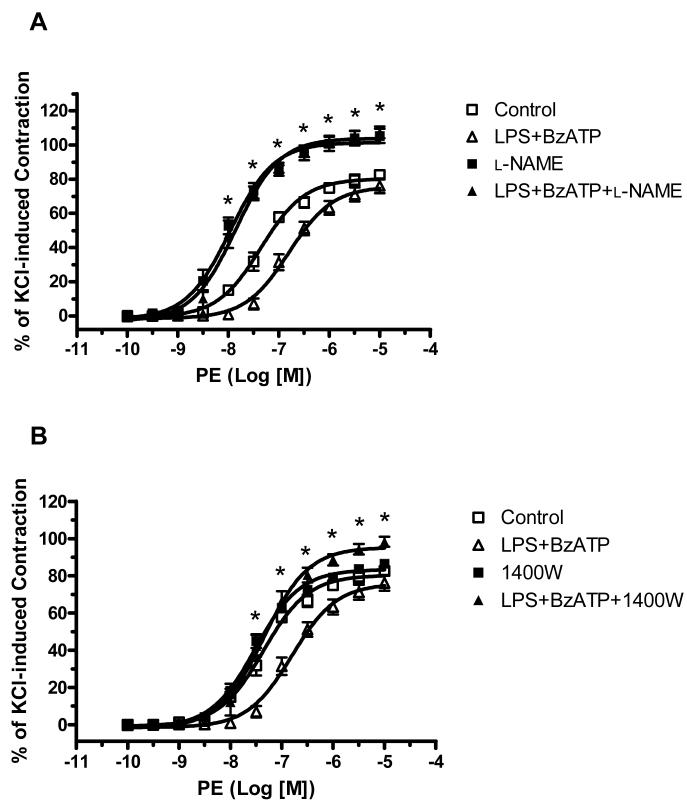
Incubation of endothelium-intact aortic rings with IL-1ra (IL-1β receptor antagonist, 100 ng/ml for 4 hours) completely prevented the attenuated contractile response to PE in aortas treated with LPS plus BzATP (LogEC50 of Control: -7.36± 0.05; LPS plus BzATP: -6.80± 0.06; LPS plus BzATP plus IL-1ra: -7.16±0.08) (Figure 2). Incubation with IL-1ra alone did not modify PE responses (LogEC50 of IL-1ra: -7.43-±0.09). To determine the role of nitric oxide in BzATP-amplified LPS-induced hyporeactivity, we tested the effects of a non-selective NOS inhibitor (l-NAME, 100 μM) and a selective iNOS inhibitor (1400W, 1 μM) on vascular function. Incubation with l-NAME for 40 minutes resulted in a leftward shift of the PE concentration-response curve in control and LPS plus BzATP arteries (Figure 3A). Incubation with 1400W for 40 minutes significantly reversed the decreased contractile response to PE only in LPS plus BzATP group (Figure 3B).
Figure 2. IL-1ra reverses the attenuated vascular contractile response to PE after incubation with LPS plus BzATP.
Endothelium-intact aortic rings were incubated with DMEM (Control; n=8), LPS plus BzATP (LPS 100 μg/ml plus BzATP 150 μM; LPS+BzATP; n=7) and LPS plus BzATP plus IL-1ra (LPS 100 μg/ml plus BzATP 150 μM plus IL-1ra 100 ng/ml; LPS+BzATP+IL-1ra; n=7). Data are plotted as the percentage of maximum contractile response to 120 mM KCl. Log EC50 to PE are represented. Data are expressed as mean±SEM of n animals. *P<0.001, LPS+BzATP vs. Control; #P<0.01, LPS+BzATP+IL-1ra vs. LPS+BzATP.
Figure 3. NOS inhibitors abolish the effects of LPS plus BzATP on PE-induced vascular reactivity.
(A) Endothelium-intact aortic rings were incubated with DMEM (Control; n=8), LPS plus BzATP (LPS 100 μg/ml plus BzATP 150 μM; LPS+BzATP; n=7), l-NAME (l-NAME 100 μM for 40 min in the organ bath after 24 h-incubation with DMEM; n=5), and LPS plus BzATP plus l-NAME (l-NAME 100 μM for 40 min in the organ bath after 24 h-incubation with LPS plus BzATP; LPS+BzATP+l-NAME; n=5). (B) The same curves were represented as (A) in DMEM and LPS plus BzATP.
