Abstract
Background and purpose:
Soluble guanylyl cyclase (sGC) is a receptor for nitric oxide that generates cGMP. This second messenger molecule has established roles in cellular physiology; however, less is known about its effects in tumour cells.
Experimental approach:
The effects of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and 4H-8-bromo-1,2,4-oxadiazolo(3,4-d)benz(b)(1,4)oxazin-1-one (NS2028), both selective sGC inhibitors on proliferation, death and migration were determined in prostate cancer cell lines.
Key results:
Western blot analysis confirmed the presence of α1 and β1 subunits of sGC in LNCaP and PC-3 cells. Sodium nitroprusside (SNP) increased cGMP accumulation in LNCaP and PC-3, but not DU-145 cells. SNP-stimulated cGMP production in LNCaP cells was dose-dependently reduced by ODQ, with more than 90% inhibition being observed at 0.1 μM. ODQ activated caspase-3 in all three cell lines, but not in normal prostate epithelial cells, at concentrations over 10 μM. High concentrations of ODQ also promoted DNA fragmentation and nucleosome accumulation in the cytosol of LNCaP cells. Interestingly, the chemically related inhibitor, NS2028 was without effect on caspase-3. In addition, ODQ inhibited LNCaP, Du145 and PC-3 cell growth. Finally, although fibroblast growth factor-2 did not enhance cGMP levels in LNCaP cells, its ability to stimulate LNCaP motility was abolished by ODQ.
Conclusions and implications:
These observations taken together suggest that the action of ODQ in LNCaP cells did not reflect sGC inhibition. We conclude that ODQ promotes cell death and inhibits growth and migration of prostate cancer cells and that these actions are independent of its effects on GMP levels.
Keywords: nitric oxide, sGC, ODQ, cell proliferation, apoptosis, prostate cancer
Introduction
Prostate cancer is the most commonly diagnosed malignancy among men in the Western world with 230 000 new cases each year in the United States (Jemal et al., 2002; Nelson et al., 2003). Neoplastic transformation of the prostate is a complex process characterized by the upregulation or constitutive activation of multiple signalling pathways that promote proliferation, inhibit apoptosis and enable prostate cells to migrate and invade into healthy tissue while evoking angiogenesis (McCarty, 2004). Androgen ablation induces, initially, a positive clinical response because of the death of androgen-dependent cancer cells. Owing to the heterogeneity of this tumour, however, it eventually progresses into a hormonally refractory state, posing a clinical problem that is resistant to further therapeutic interventions (Mitsiades and Koutsilieris, 2001; Nelson et al., 2003; Pomerantz and Kantoff, 2007). Better understanding of these molecular events is critical to identifying new targets and develop new therapies.
Nitric oxide (NO) is a small, freely diffusible, signalling molecule that has an important function in many physiological, as well as pathophysiological, processes including inflammation and cancer (Moncada et al., 1991; Lala and Chakraborty, 2001; Sessa, 2007; Ying and Hofseth, 2007). Most of its actions are exerted through the activation of soluble guanylyl cyclase (sGC), a heterodimeric (α/β subunits) protein bearing a haem group that catalyses the conversion of GTP to cGMP (Lucas et al., 2000; Friebe and Koesling, 2003). Elevated levels of the secondary messenger cGMP mediate a variety of actions including vasodilation, inhibition of platelet aggregation and neuronal signalling (Moncada et al., 1991; Lucas et al., 2000). It is known that activation of sGC and subsequent elevation of cGMP levels inhibit apoptosis in different cell types, including eosinophils, endothelium, neural and uterine epithelial cells, suggesting a function for the sGC/cGMP pathway in regulating cell survival (Dimmeler and Zeiher, 1999; Fiscus, 2002; Chan and Fiscus, 2003). Similar findings have been reported in tumour cell lines; in ovarian cancer cells, sGC promoted survival through p53 regulation (Fraser et al., 2005). In addition, increased sGC expression has been detected in bladder and breast carcinoma (Ehsan et al., 2002; Postovit et al., 2002). In prostate cancer, the sGC α1 subunit has been proposed to enhance cellular proliferation independently of cGMP generation, identifying an additional target through which prostate cancer cell growth can be inhibited (Cai et al., 2007). The aim of this study was to characterize the expression of sGC isoforms in prostate cancer cell lines and to determine the effects of sGC inhibition in cell death, migration and proliferation.
