Abstract
Background and purpose:
All-trans retinoic acid (ATRA) induces complete remission in a majority of acute promyelocytic leukaemia patients, but resistance of leukaemic cells to ATRA and its toxicity, such as hypercalcaemia, lead to a limitation of treatment. Therefore, combination therapies with differentiation-enhancing agents at non-toxic concentrations of ATRA may overcome its side effects. Here, we investigated the effect of plant-derived sesquiterpene lactone compounds and their underlying mechanisms in ATRA-induced differentiation of human leukaemia HL-60 cells.
Experimental approach:
HL-60 cells were treated with four sesquiterpene lactones (helenalin, costunolide, parthenolide and sclareolide) and cell differentiation was determined by NBT reduction, Giemsa and cytofluorometric analyses. Signalling pathways were assessed by western blotting, gel-shift assay and kinase activity determinations and intracellular calcium levels were determined using a calcium-specific fluorescent probe.
Key results:
Helenalin, costunolide and parthenolide, but not sclareolide, increased ATRA-induced HL-60 cell differentiation into a granulocytic lineage. Signalling kinases PKC and ERK were involved in the ATRA-induced differentiation enhanced by all of the effective sesquiterpene lactones, but JNK and PI3-K were involved in the ATRA-induced differentiation enhanced by costunolide and parthenolide. Enhancement of cell differentiation closely correlated with inhibition of NF-κB DNA-binding activity by all three effective compounds. Importantly, enhancement of differentiation induced by 50 nM ATRA by the sesquiterpene lactones was not accompanied by elevation of basal intracellular calcium concentrations.
Conclusions and implications:
These results indicate that plant-derived sesquiterpene lactones may enhance ATRA-mediated cell differentiation through distinct pathways.
Keywords: sesquiterpene lactones, all-trans retinoic acid, leukaemia, differentiation, NF-κB, intracellular calcium
Introduction
Most cancer cells exhibit a defect in their capacity to mature into non-replicating adult cells, thereby persisting in a highly proliferative state and thus outgrowing their normal cellular counterparts. The induction of terminal differentiation represents an alternative approach to the treatment of cancer by conventional antineoplastic agents, as cells exposed to chemical or biological inducers of differentiation do not undergo the cytodestruction produced by cytotoxic agents. Instead they acquire the phenotypic characteristics of end-stage adult cell forms with no replicative capacity and ultimately enter pathways to programmed cell death. Leukaemia cells can be induced to undergo terminal differentiation by a variety of chemical and biological agents, indicating that the malignant state is not irreversible. Certain cancers may be treated with agents that induce terminal differentiation, presumably with less morbidity than that produced by cytodestructive agents (Beere and Hickman, 1993).
Human myeloid leukaemia HL-60 cells are differentiated into cells of monocytic lineage or granulocytic lineage when treated with 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] or all-trans retinoic acid (ATRA), respectively (Breitman et al., 1980; Tanaka et al., 1983). Cultures of HL-60 cells have been employed as excellent model systems for studying cellular differentiation in vitro.
ATRA is structurally related to vitamin A and exerts different effects such as inhibition of proliferation, induction of differentiation and immuno-modulation. ATRA-induced cell differentiation was associated with the increased levels of PKC, MAPK and PI3-K (Wu et al., 1989; Yen et al., 1998; Bertagnolo et al., 1999). ATRA has been used as a therapeutic agent for acute promyelocytic leukaemia (APL) (Huang et al., 1988). ATRA effects at the level of gene transcription are mediated through nuclear retinoic acid receptors and rexinoid receptors. In addition to inducing transcription, these receptors also affect the activity of other transcription factors (Hughes et al., 2006). Even though complete remission by use of ATRA has been reported, several side effects including hypercalcaemia have been documented (Bennett et al., 2005). During the last decade, the optimization of the combination of ATRA and chemotherapy has been suggested to overcome the side-effects (Lengfelder et al., 2005).
Several sesquiterpene lactone compounds have received considerable attention in pharmacological research because of their potent anti-inflammatory activity (Mori et al., 1994; Hehner et al., 1999). Cytostatic and cytocidal effects of sesquiterpenes against tumour cells have also been reported (Ross et al., 1999). Helenalin, which can be isolated from several plant species of the Asteraceae family, is a potent anti-inflammatory and antineoplastic agent in vitro as well as in vivo and can inhibit human neutrophil migration and chemotaxis (Schmidt, 1997). Costunolide was isolated from many species such as Saussurea lappa and Magnolia grandiflora (el-Feraly and Chan, 1978). It exerts an anti-inflammatory and antiproliferation activity by inhibiting the DNA-binding activity of NF-κB as well as antibacterial and antifungal activity (Castro et al., 2000; Kim et al., 2002). Parthenolide, a major sesquiterpene lactone in Tanacetum parthenium, was demonstrated to have a significant anti-inflammatory effect associated with inhibition of the DNA-binding activity of NF-κB (Hehner et al., 1999).
Recently, we demonstrated that costunolide and parthenolide enhanced HL-60 cell differentiation (Kang et al., 2002; Kim et al., 2002). Moreover, helenalin by itself induced HL-60 cell differentiation (Kim et al., 2005). In this study, we have investigated a possible enhancement of ATRA-induced differentiation by four sesquiterpene lactone compounds (helenalin, costunolide, parthenolide and sclareolide) in the HL-60 leukaemia cell system, along with the effects of the combination treatments on the intracellular calcium levels. We also compared the mechanisms by which these different sesquiterpene lactones enhanced ATRA-induced differentiation of HL-60 cells.
Materials and methods
Determination of cell viability and proliferation
The HL-60 cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (Gibco BRL, Grand Island, NY, USA). Cell viability was determined by the trypan blue exclusion assay as described earlier (Coligan et al, 1995). Viability was calculated as the percentage of live cells in the total cell population. Cell proliferation was determined with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium (MTT) assay. In brief, after each treatment, 10 μL of MTT (5 mg mL−1) was added to each well in 96-well plates. After incubation for 4 h at 37 °C, the formazan crystals formed in viable cells were dissolved with 100 μL of dimethylsulphoxide (DMSO). The absorbance of each well was then read at 540 nm using a kinetic microplate reader.
Determination of cell differentiation
HL-60 cell differentiation was assessed by the nitroblue tetrazolium (NBT) reduction assay as described earlier (Collins et al., 1979). This assay is based on the ability of phagocytic cells to produce superoxide upon stimulation with phorbol 12-myristate 13-acetate (PMA). For this assay, 2 × 105 cells were harvested by centrifugation and incubated with an equal volume of 1% NBT dissolved in PBS containing 200 ng mL−1 freshly diluted PMA at 37 °C, for 30 min in the dark. Cytospin slides were prepared and were examined for blue–black nitroblue diformazan deposits, indicative of a PMA-stimulated respiratory burst. At least 200 cells were assessed for each experiment.
Morphological studies
Single-cell suspensions were prepared and 2 × 105 cells were loaded into a cyto-funnel and centrifuged at 27 g in a cytospin centrifuge. The slides were fixed with methanol and dried. The slides were stained with Giemsa staining solution for 20 min and rinsed in deionized water, air-dried and observed under a microscope with a camera. The stained cells were assessed for size, regularity of the cell margin and morphological characteristics of the nuclei.