Endothelium-intact aortic rings were incubated with 1400W (1400W 1 μM for 40 min in the organ bath after 24 h-incubation with DMEM; n=5), and LPS plus BzATP plus 1400W (1400W 1 μM for 40 min in the organ bath after 24 h-incubation with LPS plus BzATP; LPS+BzATP+1400W; n=5). Data are plotted as the percentage of maximum contractile response to 120 mM KCl. Data are expressed as mean±SEM of n animals. *P<0.001, LPS+BzATP vs. LPS+BzATP+NOS inhibitor (l-NAME or 1400W).
Effect of P2X7 receptor antagonist on LPS plus BzATP induced hyporeactivity to PE
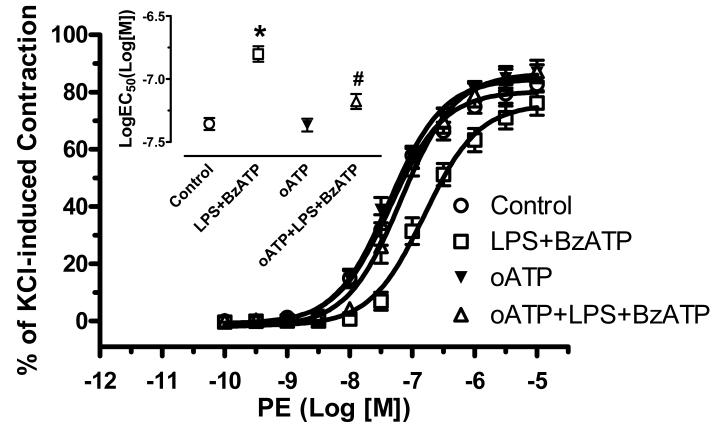
Incubation of endothelium-intact aortic rings with oATP (P2X7 receptor antagonist, 50 μM for one hour before the 24 hours incubation with LPS) completely abolished the attenuated contractile response to PE induced by LPS plus BzATP. Incubation with oATP alone did not modify the contractile responses to PE. (LogEC50 of Control: -7.36± 0.05; LPS plus BzATP: -6.80±0.06; oATP+LPS+BzATP: -7.18±0.06; oATP: -7.37±0.05) (Figure 4).
Figure 4. Oxidized ATP (oATP, P2X7 receptor antagonist) reverses the attenuated vascular contractile response to PE induced by LPS plus BzATP.
Endothelium-intact aortic rings were incubated with DMEM (Control; n=8), LPS plus BzATP (LPS 100 μg/ml plus BzATP 150 μM; LPS+BzATP; n=7), oATP (50 μM for 1 h before incubation with DMEM; n=6) and oATP plus LPS plus BzATP (oATP 50 μM for 1 h before incubation with LPS plus BzATP; oATP+LPS+BzATP; n=6). Data are plotted as the percentage of maximum contractile response to 120 mM KCl. Log EC50 to PE are represented. Data are expressed as mean±SEM of n animals. *P<0.001, LPS+BzATP vs. Control or oATP; #P<0.01, oATP+LPS+BzATP vs. LPS+BzATP.
Effects of LPS and P2X7 receptor activation on vascular IL-1β levels and iNOS protein expression
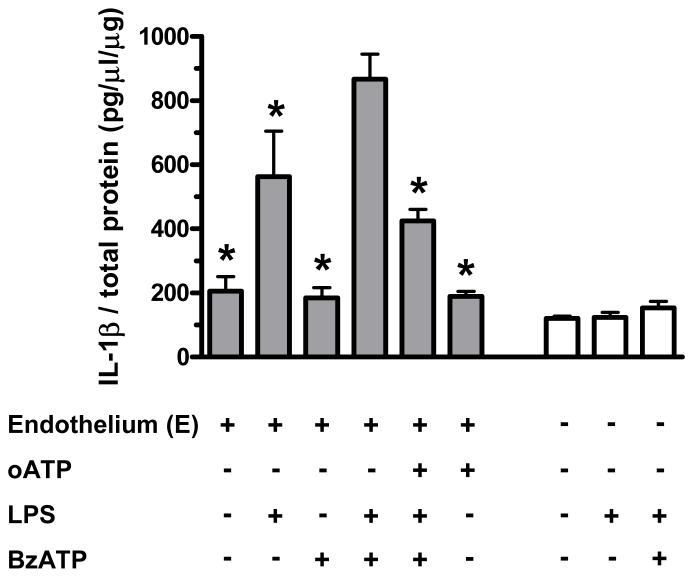
All groups were normalized by respective total protein. In endothelium-intact aortas, incubation with LPS (100 μg/ml, 24 h) increased IL-1β levels (Figure 5, left). Incubation with BzATP (150 μM, 24 h) augmented LPS-induced IL-1β release, and this effect was inhibited by the pretreatment with oATP (50 μM, one hour) (Figure 5, left). However, in endothelium-denuded aortic rings, neither LPS nor LPS plus BzATP increased IL-1β release (Figure 5, right).