Materials and methods
Cell culture
The prostate cancer-derived cell lines, DU 145, LNCaP and PC-3, were obtained from American Type Culture Collection and cultured according to the instructions provided. LNCaP cells were cultured in RPMI 1640 medium adjusted to contain 1.5 g L−1 sodium bicarbonate, 4.5 g L−1 glucose, 10 mM HEPES and 1.0 mM sodium pyruvate, and then supplemented with 2 mM L-glutamine, 10% foetal calf serum and antibiotics. PC-3 cells were grown in Ham's F12K medium supplemented with 2 mM L-glutamine, 10% foetal calf serum and antibiotics. Finally, DU-145 cells were grown in Minimum Essential Medium with Earle's salts adjusted to contain 2 mM L-glutamine, 1.5 g L−1 sodium bicarbonate, 0.1 mM non-essential amino acids and 1.0 mM sodium pyruvate and supplemented with 10% foetal calf serum and antibiotics. Normal human prostate epithelial cells (HPrECs) were cultured in Clonetics prostate growth medium following the instructions provided.
Western blotting
Cells were washed with phosphate-buffered saline and lysed using lysis buffer (1% NP-40, 0.5% Na-deoxycholic acid, 50 mM Tris-HCl, pH 7.4, 50 mM NaCl, 1 mM EDTA, 0.1 mM EGTA, 50 mM NaF, 1 mM Na3VO4). Lysates were then homogenized (Dounce homogenizer) and insoluble material was pelleted by centrifugation (12 000 g for 15 min at 4 °C). Protein concentration was determined using the Lowry method. Samples were separated by SDS-polyacrylamide gel electrophoresis in a 7.5% gel followed by an overnight transfer to polyvinylidene difluoride membranes. Membranes were blocked for 1 h at room temperature, incubated with an antibody against the α1 or β1 sGC subunit overnight. The membranes were washed and then incubated with a secondary antibody before being developed with the chemiluminescent substrate.
Determination of cGMP accumulation
To determine the responsiveness of cells to NO, cGMP accumulation was measured. After reaching confluence, cells were washed twice with Earls' balanced salt solution. When sGC inhibition was desired, cells were pretreated with ODQ for 30 min. Earls' balanced salt solution containing the phosphodiesterase inhibitor isobutylmethylxanthine (IBMX) (1 mM) was added to all wells and incubated for 5 min. Cells were then exposed to 10 or 100 μM sodium nitroprusside and cGMP accumulation was allowed to occur for 15 min. LNCaP, DU145, PC-3 or HPrECs were lysed using 200 μl per well of 0.1 N HCl. cGMP was collected and measured according to the manufacturer's instructions.
Caspase-3 activity assay
Caspase-3 activity was determined by measuring the proteolytic cleavage of the fluorogenic substrate Z-DEVD-AMC. Briefly, cells were grown to confluence and serum-starved overnight. Monolayers were then exposed to the indicated concentration of ODQ or 4H-8-bromo-1,2,4-oxadiazolo(3,4-d)benz(b)(1,4)oxazin-1-one (NS-2028); both sGC inhibitors were dissolved in dimethylsulphoxide. As a positive control, some groups of cells were treated with 100 μg mL−1 of the protein synthesis inhibitor cycloheximide. After 48 h, both floating and adherent cells were collected and washed twice with phosphate-buffered saline. Cells were then lysed in a buffer containing 10 mM Tris, pH 7.5, 100 mM NaCl, 1 mM EDTA and 0.01% Triton X-100. The fluorescence of the cleaved reporter group was measured 30 min after the addition of 100 μM substrate at 380 nm excitation and 469 nm emission. Relative fluorescence unit data were normalized per mg protein.
