Abstract
Recent advances in molecular imaging have permitted the noninvasive imaging of apoptosis, a critical process underlying the pathogenesis of many diseases of the cardiovascular system including atherosclerotic vascular disease, myocardial ischemia and reperfusion injury, chronic heart failure, myocarditis, and cardiac allograft rejection. Multiple molecular targets including phosphatidylserine, phosphatidylinositol 3-kinase, and caspases have been targeted by a variety of imaging agents and modalities such as nuclear scintigraphy, PET, MRI, and fluorescent and bioluminescent imaging. Translationally, methods utilizing radiolabeled annexin V have proven promising in several clinical trials of ischemia-reperfusion injury and cardiac allograft rejection. New approaches using novel molecular imaging agents show great potential for the ability to image apoptosis in the research and clinical setting. Ultimately the ability to detect apoptosis noninvasively would help to identify patients for emerging anti-apoptotic therapies and guide clinical management with the aim of maximal myocardial preservation.
Keywords: Noninvasive imaging, Molecular imaging, Apoptosis, Cardiovascular, Annexin
Cardiomyocyte apoptosis underlies the pathogenesis of many diseases of the cardiovascular system including atherosclerotic vascular disease, myocardial ischemia and reperfusion injury, chronic heart failure, myocarditis, and cardiac allograft rejection. In the past decade, noninvasive methods to image molecular aspects of apoptosis have grown rapidly and are now offering a wealth of information in both the biological and clinical arenas. In this review, we highlight recent advances in noninvasive molecular imaging of apoptosis in cardiovascular disease.
Molecular imaging
Molecular imaging is an emerging field that utilizes injectable imaging agents or genetically encoded reporters to assay key cells and molecules involved in a biological process of interest. While similar imaging hardware platforms can be utilized (e.g. nuclear scintigraphy, magnetic resonance imaging, optical imaging), information gleaned from molecular imaging studies is complementary to details obtained from structural and/or physiological imaging methods. As a comprehensive review of imaging agent and hardware technology is beyond the scope of this review, the interested reader is referred to several recent articles on molecular imaging in general [1–3], and the cardiovascular system [4–9].
Apoptosis biology
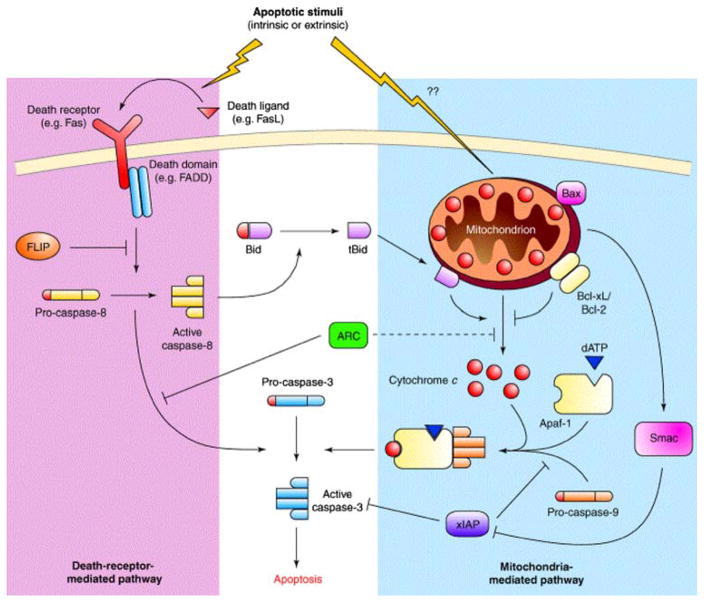
The biology of apoptosis offers a roadmap for the development and discussion of apoptosis-imaging strategies. In contrast to necrosis, apoptosis is a highly regulated, ATP-dependent, noninflammatory process resulting in fragmentation of nuclear material, membrane blebbing, and ultimately cell condensation and phagocytosis by neighboring cells [10]. Two central pathways govern this process: the extrinsic pathway that utilizes cell-surface receptors and the intrinsic pathway that involves the mitochondria and the endoplasmic reticulum (Fig. 1). The extrinsic, or death-receptor pathway, relies on the binding of specific cytokines expressed by other cells, including Fas ligand, tumor necrosis factor alpha, or other factors that bind to specific cellular receptors that result in downstream activation of a group of specialized proteases known as caspases. Caspase activation leads to a cleavage cascade that ultimately results in cellular commitment to apoptosis. In the intrinsic pathway, mitochondria responding to cellular stress, including ischemia, reperfusion injury, radiation, or toxins, release proapoptotic factors into the cell that through cellular signaling results in the activation of an enzyme complex known as the apoptosome [12, 13]. This process ultimately converges with the extrinsic pathway through activation of caspase-3 [14]. Interactions between the intrinsic and extrinsic pathways are well described and can lead to amplification of signals for apoptosis [15].
Fig. 1.
Pathways of apoptosis. Apoptosis in cardiomyocytes can be mediated by mitochondria (blue shaded area), in a pathway initiated by the release of cytochrome c into the cytosol, or through the death-receptor pathway. The net result of both these pathways is to activate caspase-3, the final effector enzyme of apoptosis. Reproduced with permission from Ref. [11]
Molecular imaging agents and targets for direct imaging of apoptotic cells
Externalized phosphatidylserine on the apoptotic cell membrane
Molecular targets present on the cell membrane can be favorable for molecular imaging due to their relative accessibility compared to cytoplasmic or nuclear targets. The vast majority of studies involving noninvasive imaging of apoptosis to date have involved the detection of phosphatidylserine (PS) on the cell membrane (Table 1). PS is a membrane aminophospholipid that is normally expressed only on the inner plasma membrane layer. Under conditions of cell stress, PS is rapidly translocated to the outer leaflet of the plasma membrane and thus becomes readily accessible to imaging agents that enter the interstitium [41]. Physiologically, PS serves as a marker for macrophages to phagocytose and remove apoptotic cells in a coordinated manner [10].
Table 1.
Noninvasive imaging of apoptosis
Several ligands with specific, high-affinity binding to PS have been exploited as agents capable of detecting apoptosis. The most investigated of these agents is the 37 kD protein annexin V, which binds with high affinity to PS in a calcium-dependent manner [16]. Annexin V is widely used for in vitro apoptosis applications, where it is commonly conjugated to a fluorescent label or biotinylated for immunohistochemical detection [42]. For noninvasive imaging, annexin V has been conjugated to radioisotopes (e.g. 99mTc) for SPECT imaging [16, 28], to magnetic nanoparticles and gadolinium-containing liposomes for magnetic resonance imaging [32, 33, 43], to positron emitters for positron emission tomography [29–31], and to fluorescent markers for optical imaging [34, 35].