Figure 5. BzATP augments IL-1β production induced by LPS in endothelium-intact vessels.
The changes in endothelium-intact and -denuded aortic IL-1β levels were assessed by ELISA, after incubation with DMEM [Control; E(+), n=6; E(-), n=3], LPS [100 μg/ml; E(+), n=3; E(-), n=3], BzATP [150 μM; E(+), n=4], LPS plus BzATP (LPS 100 μg/ml plus BzATP 150 μM; LPS+BzATP; E(+), n=4; E(-), n=3], oATP plus LPS plus BzATP [oATP 50 μM for 1 h before incubation with LPS plus BzATP; oATP+LPS+BzATP; E(+), n=3] and oATP [50 μM for 1 h before incubation with DMEM; E(+), n=3]. Data are expressed as mean±SEM of n animals. *P<0.05 vs. LPS+BzATP in endothelium-intact vessels.
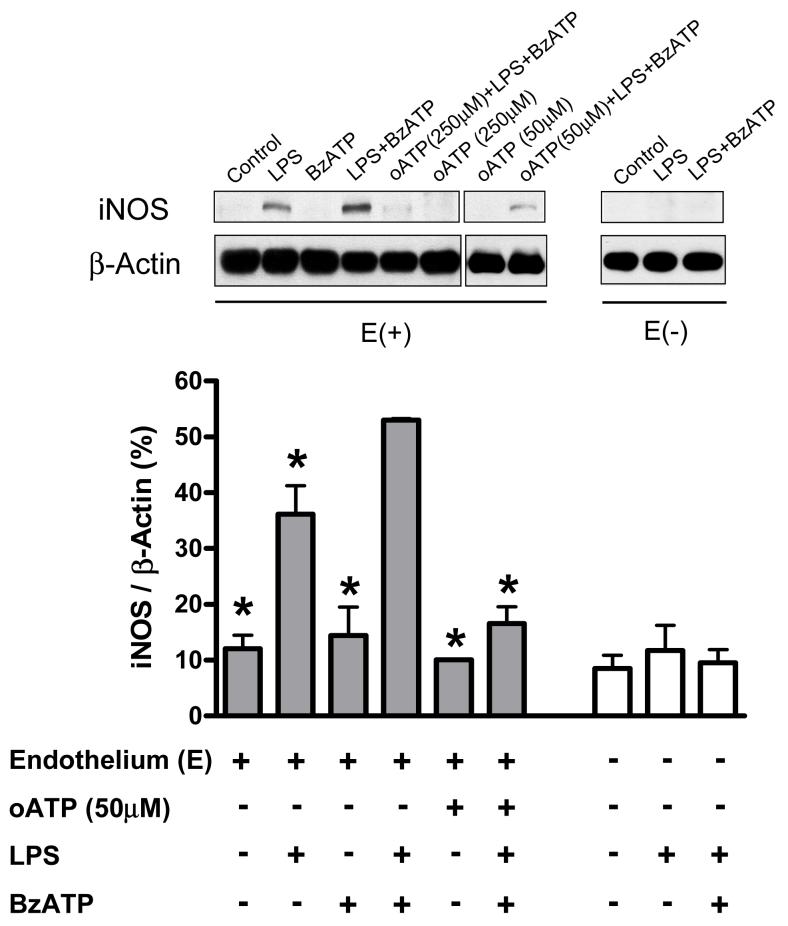
In endothelium-intact aortas, iNOS protein expression was detected in LPS (100 μg/ml) treatment, but not in control (DMEM) group (Figure 6, left). Incubation with LPS plus BzATP (150 μM) further increased iNOS protein expression (Figure 6, left), but no changes in endothelial NOS (eNOS) expression were observed after incubation with LPS or LPS plus BzATP (data not shown). Pretreatment of aortas with oATP for one hour inhibited the augmented LPS plus BzATP-induced iNOS protein expression (Figure 6, left). In endothelium-denuded aortas, iNOS protein expression did not change after incubation with LPS or LPS plus BzATP (Figure 6, right).