Cytosolic DNA extraction/electrophoresis and nucleosome detection
Confluent LNCaP cells were serum-starved overnight and exposed to ODQ (10–100 μM). As a positive control for the induction of apoptosis, some groups of cells were treated with 100 μg mL−1 of cycloheximide. After 48 h, cells were harvested using a plastic scraper. Both floating and adherent cells were collected and washed with phosphate-buffered saline. Cytosolic DNA was prepared as follows: cell pellets were resuspended in 750 μl of lysis buffer (20 mM Tris-HCl, 10 mM EDTA, 0.5% Triton X-100, pH 8.0) and occasionally shaken while on ice for 45 min. The cytosolic fraction was collected by centrifugation at 13 000 g for 20 min at 4 °C and protein concentrations were determined. Cytosol aliquots containing equal amounts of protein were extracted with a mixture of phenol and chloroform. One-tenth volume of 3 M sodium acetate (pH 5.2) was added to the supernatant, and DNA was precipitated by adding an equal volume of isopropanol. After storing at −20 °C overnight, a DNA pellet was obtained by centrifugation at 13 000 g for 15 min at 4 °C and washed twice with 75% ethanol. The DNA pellet was then resuspended in 100 μl of 20 mM Tris-HCl (pH 8.0). After digesting RNA with RNase (0.1 mg mL−1) at 37 °C for 1 h, samples (15 μl) were electrophoresed through a 1.2% agarose gel. DNA was visualized and photographed with UV light. For nucleosome determination, confluent LNCaP cells were serum-starved overnight and exposed to ODQ (1–100 μM). Cytoplasmic mono- and oligonucleosomes were measured using the Cell Death Detection ELISA kit according to the manufacturer's instructions.
RNA interference
Short hairpin RNA (shRNA) against the sGC α1 subunit was constructed using the BLOCK-iT Adenoviral RNAi Expression System targeting an area that is highly conserved between human, rat and mouse sequences. The sGC α1 shRNA-encoding sequence was created by using two complimentary oligonucleotides, each containing the target sequences for the α1 subunit (1398–1419 of the rat sequence) followed by a short spacer and an antisense sequence of the target. The oligonucleotides used were 5′-CACCGCAAGGACAAATTGTGCAAGCCGAAGCTTGCACAATTTGTCCTTGC-3′ and 5′-AAAAGCAAGGACAAATTGTGCAAGCTTCGGCTTGCACAATTTGTCCTTGC-3′. Annealed oligonucleotides were cloned into the pENTR/U6 vector and the RNAi cassette was transferred into pAd/BLOCK-iT-DEST. Adenoviruses were produced with the help of HEK293 packaging cell line, titered and used at four multiplicities of infection.
Cell migration
LNCaP cells were serum-starved overnight. After trypsinization, 1 × 105 cells were added to transwells (8 μm pore size) in 100 μl of serum-free medium containing 0.25% BSA. To inhibit sGC, cells were pretreated with ODQ (1 or 50 μM, 2 h). Fibroblast growth factor-2 (FGF-2; 10 ng mL−1) was then added to the well containing the transwell inserts in 600 μL of media. The sGC inhibitor was added in both the upper and lower compartments of the transwell setup. LNCaP cells were allowed to migrate for 4 h after which non-migrated cells at the top of the transwell filter were removed with a cotton swab. The migrated cells were fixed in Carson's solution for at least 30 min at room temperature and then stained in toluidine blue for 20 min. Migrated cells were scored in eight random fields.
Cell proliferation
For proliferation assays, LNCaP cells were seeded in 96-well plates at 4 × 104 cells cm−2 (2 × 104 cells cm−2 for PC-3 and DU 145 cells) in full growth medium. They were incubated with the indicated concentration of ODQ, NS-2028 or vehicle and allowed to proliferate for 24 or 48 h. Cell number was quantified using the CyQUANT kit following the manufacturer's protocol. Reference standard curves with known number of cells were used to convert relative fluorescence units of each sample into cell number.