Recently, a novel PS-binding peptide and possible alternative to annexin V has been described. The C2A domain of synaptotagmin is a 128-amino acid residue peptide that also binds to PS in a highly specific, calcium-dependent manner [44]. Synaptotagmin has been successfully conjugated to magnetic nanoparticles for MRI as well as 99mTc for apoptosis SPECT imaging [36, 37]. The experience with this agent, however, is considerably less than that with annexin, and its safety and efficacy for use is humans has yet to be established. Other novel approaches to targeting PS have employed the use of surface-modified nanoparticles and phage-derived affinity peptides. The surface functionalized nanoparticle CL-45, for instance, has been shown to co-localize strongly with Annexin FITC on apoptotic Jurkat T-cells [45]. The conjugation of Zn(II) di-2-picolylamine (DPA) complexes to magnetic nanoparticles in multivalent fashion produced preferential attachment of the nanoparticle to the cell membranes of apoptotic cells [46].
Novel targets and agents for imaging apoptosis and cell survival
The experience with annexin has been extensive and its safety record in humans is strongly established [18,20, 21]. However, some concerns have been raised regarding the use of annexin for apoptosis imaging. Annexin binds to some necrotic cells and in the milieu of complex injury may reduce its specificity for apoptotic cells. A solution to this problem in the experimental setting has been to use animal models characterized by purely apoptotic cell death or to use a second co-injected probe specific for cardiomyocyte necrosis, such as an anti-myosin or anti-actin agent [28]. It should be noted, however, that while the use of annexin in the setting of complex injury may not allow a mechanistically specific diagnosis of apoptosis to be obtained, the imaging of composite cell death (apoptosis and necrosis) could still provide useful diagnostic and prognostic information. Noninvasive imaging of annexin uptake could allow disease severity and treatment response to be assessed and play an important role in guiding treatment and in the development of novel cardioprotective pharmaceuticals.
There is however significant interest in the development of complementary approaches to PS-based imaging to detect specific components in the apoptotic cascade. Several of the central enzymes in the apoptotic cascade, in particular, are being targeted with novel imaging strategies. Thus while it is likely that annexin-based imaging will remain the central technique in the molecular imaging of apoptosis for the significant future, the role of complementary approaches is also likely to grow. A brief description of some these novel and experimental approaches is provided below.
Probes targeting altered membrane permeability
Another promising cell membrane-based approach to image apoptosis exploits changes in membrane permeability, a common feature of apoptotic and necrotic cells. Recently, a small molecule (MW = 368) amphipathic apoptosis marker that selectively and rapidly accumulates within apoptotic and necrotic cells was demonstrated to effectively image apoptosis in vivo. This molecule contains an 18F atom to permit noninvasive PET imaging [47]. This compound is rapidly cleared from blood and concentrates within damaged cells (as opposed to the cell surface), thus potentially providing superior signal to noise and imaging characteristics compared to annexin V.
PI3 kinase
The enzyme phosphatidylinositol (PI) 3-kinase plays a central role in the regulation of cell survival and death, and when activated appears to exert anti-apoptotic, cardiomyocyte-protective effects [48]. Wortmannin is a cell-permeable, steroid-like compound that binds to and inhibits PI3 kinase at nanomolar concentrations without significant interference to other signal transduction pathways [49], and can be labeled and used as a ligand to image PI3 kinase levels. Radiolabeled and fluorescent conjugates of wortmannin have been developed; however, meticulous conjugation chemistry is needed to avoid inactivation of the compound [50]. While current wortmannin-based agents assay total levels of PI3 kinase, future constructs may be able to differentiate between the phosphorylated and nonphosphorylated forms of the enzyme [50].
Caspases
Caspases are a family of proteases produced as inactive zymogens that when activated are instrumental in the signal transduction of apoptosis, both upstream (initiator caspases) and downstream (effector or executioner caspases) [10]. Under apoptotic conditions, activation of caspases leads to a cascade of caspase activation that ultimately results in cellular apoptosis. Several optical imaging approaches have been developed to image caspase expression and activity in vivo.
In experimental systems, several models have been developed that involve the genetic encoding of reporter agents that are transcribed and activated intracellularly. Most commonly, these agents are detected by the intrinsic fluorescence of the reporter (fluorescence) or the enzymatic activation of an inactive substrate by the reporter in a light-producing reaction (bioluminescence) [51]. For the purposes of apoptosis imaging, these probes can be designed to be produced in an inactive form that is subsequently activated by enzymatic activity of the enzyme of interest. For example, a human glioma cell line expressing an inactivated firefly luciferase reporter that is activated by cleavage by caspase-3 was used to image tumor apoptosis in mice [40]. A recent strategy utilized a cell-surface-targeted single-chain antibody fused to a Golgi retention signal with a sequence recognized by caspase-3; induction of apoptosis causes cleavage of the protein resulting in transport of the antibody from the Golgi to the cell surface where it is detectable by immunofluorescence microscopy [52]. From a research standpoint, these approaches are helpful in further delineating the pathways of apoptosis and identifying agents that promote or attenuate signals for apoptosis. To these ends, one novel approach using a two-fluorescence resonance energy transfer probe was able to explore the timing of caspase-2 and caspase-3 activation within a single living cell [53].
Though many efforts to image caspase rely upon genetically encoded reporters, several techniques using exogenous fluorescent and bioluminescent probes are emerging. One strategy utilizes a polymer conjugated to a near-infrared fluorescence effector caspase-specific peptide to create a biocompatible, cell-permeable, autoquenched nanoparticle to image apoptosis in real time in living cells [54]. Using a caspase-3 and -7 specific luciferase-based reagent, apoptosis was successfully imaged within human adenocarcinoma cells [55]. One approach employs a novel caspase-1 (ICE)-specific, activatable, near-infrared fluorescent probe as a means to image apoptosis in multiple models of apoptosis; this strategy was used to successfully image apoptosis noninvasively in mice [39]. In a combined diagnostic and therapeutic approach, a targeted photodynamic therapy agent with a caspase-3 activated fluorophore was able to induce and then detect apoptosis in tumors in vivo [56].
Caspase activity has also been imaged using exogenous radiolabeled imaging agents. In a series of cell-based experiments, two radioiodinated peptides selective for caspase-3 were found to have increased uptake and retention within apoptotic cells [57]. Further modifications of this probe may yield a viable scintigraphic imaging agent for in vivo use. One successful in vivo strategy employed an 18F-radiolabeled isatin sulfonamide analog that targets activated caspase-3 [38]. This agent was used with micro-PET imaging to visualize cyclohexamide induced hepatic apoptosis in a well-established rat model. Ultimately, the development of biocompatible caspase imaging agents for clinical optical or scintigraphic imaging would be a major advance in apoptosis imaging.