Figure 6. BzATP augments inducible nitric oxide synthase (iNOS) protein expression induced by LPS in endothelium-intact vessels.
The figure depicts a typical western blot image of iNOS protein expression (upper figure) and the statistical analysis of changes of iNOS protein expression in mouse endothelium-intact and -denuded aortic rings, which were incubated with DMEM [Control; E(+), n=3; E(-), n=3], LPS [100 μg/ml; E(+), n=3; E(-), n=3], BzATP [150 μM; E(+), n=3], LPS plus BzATP [LPS 100 μg/ml plus BzATP 150 μM; LPS+BzATP; E(+), n=3; E(-), n=3], oATP plus LPS plus BzATP [oATP 50 μM or 250 μM for 1 h before incubation with LPS plus BzATP; oATP (50 μM)+BzATP+LPS; E(+), n=3 or oATP (250 μM) +BzATP+LPS; E(+), n=3] and oATP [50 μM or 250 μM for 1 h before incubation with DMEM; oATP (50 μM); E(+), n=3 or oATP (250 μM); E(+), n=3]. Data are expressed as mean±SEM of n animals. *P<0.05 vs. LPS+BzATP in endothelium-intact vessels.
Effects of IL-1β treatment on vascular iNOS protein expression
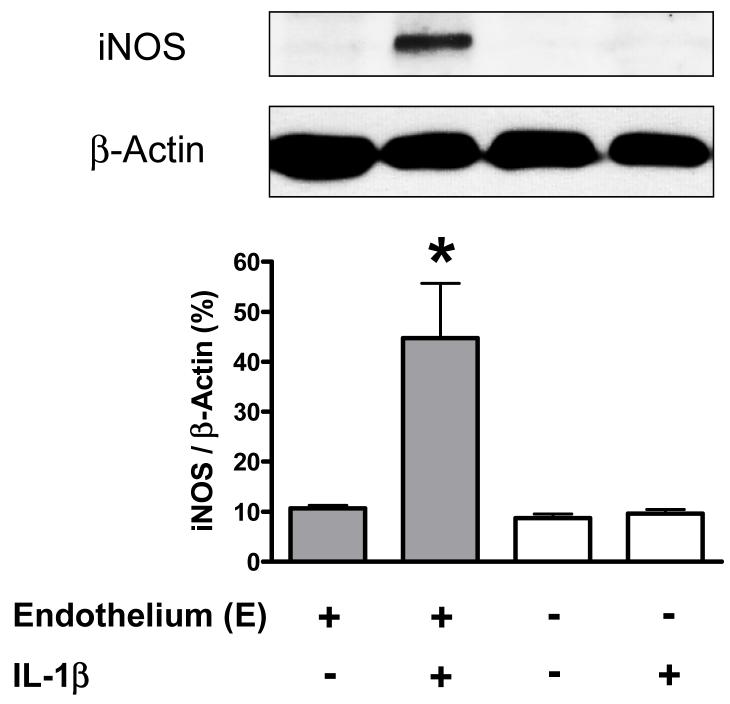
Incubation of endothelium-intact aortic rings with IL-1β (20 ng/ml) for 24 hours increased iNOS protein expression, but not in endothelium-denuded aortic rings(Figure 7).
Figure 7. IL-1β induces iNOS protein expression in endothelium-intact vessels.
The figure depicts a typical western blot image of iNOS protein expression (upper figure) and the statistical analysis of changes of iNOS protein expression in mouse endothelium-intact and -denuded aortic rings, which were incubated with DMEM [Control; E(+), n=3; E(-), n=3], IL-1β [20 ng/ml; E(+), n=3; E(-), n=3]. Data are expressed as mean±SEM of n animals. *P<0.01, IL-1β vs. Control in endothelium-intact vessels.