Data analysis and statistics
Data are presented as means±s.e.m. of the indicated number of observations. Statistical comparisons between groups were performed using the one-way ANOVA followed by the Dunnett's least significance difference or Newman–Keuls post hoc test. Differences among means were considered significant when P<0.05.
Materials
Tissue culture plastic ware was obtained from Corning-Costar (Corning, NY, USA). Cell culture media and supplements, foetal calf serum, trypsin, penicillin and streptomycin were obtained from GIBCO-BRL (Paisley, UK). The polyvinylidene difluoride membrane was obtained from Macherey-Nagel GmbH (Düren, Germany) and the SuperSignal West Pico Chemiluminescent substrate from Pierce (Rockford, IL, USA). ODQ and NS-2028 were purchased from Calbiochem (San Diego, CA, USA). The antibody against sGC β1 subunit was obtained from Cayman Chemical (Ann Arbor, MI, USA). The anti-rabbit and anti-mouse horseradish peroxidase-labelled secondary antibodies were purchased from NEN Life Science (Boston, MA, USA). DC Protein assay kit and other reagents for SDS-polyacrylamide gel electrophoresis were obtained from Bio-Rad (Hercules, CA, USA). The CyQUANT cell proliferation assay kit and EnzChek kit for determination of caspace-3 activity were purchased from Molecular Probes Inc. (Eugene, OR, USA). The BLOCK-iT Adenoviral RNAi Expression System was purchased from Invitrogen (Carlsbad, CA, USA). cGMP enzyme immunoassay kits were obtained from R&D Systems (Minneapolis, MN, USA). FGF-2 was purchased from Peprotech (Rocky Hill, NJ, USA). The CytoTox 96 Non-Radioactive Cytotoxicity Assay kit was obtained from Promega (Madison, WI, USA), whereas the Cell Death Detection ELISA kit was obtained from Roche Diagnostics (Mannheim, Germany). HPrECs were purchased from Lonza Verviers, S.p.r.l. (Verviers, Belgium). All other reagents including the antibody for sGC α1 subunit, N(G)-nitro-L-arginine methyl ester (L-NAME), sodium nitroprusside (SNP), IBMX, phenylmethylsulphonyl fluoride, aprotinin, EGTA, EDTA and pepstatin were obtained from Sigma (St Louis, MO, USA).
Results
Expression of sGC in prostate cancer cell lines
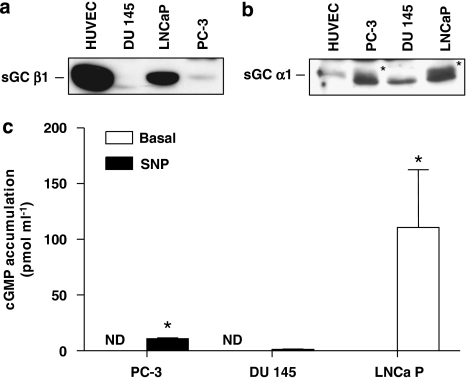
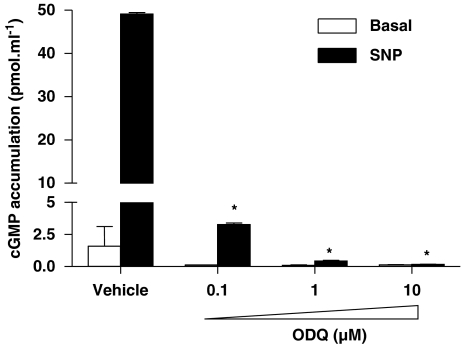
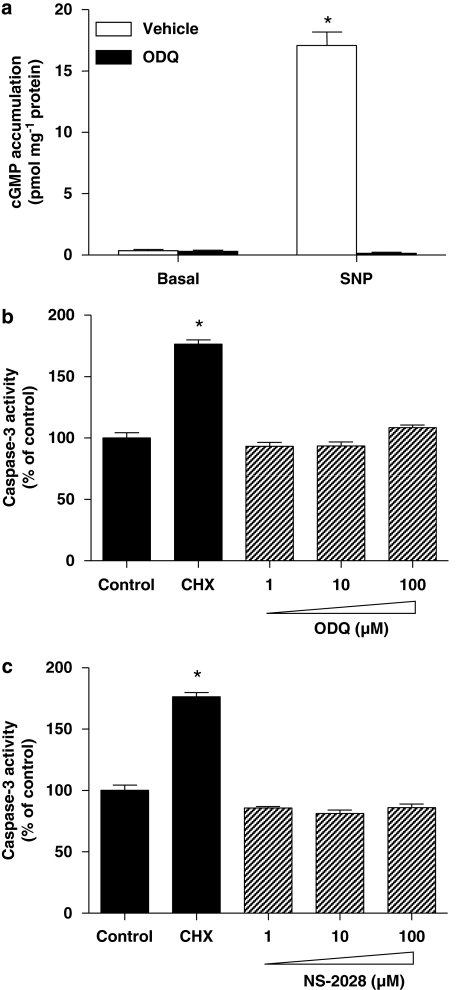