Immunofluorescent staining and cytofluorometric measurements
Quantitative immunofluorescence measurements were performed in an Epic XL flow cytofluorograph (Coulter Electronics, Hialeah, FL, USA) equipped with a multi-parameter data acquisition and display system. Briefly, a single-cell suspension was collected from the various cultures and washed twice with ice-cold PBS (pH 7.4). Afterwards, phytoerythrin (PE)-conjugated anti-human CD11b or fluorescein isothiocyanate-conjugated anti-human CD14 monoclonal antibodies (Becton Dickinson, San Jose, CA, USA) were added, followed by incubation for 1h at 4 °C. After incubation, the cells were washed with PBS and were fixed in PBS containing 1% paraformaldehyde, and cytofluorometric analysis was performed. Background staining was determined by staining the cells with PE- or fluorescein isothiocyanate (FITC)-conjugated isotype control monoclonal antibodies. One parameter fluorescence histograms were generated by analysing at least 1 × 104 cells.
Cell cycle analysis
Cells were harvested by centrifugation and washed twice with ice-cold PBS (pH 7.4). Cells were fixed in ice-cold 70% ethanol and frozen at −20 °C. Next, cells were washed two times with PBS and resuspended in 1 mL of DNA-staining solution (50 μg mL−1 propidium iodide and 100 μg mL−1 RNase A in PBS) for 30 min. The cells were analysed using a FACSCalibur flow cytometer (BD Bioscience, San Jose, CA, USA). Cell cycle distribution was determined by a ModFit LT computer program (Verity Software House, Topsham, ME, USA).
Preparation of cell lysates and western blot analysis
Cells were lysed in lysis buffer (50 mM Tris buffer, pH 7.5 containing 100 mM NaCl, 1% Nonidet P-40, 10% glycerol, 1 mM EDTA, 1 mM NaF, 1 mM sodium orthovanadate, 50 μg mL−1 leupeptin, 50 μg mL−1 aprotinin and 50 μg mL−1 phenylmethylsulphonyl fluoride by incubation on ice for 30 min. Lysates were then centrifuged at 16 650 g at 4 °C for 10 min. The proteins (15 μg) of the supernatants were separated using a 10% SDS-PAGE and transferred to the nitrocellulose membrane. The blots were probed with rabbit anti-human protein kinase C (PKC) isoforms, mouse antiphosphorylated extracellular signal-regulated kinase, rabbit anti-ERK2, mouse anti-phosphorylated c-Jun N-terminal kinase, rabbit anti-JNK or rabbit anti-phosphatidylinositol 3-kinase (PI3-K) subunit p85 antibodies, washed and exposed to horseradish peroxidase-conjugated anti-mouse IgG2a or rabbit IgG antibodies. Immunoreactive bands were visualized by the enhanced chemiluminescence system (Amersham, Buckinghamshire, UK).
Protein kinase C activity assay
HL-60 cells were lysed in lysis buffer containing 50 mM Tris (pH 7.5), 2 mM EDTA, 1 mM EGTA, 1% Triton X-100, 150 mM NaCl, 1 μM dithiothreitol, 1 mM phenylmethylsulphonyl fluoride, 50 mM NaF, 1 mM sodium orthovanadate, 50 μg mL−1 leupeptin and 50 μg mL−1 aprotinin by incubation on ice for 30 min. Lysates were then centrifuged at 19 350 g at 4 °C, for 20 min. The proteins (200 μg) of the supernatants were incubated with PKC antibody at 4 °C for 2 h. After protein A was added, the mixture was shaken at 4 °C, for 1 h and washed with lysis buffer. The antibody-coupled proteins were centrifuged at 2500 g for 1 min and reacted with 5 μg myelin basic protein and 0.5 μL [32P]-ATP (2.5 μCi) in reaction buffer (0.5 mM EGTA, 10 mM MgCl2, 20 mM HEPES (pH 7.4), 50 mM ATP, 2 mM dithiothreitol, 2 mM NaF and 2 mM sodium orthovanadate) at room temperature, for 30 min. The reaction mixture was analysed by electrophoresis on a 15% SDS-PAGE. Radioactive bands were visualized by an X-ray film and the intensities were assessed by densitometric analysis.
Electrophoretic mobility shift assay
Nuclear extracts were prepared from the cells, as described earlier (Kim et al., 2006). A double-stranded oligonucleotide containing an NF-κB-binding site within the Igκ-chain (5′ CCGGTTAACAGAGGGGGCTTTCCGAG 3′) was utilized as a probe. The 32P-labelled oligonucleotide (10 000 c.p.m.) was incubated for 30 min at room temperature, along with nuclear extracts (10 μg protein), in 20 μL of binding buffer (10 mM Tris-HCl (pH 7.6), 500 mM KCl, 10 mM EDTA, 50% glycerol, 100 ng of poly dI-dC and 1 mM dithiothreitol). The reaction mixture was then analysed by electrophoresis on a 4% polyacrylamide gel in 0.5 × Tris-borate EDTA buffer (45 mM Tris-borate, 1 mM EDTA, pH 8.0). Specific binding was verified by competition experiments with a 50-fold excess of unlabelled ‘self' oligonucleotide or the unrelated cAMP response element-containing oligonucleotide. For super-shift assay, 5 μg of nuclear extracts were incubated with antibodies against p50, p52, c-Rel or p65 subunit of NF-κB for 30 min at room temperature before the complex was analysed by EMSA.
Determination of basal [Ca2+]i
Basal [Ca2+]i in HL-60 cells was measured using calcium-specific fluorescent probe Fluo-4 as previously described (Wang et al., 2005). Cells were collected and washed with Hank's buffered salt solution containing 10 mM HEPES (pH 7.4) and 1 mg mL−1 BSA (buffer A). Cells were then loaded with 2 μM Fluo-4 (TEF labs, Austin, TX, USA) at 37°C for 30 min in buffer A containing 2.5 mM probenecid (buffer B). Parallel samples were incubated under the same conditions in the absence of Fluo-4 and used for measuring non-specific cell autofluorescence. Following incubations, cells were washed twice with buffer B and resuspended at 1 × 106 cells per mL−1 in the same buffer. Triplicate aliquots of 2 × 105 cells (200 μL) were then transferred to a black-walled 96-well plate. The intensities of Fluo-4 fluorescence and auto-fluorescence were measured at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using a Spectrafluor Plus multi-well luminometer/fluorimeter (Tecan, Maennedorf, Switzerland). The measurements were made every 20 s for a period of 90 s. Averaged auto-fluorescence data collected from triplicate wells were then subtracted from corresponding averaged Fluo-4 fluorescence data at each time point. The resulting fluorescence values of all time points of each 90 s scan were integrated and used as the relative measure of basal [Ca2+]i.
Statistical analysis
The Student's t-test or one-way analysis of variance followed by the Bonferroni post-test were used to determine the statistical significance of differences between values for various experimental and control groups. A P-value of <0.05 was considered as significant.
Materials
Helenalin and SP 600125 were from Calbiochem (San Diego, CA, USA). Costunolide was from Wako Pure Chemical Ind. (Osaka, Japan). Sclareolide was from Aldrich Chemical Co (St Louis, MO, USA). Bisindolylmaleimide (GF 109203X), H 7 and PD 98059 were purchased from Tocris Cookson (UK). Parthenolide, ATRA, PMA, LY 294002, wortmannin and all other reagents were from Sigma Chemical (St Louis, MO, USA). Stock solutions of helenalin, costunolide, parthenolide and ATRA were made in DMSO. The solutions were diluted at least 1000-fold in the growth medium such that the final concentration of DMSO had no effect on the differentiation and proliferation of HL-60 cells. All manipulations were performed in subdued light.