Discussion
Previous studies have shown that endotoxin induces a variety of biological responses and disease. Hypotension, tachycardia and vascular hyporeactivity to constrictor stimuli are common in animals with endotoxic shock (Szabo, et al., 1993). In addition, pro-inflammatory cytokines are thought to be involved. Several studies have suggested that the P2X7 receptor is an important regulator of inflammatory cell responsiveness to LPS (Falzoni, et al., 1995; Solle, et al., 2001; Guerra, et al., 2003). LPS-activated isolated macrophages, as well as blood from the P2X7 receptor-deficient mouse are incapable of releasing mature IL-1β in response to ATP challenge (Solle, et al., 2001; Labasi, et al., 2002). Our current study extends these initial findings by showing that P2X7 receptor activation facilitates LPS-induced vascular hyporeactivity. Incubation of endothelium-intact aortic rings with LPS plus BzATP significantly attenuated PE-induced contractile responses. oATP, a P2X7 receptor inhibitor, effectively blocked the LPS plus BzATP-induced attenuation of PE contractile responses. It has been previously shown that incubation of rat aorta with IL-1β for 24 hours attenuates PE-induced smooth muscle contraction (Takizawa, et al., 1999). Here, we also demonstrated that IL-1β and iNOS-derived nitric oxide are involved in this attenuated contractile response to PE induced by LPS plus BzATP. Thus, these studies implicated the P2X7 receptor as a critical factor to regulate vascular reactivity in inflammation or inflammatory-related conditions.
ATP, the natural agonist for P2X7 receptor activation, was initially considered an intracellular energy source or enzyme cofactor involved in biosynthetic function. Later, ATP was recognized as a neurotransmitter involved on non-adrenergic, non-cholinergic nerve responses (Burnstock, et al., 1970; Burnstock, et al., 1972). P1 receptors and P2 receptors constitute the two major families of purinergic receptors. Adenosine, which is formed by breakdown of ATP by ubiquitous intracellular or extracelllular ectonucleotidases, exerts its effects by activating P1 receptors. ATP achieves its effects by activating the P2 receptor family, which based on structural and mechanistic differences was divided into two subsets-P2X and P2Y. The P2X receptors are ligand-gated ion channels classified in seven subtypes, P2X1-7, some of which seem to modulate vascular tone, contraction of muscle cells, and signal transduction in cardiac cells (Burnstock and Knight, 2004). However, an important role in inflammation was inferred from the finding that ATP also elicited the processing and release of pro-inflammatory cytokines from LPS-primed macrophages by acting on a receptor (North, 2002). Until gene cloning, this receptor was recognized to have similar properties to the P2X7 receptor (Surprenant, et al., 1996). The putative LPS-binding region is also present in the P2X7 receptor cytosolic domain (Khakh and North, 2006). Expression of P2X7 receptor message and protein have been reported in different cell types, such as macrophages (Falzoni, et al., 1995; Solle, et al., 2001), leukocytes (Labasi, et al., 2002), microglia (Bianco, et al., 2006), epithelial cells (Groschel-Stewart, et al., 1999), endothelial cells (Ramirez and Kunze, 2002) and fibroblasts (Solini, et al., 1999). In the present study, we tested the hypothesis that P2X7 receptor activation plays a role in the functional vascular changes induced by LPS. Our results showed that simultaneous incubation of mouse endothelium-intact aortic rings with LPS plus the P2X7 receptor agonist BzATP, but not LPS or BzATP alone, significantly attenuated PE-induced vessel constriction (Figure 1), indicating that P2X7 receptor activation does play an important role in LPS-mediated vascular effects. Our results are in accordance with previous work showing that rat aortic rings after 20-24 hours incubation with LPS and media alone displayed similar response to PE (Wylam, et al., 2001). It is important to mention that incubation with media alone may elicit vascular synthesis of cytokines (Newman, et al., 1996) and it is possible to prolong exposure to include very small amounts of LPS in nominally sterile media (McKenna, 1990). We were able to detect IL-1β in aortas incubated with DMEM, although in smaller concentration than observed after LPS incubation (Figure 5).
In a previous study, Takizawa et al. showed that incubation of rat aortas with IL-1β for 24 hours decreases contractile responses to PE (Takizawa, et al., 1999). In addition, IL-1β has been shown to increase in plasma 3 hours after LPS was injected into rats (Izeboud, et al., 2004). Accordingly, in our current study, addition of IL-1ra reversed vascular hyporeactivity induced by LPS plus BzATP incubation (Figure 2) and we suggested that vascular hyporeactivity from LPS plus BzATP was dependent on the IL-1β release. The IL-1ra is a competitive inhibitor of IL-1α and IL-1β binding to the IL-1 receptor, and blocks IL-1 dependent signal transduction, thus functioning as an endogenous, IL-1-selective inhibitor of inflammation (Dinarello, 2000). Conversely, it can be noted that in human vascular endothelial cells, LPS treatment followed by BzATP stimulation, results in a maximal release of IL-1ra into the extracellular medium (Wilson, et al., 2004). The balance of pro-inflammatory IL-1β and anti-inflammatory IL-1ra may determine the overall inflammatory response.
