Abstract
Background
Environmental tobacco smoke (ETS) causes increased morbidity among children with asthma, however pediatricians do not consistently screen and counsel families of asthmatic children regarding ETS. An index score based on parent report of exposure could help providers efficiently screen for ETS.
Objective
1) To develop an index measure of ETS based on parent self-report of smoking behaviors; 2) To determine whether the index score is associated with children’s present and future cotinine levels.
Methods
Data were drawn from a community intervention for inner-city children with persistent asthma (n=226, response rate 72%). Measures of child salivary cotinine and parent self-reported ETS-related behaviors were obtained at baseline and 7–9 months later. To develop the index score, we used a 15-fold cross-validation method on 70% of our data that considered combinations of smoke exposure variables, controlling for demographics. We chose the most parsimonious model that minimized the mean square predictive error. The resulting index score included primary caregiver smoking and home smoking ban status. We validated our model on the remaining 30% of data. ANOVA and multivariate analyses were used to determine the association of the index score with children’s cotinine levels.
Results
54% of asthmatic children lived with ≥1 smoker and 51% of caregivers reported a complete home smoking ban. The children’s mean baseline cotinine was 1.55ng/ml (range 0.0–21.3). Children’s baseline and follow-up cotinine levels increased as scores on the index measure increased. In a linear regression, the index score was significantly and positively associated with children’s cotinine measurements at baseline (p<.001, model R2=.37) and 7–9 months later (p<.001, R2=.38).
Conclusion
An index measure with combined information regarding primary caregiver smoking and household smoking restrictions helps to identify asthmatic children with the greatest exposure to ETS, and can predict children who will have elevated cotinine levels 7–9 months later.
Keywords: Environmental tobacco smoke, asthma, children, primary care, screening
INTRODUCTION
Environmental tobacco smoke (ETS) exposure is a known risk to health and a preventable cause of morbidity among children.1,2 More than one quarter of children in the US live with at least one smoker.2 While many states have restrictions on smoking in public places, the greatest source of ETS exposure for children occurs in the home.3–5
ETS exposure is of particular concern for children with asthma. Children with asthma who are exposed to ETS experience more severe symptoms and more frequent exacerbations compared to children without exposure.6,7 While parents describe a variety of intended ETS harm-reduction strategies when describing their smoking practices, many children, even those with significant asthma, continue to experience exposure to ETS.8 Not surprisingly, avoiding ETS is recommended as an important component of asthma management.9
Exposure to ETS is best measured through the biomarker cotinine. Cotinine is a metabolite of nicotine, which is measured from samples of urine, serum, saliva, and hair. Under conditions of sustained smoke exposure, cotinine levels provide valid and quantitative measures of average ETS exposure.10–12 Cotinine levels have been positively correlated with risk of ETS-related health complications in children.10,11 While cotinine measurements now are commonly used in research studies, such measurements are not currently feasible in routine pediatric health care due to cost and time constraints.
Pediatric clinicians are in an ideal position to screen and counsel families regarding childhood ETS exposure. However these practices do not occur consistently during health care visits,13,14 in part due to limited time and competing priorities.14 Researchers have designed tools to systematically and accurately assess children’s smoke exposure. For example, Johansson et al found that urine cotinine/creatinine levels in children were significantly related to parents’ self-reported smoking behaviors on a written questionnaire.15 In addition, Groner et al derived and validated an office-based tool to identify non-asthmatic young children at risk for ETS exposure.16 The usefulness of these types of tools for parents of children with asthma has not been established.
The objective of this study was to develop a simple algorithm, based on parent self-report of smoking behaviors, that provides quick and accurate information on ETS exposure and that, unlike biologic measures of exposure, can be easily integrated into clinical practice. We further sought to determine whether the algorithm could be used to predict which children would continue to have high levels of exposure 7–9 months later.
METHODS
Study Population
We analyzed data from 226 children 3–10 years of age with asthma who were participating in a school-based asthma treatment program (the School-Based Asthma Therapy Trial). Children whose parents indicated that they have asthma on their school screening forms were identified for potential enrollment. Parents of these children received a telephone survey to determine if the child met study criteria.
Eligibility for the program required children to have physician-diagnosed asthma with mild persistent to severe persistent symptoms.9 All children attended school or preschool in the Rochester City School district, and their primary care provider authorized their participation. We excluded children with congenital heart disease, cystic fibrosis, or other chronic lung disease, that could interfere with the assessment of asthma-related outcome measures. We also excluded children whose families were planning to move within the next 6 months. Two hundred and twenty-six out of 313 eligible children were enrolled (response rate:72%) from August 2006-November 2006.