To determine whether prostate cancer cells express sGC subunits, western blot analysis was performed using lysates from LNCaP, PC3 and DU-145 cell lines. Human vascular endothelial cells, known to express sGC (Papapetropoulos et al., 1996; Pyriochou et al., 2006), were used as positive controls. Figures 1a and b show that LNCaP exhibited the highest levels of α1 and β1 expression among the prostate cancer cell lines, whereas PC3 exhibited very low levels and DU-145 lacked sGC subunits. To determine if the cells expressing sGC immunoreactivity also expressed catalytically active sGC, we examined their capability to increase cGMP production in response to a NO donor, SNP. Stimulation of LNCaP cells resulted in a marked increase in cGMP production (Figure 1c). In line with what we observed in our western blot analysis, no cGMP synthesis was measured in DU-145 cells, whereas in PC3 cells, there was a small, but significant, increase in cGMP accumulation after the SNP treatment. Moreover, pretreating LNCaP cells with the sGC inhibitor ODQ inhibited SNP-induced cGMP accumulation; a concentration as low as 0.1 μM of ODQ caused a greater than 90% inhibition of sGC activity and a concentration of 10 μM abolished the response to SNP (Figure 2).
Figure 1.
Soluble guanylyl cyclase (sGC) expression in prostate cancer cells. Lysates from PC3, DU-145 and LNCaP cells were prepared as described in Materials and methods. Cellular proteins (∼40 μg) were separated by electrophoresis on 7.5% polyacrylamide gels, transferred to a nitrocellulose membrane and blotted with a β1 (a) or α1 (b) sGC antibody. The asterisks in (b) show the α1 immunoreactive band. A lower non-specific band is present in prostate cancer cells, but not in human umbilical vein endothelial cells. Similar results were obtained in three experiments. (c) Enzymically active sGC is present in LNCaP and PC-3 prostate cancer cells. Cells were incubated with isobutylmethylxanthine (IBMX; 1 mM) for 5 min. They were then stimulated with sodium nitroprusside (SNP) or exposed to vehicle for an additional 15 min. cGMP was extracted with HCl and measured using EIA. Values are means±s.e.m., n=4 wells; *P<0.05 from basal.
Figure 2.
Inhibition of cGMP accumulation by ODQ. LNCaP cells were treated with the indicated concentration of ODQ for 30 min. Cells were then stimulated with 10 μM SNP for 15 min, in the presence of the phosphodiesterase inhibitor isobutylmethylxanthine (IBMX; 1 mM), before extraction of cGMP with HCl. Values are means±s.e.m., n=4 wells; *P<0.05 from the vehicle SNP group.