We observed significant attenuation of PE-induced contractile response in endothelium-intact aortic rings incubated with LPS plus BzATP (Figure 1), but not in endothelium-denuded aortic rings. Thus, this implicates that the endothelium is necessary for P2X7 receptors activation by BzATP to amplify the LPS-induced vascular hyporeactivity. In addition, P2X7 receptor immunoreactivity has been detected in aorta, but a weak level of P2X7 receptor immunoreactivity was associated with the smooth muscle layer (Lewis and Evans, 2001). In our results, we found that BzATP significantly enhanced iNOS protein expression induced by LPS in endothelium-intact, but not in endothelium-denuded aortic rings (Figure 6). oATP not only reversed vascular hyporeactivity to PE (Figure 4), but also inhibited the enhanced IL-1β level and iNOS expression in aorta treated with LPS plus BzATP (Figure 5, 6). The endothelium is necessary for BzATP-induced amplification of LPS-induced hyporeactivity in mouse aorta. We also found that increased IL-1β level and iNOS protein expression are related to the presence of endothelium (Figure 5, 6). BzATP may activate P2X7 receptor on the endothelium, stimulating the conversion of pro-IL-1β into mature IL-1β, which is then released from the endothelial cells, affecting adjacent cells. Accordingly, Gui et al. suggested that IL-1β plays an autocrine/paracrine role in the induction of iNOS in rat aorta tissue (Gui, et al., 2000). We have detected increased IL-1β following 24 hours incubation in endothelium-intact aortic tissue in LPS plus BzATP treatment (Figure 5, left). However, IL-1β levels did not change in endothelium-denuded aortas treated with LPS plus BzATP (Figure 5, right) and no IL-1β was detectable in the medium (data not shown). In addition, in order to understand the signaling relationship between IL-1β and iNOS, we directly incubated aortic rings with IL-1β for 24 hours. iNOS protein expression was significantly increased in the endothelium-intact, but not in the endothelium-denuded aortic tissue (Figure 7). Thus, these results demonstrate that IL-1β plays an autocrine role acting on the adjacent vascular endothelial cells to induce iNOS production. Another study has shown that during LPS-mediated acute inflammation, an increase in ATP release was measured from endothelial cells (Bodin and Burnstock, 1998). The endothelium may be exposed to high levels of extracellular ATP under inflammatory conditions, due to release from degranulating platelets, via sympathetic nerve stimulation, as well as damaged cells in atherosclerosis, hypertension, restenosis and ischemia (Burnstock, 2002). Although we did not observe vascular functional changes in LPS alone-treated vessels (Figure 1), we found an increase in IL-1β level and iNOS protein expression in endothelium-intact aorta (Figure 5, 6). LPS-induced endogenous ATP release from endothelium may not be high enough to enhance the vascular hyporeactivity by LPS until extra-additional ATP analog (BzATP) is added. Thus, it will be important to understand the main source of ATP in systemic LPS treatment, since systemic vascular hyporeactivity to NE is evident in LPS alone-treated animals (Chiao, et al., 2005). Moreover, our results showed that a P2X7 receptor antagonist, oATP, decreased the expression of iNOS induced by LPS plus BzATP to a level below that obtained with LPS alone in endothelium-intact aortic tissue (Figure 5, 6). Since P2X7 receptor immunoreactivity associated with the smooth muscle layer of the arteries is weak (Lewis and Evans, 2001), no activity of the P2X7 receptor was shown in endothelium-denuded vessels, even those treated with LPS or LPS plus BzATP, implicating that the action of LPS involves activation of P2X7 receptors in endothelial cells. Accordingly, it had been demonstrated that oATP inhibits LPS-stimulated nitric oxide production and iNOS expression, and attenuates LPS-induced activation of NF-κB and ERK-1/2 in macrophages (Hu, et al., 1998). P2X7 receptors can cross-talk with the LPS-dependent pathways, since LPS signals through CD14/MD2/TLR-dependent pathways as well as through CD14/P2X7-dependent pathways (Guerra, et al., 2003). In addition, the putative LPS-binding region is present in the P2X7 receptor cytosolic domain (Khakh and North, 2006). Thus, this evidence implicates that the vascular action of LPS may involve the activation of P2X7 receptor.