During a baseline evaluation for this program at the beginning of the school year, we conducted extensive home-based interviews with parents or caregivers (hereafter referred to as ‘parents’) to obtain information regarding demographics, asthma symptoms and medications, and household smoking behaviors. Saliva samples also were obtained from every child (n=226) for cotinine measurement during the same time period. A follow-up assessment for each child was done 7–9 months after baseline (at the end of the school year, in June/July) during which an additional cotinine measurement was obtained. We were able to obtain follow-up cotinine measurements for 216 children (96%); 7 children had withdrawn and 3 missed the follow-up collection. Because some of the children were eligible for an ETS reduction program as part of their participation in the intervention, we only used follow-up data from the children who did not receive the ETS reduction intervention (n=162). The University of Rochester Institutional Review Board approved the study protocol, and informed consent was obtained from all caregivers.
Smoke Exposure
We assessed ETS exposure by salivary cotinine measurements and parent self-report. Salivary cotinine was used because it is well accepted for the measurement of ETS exposure, non-invasive, and can be done quickly and easily in young children.17 We collected saliva samples with a sorbette (a wand with a small sponge) using a standard protocol developed for children. Study staff collected three sorbettes from each child to obtain sufficient saliva for analysis. Cotinine analyses were performed with Enzyme Immunoassay techniques by Salimetrics LLC, State College, PA, and reported in ng/ml. The lower limit of cotinine sensitivity is 0.05 ng/ml. Undetectable cotinine values were recorded as 0 (baseline; n=9, follow-up; n=6).
Parent report of children’s exposure to ETS in the home was measured by responses to detailed questions asked at the baseline home interview. We asked caregivers how often they smoked (never, occasionally, daily; dichotomized as never vs. occasionally or daily) and the number of smokers living in the child’s home (0, 1, 2, 3, ≥4; dichotomized as 0 vs. ≥1).18 We measured home smoking restrictions19 by asking parents “which option best describes the situation regarding smoking in this child’s home”; 1)smoking is allowed in any common room of the home, 2)smoking is limited to a part of the house where the child rarely goes, and 3)there is no smoking at all. If the parent reports no smoking at all, then we asked if there are any exceptions to this situation. A complete home smoking ban was defined as a parent who reported no smoking at all with no exceptions.19 Similarly, we asked about smoking rules in the car (for those who owned a car); families with no smoking in the car with no exceptions were defined as having a car smoking ban.
Demographic Measures
Demographic variables included age (preschool, 3–5 years vs. school-age, 6–10 years), gender, race (White, Black, or other), ethnicity (Hispanic or not Hispanic) and insurance status (Medicaid or other). Parental characteristics included parent age (<30 or ≥30 years) and education (< high school or ≥ high school).
Psychosocial Measures
We used the previously validated 10-item Kessler Psychological Distress Scale (K-10)20 to assess parental symptoms of depression. Parents were asked to think about the prior 30 days and answer on a 5-point likert scale. Scores were summed and categorized into well or mild symptoms of depression (score 10–24) vs. moderate or severe symptoms (score 25–50). We measured parent stress using questions from the Parenting Stress Index.21 Five items were scored on a 5-point scale including questions such as: “being a parent is harder than I thought it would be” and “I can’t handle making decisions without help”. Responses were summed for a total parent stress score (range 0–20) with higher scores indicating increased stress.
Analysis
We performed analyses using SPSS version 15.0 (Statistical Product and Service Solutions 15.0; SPSS Inc, Chicago, Ill), and STATA (version 9, StataCorp, College Station, Texas). We used t-test and ANOVA statistics to compare cotinine values between children of different demographic populations and ages and with different household smoking behaviors. To develop the index score, we used a 15-fold cross-validation method22 on 70% of our data (158 observations) that looked at the different combinations of the exposure variables, controlling for demographics (age, race and ethnicity). We chose the most parsimonious model that minimized the mean square predictive error. We then validated our model on the remaining 30% of data (68 observations). We ended up with a 2-item index measure, and then evaluated the relationship between the index measure and cotinine values using ANOVA statistics. We determined the relationship between the index score and cotinine values at baseline and follow-up using regression analyses, controlling for the child’s age, race, Hispanic ethnicity, parent education, stress and depressive symptoms. Due to the non-normal distribution of cotinine data, we used the natural log function to transform the data prior to analysis. A 2-sided alpha <.05 was considered statistically significant.