Other targets identified by expression profiling and screening
Extensive efforts have been directed towards detecting altered gene and protein expression related to pathologic conditions affecting the cardiovascular system. Many of these genes play a direct or indirect role in apoptotic pathways. For example, a study using right atrial tissue of patients undergoing cardioplegic arrest with cardiopulmonary bypass, showed activation of upstream and downstream mediators of apoptosis in both the caspase-dependent and caspase-independent pathways [58]. An examination of mRNA expression in human atherosclerotic plaques showed that proapoptotic genes were significantly more expressed in the plaques of acute coronary syndrome patients, whereas anti-apoptotic genes were more transcribed in plaques of stable angina patients [59]. A full review of expression profiling in response to cardiovascular pathology is beyond the scope of this review, but it is worthy to note that many of these genes and gene products may be harnessed in the future as potential targets for molecular imaging agents.
Noninvasive imaging of apoptosis in cardiovascular disease
Atherosclerosis
Atherosclerotic vascular disease is a leading cause of myocardial infarction, heart failure, and death. It is now appreciated that various cells within atheroma such as smooth muscle cells and macrophages undergo apoptosis within unstable atherosclerotic plaques [60]. Loss of smooth muscle cells may promote breakdown of the fibrous cap and thus increase susceptibility to rupture of the fibrous cap. Though apoptosis of macrophages is theoretically beneficial for plaque stability through resolution of inflammation, poor clearance of apoptotic macrophages may lead to accumulation of cellular debris within the lipid-rich core of atherosclerotic plaque, thus in fact contributing to plaque progression and rupture [61, 62].
Molecular imaging of atherosclerosis is an area of intense research, and may offer unique opportunities to assay the in vivo biology underlying acute coronary syndromes, strokes and their sequelae [1, 7, 23]. Several preclinical and clinical apoptosis imaging strategies have emerged recently. One area of extensive investigation utilizes 99mTc-radiolabeled annexin V for SPECT imaging. In experimental models, the imaging agent has visualized apoptosis in both a rabbit balloon injury model of atherosclerosis with apoptotic macrophages [63] as well as a porcine model of coronary atherosclerosis with high levels of smooth muscle cell apoptosis [26]. Using annexin V-enhanced micro-SPECT combined with subsequent micro-CT, murine atheroma models demonstrated excellent correlation between noninvasive and histopathological assessment of macrophage infiltration and the extent of apoptosis [27].
Radiolabeled annexin V has also demonstrated applicability to clinical imaging of apoptosis in carotid atherosclerosis. In a pilot clinical study, 99mTc SPECT imaging demonstrated higher uptake in carotid artery plaques of patients with recent TIA or stroke symptoms compared to patients with remote symptoms. Resected endarterectomy specimens validated this difference, showing significantly greater immunoreactive annexin V staining of histological sections [23]. Ultimately, clinical noninvasive imaging of apoptosis within atherosclerosis might provide not only a risk assessment at a single time point, but also be able to assess the effectiveness of local or systemic therapies that stabilize plaques and reduce coronary risk. To this end, Hartung and colleagues randomized balloon injured rabbits to high cholesterol diet (HCD), HCD for three months followed by one month of standard chow, and HCD for three months followed by HCD with statin treatment. Noninvasive imaging using radiolabeled annexin V demonstrated maximum signal in untreated HCD animals, with significantly less signal in the HCD withdrawal and statin-treated groups [25].
Myocardial ischemia/reperfusion injury
The regenerative capacity of the myocardium is limited and there is thus intense interest in the prevention of cardiomyocyte loss during ischemia and reperfusion [64]. Cell death following myocardial ischemia is primarily mediated through apoptosis during the first 4–6 h of injury [65]. Thereafter, ongoing myocardial ischemia typically results in a central zone of cardiomyocyte necrosis surrounded by an ischemic penumbra where cardiomyocyte apoptosis remains dominant [66]. Reperfusion injury following cardiac ischemia results in a brisk period of cardiomyocyte apoptosis mediated by the intrinsic and extrinsic pathways [67]. Various animal models of ischemia-reperfusion injury mediated apoptosis using animals deficient in critical apoptotic regulatory proteins or overexpressing anti-apoptotic signaling molecules have demonstrated a reduction of infarct size between 48% and 68% [13]. From a clinical standpoint, therapies designed to minimize the extent of apoptosis following ischemia-reperfusion injury are an attractive goal to reduce left ventricular damage and dysfunction after acute coronary syndromes [68].
Noninvasive molecular imaging of apoptosis has permitted further insight into the pathogenesis of experimental myocardial infarction. For example, real-time intravital microscopy has demonstrated binding of fluorescently conjugated annexin V to individual myocytes following ischemia-reperfusion injury [69] This method allows detailed study of the kinetics of apoptosis in the ischemia-reperfusion injury microenvironment and may offer an efficient mode of screening agents for anti-apoptosis properties. Apoptosis imaging has shed light on the spatial and temporal evolution of apoptosis in models of ischemia-reperfusion injury. In a rat model of ischemia-reperfusion injury, autoradiography of resected hearts following 99mTc-radiolabeled annexin V administration revealed that the zone of apoptosis initially begins in the mid-myocardium 30 min after reperfusion, extends into the subendocardium and subepicardium 6 h after reperfusion, and then eventually regresses over 3 days [70].
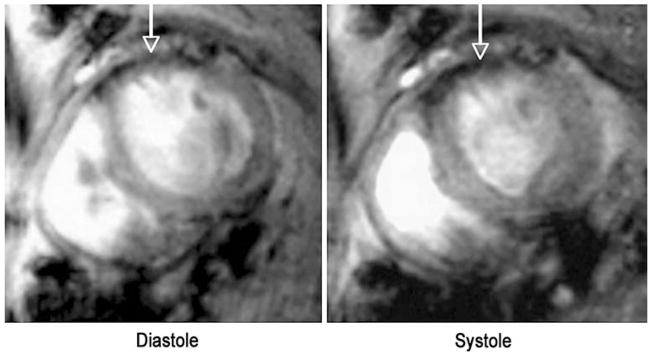
Cardiac MRI allows for high-resolution anatomical and functional images of the myocardium. Molecular MR images can thus be correlated with MR images of myocardial function, contractility, strain, perfusion and viability in a single integrated dataset. In a recent high-resolution, noninvasive MR imaging approach, a novel annexin V-based magnetofluorescent iron oxide nanoparticle was used to quantitatively image myocardial apoptosis in vivo in an ischemia-reperfusion injury mouse model. Cine MRI of the mouse heart allowed the molecular image of cardiomyocyte apoptosis (Fig. 2) to be correlated with global left ventricular function as well as regional myocardial contractility [32]. The distribution of the magnetofluorescent annexin suggested a midmyocardial predominance of the agent, in accordance with prior observations by other investigators [70, 71]. The dual modality nature of the probe allowed the in vivo MRI findings to be confirmed by ex vivo fluorescent imaging [32]. The results of this study show that high-resolution serial quantitative imaging of cardiomyocyte apoptosis can be performed in vivo by MRI. In addition the molecular MR image could be integrated with MR images of myocardial function in a single integrated dataset. Further integration of molecular MR images of cardiomyocyte apoptosis with MR images of myocardial perfusion and viability would be highly feasible and demonstrate the breadth and flexibility of a molecular MR approach to apoptosis imaging. MRI has also been used to image apoptosis in an isolated, perfused rat heart model with the use of a different contrast agent consisting of annexin V linked to gadolinium diethylenetriamine penta-acetate (Gd-DTPA)-coated liposomes [33]. The utility of this agent in vivo, however, remains to be determined.