In summary, our results demonstrate for the first time that activation of P2X7 receptor amplifies LPS-induced vascular hyporeactivity. P2X7 receptor-mediated effects on vascular reactivity are due to IL-1β release from endothelial cells, which, in turn, induces downstream nitric oxide production by iNOS. Finally, we suggest that the P2X7 receptor is an important regulator for vascular hypotensive responses in inflammation or inflammatory-related disease.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (HL-74167).
The abbreviations used are
- 1400W
N-([3-(aminomethyl)phenyl]methyl)ethanimidamide
- ANOVA
analysis of variance
- BzATP
benzoylbenzoyl-ATP
- DMEM
Dulbecco’s Modified Eagle’s Medium
- ELISA
enzyme-linked immunoadsorbent assay
- eNOS
endothelial nitric oxide synthase
- HRP
horseradish peroxidase
- IL
interleukin
- IL-1ra
IL-1β receptor antagonist
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- l-NAME
NG-nitro-l-arginine methyl ester
- NOS
nitric oxide synthase
- oATP
oxidized ATP
- PE
phenylephrine
- SDS
sodium dodecyl sulfate
- TBS
Tris buffer solution
- TNF-α
tumor necrosis factor-alpha
Footnotes
Recommended section: Cardiovascular
References
- Beasley D, Cohen RA, Levinsky NG. Interleukin 1 inhibits contraction of vascular smooth muscle. J Clin Invest. 1989;83:331–335. doi: 10.1172/JCI113879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, Ceruti S, Colombo A, Fumagalli M, Ferrari D, Pizzirani C, Matteoli M, Di VF, Abbracchio MP, Verderio C. A role for P2X7 in microglial proliferation. J Neurochem. 2006;99:745–758. doi: 10.1111/j.1471-4159.2006.04101.x. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Increased release of ATP from endothelial cells during acute inflammation. Inflamm Res. 1998;47:351–354. doi: 10.1007/s000110050341. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Satchell DG, Smythe A. A comparison of the excitatory and inhibitory effects of non-adrenergic, non-cholinergic nerve stimulation and exogenously applied ATP on a variety of smooth muscle preparations from different vertebrate species. Br J Pharmacol. 1972;46:234–242. doi: 10.1111/j.1476-5381.1972.tb06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami A, Beutler B. The role of cachectin/TNF in endotoxic shock and cachexia. Immunol Today. 1988;9:28–31. doi: 10.1016/0167-5699(88)91353-9. [DOI] [PubMed] [Google Scholar]
- Chiao CW, Lee SS, Wu CC, Su MJ. Thaliporphine increases survival rate and attenuates multiple organ injury in LPS-induced endotoxaemia. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:34–43. doi: 10.1007/s00210-004-1014-6. [DOI] [PubMed] [Google Scholar]
- Di VF. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343:732–734. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Falzoni S, Munerati M, Ferrari D, Spisani S, Moretti S, Di VF. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest. 1995;95:1207–1216. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal SM, Melchiorri L, Baricordi OR, Di VF. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di VF. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- French JF, Lambert LE, Dage RC. Nitric oxide synthase inhibitors inhibit interleukin-1 beta-induced depression of vascular smooth muscle. J Pharmacol Exp Ther. 1991;259:260–264. [PubMed] [Google Scholar]
- Groschel-Stewart U, Bardini M, Robson T, Burnstock G. Localisation of P2X5 and P2X7 receptors by immunohistochemistry in rat stratified squamous epithelia. Cell Tissue Res. 1999;296:599–605. doi: 10.1007/s004410051321. [DOI] [PubMed] [Google Scholar]
- Guerra AN, Fisette PL, Pfeiffer ZA, Quinchia-Rios BH, Prabhu U, Aga M, Denlinger LC, Guadarrama AG, Abozeid S, Sommer JA, Proctor RA, Bertics PJ. Purinergic receptor regulation of LPS-induced signaling and pathophysiology. J Endotoxin Res. 2003;9:256–263. doi: 10.1179/096805103225001468. [DOI] [PubMed] [Google Scholar]