RESULTS
Table 1 shows the overall demographic characteristics and household smoking behaviors for the children. The mean age was 7 years (SD 1.9). Most children were male (58%), Black (65%) and had Medicaid insurance (74%). More than half of the parents had a high school degree. Thirty-nine percent of caregivers reported smoking either occasionally or daily and 54% of children lived in a home with ≥1 smoker. Complete smoking bans were reported in 51% of children’s homes.
Table 1.
Population Demographics and Mean Cotinine Values
| Population Demographics | Overall N (%) | Cotinine (χ̄ng/ml±sd) | p-value |
|---|---|---|---|
| Child Age in years | |||
| 3–5 | 48 (21.2) | 1.98 ± 3.71 | .328 |
| 6–10 | 178 (78.8) | 1.43 ± 2.09 | |
| Child Gender | |||
| Male | 131 (58.0) | 1.34 ± 2.05 | .145 |
| Female | 95 (42.0) | 1.84 ± 3.05 | |
| Child Race | |||
| White | 24 (10.6) | 2.44 ± 2.43* | |
| Black | 147 (65.0) | 1.67 ± 2.87 | .019 |
| Other | 55 (24.3) | 0.82 ± 0.95 | |
| Child Ethnicity | |||
| Hispanic | 63 (27.9) | 1.22 ± 1.43 | .223 |
| Non-Hispanic | 163 (72.1) | 1.68 ± 2.83 | |
| Medicaid | |||
| Yes | 168 (74.3) | 1.67 ± 2.58 | .229 |
| No | 58 (25.7) | 1.20 ± 2.36 | |
| Parent Age | |||
| <30 | 76 (33.6) | 1.80 ± 3.08 | .308 |
| ≥30 | 148 (65.5) | 1.43 ± 2.20 | |
| Parent Education | |||
| <High school | 77 (34.1) | 2.01 ± 3.29 | .087 |
| ≥High school | 149 (65.9) | 1.31 ± 1.99 | |
| Parent Depression | |||
| None/Mild | 159 (70.4) | 1.51 ± 2.56 | .739 |
| Mod/Severe | 67 (29.6) | 1.63 ± 2.45 | |
| Smoker in the Home | |||
| Yes | 121 (53.5) | 2.40 ± 3.15 | <.001 |
| No | 105 (46.5) | 0.56 ± 0.73 | |
| Primary Caregiver is a Smoker | |||
| Yes | 89 (39.4) | 2.82 ± 3.42 | <.001 |
| No | 137 (60.6) | 0.72 ± 1.10 | |
| Home Smoking Ban | |||
| Yes | 115 (50.9) | 0.63 ± 0.74 | <.001 |
| No | 111 (49.1) | 2.50 ± 3.27 | |
| Car Smoking Ban | |||
| Yes | 146 (64.6) | 1.28 ± 2.67 | .033 |
| No | 80 (35.4) | 2.03 ± 2.18 | |
Table 1 also shows bivariate comparisons of demographic variables and smoking characteristics by children’s cotinine scores. Overall, the children’s mean baseline cotinine was 1.55ng/ml (range 0.0–21.3). White children in this sample had the highest mean cotinine values, as compared to black children and children of other racial backgrounds (2.44ng/ml, 1.67ng/ml, 0.82ng/ml respectively, p=.019). The mean cotinine values were significantly higher in children of caregivers who smoked (2.82ng/ml) vs. children whose parents did not smoke (0.72ng/ml, p<.001). Children from households with no smoking bans had higher cotinine levels (2.50ng/ml) than children from household with complete smoking bans (0.63ng/ml, p<.001).
The model that minimized the mean square prediction error from our cross-validation procedure included the exposure variables primary caregiver smoking and smoking ban status. This model significantly increased prediction of cotinine level from our base model that just included demographic information (p=.0003). We used this model to create our 2-item index score. We validated the index score in the 30% of the sample that were not included in the original development of the measure. Validation of this model suggested little bias.