Results
Effects of sesquiterpene lactone compounds on ATRA-induced HL-60 cell differentiation and cell cycle arrest
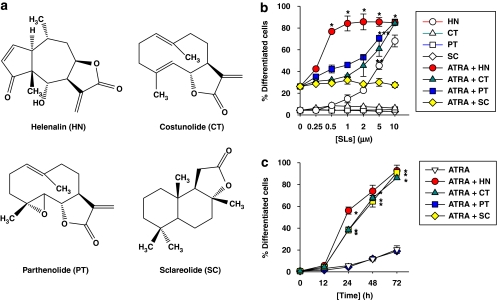
To determine the effects of plant-derived sesquiterpene lactone compounds on ATRA-induced HL-60 cell differentiation and proliferation, HL-60 cells were treated with each of sesquiterpene lactone compounds (see structures in Figure 1a) in combination with a non-toxic concentration of ATRA, and the extent of myeloid cell differentiation was assessed by the NBT reduction assay. For comparison, the cells were treated with ATRA alone.
Figure 1.
Effects of sesquiterpene lactone compounds on ATRA-induced HL-60 cell differentiation. (a) Chemical structures of sesquiterpene lactone compounds used in this study. (b) HL-60 cells were treated for 72 h with 50 nM ATRA alone or in combination with increasing concentrations of each of the sesquiterpene lactones. (c) Cells were treated with 50 nM ATRA alone or in combination with 1 μM helenalin (HT) or 10 μM of costunolide (CT), parthenolide (PT) or sclareolide (SC), for various time periods. Cell differentiation was assessed by the NBT reduction assay. Each value represents the mean±s.e.mean (n=3). *P<0.01, relative to a group treated with ATRA alone. SLs, sesquiterpene lactones.
As shown in Figures 1b and c, the addition of helenalin, costunolide and parthenolide to cultures exposed to a suboptimal concentration of ATRA (50 nM), which by itself caused a relatively low level of cell differentiation, resulted in a marked dose- and time-dependent increase in the degree of differentiation. In contrast, sclareolide, a sesquiterpene lactone found in the leaf surface of Nicotiana spp., had no enhancing effect in HL-60 cell differentiation.
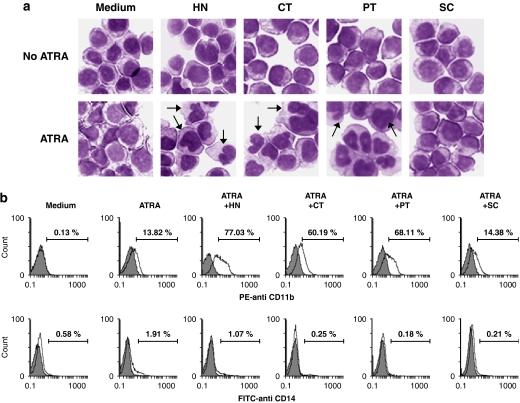
To further characterize the enhancing effect of sesquiterpene lactone compounds on ATRA-induced HL-60 cell differentiation, the morphologic phenotypes and the expression of cell surface antigens in control and treated cells were analysed. As shown in Figure 2a, Giemsa-stained undifferentiated control HL-60 cells were predominantly myelocytes with round and regular cell margins, and large nuclei. Cells treated with 1 μM helenalin, 10 μM costunolide, 10 μM parthenolide or 50 nM ATRA exhibited relatively small changes in cell morphology such as irregular cell margins. Combined treatment of HL-60 cells with 50 nM ATRA and 1 μM helenalin, 10 μM costunolide or 10 μM parthenolide resulted in significantly decreased cell size, denser chromatin and an increased cytoplasm to nuclear ratio, which suggested less DNA synthesis. As shown in Figure 2a, some cells showed a multilobed nucleus, which is a sign of cell differentiation into a granulocytic lineage.
Figure 2.
Morphological and cytofluorometric analysis of four sesquiterpene lactone compounds on ATRA-induced HL-60 cell differentiation. HL-60 cells were treated for 72 h with medium alone (control), 50 nM ATRA alone or in combination with 1 μM helenalin (HT) or 10 μM of costunolide (CT), parthenolide (PT) or sclareolide (SC). (a) Cell morphology was assessed with Giemsa staining and analysed under a light microscope (magnification: × 400). The arrows indicate differentiated HL-60 cells with multilobed nuclei. (b) Cells were subjected to cytofluorometric analysis using PE-conjugated anti-CD11b mAb or FITC-conjugated anti-CD14 mAb (unshaded area) or using the isotype control mAb (shaded area). The data are representative of three independent experiments.
Cytofluorometric analysis of ATRA and sesquiterpene lactone-treated HL-60 cells was performed using fluorophore-conjugated antibodies for two cell surface antigens, CD11b and CD14, which are differentially expressed on myeloid cells differentiating into granulocytic and monocytic lineages. CD11b, a general myeloid differentiation marker, is expressed on both monocytes and granulocytes (Kansas et al., 1990), whereas CD14 is a specific monocytic differentiation marker (van der Schoot et al., 1987). As shown in Figure 2b (upper panels), combination treatment with ATRA and helenalin, costunolide or parthenolide resulted in an increase in the number of CD11b-positive cells exhibiting high fluorescence intensities. In contrast, the treated cells showed little staining with anti-CD14 monoclonal antibody (Figure 2b, bottom panels). These results indicate that the effective sesquiterpene lactone compounds stimulated ATRA-induced HL-60 cell differentiation along the granulocytic pathway.
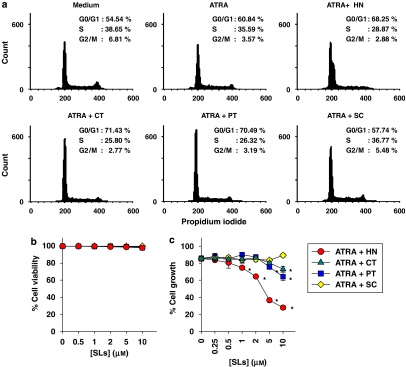
As several reports demonstrated that ATRA-induced differentiation of HL-60 cell is associated with G0/G1 arrest and reduction of S phase (Yen et al., 1998), we analysed the effect of the sesquiterpene lactones on the cell cycle distribution in ATRA-treated HL-60 cells. A typical experiment shown in Figure 3a illustrates that treatment with 50 nM ATRA alone resulted in a slight increase in the percentage of cells in G0/G1 phase as compared with untreated control cells (60.8 vs 54.5%) without affecting the S phase population (38.7 vs 35.6%). When ATRA was combined with helenalin, costunolide or parthenolide, a greater number of cells were found in the G0/G1 phase (68.3–71.4%) and correspondingly fewer cells appeared in the S phase (25.8–28.9%). This indicates that HL-60 cells treated with ATRA and sesquiterpene lactones were partially arrested in G0/G1 phase.
Figure 3.