- Gui Y, Zheng XL, Hollenberg MD. Interleukin-1beta, Src- and non-Src tyrosine kinases, and nitric oxide synthase induction in rat aorta in vitro. Am J Physiol Heart Circ Physiol. 2000;279:H566–H576. doi: 10.1152/ajpheart.2000.279.2.H566. [DOI] [PubMed] [Google Scholar]
- Hu Y, Fisette PL, Denlinger LC, Guadarrama AG, Sommer JA, Proctor RA, Bertics PJ. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in RAW 264.7 macrophages. J Biol Chem. 1998;273:27170–27175. doi: 10.1074/jbc.273.42.27170. [DOI] [PubMed] [Google Scholar]
- Izeboud CA, Hoebe KH, Grootendorst AF, Nijmeijer SM, van Miert AS, Witkamp RR, Rodenburg RJ. Endotoxin-induced liver damage in rats is minimized by beta 2-adrenoceptor stimulation. Inflamm Res. 2004;53:93–99. doi: 10.1007/s00011-003-1228-y. [DOI] [PubMed] [Google Scholar]
- Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Evans RJ. P2X receptor immunoreactivity in different arteries from the femoral, pulmonary, cerebral, coronary and renal circulations. J Vasc Res. 2001;38:332–340. doi: 10.1159/000051064. [DOI] [PubMed] [Google Scholar]
- McCabe WR, Treadwell TL, De MA., Jr. Pathophysiology of bacteremia. Am J Med. 1983;75:7–18. doi: 10.1016/0002-9343(83)90067-0. [DOI] [PubMed] [Google Scholar]
- McKenna TM. Prolonged exposure of rat aorta to low levels of endotoxin in vitro results in impaired contractility. Association with vascular cytokine release. J Clin Invest. 1990;86:160–168. doi: 10.1172/JCI114679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DC, Ulevitch RJ. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978;93:526–617. [PMC free article] [PubMed] [Google Scholar]
- Newman WH, Zhang LM, Leeper-Woodford SK, Castresana MR. Human blood vessels release tumor necrosis factor-alpha from a smooth muscle cell source. Crit Care Med. 1996;24:294–297. doi: 10.1097/00003246-199602000-00019. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- Pinsky MR. Organ-specific therapy in critical illness: interfacing molecular mechanisms with physiological interventions. J Crit Care. 1996;11:95–107. doi: 10.1016/s0883-9441(96)90024-6. [DOI] [PubMed] [Google Scholar]
- Ramirez AN, Kunze DL. P2X purinergic receptor channel expression and function in bovine aortic endothelium. Am J Physiol Heart Circ Physiol. 2002;282:H2106–H2116. doi: 10.1152/ajpheart.00892.2001. [DOI] [PubMed] [Google Scholar]
- Sanz JM, Di VF. Kinetics and mechanism of ATP-dependent IL-1 beta release from microglial cells. J Immunol. 2000;164:4893–4898. doi: 10.4049/jimmunol.164.9.4893. [DOI] [PubMed] [Google Scholar]
- Solini A, Chiozzi P, Morelli A, Fellin R, Di VF. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. J Cell Sci. 1999;112(Pt 3):297–305. doi: 10.1242/jcs.112.3.297. [DOI] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Szabo C, Mitchell JA, Thiemermann C, Vane JR. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993;108:786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa S, Ozaki H, Karaki H. Possible involvement of K+ channel opening to the interleukin-1 beta-induced inhibition of vascular smooth muscle contraction. J Vet Med Sci. 1999;61:357–360. doi: 10.1292/jvms.61.357. [DOI] [PubMed] [Google Scholar]
- Wilson HL, Francis SE, Dower SK, Crossman DC. Secretion of intracellular IL-1 receptor antagonist (type 1) is dependent on P2X7 receptor activation. J Immunol. 2004;173:1202–1208. doi: 10.4049/jimmunol.173.2.1202. [DOI] [PubMed] [Google Scholar]
- Wylam ME, Metkus AP, Umans JG. Nitric oxide dependent and independent effects of in vitro incubation or endotoxin on vascular reactivity in rat aorta. Life Sci. 2001;69:455–467. doi: 10.1016/s0024-3205(01)01137-7. [DOI] [PubMed] [Google Scholar]