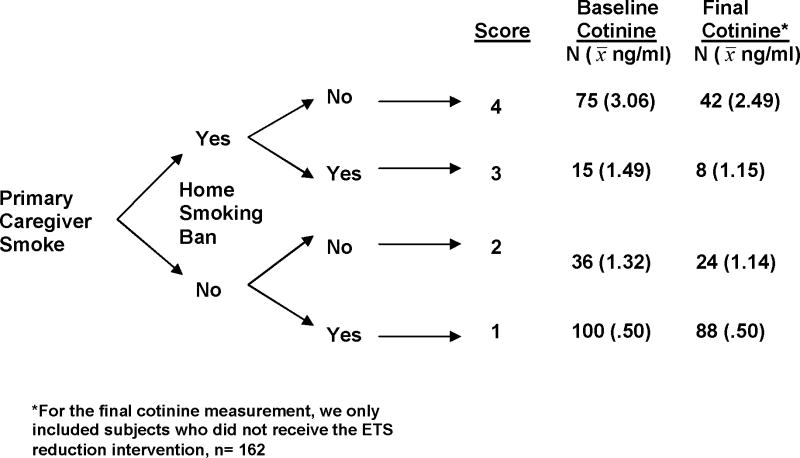
Figure 1 illustrates the distribution of the index score values and its relationship with children’s cotinine measurements. Primary caregiver smoking was given the greatest weight on the algorithm, since it had the lowest mean square prediction error, and smoking ban status was included on the second tier. The index score values range from 1–4. Children whose primary caregiver did not smoke and who had a complete home smoking ban had an index score of 1 (44% of the sample). Children with a non-smoking primary caregiver but no smoking ban in the home received a score of 2 (16%). Children whose primary caregiver smoked and who lived in a home with a complete smoking ban received a 3 (7%). The maximum score (4) was applied to those children with a primary caregiver who smoked and reported no smoking ban in the home (33%).
Figure 1.
Algorithm for the Index Score and Associated Mean Cotinine Values
We found that mean baseline cotinine values increased as children’s scores on the index measure increased. Children with a primary caregiver who smoked who were not protected by a household smoking ban (score of 4) had the highest mean cotinine value (3.06ng/ml), and children with a non-smoking primary caregiver who reported a household smoking ban (score of 1) had the lowest mean cotinine levels (.50ng/ml). Follow-up cotinine values overall were slightly lower than at baseline, due in part to the exclusion of some smoke-exposed children who participated in an ETS reduction program, and the lower levels of exposure often seen during the summer months.23, 24 Despite this, we found that the follow-up cotinine values 7–9 months after the initial assessment increased as children’s scores on the index measure increased. Children with a primary caregiver who smoked and were not protected by a smoking ban at baseline had the highest cotinine values 7–9 months later (2.49ng/ml)
In a multivariate linear regression model estimating baseline cotinine values, the index score was the most significant variable with the largest standardized coefficient (β=.552), even when controlling for demographic characteristics, parent depression and stress (p<.001, model R2=0.37). These findings were substantiated in a similar model with the baseline index score predicting cotinine values 7–9 months following the baseline assessment (p<.001, model R2=0.38).
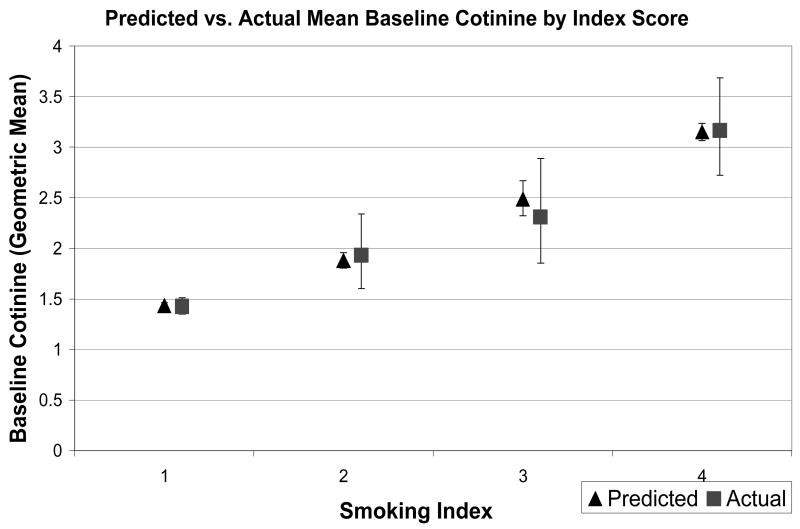
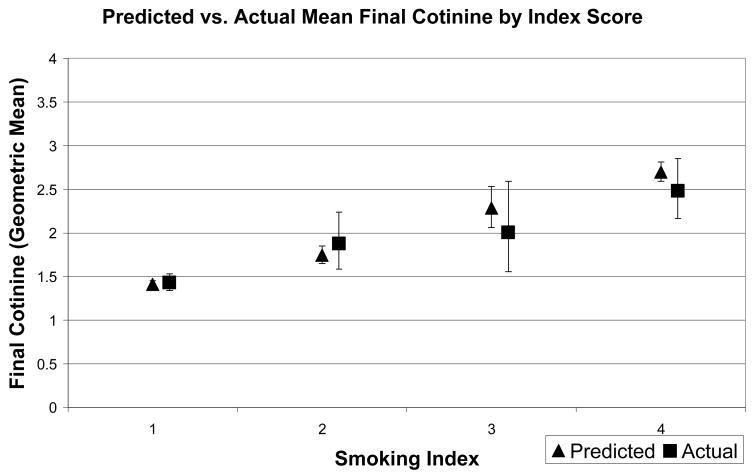
Figure 2 shows the actual cotinine values (geometric means) by index score, with associated 95% confidence intervals, as well as the cotinine values predicted from the multivariate regression model. Predictions are reasonably accurate, demonstrating the same trend of increasing cotinine values as scores on the index measure increase. There was a similar pattern for follow-up cotinine values (Figure 3).