Fig. 2.
In vivo imaging of cardiomyocyte apoptosis in mice by MRI. Apoptosis was induced by transient coronary ligation, following which reperfusion was established. The annexin V-labeled magnetofluorescent nanoparticle, AnxCLIO-Cy5.5, was injected at 2 mg Fe/kg into the tail vein of the mice upon coronary reperfusion. Accumulation of the probe in the hypokinetic anterolateral wall produced strong negative contrast enhancement (arrows). No evidence of probe accumulation was seen when the mice where injected with the unlabeled CLIO-Cy5.5 probe. Reproduced with permission from Ref. [32]
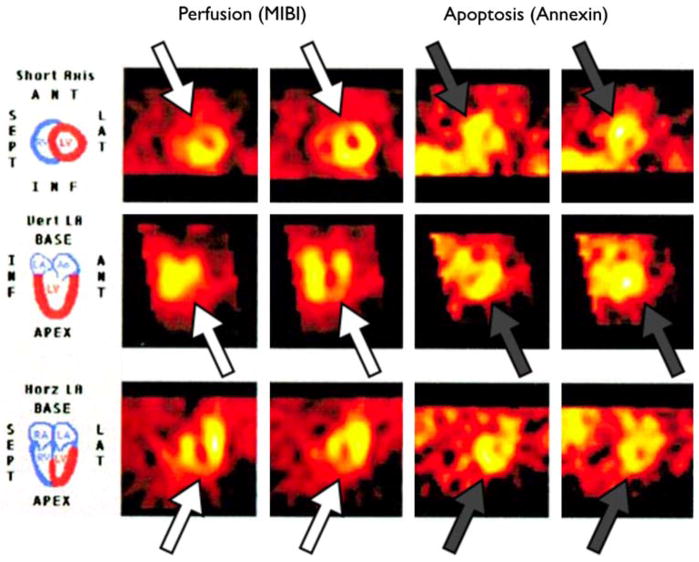
Several scintigraphic imaging agents have demonstrated utility for visualization of apoptosis in clinical studies of myocardial infarction. In a seminal study, seven patients presenting with myocardial infarction treated with percutaneous transluminal coronary angioplasty underwent SPECT imaging of radiolabeled 99mTc annexin V at two time points post-infarction; images showed increased uptake in infarcted areas with a matching perfusion defect [18]. Later, this group employed a modified, higher sensitivity 99mTc annexin V conjugate to image nine patients presenting with acute myocardial infarction [21] (Fig. 3). Again, areas of increased uptake were well defined within the ischemic zone with optimum signal intensity seen at 15 h post-infarction. These types of clinical approaches to noninvasively image the extent of apoptosis resulting from acute coronary syndromes could be an important tool to help guide revascularization strategies, optimize heart failure therapies, and identify patients for emerging anti-apoptotic specific agents. Ultimately these strategies might be used with the aim of preventing left ventricular dysfunction, monitoring ventricular remodeling following injury, and identifying patients at high risk for future cardiac events.
Fig. 3.
Imaging of cardiomyocyte apoptosis in acute myocardial infarction with 99mTc-annexin V. Combination of acute 99mTc-MIBI (left 2 columns) and 99mTc annexin V uptake (right 2 columns) in area at risk on day 1 in a patient presenting with acute MI. 99mTc-MIBI perfusion defects in anteroseptal and apical region (open arrows) correlate well with 99mTc-annexin V activity (grey arrows). Adapted and reproduced with permission from Ref. [21]
Heart failure and myocarditis
In chronic heart failure, the connection between cardiomyocyte apoptosis and cardiomyopathy is complex but emerging. Data suggest that patients with advanced heart failure have higher rates of cardiomyocyte apoptosis than normal subjects, 0.08–0.25% versus 0.001–0.002% [72–74]. Genetic and pharmacological studies from animal models suggest that apoptosis may play a causal role in the development and progression of heart failure [13]. Thus, while cardiomyocyte apoptosis in established heart failure occurs at an extremely low frequency, it is a persistent process, and thereby results in a large loss in the number of functional cardiomyocytes. Imaging very low levels of apoptosis with targeted probes is challenging and may require the use of activatable imaging strategies that are chemically engineered to produce high levels of signal amplification in the presence of their molecular target, e.g. with caspase-activatable molecular probes [4, 39].
Apoptosis however has been imaged in several models of heart failure and cardiomyopathy with higher constitutive levels of apoptosis. One such model utilizes mice genetically engineered to overexpress Gαq, a subunit of the cell-surface receptors involved in promoting cardiac myocyte hypertrophy (α1-adrenergic receptor, angiotensin II type 1 receptor, and endothelin-1 receptor) [75]. In the peripartum period, 30–50% of females develop lethal heart failure with apoptosis in 1–2% of cardiomyocytes and little or no necrosis or inflammation [76]. Using this model, the magnetofluorescent nanoparticle AnxCLIO-Cy5.5 has been used to image apoptosis in vivo in postpartum Gaq overexpressing mice by MRI [32, 77]. The ability to successfully image apoptosis in this model of heart failure shows that AnxCLIO-Cy5.5 crosses an intact capillary membrane, penetrates the interstitium of the myocardium, and detects relatively low levels of apoptosis in vivo.
Imaging of apoptosis has also been performed in diseases inducing acute heart failure. Recently, a rat model of lipopolysaccharide-induced systemic inflammatory response syndrome was used to demonstrate cardiomyocyte apoptosis by uptake of radioiodinated annexin V [78]. In a rat model of subacute catecholamine-induced myocarditis using an inbred strain of Wistar Bonn/Kobori rats that spontaneously develop myocarditis at age 18 months, 99mTc-labeled annexin V showed increased uptake compared to control animals and showed a strong correlation with imuunohistochemical evidence of apoptosis [24]. This finding lays the groundwork for clinical imaging of apoptosis in myocarditis, a technique that would offer great benefit in confirming the diagnosis of myocarditis, determining the extent of involvement, selecting patients for anti-apoptotic therapies, and potentially identifying patients with a heavy disease burden that may benefit from ventricular-assist devices as a bridge to recovery or transplant.