Cell cycle distribution and growth of HL-60 cells treated with ATRA and sesquiterpene lactone compounds. HL-60 cells were treated for 72 h with 50 nM ATRA alone or in combination with 1 μM helenalin (HT) or 10 μM of costunolide (CT), parthenolide (PT) or sclareolide (SC). The cell cycle distribution was determined by a flow cytometry followed by propidium iodide staining (a). Cell viability and proliferation were determined by the Trypan blue exclusion (b) and MTT assay (c), respectively. The experiment was repeated more than three times with similar results. *P<0.01, relative to a group treated with ATRA alone.
The inhibition of cell cycle progression by combinations of ATRA and sesquiterpene lactone compounds was accompanied by a moderate decrease in cell proliferation. As shown in Figure 3c, 50 nM ATRA alone inhibited cell proliferation approximately by 17.1%, whereas its combinations with 1 μM helenalin, 10 μM costunolide or 10 μM parthenolide significantly decreased cell growth by 28.1–38.3%, as determined by MTT assay. For all treatments, cell viability was greater than 98% throughout the incubation period, as demonstrated by the trypan blue exclusion assay (Figure 3b).
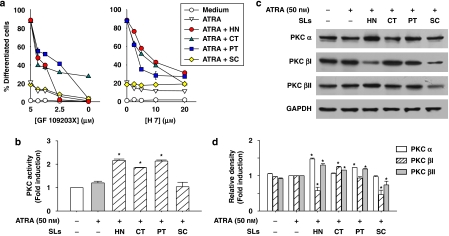
Involvement of PKC, ERK, PI3-K or JNK on HL-60 cell differentiation induced by sesquiterpene lactone compounds in combination with ATRA
Previous studies have provided evidence that activation of PKC is necessary for differentiation of HL-60 cells (Wu et al., 1989; Pan et al., 1997). To determine any relationship between the effect of sesquiterpene lactones on ATRA-induced cell differentiation and PKC activation, HL-60 cells were pretreated with the specific PKC inhibitors, GF 109203X and H 7, followed by incubation for 72 h with ATRA alone or in combination with sesquiterpene lactones. Afterwards, the degree of cell differentiation was assessed by the NBT reduction assay. As shown in Figure 4a, PKC inhibitors significantly reduced the extent of HL-60 cell differentiation induced by ATRA in combination with helenalin, costunolide or parthenolide. To further investigate the involvement of PKC in ATRA-induced HL-60 cell differentiation enhanced by sesquiterpene lactone compounds, PKC activity was measured in HL-60 cells treated with ATRA alone or in combination with these plant agents. As shown in Figure 4b, helenalin, costunolide and parthenolide increased PKC activity in ATRA-treated HL-60 cells, but sclareolide did not. In an attempt to determine the PKC isoforms involved in the differentiation enhancement, we focused on the conventional PKC isoforms such as α, βI, βII, which are known to be the most abundant in leukaemia cells, and their expression levels have been correlated closely with HL-60 cell differentiation (Komada et al., 1991). HL-60 cells were treated with ATRA alone or in combination with sesquiterpene lactone compounds, and the protein levels of PKC isoforms were determined by western blot analysis. As shown in Figures 4c and d, helenalin and parthenolide increased the protein levels of PKCα and βI in ATRA-induced HL-60 cells, whereas costunolide increased the levels of PKCβI and βII. In contrast, sclareolide did not increase but rather decreased the expression of PKC isoforms.
Figure 4.
Differential involvement of PKC in HL-60 cell differentiation induced by ATRA and sesquiterpene lactone compounds. (a) HL-60 cells were treated for 40 min with various concentrations of PKC inhibitors (GF 109203X or H 7), followed by incubation for 72 h with 50 nM ATRA alone, or in combination with 1 μM helenalin (HT) or 10 μM of costunolide (CT), parthenolide (PT) or sclareolide (SC). Cell differentiation was assessed by the NBT reduction assay. Each value represents the mean±s.e.mean (n=3). (b) HL-60 cells were treated with 50 nM ATRA alone or in combination with 1 μM HN, or 10 μM of CT, PT or SC and total PKC activity in the treated cells was measured. (c) The levels of conventional PKC isoforms were determined by western blot analysis. The experiment was repeated at least two times with similar results. (d) Densitometric analysis of the protein band intensities in immunoblots shown in panel c. The data are expressed as a relative density of the bands representing the ATRA+sesquiterpene lactone combination groups to that of the corresponding ATRA alone treatment groups. The values are presented as the means±s.e.mean (n=3). *P<0.05, relative to a group treated with ATRA alone.
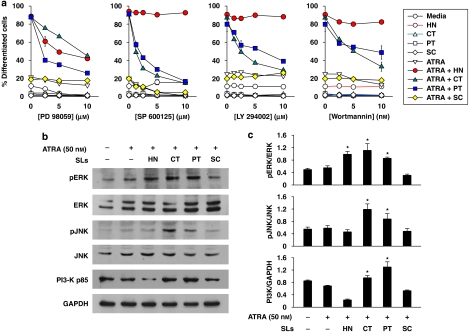
It has been suggested that the mitogen-activated protein kinases (MAPKs) are downstream elements in the PKC-signalling pathway of HL-60 cells (Marcinkowska et al., 1997). To determine the involvement of ERK in ATRA-induced cell differentiation enhanced by sesquiterpene lactone compounds, HL-60 cells were treated with ATRA alone or in combinations of sesquiterpene lactones after pretreatment with PD 98059, which inhibits mitogen activated protein kinase kinase (MEK), the upstream activator of ERK (Alessi et al., 1995). As shown in Figure 5a, PD 98059 significantly inhibited HL-60 cell differentiation induced by combinations of sesquiterpene lactone compounds with ATRA. Moreover, the protein levels of phosphorylated/activated ERK were observed to increase following treatment with effective sesquiterpene lactone compounds on ATRA-induced HL-60 cells (Figures 5b and c).
Figure 5.
Involvement of ERK, JNK and PI3-K in HL-60 cell differentiation induced by ATRA and sesquiterpene lactone compounds. (a) HL-60 cells were treated for 40 min with various concentrations of ERK inhibitor (PD 98059), PI3-K inhibitors (LY 294002 or wortmannin) or JNK inhibitor (SP 600125) followed by incubation for 72 h with ATRA alone or in combination with each of sesquiterpene lactones (1 μM helenalin (HT) or 10 μM of costunolide (CT), parthenolide (PT) or sclareolide (SC). Cell differentiation was assessed by the NBT reduction assay. (b) HL-60 cells were treated for 30 min (ERK and PI3-K) or 3 h (JNK) with 50 nM ATRA alone or in combination with 1 μM HN, or 10 μM of CT, PT or SC. This experiment was repeated more than two times with similar results. A representative blot from three independent experiments is shown and (c) represents the densitometry quantification of protein expression. *P<0.05, relative to a group treated with ATRA alone.
Another kinase, PI3-K, has an important function in differentiation of HL-60 cells (Bertagnolo et al., 1999). To determine whether or not PI3-K was involved in HL-60 cell differentiation induced by combined treatments with ATRA and sesquiterpene lactone compounds, HL-60 cells were treated with these combinations after pretreatment with the specific PI3-K inhibitors, wortmannin or LY294002. These PI3-K inhibitors exerted a dose-dependent inhibition of HL-60 cell differentiation induced by ATRA in combination with costunolide and parthenolide, whereas helenalin plus ATRA-induced HL-60 cell differentiation was not inhibited by PI3-K inhibition (Figure 5a). To further characterize the involvement of PI3-K in sesquiterpene laconte-enhanced HL-60 cell differentiation, the levels of p85, a regulatory subunit of PI3-K, were determined by western blotting. As shown in Figures 5b and c, the levels of p85 subunit were elevated after treatment with ATRA combined with costunolide or parthenolide, whereas helenalin or sclareolide decreased p85 levels in ATRA-induced HL-60 cells, as compared with the effect of ATRA alone.