Figure 2.
Predicted and Actual Baseline Cotinine Values by Index Score.
Figure 3.
Predicted and Actual Final Cotinine Values by Baseline Index Score
DISCUSSION
Many children with persistent asthma continue to be exposed to ETS. In this study we found that combined information regarding primary caregiver smoking and household smoking restrictions helps to identify asthmatic children with the greatest exposure to ETS, and can predict those children who will have elevated cotinine levels 7–9 months later. This information could be used to help clinicians estimate risk of exposure among their asthmatic patients and guide their management with two simple questions.
Other studies have similarly shown that questionnaire information can be useful to determine a child’s ETS exposure category.3,16 For example, Groner et al enrolled young children from a primary care office and developed a model based on mother’s smoking status, exposure to others smoking, and whether others smoked inside or outside.16 These questions related to children’s cotinine levels; however children with asthma were excluded from the study. Our study builds on this work by specifically considering a group of children with asthma who are at elevated risk from the deleterious effects of ETS, and by also using the index score to predict future cotinine levels. We found that a similar method of inquiry to parents regarding smoking behaviors is useful among urban children with asthma.
Other research supports our finding of the importance of primary caregiver smoking and home smoking rules as significant contributors to children’s ETS exposure levels. Prior studies have demonstrated that maternal smoking contributes more to a child’s ETS exposure than smoking by others in the household.25–29 Additionally, a study by Wakefield et al19 showed that children living in homes with absolute smoking bans had lower cotinine levels than children from homes with smoking bans to which exceptions were made, and this observation has been substantiated in other studies.3,29 The prevalence of primary caregiver smoking and smoking bans found in this study is consistent with other studies of young children with asthma. In the inner-city asthma study 59% of children lived with a smoker,30 and Berman et al29 found that 47% of smoking caregivers of asthmatic children reported absolute smoking restrictions in the home.
Cotinine is a marker of recent ETS exposure with a half-life of 16–20 hours. Thus cotinine measurements reflect smoke exposure over the past several days. Reported information on past smoking habits has been associated with present cotinine levels in children, suggesting that children’s exposure to nicotine is relatively stable.31 The data presented here demonstrate that parent report of smoking behaviors is strongly associated with children’s cotinine levels 7–9 months later, suggesting that smoke exposure among asthmatic children remains relatively stable over time.
There is no level of smoke exposure that is known to be safe for children, and the clinical significance of small differences in cotinine is not clear. Therefore we considered cotinine as a continuous measure in this study. Current methods allow for detection of cotinine as low as .05ng/ml to quantify ETS exposure. While children with the lowest scores on our index of household smoking behaviors clearly had lower cotinine values compared to children with higher scores, it is important to note that many children in the lowest categories still experienced some exposure to smoke. This suggests the need for ongoing public health efforts around the deleterious effects of ETS exposure, particularly for children with chronic illnesses such as asthma.