Chemotherapeutic-related cardiotoxicity remains a significant clinical problem, and there is an unmet need to identify susceptible patients [79]. Current clinical approaches utilize serial determinations of left ventricular ejection fraction to identify cardiotoxicity. In a rat model of doxorubicin cardiotoxicity, radiolabeled annexin V was able to detect high levels of cardiomyocyte apoptosis [22]. Ultimately, this approach could serve as a more sensitive early marker of anthracylicne toxicity than studies based on macroscopic left ventricular dysfunction, providing the opportunity to modify or stop administration of the agent before clinically overt heart failure.
Cardiac transplant rejection
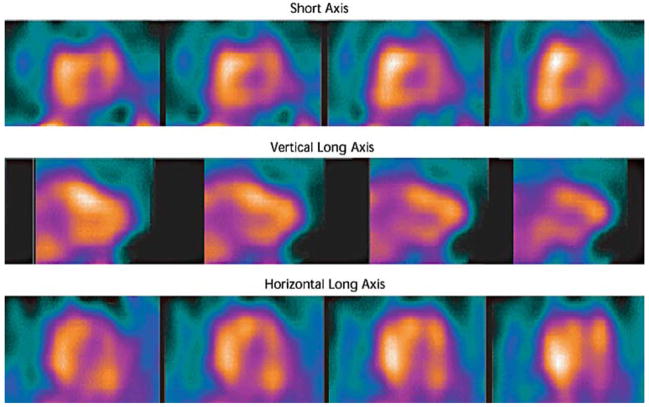
Current monitoring of cardiac allograft rejection relies heavily on endomyocardial biopsy, an invasive procedure with attendant risks. Allograft rejection is characterized by varying degrees of inflammation with associated necrosis and apoptosis. Apoptosis imaging therefore has the potential to noninvasively identify patients with transplant rejection and monitor response to immune modulation therapy. Early efforts with a rat model of cardiac allograft rejection showed increased uptake of 99mTc annexin V that correlated well with histologic evidence of apoptosis associated with a mononuclear inflammatory infiltrate [16]. In a study of 18 cardiac transplant recipients using 99mTc annexin V SPECT for apoptosis imaging, 13 patients with negative scans had no histologic evidence of rejection, whereas 5 patients with positive scans had histologically verified transplant rejection of ISHLT grade 2/4 or higher [20] (Fig. 4). In a separate study of 10 transplant recipients using radiolabeled annexin V, two patients with moderate acute rejection by biopsy were correctly identified by SPECT imaging; however, specificity in this study was suboptimal with half of the patients with grade IA rejection or less having two foci of uptake on imaging [19]. With further refinements, these encouraging clinical efforts may reduce the need for routine surveillance endomyocardial biopsy in transplant recipients.
Fig. 4.
Diffuse myocardial uptake of 99mTc-annexin V in cardiac allograft rejection. SPECT imaging 3 h after intravenous injection of radiolabeled annexin V demonstrated diffuse myocardial uptake of radiotracer, suggesting extensive apoptosis in the myocardium and transplant rejection. Reproduced with permission from Ref. [20]
Future directions
Advances in noninvasive apoptosis imaging have the potential to offer tremendous insight to clinicians. By their nature, apoptotic cells indicate an ongoing pathophysiologic process that is at a tipping point, with the opportunity for cell death to be contained or even reversed outright through the elimination of inciting stressors or, in the future, through therapies that aim to inhibit cellular pathways leading to apoptosis. Apoptosis imaging may prove a valuable tool for identifying vulnerable atherosclerotic plaque with potential for screening of coronary and carotid arteries for risk stratification and treatment. For ischemia-reperfusion injury, apoptosis imaging has demonstrated the ability to identify regions of apoptosis and may eventually be used to guide revascularization strategies and identify patients who may benefit from apoptosis inhibitors to prevent extension of left ventricular damage. Whereas current clinical practice focuses on cardiac biomarkers that reflect cardiomyocyte lysis well after the damage is complete, apoptosis imaging provides a window onto areas with ongoing cellular damage that presages functional and structural impairment. In the setting of chronic heart failure, significant work remains to translate in vitro studies of apoptosis to clinical imaging, but these techniques may become extremely useful in assessing response to heart failure therapies and progression of the disease. Finally, apoptosis imaging may be a viable alternative to current practice of routine endomyocardial biopsy to identify cardiac allograft rejection. While annexin V imaging has proved to be an effective strategy that will no doubt continue to be a highly useful technique, future methods targeting other cell membrane components or intracellular signaling processes such as caspases may further enhance our capabilities to image apoptosis noninvasively.
Acknowledgments
Support Sources: 1. National Institutes of Health, Bethesda, Maryland: (a) NIH K08 HL079984 (DS); (b) NIH UO1-HL080731 (FJ, DS, RW); 2. Donald W. Reynolds Foundation, Las Vegas, Nevada (FJ, RW).
Contributor Information
Ethan Chauncey Korngold, Center for Molecular Imaging Research, Massachusetts General Hospital, Harvard Medical School, 149 13th St., 5th floor, Boston, MA 02129, USA; Cardiology Division, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Farouc Amin Jaffer, Center for Molecular Imaging Research, Massachusetts General Hospital, Harvard Medical School, 149 13th St., 5th floor, Boston, MA 02129, USA; Cardiology Division, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Ralph Weissleder, Center for Molecular Imaging Research, Massachusetts General Hospital, Harvard Medical School, 149 13th St., 5th floor, Boston, MA 02129, USA.