The JNK signalling module is also known to participate in myeloid cell differentiation (Wang et al., 2003; Mathieu and Besançon, 2006). To determine the involvement of JNK in cell differentiation induced by combinations of ATRA and sesquiterpene lactone compounds, HL-60 cells were pretreated with SP 600125, a specific JNK inhibitor, followed by incubation with ATRA alone or in combination of sesquiterpene lactone compounds. As shown in Figure 5a, SP 600125 decreased the enhancing effects of costunolide and parthenolide on ATRA-induced differentiation, whereas this inhibitor has no effects on helenalin and ATRA-induced differentiation. Furthermore, cells treated with ATRA together with costunolide or parthenolide demonstrated elevated levels of phosphorylated/activated form, phosphorylated c-Jun N-terminal kinase (Figures 5b and c). Consistent with our result that ATRA alone had no effect on JNK activation in protein levels, others have reported that retinoic acid did not induce JNK activation in HL-60 cells (Yen et al., 1999).
Enhancement of ATRA-induced HL-60 cell differentiation by sesquiterpene lactone compounds may result from the inhibition of NF-κB DNA-binding activity
NF-κB is a ubiquitous transcription factor complex that is involved in the activation of a large number of cellular and viral genes. A large number of reports have provided evidence that NF-κB is involved in the development of cancer (Sharma and Narayanan, 1996). Interference with the activation of NF-κB appears to be a common feature for agents that regulate growth and enhance differentiation of cells (Kopp and Ghosh, 1994).
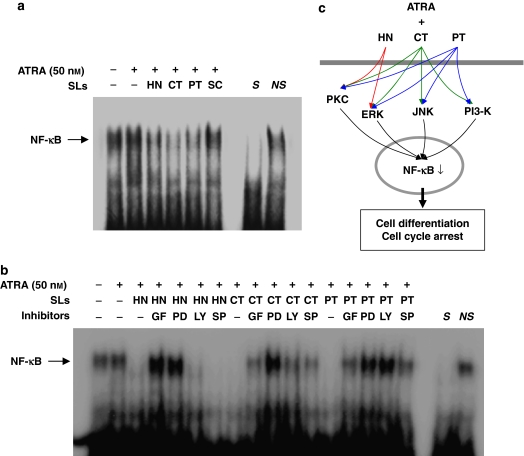
In order to ascertain whether or not the levels of sesquiterpene lactone compounds employed in this study were capable of affecting NF-κB in ATRA-treated HL-60 cells, we analysed nuclear extracts from control and treated cells for NF-κB-binding activity by an electrophoretic mobility shift assay (EMSA), using a probe specific to the NF-κB DNA-binding motif.
As shown in Figure 6, HL-60 cells exhibited relatively high levels of NF-κB DNA-binding activity under both control and ATRA-treated conditions. This binding was specific as it was competed with an unlabelled, identical oligonucleotide, but not with unrelated, non-specific oligonucleotide. A 24 h exposure to helenalin, costunolide and parthenolide induced a marked reduction in the NF-κB DNA-binding activity in the presence of ATRA, but sclareolide exerted no significant effect (Figure 6a).
Figure 6.
Effects of sesquiterpene lactone compounds on NF-κB DNA-binding activity in ATRA-induced HL-60 cells. (a) HL-60 cells were treated for 24 h with medium alone, 50 nM ATRA alone or combinations of ATRA and 1 μM helenalin (HT) or 10 μM of costunolide (CT), parthenolide (PT) or sclareolide (SC). (b) HL-60 cells were treated for 40 min with 5 μM of GF 109203X (GF), 10 μM of PD 98059 (PD), 10 μM of LY 294002 (LY) or 10 μM of SP 600125 (SP), followed by incubation for 24 h with ATRA alone or in combination with each of sesquiterpene lactone compounds. Following incubations (a, b), nuclear extracts were analysed by EMSA for NF-κB DNA-binding activity using a labelled oligonucleotide containing a consensus I-κB site. S and NS indicate the presence of an unlabelled, identical (specific) oligonucleotide and non-specific oligonucleotide, respectively. The experiments shown in panels a and b were repeated two times with similar results. (c) A proposed mechanism for the enhanced HL-60 cell differentiation induced by combined treatments with sesquiterpene lactone compounds and ATRA. HN may enhance ATRA-mediated HL-60 cell differentiation through PKC/ERK/NF-κB pathway without involvement of PI3-K and JNK, whereas CT and PT may act through inhibition of NF-κB with the involvement of all four kinases to varying extents.
To determine the involvement of PKC, ERK, PI3-K and JNK in NF-κB activation of HL-60 cells treated with ATRA and sesquiterpene lactone compounds, the cells were preincubated for 40 min with each of the kinase inhibitors, followed by treatment for 24 h with ATRA alone or in combinations with each of the sesquiterpene lactone compounds. The levels of NF-κB DNA-binding activity were then determined by EMSA. As illustrated in Figure 6b, PKC and ERK inhibitors clearly restored the NF-κB-binding activity in helenalin and ATRA-treated HL-60 cells, whereas the PI3-K and JNK inhibitors exerted no such effects. On the other hand, all of the inhibitors increased NF-κB-binding activity in cells treated with combinations of ATRA and costunolide or parthenolide. These results indicate that some, if not all, of PI3-K, JNK, PKC and ERK might be upstream components involved in the inhibition of NF-κB-binding activity induced by sesquiterpene lactone compounds.
Collectively, the results suggest that sesquiterpene lactones such as helenalin, costunolide and parthenolide may potentiate ATRA-induced HL-60 cell differentiation through distinct pathways. Helenalin may enhance ATRA-mediated HL-60 cell differentiation through PKC/ERK/NF-κB pathway without involvement of PI3-K and JNK, whereas costunolide and parthenolide may act through inhibition of NF-κB with the involvement of all four kinases to different extents (Figure 6c).
Effects of ATRA and sesquiterpene lactones on basal levels of intracellular calcium in differentiating HL-60 cells
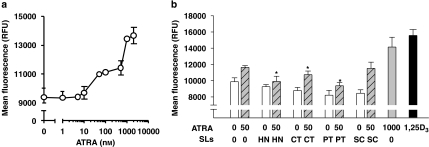
Previous studies reported that ATRA therapy for APL is associated with hypercalcaemia (Bennett et al., 2005). Disturbances of calcium homeostasis may have profound implications for any potential use of the ATRA/sesquiterpene lactone combinations in differentiation therapy of myeloid leukaemia. Therefore, we determined whether ATRA was capable of elevating the resting intracellular calcium levels ([Ca2+]i) in HL-60 cells and, if so, whether sesquiterpene lactones potentiate this effect along with their enhancement of the differentiating and antiproliferating effects of ATRA. Cell treatment with increasing ATRA concentrations (1–2000 nM) for 72 h resulted in a dose-dependent increase in the mean fluorescence intensity of Fluo-4, which reflects elevation of basal [Ca2+]i (Figure 7a). The maximal increase in [Ca2+]i (by about 45%, as compared with untreated control cells) was observed at 1000–2000 nM ATRA with a half-maximal effect at 85.9±11.1 nM. While the apparent ATRA-induced Fluo-4 signal may be diminished because of the inner filter effect, that is, as a result of reabsorption of emitted fluorescence by cellular components, the fact that 100 nM 1,25-(OH)2D3, which is known to induce both a systemic hypercalcaemia and elevation of [Ca2+]i in HL-60 cells (Barnea et al., 1990; Wang et al., 1997, 2005; Danilenko et al., 2003), caused stronger Ca2+ elevation than the maximally effective ATRA concentration (Figure 7b) indicates relatively lower potency of the latter as a calcium-elevating agent.