Health care provider counseling regarding smoking cessation can be effective in helping smokers quit.32–36 However there is significant room for improvement in provider counseling and intervention regarding smoke exposure.14,37–41 Simple screening methods to identify children at high risk could help providers identify those families in greatest need for intervention. We propose the use of two simple questions in the primary care setting: ‘How much does the child’s primary caregiver smoke’; and ‘what are the rules regarding smoking in this child’s home’; to help identify asthmatic children’s present and future risk for significant smoke exposure. Families with either a primary caregiver who smokes or a lack of complete smoking restrictions in the home should be counseled on both smoking cessation and ETS reduction and referred to services as appropriate. New training programs to improve resident physician’s tobacco intervention skills are now being evaluated,37 and these skills will be particularly useful for families of smoke exposed children with asthma.
There are some potential limitations to this study. First, household smoking information was obtained from parents as part of an intervention study to improve preventive care for asthma. Parents may have been subject to social desirability bias and underreported smoking behaviors. Despite this possibility, we still found strong associations between the index measure and children’s cotinine levels. All of the children in this study had significant asthma, and therefore we were unable to correlate smoke exposure with symptom severity in this sample. We purposely limited the number of factors in the index score to assure simplicity of use for a busy practitioner. While additional information such as household size might have strengthened the association further, we found that the addition of more variables from our dataset did not change the least mean squared estimate significantly. Further, the majority of children included in the study were African-American, and data suggests that cotinine levels in African-American children may be higher than in children from other racial backgrounds with similar exposure to ETS.42 Lastly, this sample included children with persistent asthma living in an urban community, and the results can only be generalized to similar populations.
Implications
Pediatricians have an important role to play in protecting children from ETS exposure. The brief index presented here has potential use to help identify children at risk of exposure, and could serve as a guide for counseling in clinical settings. Consistent screening and counseling regarding smoke exposure likely will help to reduce the burden of smoke-related morbidity for all children and in particular for children with asthma.
Table 2.
Multivariate Regression Model Predicting Baseline and Final Cotinine Values
| Baseline Index Predicting Baseline Cotinine (N=226) | Baseline Index Predicting Final Cotinine (N=162) | |||
|---|---|---|---|---|
| Standardized Beta | P-value | Standardized Beta | P-value | |
| Child’s Age | −.105 | .06 | −.213 | .001 |
| Child’s Race | ||||
| Black | ----- | ----- | ----- | ----- |
| White | .050 | .41 | .050 | .49 |
| Other | −.079 | .24 | .046 | .57 |
| Child Hispanic | −.041 | .54 | −.180 | .03 |
| Parent Education | −.116 | .04 | −.174 | .01 |
| Parenting Stress | .029 | .61 | .036 | .60 |
| Parental Depression | .037 | .52 | −.039 | .59 |
| Smoking Index Score | .552 | <.001 | .530 | <.001 |
Acknowledgments
The research for this article was funded by the National Heart, Lung, and Blood Institute (HL079954) and the Halcyon Hill Foundation.
We thank the School Based Asthma Therapy team for their hard work enrolling subjects for this study, Andrew MacGowan and the Rochester City School District for their partnership and continued support of our work, Aaron Blumkin, MS for his statistical consultation, and Deste Relyea and Nicholas Shippers for their help with manuscript preparation.
Abbreviations
- ETS
Environmental Tobacco Smoke
References
- 1.Mannino DM, Moorman JE, Kingsley B, Rose D, Repace J. Health effects related to environmental tobacco smoke exposure in children in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2001 Jan;155(1):36–41. doi: 10.1001/archpedi.155.1.36. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2006; 2006. [Google Scholar]