David Edwin Sosnovik, Center for Molecular Imaging Research, Massachusetts General Hospital, Harvard Medical School, 149 13th St., 5th floor, Boston, MA 02129, USA, e-mail: sosnovik@nmr.mgh.harvard.edu; Cardiology Division, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
References
- 1.Jaffer FA, Weissleder R. Molecular imaging in the clinical arena. JAMA. 2005;293(7):855–862. doi: 10.1001/jama.293.7.855. [DOI] [PubMed] [Google Scholar]
- 2.Sosnovik DE, Weissleder R. Emerging concepts in molecular MRI. Curr Opin Biotechnol. 2006 doi: 10.1016/j.copbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Weissleder R. Molecular imaging in cancer. Science. 2006;312(5777):1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 4.Jaffer FA, Weissleder R. Seeing within: molecular imaging of the cardiovascular system. Circ Res. 2004;94(4):433–445. doi: 10.1161/01.RES.0000119321.18573.5A. [DOI] [PubMed] [Google Scholar]
- 5.Narula J, Kietselaer B, Hofstra L. Role of molecular imaging in defining and denying death. J Nucl Cardiol. 2004;11(3):349–357. doi: 10.1016/j.nuclcard.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Chang GY, Xie X, Wu JC. Overview of stem cells and imaging modalities for cardiovascular diseases. J Nucl Cardiol. 2006;13(4):554–569. doi: 10.1016/j.nuclcard.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Jaffer FA, Libby P, Weissleder R. Molecular and cellular imaging of atherosclerosis: emerging applications. J Am Coll Cardiol. 2006;47(7):1328–1338. doi: 10.1016/j.jacc.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Jaffer FA, Sosnovik DE, Nahrendorf M, Weissleder R. Molecular imaging of myocardial infarction. J Mol Cell Cardiol. 2006;41(6):921–933. doi: 10.1016/j.yjmcc.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Wickline SA, Neubauer AM, Winter P, Caruthers S, Lanza G. Applications of nanotechnology to atherosclerosis, thrombosis, and vascular biology. Artherioscler Thromb Vasc Biol. 2006;26(3):435–441. doi: 10.1161/01.ATV.0000201069.47550.8b. [DOI] [PubMed] [Google Scholar]
- 10.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 11.Kang PM, Izumo S. Apoptosis in heart: basic mechanisms and implications in cardiovascular diseases. Trends Mol Med. 2003;9(4):177–182. doi: 10.1016/s1471-4914(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 12.Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112(4):481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 13.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115(3):565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brauer M. In vivo monitoring of apoptosis. Prog Neuro-psychopharmacol Biol Psychiatr. 2003;27(2):323–331. doi: 10.1016/S0278-5846(03)00026-5. [DOI] [PubMed] [Google Scholar]
- 15.Hatano E, Bradham CA, Stark A, Iimuro Y, Lemasters JJ, Brenner DA. The mitochondrial permeability transition augments Fas-induced apoptosis in mouse hepatocytes. J Biol Chem. 2000;275(16):11814–11823. doi: 10.1074/jbc.275.16.11814. [DOI] [PubMed] [Google Scholar]
- 16.Blankenberg FG, Katsikis PD, Tait JF, Davis RE, Naumovski L, Ohtsuki K, Kopiwoda S, Abrams MJ, Darkes M, Robbins RC, Maecker HT, Strauss HW. In vivo detection and imaging of phosphatidylserine expression during programmed cell death. Proc Natl Acad Sci USA. 1998;95(11):6349–6354. doi: 10.1073/pnas.95.11.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blankenberg FG, Katsikis PD, Tait JF, Davis RE, Naumovski L, Ohtsuki K, Kopiwoda S, Abrams MJ, Strauss HW. Imaging of apoptosis (programmed cell death) with 99mTc annexin V. J Nucl Med. 1999;40(1):184–191. [PubMed] [Google Scholar]
- 18.Hofstra L, Liem IH, Dumont EA, Boersma HH, van Heerde WL, Doevendans PA, De Muinck E, Wellens HJ, Kemerink GJ, Reutelingsperger CP, Heidendal GA. Visualisation of cell death in vivo in patients with acute myocardial infarction. Lancet. 2000;356(9225):209–212. doi: 10.1016/s0140-6736(00)02482-x. [DOI] [PubMed] [Google Scholar]
- 19.Kown MH, Strauss HW, Blankenberg FG, Berry GJ, Stafford-Cecil S, Tait JF, Goris ML, Robbins RC. In vivo imaging of acute cardiac rejection in human patients using (99 m)technetium labeled annexin V. Am J Transplant. 2001;1(3):270–277. doi: 10.1034/j.1600-6143.2001.001003270.x. [DOI] [PubMed] [Google Scholar]
- 20.Narula J, Acio ER, Narula N, Samuels LE, Fyfe B, Wood D, Fitzpatrick JM, Raghunath PN, Tomaszewski JE, Kelly C, Steinmetz N, Green A, Tait JF, Leppo J, Blankenberg FG, Jain D, Strauss HW. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med. 2001;7(12):1347–1352. doi: 10.1038/nm1201-1347. [DOI] [PubMed] [Google Scholar]
- 21.Thimister PW, Hofstra L, Liem IH, Boersma HH, Kemerink G, Reutelingsperger CP, Heidendal GA. In vivo detection of cell death in the area at risk in acute myocardial infarction. J Nucl Med. 2003;44(3):391–396. [PubMed] [Google Scholar]
- 22.Bennink RJ, van den Hoff MJ, van Hemert FJ, de Bruin KM, Spijkerboer AL, Vanderheyden JL, Steinmetz N, van Eck-Smit BL. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J Nucl Med. 2004;45(5):842–848. [PubMed] [Google Scholar]
- 23.Kietselaer BL, Reutelingsperger CP, Heidendal GA, Daemen MJ, Mess WH, Hofstra L, Narula J. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med. 2004;350(14):1472–1473. doi: 10.1056/NEJM200404013501425. [DOI] [PubMed] [Google Scholar]
- 24.Peker C, Sarda-Mantel L, Loiseau P, Rouzet F, Nazneen L, Martet G, Vrigneaud JM, Meulemans A, Saumon G, Michel JB, Le Guludec D. Imaging apoptosis with (99m)Tc-annexin-V in experimental subacute myocarditis. J Nucl Med. 2004;45(6):1081–1086. [PubMed] [Google Scholar]
- 25.Hartung D, Sarai M, Petrov A, Kolodgie F, Narula N, Verjans J, Virmani R, Reutelingsperger C, Hofstra L, Narula J. Resolution of apoptosis in atherosclerotic plaque by dietary modification and statin therapy. J Nucl Med. 2005;46(12):2051–2056. [PubMed] [Google Scholar]
- 26.Johnson LL, Schofield L, Donahay T, Narula N, Narula J. 99mTc-annexin V imaging for in vivo detection of atherosclerotic lesions in porcine coronary arteries. J Nucl Med. 2005;46(7):1186–1193. [PubMed] [Google Scholar]
- 27.Isobe S, Tsimikas S, Zhou J, Fujimoto S, Sarai M, Branks MJ, Fujimoto A, Hofstra L, Reutelingsperger CP, Murohara T, Virmani R, Kolodgie FD, Narula N, Petrov A, Narula J. Noninvasive imaging of atherosclerotic lesions in apolipoprotein E-deficient and low-density-lipoprotein receptor-deficient mice with annexin A5. J Nucl Med. 2006;47(9):1497–1505. [PubMed] [Google Scholar]