Figure 7.
The resting [Ca2+]i in HL-60 cells treated with sesquiterpene lactone compounds and ATRA. (a) HL-60 cells were treated for 72 h with increasing concentrations of ATRA (b) Cells were treated (72 h) separately with medium alone, 50 nM ATRA, 1 μM helenalin (HT) or 10 μM of costunolide (CT), parthenolide (PT) or sclareolide (SC), or with combinations of 50 nM ATRA and the sesquiterpene lactones. High concentrations of ATRA (1000 nM) and 1,25-(OH)2D3 (1,25D3, 100 nM) were used as the positive controls. The cells were loaded with Fluo-4 fluorescent probe and the fluorescence intensity was measured by a fluorescence microplate reader. Each value represents the mean±s.e.mean obtained in at least three independent experiments. *P<0.05, relative to the 50 nM ATRA-treated group.
We further examined [Ca2+]i in cells treated with 50 nM ATRA alone or in combinations with four sesquiterpene lactones. High doses of ATRA (1000 nM) and 1,25-(OH)2D3 (100 nM) were used as the positive controls for the intracellular ‘hypercalcaemic' effect. As shown in Figure 7b, in contrast to ATRA, the sesquiterpene lactones alone did not significantly affect the basal levels of intracellular calcium. Moreover, when combined with 50 nM ATRA, these compounds did not potentiate but rather tended to decrease the calcium levels elevated by ATRA.
Discussion
In this study, we examined the ability of several sesquiterpene lactone compounds to enhance the differentiation signal provided by a low concentration of ATRA. HL-60 cells were synergistically differentiated into granulocytes when treated with helenalin, costunolide and parthenolide in combination with low doses of ATRA. Previous studies have also described ATRA combinations with other agents, such as histone deacetylase inhibitors (Savickiene et al., 2006), sodium butyrate, DMSO or hexamethylene bisacetamide (Breitman and He, 1990) and the rosemary polyphenol, carnosic acid (Danilenko et al., 2001; Steiner et al., 2001), which exerted an additive or synergistic effects on HL-60 cell differentiation.
Induction of HL-60 cell differentiation requires the activation of a variety of signal transduction systems, such as the PI3-K (Bertagnolo et al., 1999), PKC (Komada et al., 1991) and MAPK pathways (Marcinkowska et al., 1997). Previous reports demonstrated the involvement of the MEK/ERK MAP kinase pathway in PMA-, ATRA- and G-CSF-induced myeloid differentiation (Hu et al., 2000; Miranda et al., 2002). In addition, we have shown that treatment with a combination of 1,25-(OH)2D3 and costunolide or parthenolide induced monocytic differentiation of HL-60 cells through the PKC and ERK pathways (Kang et al., 2002; Kim et al., 2002). We found here, that all of the three effective sesquiterpene lactones (helenalin, costunolide and parthenolide) contributed to the activation of the PKC and ERK pathways as demonstrated by western blot analyses and kinase inhibitor studies, although the protein levels of conventional PKC isoforms such as α, βI and βII were increased by different amounts (Figures 4 and 5). Interestingly, the PI3-K and JNK pathways were differently involved in ATRA-induced cell differentiation enhanced by sesquiterpene lactone compounds. PI3-K and JNK might not be involved in ATRA-induced HL-60 cell differentiation in combination with helenalin, but these signalling molecules were involved in the combined effects of ATRA and either parthenolide or costunolide (Figure 5). In contrast, sclareolide did not affect either protein levels or activity of all the signalling kinases measured in this study.
In previous studies, we have demonstrated that interference with NF-κB activation appears to be a common feature of agents that enhance differentiation in HL-60 cells (Kim et al., 2006). Consistent with reports from others (Sokoloski et al., 1993), our NF-κB EMSA indicated that HL-60 leukaemia cells showed relatively high levels of NF-κB activity. Constitutively high levels of NF-κB activity have been detected in many tumour cells because the aberrant expression of NF-κB was associated with oncogenesis and carcinogenesis (Yen et al., 1999). Furthermore, we (Kang et al., 2002; Kim et al., 2002) and others (Sokoloski et al., 1997, 1998) previously reported that inhibition of NF-κB activity sensitized the HL-60 cells to differentiation-inducing agents such as 1,25-(OH)2D3. The three effective sesquiterpene lactones with an inhibitory activity on NF-κB enhanced the ATRA-induced cell differentiation whereas sclareolide, a sesquiterpene lactone without effect on NF-κB, did not (Figure 6a). Furthermore, our kinase inhibitor study showed that both PKC and ERK may be, at least in part, involved in NF-κB inhibition of HL-60 cell differentiation by sesquiterpene lactone compounds (Figure 6b). Taken together, these results suggest a possible involvement of NF-κB inhibition in the enhanced HL-60 cell differentiation induced by sesquiterpene lactone compounds and ATRA. However, the precise role of NF-κB inhibition in this enhancement should be further investigated.
ATRA is an active derivative of vitamin A which has been used for the treatment of APL patients (Huang et al., 1988; Lengfelder et al., 2005) with a high rate of complete remission. ATRA has an advantage in the treatment of leukaemia, but it also has side effects. Particularly, previous studies reported on hypercalcaemia associated with ATRA treatment (Lemez, 1995; Bennett et al., 2005). Although hypercalcaemia that occurs in ATRA-treated human subjects is the systemic elevation of serum calcium levels, this study shows that ATRA can also increase the basal [Ca2+]i in HL-60 human leukaemia cells in a dose-dependent manner. Although this [Ca2+]i elevation does not necessarily reflect systemic calcaemia, we (Danilenko et al., 2003; Wang et al., 2005) and others (Barnea et al., 1990; Wang et al., 1997) have demonstrated that 1,25-(OH)2D3, which induces systemic hypercalcaemia at supra-physiological doses, also elevates resting [Ca2+]i in HL-60 cells. Furthermore, it has been reported that 1,25-(OH)2D3 analogues with low calcaemic potency in animals also produced lower elevations in intracellular calcium (Wang et al., 1997). Although the biological significance of this interesting correlation between systemic and cellular ‘calcaemic' effects of vitamin 1,25D3 remains unclear, our current data imply a similar correlation for ATRA. Therefore, we suggest that [Ca2+]i assays may be used as part of initial screening for potential antileukaemic agents with low calcaemic activity.
Importantly, despite the strong differentiation-enhancing effect of the sesquiterpene lactones, these compounds did not potentiate the elevation of [Ca2+]i induced by a low dose of ATRA (Figure 7b). Along with the previously reported data (Danilenko et al., 2003; Wang et al., 2005), these results further support the suggestion that the raised [Ca2+]i is not necessary for the enhanced myeloid differentiation of HL-60 cells induced by combinations of low concentrations of differentiation inducers, such as 1,25-(OH)2D3 and ATRA, and active plant-derived agents (polyphenols and sesquiterpene lactones).