- 3.Johansson A, Hermansson G, Ludvigsson J. How should parents protect their children from environmental tobacco-smoke exposure in the home? Pediatrics. 2004 Apr;113(4):e291–295. doi: 10.1542/peds.113.4.e291. [DOI] [PubMed] [Google Scholar]
- 4.Scherer G, Meger-Kossien I, Riedel K, Renner T, Meger M. Assessment of the exposure of children to environmental tobacco smoke (ETS) by different methods. Hum Exp Toxicol. 1999 Apr;18(4):297–301. doi: 10.1191/096032799678840075. [DOI] [PubMed] [Google Scholar]
- 5.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006 Jun;114(6):853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chilmonczyk BA, Salmun LM, Megathlin KN, et al. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. N Engl J Med. 1993 Jun 10;328(23):1665–1669. doi: 10.1056/NEJM199306103282303. [DOI] [PubMed] [Google Scholar]
- 7.Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002 Aug;122(2):409–415. doi: 10.1378/chest.122.2.409. [DOI] [PubMed] [Google Scholar]
- 8.Halterman JS, Fagnano M, Conn KM, Szilagyi PG. Do parents of urban children with persistent asthma ban smoking in their homes and cars? Ambul Pediatr. 2006 Mar-Apr;6(2):115–119. doi: 10.1016/j.ambp.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.National Asthma Education and Prevention Program. Expert Panel Report II: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute; 1997. NIH Publication No. 97–4051. [Google Scholar]
- 10.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 11.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999 May;107(Suppl 2):349–355. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook DG, Whincup PH, Jarvis MJ, Strachan DP, Papacosta O, Bryant A. Passive exposure to tobacco smoke in children aged 5–7 years: individual, family, and community factors. Bmj. 1994 Feb 5;308(6925):384–389. doi: 10.1136/bmj.308.6925.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hymowitz N. A survey of pediatric office-based interventions on smoking. N J Med. 1995 Oct;92(10):657–660. [PubMed] [Google Scholar]
- 14.Zapka JG, Fletcher K, Pbert L, Druker SK, Ockene JK, Chen L. The Perceptions and Practices of Pediatricians: Tobacco Intervention. Pediatrics. 1999;103(5):e65. doi: 10.1542/peds.103.5.e65. [DOI] [PubMed] [Google Scholar]
- 15.Johansson A, Halling A, Hermansson G, Ludvigsson J. Assessment of smoking behaviors in the home and their influence on children’s passive smoking: development of a questionnaire. Ann Epidemiol. 2005 Jul;15(6):453–459. doi: 10.1016/j.annepidem.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Groner JA, Hoshaw-Woodard S, Koren G, Klein J, Castile R. Screening for Children’s Exposure to Environmental Tobacco Smoke in a Pediatric Primary Care Setting. Arch Pediatr Adolesc Med. 2005 May;159:450–455. doi: 10.1001/archpedi.159.5.450. [DOI] [PubMed] [Google Scholar]
- 17.Bernert JT, Jr, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol. 2000 Jul-Aug;24(5):333–339. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 18.McMillen RC, Winickoff JP, Klein JD, Weitzman M. US adult attitudes and practices regarding smoking restrictions and child exposure to environmental tobacco smoke: changes in the social climate from 2000–2001. Pediatrics. 2003 Jul;112(1 Pt 1):e55–60. doi: 10.1542/peds.112.1.e55. [DOI] [PubMed] [Google Scholar]
- 19.Wakefield M, Banham D, Martin J, Ruffin R, McCaul K, Badcock N. Restrictions on smoking at home and urinary cotinine levels among children with asthma. Am J Prev Med. 2000 Oct;19(3):188–192. doi: 10.1016/s0749-3797(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Barker PR, Colpe LJ, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003 Feb;60(2):184–189. doi: 10.1001/archpsyc.60.2.184. [DOI] [PubMed] [Google Scholar]
- 21.Abidin RR. Parenting Stress Index. 3. Odessa, FL: Psychological Assessment Resources, Inc; 1995. [Google Scholar]
- 22.Burman P. A comparitive study of ordinary cross-validation, v-fold cross-validation and the repeated learning-testing methods. Biometrika. 1989 Sept;76(3):503–514. [Google Scholar]
- 23.Chilmonczyk BA, Knight GJ, Palomaki GE, Pulkkinen AJ, Williams J, Haddow JE. Environmental tobacco smoke exposure during infancy. Am J Public Health. 1990 Oct;80(10):1205–1208. doi: 10.2105/ajph.80.10.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ronchetti R, Bonci E, de Castro G, et al. Relationship between cotinine levels, household and personal smoking habit and season in 9–14 year old children. Eur Respir J. 1994 Mar;7(3):472–476. doi: 10.1183/09031936.94.07030472. [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich R, Kattan M, Godbold J, et al. Childhood asthma and passive smoking. Urinary cotinine as a biomarker of exposure. Am Rev Respir Dis. 1992 Mar;145(3):594–599. doi: 10.1164/ajrccm/145.3.594. [DOI] [PubMed] [Google Scholar]