- 28.Sarda-Mantel L, Michel JB, Rouzet F, Martet G, Louedec L, Vanderheyden JL, Hervatin F, Raguin O, Vrigneaud JM, Khaw BA, Le Guludec D. (99 m)Tc-annexin V and (111)In-antimyosin antibody uptake in experimental myocardial infarction in rats. Eur J Nucl Med Mol Imaging. 2006;33(3):239–245. doi: 10.1007/s00259-005-1900-2. [DOI] [PubMed] [Google Scholar]
- 29.Grierson JR, Yagle KJ, Eary JF, Tait JF, Gibson DF, Lewellen B, Link JM, Krohn KA. Production of [F-18]fluoroannexin for imaging apoptosis with PET. Bioconjug Chem. 2004;15(2):373–379. doi: 10.1021/bc0300394. [DOI] [PubMed] [Google Scholar]
- 30.Keen HG, Dekker BA, Disley L, Hastings D, Lyons S, Reader AJ, Ottewell P, Watson A, Zweit J. Imaging apoptosis in vivo using 124I-annexin V and PET. Nucl Med Biol. 2005;32(4):395–402. doi: 10.1016/j.nucmedbio.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Cauchon N, Langlois R, Rousseau JA, Tessier G, Cadorette J, Lecomte R, Hunting DJ, Pavan RA, Zeisler SK, van Lier JE. PET imaging of apoptosis with (64)Cu-labeled streptavidin following pretargeting of phosphatidylserine with biotinylated annexin-V. Eur J Nucl Med Mol Imaging. 2006 doi: 10.1007/s00259-006-0199-y. [DOI] [PubMed] [Google Scholar]
- 32.Sosnovik DE, Schellenberger EA, Nahrendorf M, Novikov MS, Matsui T, Dai G, Reynolds F, Grazette L, Rosenzweig A, Weissleder R, Josephson L. Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto-optical nanoparticle. Magn Reson Med. 2005;54(3):718–724. doi: 10.1002/mrm.20617. [DOI] [PubMed] [Google Scholar]
- 33.Hiller KH, Waller C, Nahrendorf M, Bauer WR, Jakob PM. Assessment of cardiovascular apoptosis in the isolated rat heart by magnetic resonance molecular imaging. Mol Imaging. 2006;5(2):115–121. [PubMed] [Google Scholar]
- 34.Petrovsky A, Schellenberger E, Josephson L, Weissleder R, Bogdanov A., Jr Near-infrared fluorescent imaging of tumor apoptosis. Cancer Res. 2003;63(8):1936–1942. [PubMed] [Google Scholar]
- 35.Ntziachristos V, Schellenberger EA, Ripoll J, Yessayan D, Graves E, Bogdanov A, Jr, Josephson L, Weissleder R. Visualization of antitumor treatment by means of fluorescence molecular tomography with an annexin V-Cy5.5 conjugate. Proc Natl Acad Sci USA. 2004;101(33):12294–12299. doi: 10.1073/pnas.0401137101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao M, Zhu X, Ji S, Zhou J, Ozker KS, Fang W, Molthen RC, Hellman RS. 99mTc-labeled C2A domain of synaptotagmin I as a target-specific molecular probe for noninvasive imaging of acute myocardial infarction. J Nucl Med. 2006;47(8):1367–1374. [PubMed] [Google Scholar]
- 37.Zhao M, Beauregard DA, Loizou L, Davletov B, Brindle KM. Non-invasive detection of apoptosis using magnetic resonance imaging and a targeted contrast agent. Nat Med. 2001;7(11):1241–1244. doi: 10.1038/nm1101-1241. [DOI] [PubMed] [Google Scholar]
- 38.Zhou D, Chu W, Rothfuss J, Zeng C, Xu J, Jones L, Welch MJ, Mach RH. Synthesis, radiolabeling, and in vivo evaluation of an 18F-labeled isatin analog for imaging caspase-3 activation in apoptosis. Bioorg Med Chem Lett. 2006;16(19):5041–5046. doi: 10.1016/j.bmcl.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 39.Messerli SM, Prabhakar S, Tang Y, Shah K, Cortes ML, Murthy V, Weissleder R, Breakefield XO, Tung CH. A novel method for imaging apoptosis using a caspase-1 near-infrared fluorescent probe. Neoplasia. 2004;6(2):95–105. doi: 10.1593/neo.03214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laxman B, Hall DE, Bhojani MS, Hamstra DA, Chenevert TL, Ross BD, Rehemtulla A. Noninvasive real-time imaging of apoptosis. Proc Natl Acad Sci USA. 2002;99(26):16551–16555. doi: 10.1073/pnas.252644499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149(12):4029–4035. [PubMed] [Google Scholar]
- 42.van Heerde WL, Robert-Offerman S, Dumont E, Hofstra L, Doevendans PA, Smits JF, Daemen MJ, Reutelingsperger CP. Markers of apoptosis in cardiovascular tissues: focus on Annexin V. Cardiovasc Res. 2000;45(3):549–559. doi: 10.1016/s0008-6363(99)00396-x. [DOI] [PubMed] [Google Scholar]
- 43.Schellenberger EA, Sosnovik D, Weissleder R, Josephson L. Magneto/optical annexin V, a multimodal protein. Bioconjug Chem. 2004;15(5):1062–1067. doi: 10.1021/bc049905i. [DOI] [PubMed] [Google Scholar]
- 44.Davletov BA, Sudhof TC. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J Biol Chem. 1993;268(35):26386–26390. [PubMed] [Google Scholar]
- 45.Schellenberger EA, Reynolds F, Weissleder R, Josephson L. Surface-functionalized nanoparticle library yields probes for apoptotic cells. Chembiochem. 2004;5(3):275–279. doi: 10.1002/cbic.200300713. [DOI] [PubMed] [Google Scholar]
- 46.Quinti L, Weissleder R, Tung CH. A fluorescent nano-sensor for apoptotic cells. Nano Lett. 2006;6(3):488–490. doi: 10.1021/nl0524694. [DOI] [PubMed] [Google Scholar]
- 47.Aloya R, Shirvan A, Grimberg H, Reshef A, Levin G, Kidron D, Cohen A, Ziv I. Molecular imaging of cell death in vivo by a novel small molecule probe. Apoptosis. 2006;11(12):2089–2101. doi: 10.1007/s10495-006-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101(6):660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16(4):1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan H, Luo J, Field S, Weissleder R, Cantley L, Josephson L. Synthesis and activity of C11-modified wortmannin probes for PI3 kinase. Bioconjug Chem. 2005;16(3):669–675. doi: 10.1021/bc049714f. [DOI] [PubMed] [Google Scholar]
- 51.Gross S, Piwnica-Worms D. Spying on cancer: molecular imaging in vivo with genetically encoded reporters. Cancer Cell. 2005;7(1):5–15. doi: 10.1016/j.ccr.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Bhojani MS, Hamstra DA, Chang DC, Coppola JM, Khan AP, Reddy GR, Ross BD, Rehemtulla A. Imaging of proteolytic activity using a conditional cell surface receptor. Mol Imaging. 2006;5(2):129–137. [PubMed] [Google Scholar]
- 53.Lin J, Zhang Z, Yang J, Zeng S, Liu BF, Luo Q. Real-time detection of caspase-2 activation in a single living HeLa cell during cisplatin-induced apoptosis. J Biomed Opt. 2006;11(4):049801. doi: 10.1117/1.2187013. [DOI] [PubMed] [Google Scholar]