In conclusion, helenalin, costunolide and parthenolide, lactones of plant origin, enhanced ATRA-mediated HL-60 cell differentiation through distinct pathways without significantly affecting the basal intracellular calcium levels. These results suggest a possible use of combinations of some sesquiterpene lactone compounds and low doses of ATRA in the treatment of myeloid leukaemia, which may produce a greater therapeutic response than ATRA alone, possibly with lesser side effects such as hypercalcaemia.
Acknowledgments
This study was supported by a grant from the Korea–Israel Joint Program, Ministry of Science and Technology (Korea: M6-0510-00-0046; Israel: 16-2005), and also by a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (01-PJ10-PG6-01GN16-0005).
Abbreviations
- APL
acute promyelocytic leukaemia
- ATRA
all-trans retinoic acid
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun N-terminal kinase
- LY294002
2-(4-Morpholinyl)-8-phenyl-1(4H)-1-benzopyran-4-one
- NBT
nitroblue tetrazolium
- NF-κB
nuclear transcription factor kappaB
- PD 98059
2-(2′-amino-3′-methoxyphenyl)-oxanaphthalen-4-one
- PI3-K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- Wortmannin
3-furo[4,3,2-de]indeno[4,5-h]-2-benzopyran-3,6,9-trione
Conflict of interest
The authors state no conflict of interest.
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Barnea E, Levy R, Zimlichman R, Shany S. 1,25-Dihydroxyvitamin D3 enhances cytosolic free calcium in HL-60 cells. Exp Hematol. 1990;18:1147–1151. [PubMed] [Google Scholar]
- Beere HM, Hickman JA. Differentiation: a suitable strategy for cancer chemotherapy. Anticancer Drug Des. 1993;8:299–322. [PubMed] [Google Scholar]
- Bennett MT, Sirrs S, Yeung JK, Smith CA. Hypercalcemia due to all trans retinoic acid in the treatment of acute promyelocytic leukemia potentiated by voriconazole. Leuk Lymphoma. 2005;46:1829–1831. doi: 10.1080/10428190500235298. [DOI] [PubMed] [Google Scholar]
- Bertagnolo V, Neri LM, Marchisio M, Mischiati C, Capitani S. Phosphoinositide 3-kinase activity is essential for all-trans-retinoic acid-induced granulocytic differentiation of HL-60 cells. Cancer Res. 1999;59:542–546. [PubMed] [Google Scholar]
- Breitman TR, He RY. Combinations of retinoic acid with either sodium butyrate, dimethyl sulfoxide, or hexamethylene bisacetamide synergistically induce differentiation of the human myeloid leukemia cell line HL60. Cancer Res. 1990;50:6268–6273. [PubMed] [Google Scholar]
- Breitman TR, Selonick SE, Collins SJ. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci USA. 1980;77:2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro V, Murillo R, Klaas CA, Meunier C, Mora G, Pahl HL, et al. Inhibition of the transcription factor NF-κB by sesquiterpene lactones from Podachaenium eminens. Planta Med. 2000;66:591–595. doi: 10.1055/s-2000-8649. [DOI] [PubMed] [Google Scholar]
- Coligan JE, Kruisbeck AM, Margulies DH, Shevach EM, Strober W. Current protocols in immunology. Wiley-Liss: New York; 1995. [Google Scholar]
- Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979;149:969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilenko M, Wang Q, Wang X, Levy J, Sharoni Y, Studzinski GP. Carnosic acid potentiates the antioxidant and prodifferentiation effects of 1α,25-dihydroxyvitamin D3 in leukemia cells, but does not promote elevation of basal levels of intracellular calcium. Cancer Res. 2003;63:1325–1332. [PubMed] [Google Scholar]
- Danilenko M, Wang X, Studzinski GP. Carnosic acid and promotion of monocytic differentiation of HL60-G cells initiated by other agents. J Natl Cancer Inst. 2001;93:1224–1233. doi: 10.1093/jnci/93.16.1224. [DOI] [PubMed] [Google Scholar]
- el-Feraly FS, Chan YM. Isolation and characterization of the sesquiterpene lactones costunolide, parthenolide, costunolide diepoxide, santamarine, and reynosin from Magnolia grandiflora L. J Pharm Sci. 1978;67:347–350. doi: 10.1002/jps.2600670319. [DOI] [PubMed] [Google Scholar]
- Hehner SP, Hofmann TG, Droge W, Schmitz ML. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-κB by targeting the IκB kinase complex. J Immunol. 1999;163:5617–5623. [PubMed] [Google Scholar]
- Hu X, Moscinski LC, Valkov NI, Fisher AB, Hill BJ, Zuckerman KS. Prolonged activation of the mitogen-activated protein kinase pathway is required for macrophage-like differentiation of a human myeloid leukemic cell line. Cell Growth Differ. 2000;11:191–200. [PubMed] [Google Scholar]
- Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. [PubMed] [Google Scholar]
- Hughes PJ, Zhao Y, Chandraratna RA, Brown G. Retinoid-mediated stimulation of steroid sulfatase activity in myeloid leukemic cell lines requires RARalpha and RXR and involves the phosphoinositide 3-kinase and ERK-MAP kinase pathways. J Cell Biochem. 2006;97:327–350. doi: 10.1002/jcb.20579. [DOI] [PubMed] [Google Scholar]
- Kang SN, Kim SH, Chung SW, Lee MH, Kim HJ, Kim TS. Enhancement of 1 alpha,25-dihydroxyvitamin D3-induced differentiation of human leukaemia HL-60 cells into monocytes by parthenolide via inhibition of NF-κB activity. Br J Pharmacol. 2002;135:1235–1244. doi: 10.1038/sj.bjp.0704573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas GS, Muirhead MJ, Dailey MO. Expression of the CD11/CD18, leukocyte adhesion molecule 1, and CD44 adhesion molecules during normal myeloid and erythroid differentiation in humans. Blood. 1990;76:2483–2492. [PubMed] [Google Scholar]
- Kim SH, Kang SN, Kim HJ, Kim TS. Potentiation of 1,25-dihydroxyvitamin D3-induced differentiation of human promyelocytic leukemia cells into monocytes by costunolide, a germacranolide sesquiterpene lactone. Biochem Pharmacol. 2002;64:1233–1242. doi: 10.1016/s0006-2952(02)01292-3. [DOI] [PubMed] [Google Scholar]
- Kim SH, Oh SM, Kim TS. Induction of human leukemia HL-60 cell differentiation via a PKC/ERK pathway by helenalin, a pseudoguainolide sesquiterpene lactone. Eur J Pharmacol. 2005;511:89–97. doi: 10.1016/j.ejphar.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Kim SH, Song JH, Choi BG, Kim HJ, Kim TS. Chemical modification of santonin into a diacetoxy acetal form confers the ability to induce differentiation of human promyelocytic leukemia cells via the down-regulation of NF-κB DNA binding activity. J Biol Chem. 2006;281:13117–13125. doi: 10.1074/jbc.M510944200. [DOI] [PubMed] [Google Scholar]