- 26.Infante-Rivard C. Childhood asthma and indoor environmental risk factors. Am J Epidemiol. 1993 Apr 15;137(8):834–844. doi: 10.1093/oxfordjournals.aje.a116745. [DOI] [PubMed] [Google Scholar]
- 27.Martinez FD, Cline M, Burrows B. Increased incidence of asthma in children of smoking mothers. Pediatrics. 1992 Jan;89(1):21–26. [PubMed] [Google Scholar]
- 28.Oddoze C, Dubus JC, Badier M, et al. Urinary cotinine and exposure to parental smoking in a population of children with asthma. Clin Chem. 1999 Apr;45(4):505–509. [PubMed] [Google Scholar]
- 29.Berman BA, Wong GC, Bastani R, et al. Household smoking behavior and ETS exposure among children with asthma in low-income, minority households. Addict Behav. 2003 Jan-Feb;28(1):111–128. doi: 10.1016/s0306-4603(01)00221-0. [DOI] [PubMed] [Google Scholar]
- 30.Kattan M, Mitchell H, Eggleston P, et al. Characteristics of inner-city children with asthma: the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997 Oct;24(4):253–262. doi: 10.1002/(sici)1099-0496(199710)24:4<253::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 31.Willers S, Axmon A, Feyerabend C, Nielsen J, Skarping G, Skerfving S. Assessment of environmental tobacco smoke exposure in children with asthmatic symptoms by questionnaire and cotinine concentrations in plasma, saliva, and urine. J Clin Epidemiol. 2000 Jul;53(7):715–721. doi: 10.1016/s0895-4356(99)00212-7. [DOI] [PubMed] [Google Scholar]
- 32.Wall MA, Severson HH, Andrews JA, Lichtenstein E, Zoref L. Pediatric office-based smoking intervention: impact on maternal smoking and relapse. Pediatrics. 1995 Oct;96(4 Pt 1):622–628. [PubMed] [Google Scholar]
- 33.Fiore MC, Bailey WC, Cohen SJ, et al. Clinical Practice Guideline. US Department of Health and Human Services, Public Health Service; Rockville, MD: 2000. Treating tobacco use and dependence. [Google Scholar]
- 34.Silagy C. Physician advice for smoking cessation. Cochrane Library. 1999 doi: 10.1002/14651858.CD000165. [DOI] [PubMed] [Google Scholar]
- 35.Winickoff JP, Buckley VJ, Palfrey JS, Perrin JM, Rigotti NA. Intervention with parental smokers in an outpatient pediatric clinic using counseling and nicotine replacement. Pediatrics. 2003 Nov;112(5):1127–1133. doi: 10.1542/peds.112.5.1127. [DOI] [PubMed] [Google Scholar]
- 36.Borrelli B, Novak S, Hecht J, Emmons K, Papandonatos G, Abrams D. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community-nurse Assisted Research and Education on Smoking) Prev Med. 2005 Nov-Dec;41(5–6):815–821. doi: 10.1016/j.ypmed.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Hymowitz N, Schwab J, Haddock C, Pyle S, Moore G, Meshberg S. The pediatric resident training on tobacco project: baseline findings from the Parent/Guardian Tobacco Survey. Prev Med. 2005 Jul;41(1):334–341. doi: 10.1016/j.ypmed.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Tanski SE, Klein JD, Winickoff JP, Auinger P, Weitzman M. Tobacco counseling at well-child and tobacco-influenced illness visits: opportunities for improvement. Pediatrics. 2003 Feb;111(2):E162–167. doi: 10.1542/peds.111.2.e162. [DOI] [PubMed] [Google Scholar]
- 39.Winickoff JP, Tanski SE, McMillen RC, Klein JD, Rigotti NA, Weitzman M. Child health care clinicians’ use of medications to help parents quit smoking: a national arent survey. Pediatrics. 2005 Apr;115(4):1013–1017. doi: 10.1542/peds.2004-1372. [DOI] [PubMed] [Google Scholar]
- 40.Winickoff JP, McMillen RC, Carroll BC, et al. Addressing parental smoking in pediatrics and family practice: a national survey of parents. Pediatrics. 2003 Nov;112(5):1146–1151. doi: 10.1542/peds.112.5.1146. [DOI] [PubMed] [Google Scholar]
- 41.Borrelli B, McQuaid EL, Becker B, et al. Motivating parents of kids with asthma to quit smoking: the PAQS project. Health Educ Res. 2002 Oct;17(5):659–669. doi: 10.1093/her/17.5.659. [DOI] [PubMed] [Google Scholar]
- 42.Wilson SE, Kahn RS, Khoury J, Lanphear BP. The role of air nicotine in explaining racial differences in cotinine among tobacco-exposed children. Chest. 2007 Mar;131(3):856–862. doi: 10.1378/chest.06-2123. [DOI] [PubMed] [Google Scholar]