- 54.Kim K, Lee M, Park H, Kim JH, Kim S, Chung H, Choi K, Kim IS, Seong BL, Kwon IC. Cell-permeable and biocompatible polymeric nanoparticles for apoptosis imaging. J Am Chem Soc. 2006;128(11):3490–3491. doi: 10.1021/ja057712f. [DOI] [PubMed] [Google Scholar]
- 55.Liu JJ, Wang W, Dicker DT, El-Deiry WS. Bioluminescent imaging of TRAIL-induced apoptosis through detection of caspase activation following cleavage of DEVD-aminoluciferin. Cancer Biol Ther. 2005;4(8):885–892. doi: 10.4161/cbt.4.8.2133. [DOI] [PubMed] [Google Scholar]
- 56.Stefflova K, Chen J, Li H, Zheng G. Targeted photodynamic therapy agent with a built-in apoptosis sensor for in vivo near-infrared imaging of tumor apoptosis triggered by its photosensitization in situ. Mol Imaging. 2006;5(4):520–532. [PubMed] [Google Scholar]
- 57.Bauer C, Bauder-Wuest U, Mier W, Haberkorn U, Eisenhut M. 131I-labeled peptides as caspase substrates for apoptosis imaging. J Nucl Med. 2005;46(6):1066–1074. [PubMed] [Google Scholar]
- 58.Ramlawi B, Feng J, Mieno S, Szabo C, Zsengeller Z, Clements R, Sodha N, Boodhwani M, Bianchi C, Sellke FW. Indices of apoptosis activation after blood cardioplegia and cardiopulmonary bypass. Circulation. 2006;114(1 Suppl):I257–I263. doi: 10.1161/CIRCULATIONAHA.105.000828. [DOI] [PubMed] [Google Scholar]
- 59.Rossi ML, Marziliano N, Merlini PA, Bramucci E, Canosi U, Belli G, Parenti DZ, Mannucci PM, Ardissino D. Different quantitative apoptotic traits in coronary atherosclerotic plaques from patients with stable angina pectoris and acute coronary syndromes. Circulation. 2004;110(13):1767–1773. doi: 10.1161/01.CIR.0000142865.04816.89. [DOI] [PubMed] [Google Scholar]
- 60.Kockx MM, Knaapen MW. Pathological changes in the coronary arteries in the acute coronary syndromes. Heart. 2006;92(11):1557–1558. doi: 10.1136/hrt.2005.086942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bjorkerud S, Bjorkerud B. Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am J Pathol. 1996;149(2):367–380. [PMC free article] [PubMed] [Google Scholar]
- 62.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 63.Kolodgie FD, Petrov A, Virmani R, Narula N, Verjans JW, Weber DK, Hartung D, Steinmetz N, Vanderheyden JL, Vannan MA, Gold HK, Reutelingsperger CP, Hofstra L, Narula J. Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V: a technique with potential for noninvasive imaging of vulnerable plaque. Circulation. 2003;108(25):3134–3139. doi: 10.1161/01.CIR.0000105761.00573.50. [DOI] [PubMed] [Google Scholar]
- 64.Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95(2):125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 65.Anversa P, Cheng W, Liu Y, Leri A, Redaelli G, Kajstura J. Apoptosis and myocardial infarction. Basic Res Cardiol. 1998;93(Suppl 3):8–12. doi: 10.1007/s003950050195. [DOI] [PubMed] [Google Scholar]
- 66.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1996;28(9):2005–2016. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 67.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94(4):1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garg S, Hofstra L, Reutelingsperger C, Narula J. Apoptosis as a therapeutic target in acutely ischemic myocardium. Curr Opin Cardiol. 2003;18(5):372–377. doi: 10.1097/00001573-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 69.Dumont EA, Reutelingsperger CP, Smits JF, Daemen MJ, Doevendans PA, Wellens HJ, Hofstra L. Real-time imaging of apoptotic cell-membrane changes at the single-cell level in the beating murine heart. Nat Med. 2001;7(12):1352–1355. doi: 10.1038/nm1201-1352. [DOI] [PubMed] [Google Scholar]
- 70.Taki J, Higuchi T, Kawashima A, Tait JF, Kinuya S, Muramori A, Matsunari I, Nakajima K, Tonami N, Strauss HW. Detection of cardiomyocyte death in a rat model of ischemia and reperfusion using 99mTc-labeled annexin V. J Nucl Med. 2004;45(9):1536–1541. [PubMed] [Google Scholar]
- 71.Dumont EA, Hofstra L, van Heerde WL, van den Eijnde S, Doevendans PA, DeMuinck E, Daemen MA, Smits JF, Frederik P, Wellens HJ, Daemen MJ, Reutelingsperger CP. Cardiomyocyte death induced by myocardial ischemia and reperfusion: measurement with recombinant human annexin-V in a mouse model. Circulation. 2000;102(13):1564–1568. doi: 10.1161/01.cir.102.13.1564. [DOI] [PubMed] [Google Scholar]
- 72.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336(16):1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 73.Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA, Kajstura J, Anversa P. Myocyte death in the failing human heart is gender dependent. Circ Res. 1999;85(9):856–866. doi: 10.1161/01.res.85.9.856. [DOI] [PubMed] [Google Scholar]
- 74.Saraste A, Pulkki K, Kallajoki M, Heikkila P, Laine P, Mattila S, Nieminen MS, Parvinen M, Voipio-Pulkki LM. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur J Clin Invest. 1999;29(5):380–386. doi: 10.1046/j.1365-2362.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 75.Hayakawa Y, Chandra M, Miao W, Shirani J, Brown JH, Dorn GW, 2nd, Armstrong RC, Kitsis RN. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation. 2003;108(24):3036–3041. doi: 10.1161/01.CIR.0000101920.72665.58. [DOI] [PubMed] [Google Scholar]
- 76.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW., 2nd Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA. 1998;95(17):10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sosnovik D, Nahrendorf M, Grazette L, Reynolds F, Rosenzweig A, Weissleder R, Josephson L. Molecular imaging of low levels of cardiomyocyte apoptosis with a targeted magneto-fluorescent contrast agent. In: American Heart Association Scientific Sessions Series. Molecular imaging of low levels of cardiomyocyte apoptosis with a targeted magneto-fluorescent contrast agent 2005 [Google Scholar]
- 78.Petillot P, Lahorte C, Bonanno E, Signore A, Lancel S, Marchetti P, Vallet B, Slegers G, Neviere R. Annexin V detection of lipopolysaccharide-induced cardiac apoptosis. Shock. 2007;27(1):69–74. doi: 10.1097/01.shk.0000235085.56100.38. [DOI] [PubMed] [Google Scholar]
- 79.Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, Durand JB, Gibbs H, Zafarmand AA, Ewer MS. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109(25):3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]