- Komada F, Nishikawa M, Uemura Y, Morita K, Hidaka H, Shirakawa S. Expression of three major protein kinase C isozymes in various types of human leukemic cells. Cancer Res. 1991;51:4271–4278. [PubMed] [Google Scholar]
- Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Lemez P. Hypercalcaemia caused by all-trans retinoic acid (ATRA) treatment in a case of acute promyelocytic leukaemia was manageable after decreasing the ATRA dose to 27 mg/m2/day. Eur J Haematol. 1995;55:275–276. [PubMed] [Google Scholar]
- Lengfelder E, Saussele S, Weisser A, Buchner T, Hehlmann R. Treatment concepts of acute promyelocytic leukemia. Crit Rev Oncol Hematol. 2005;56:261–274. doi: 10.1016/j.critrevonc.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Marcinkowska E, Wiedlocha A, Radzikowski C. 1,25-Dihydroxyvitamin D3 induced activation and subsequent nuclear translocation of MAPK is upstream regulated by PKC in HL-60 cells. Biochem Biophys Res Commun. 1997;241:419–426. doi: 10.1006/bbrc.1997.7832. [DOI] [PubMed] [Google Scholar]
- Mathieu J, Besançon F. Arsenic trioxide represses NF-kappaB activation and increases apoptosis in ATRA-treated APL cells. Ann NY Acad Sci. 2006;1090:203–208. doi: 10.1196/annals.1378.022. [DOI] [PubMed] [Google Scholar]
- Miranda MB, McGuire TF, Johnson DE. Importance of MEK-1/-2 signaling in monocytic and granulocytic differentiation of myeloid cell lines. Leukemia. 2002;16:683–692. doi: 10.1038/sj.leu.2402400. [DOI] [PubMed] [Google Scholar]
- Mori H, Kawamori T, Tanaka T, Ohnishi M, Yamahara J. Chemopreventive effect of costunolide, a constituent of oriental medicine, on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1994;83:171–175. doi: 10.1016/0304-3835(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Pan Q, Granger J, O'Connell TD, Somerman MJ, Simpson RU. Promotion of HL-60 cell differentiation by 1,25-dihydroxyvitamin D3 regulation of protein kinase C levels and activity. Biochem Pharmacol. 1997;54:909–915. doi: 10.1016/s0006-2952(97)00286-4. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Arnason JT, Birnboim HC. Low concentrations of the feverfew component parthenolide inhibit in vitro growth of tumor lines in a cytostatic fashion. Planta Med. 1999;65:126–129. doi: 10.1055/s-1999-13972. [DOI] [PubMed] [Google Scholar]
- Savickiene J, Treigyte G, Borutinskaite V, Navakauskiene R, Magnusson KE. The histone deacetylase inhibitor FK228 distinctly sensitizes the human leukemia cells to retinoic acid-induced differentiation. Ann NY Acad Sci. 2006;1091:368–384. doi: 10.1196/annals.1378.081. [DOI] [PubMed] [Google Scholar]
- Schmidt TJ. Helenanolide-type sesquiterpene lactones-III. Rates and stereochemistry in the reaction of helenalin and related helenanolides with sulfhydryl containing biomolecules. Bioorg Med Chem. 1997;5:645–653. doi: 10.1016/s0968-0896(97)00003-5. [DOI] [PubMed] [Google Scholar]
- Sharma HW, Narayanan R. The NF-kappaB transcription factor in oncogenesis. Anticancer Res. 1996;16:589–596. [PubMed] [Google Scholar]
- Sokoloski JA, Hodnick WF, Mayne ST, Cinquina C, Kim CS, Sartorelli AC. Induction of the differentiation of HL-60 promyelocytic leukemia cells by vitamin E and other antioxidants in combination with low levels of vitamin D3: possible relationship to NF-κB. Leukemia. 1997;11:1546–1553. doi: 10.1038/sj.leu.2400786. [DOI] [PubMed] [Google Scholar]
- Sokoloski JA, Narayanan R, Sartorelli AC. Enhancement by antisense oligonucleotides to NF-κB of the differentiation of HL-60 promyelocytic leukemia cells induced by vitamin D3. Cancer Lett. 1998;125:157–164. doi: 10.1016/s0304-3835(97)00505-3. [DOI] [PubMed] [Google Scholar]
- Sokoloski JA, Sartorelli AC, Rosen CA, Narayanan R. Antisense oligonucleotides to the p65 subunit of NF-kappa B block CD11b expression and alter adhesion properties of differentiated HL-60 granulocytes. Blood. 1993;82:625–632. [PubMed] [Google Scholar]
- Steiner M, Priel I, Giat J, Levy J, Sharoni Y, Danilenko M. Carnosic acid inhibits proliferation and augments differentiation of human leukemic cells induced by 1,25-dihydroxyvitamin D3 and retinoic acid. Nutr Cancer. 2001;41:135–144. doi: 10.1080/01635581.2001.9680624. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Abe E, Miyaura C, Shiina Y, Suda T. 1 alpha, 25-dihydroxyvitamin D3 induces differentiation of human promyelocytic leukemia cells (HL-60) into monocyte-macrophages, but not into granulocytes. Biochem Biophys Res Commun. 1983;117:86–92. doi: 10.1016/0006-291x(83)91544-9. [DOI] [PubMed] [Google Scholar]
- van der Schoot CE, von dem Borne AE, Tetteroo PA. Characterization of myeloid leukemia by monoclonal antibodies, with an emphasis on antibodies against myeloperoxidase. Acta Haematol. 1987;78:32–40. doi: 10.1159/000205900. [DOI] [PubMed] [Google Scholar]
- Wang Q, Salman H, Danilenko M, Studzinski GP. Cooperation between antioxidants and 1,25-dihydroxyvitamin D3 in induction of leukemia HL60 cell differentiation through the JNK/AP-1/Egr-1 pathway. J Cell Physiol. 2005;204:964–974. doi: 10.1002/jcp.20355. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang X, Studzinski GP. Jun N-terminal kinase pathway enhances signaling of monocytic differentiation of human leukemia cells induced by 1,25-dihydroxyvitamin D3. J Cell Biochem. 2003;89:1087–1101. doi: 10.1002/jcb.10595. [DOI] [PubMed] [Google Scholar]
- Wang X, Gardner JP, Kheir A, Uskokovic MR, Studzinski GP. Synergistic induction of HL60 cell differentiation by ketoconazole and 1-desoxy analogues of vitamin D3. J Natl Cancer Inst. 1997;89:1199–1206. doi: 10.1093/jnci/89.16.1199. [DOI] [PubMed] [Google Scholar]
- Wu XZ, Shao GY, Chen S, Wang XW, Wang ZY. Studies on the relationship between protein kinase C and differentiation of human promyelocytic leukemia cells induced by retinoic acid. Leuk Res. 1989;13:869–874. doi: 10.1016/0145-2126(89)90039-8. [DOI] [PubMed] [Google Scholar]
- Yen A, Roberson MS, Varvayanis S. Retinoic acid selectively activates the ERK2 but not JNK/SAPK or p38 MAP kinases when inducing myeloid differentiation. In vitro Cell Dev Biol Anim. 1999;35:527–532. doi: 10.1007/s11626-999-0063-z. [DOI] [PubMed] [Google Scholar]
- Yen A, Roberson MS, Varvayanis S, Lee AT. Retinoic acid induced mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent MAP kinase activation needed to elicit HL-60 cell differentiation and growth arrest. Cancer Res. 1998;58:3163–3172. [PubMed] [Google Scholar]