ODQ promotes cell death in prostate cancer cells
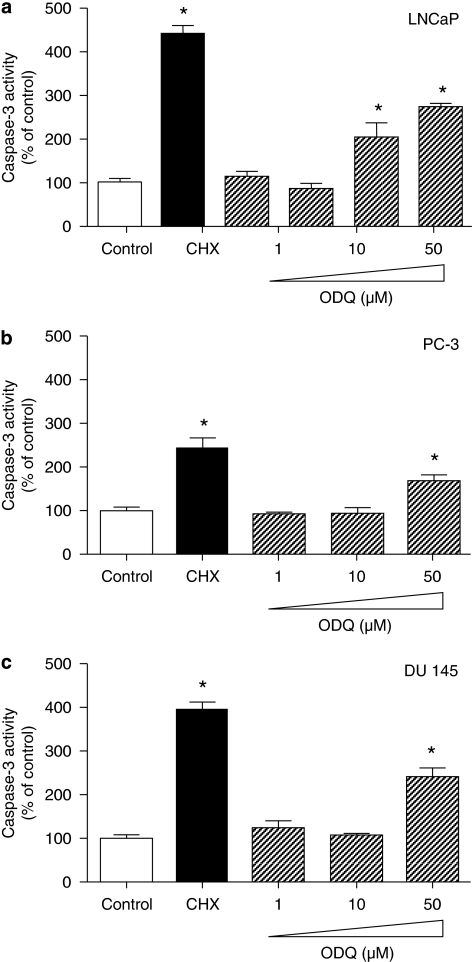
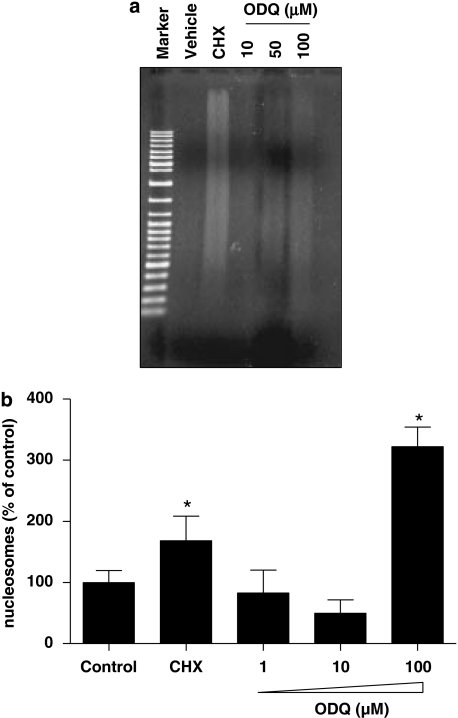
Activation of caspase-3 is recognized as a critical step in the execution phase of programmed cell death, eventually leading to degradation of cellular structures that is characteristic of apoptotic cells (Reed, 2000). To examine whether inhibition of sGC activity induces biochemical changes associated with apoptosis, we incubated all three cell lines with ODQ for 48 h and caspase-3 activity was measured. In LNCaP cells (Figure 3a), ODQ upregulated caspase-3 activity when used at 50 and 100 μM. Interestingly, in PC3 and DU-145 cells, which exhibit low or no expression of sGC respectively, ODQ caused a similar effect when used at 50 μM (Figures 3b and c). Taken together, the above data suggest that ODQ might be inducing cell death in an sGC/cGMP-independent manner in these prostate cancer cell lines. To determine whether high concentrations of ODQ lead to changes in DNA structure in LNCaP cells, we performed DNA fragmentation analysis. In agreement with the results obtained with caspase-3, exposure of cells to lower ODQ concentrations (up to 10 μM) did not cause DNA laddering, whereas higher concentrations (100 μM) triggered DNA fragmentation (Figure 4a). In addition, treatment of cells with the highest ODQ concentration led to enhanced nucleosome formation (Figure 4b). In experiments in which the Trypan blue exclusion assay was used to assess toxicity, we observed a small percentage of Trypan blue-positive cells (less than 3%) in all groups, which was not different between the control and 100 μM ODQ group (data not shown), suggesting that incubation with high ODQ concentrations does not result in overt toxicity. However, lactate dehydrogenase release from cells treated with ODQ (100 μM) or cycloheximide (50 μg mL−1) for 48 h was greater than that observed in control cultures. Lactate dehydrogenase in the culture supernatants expressed as % of control was as follows: cycloheximide, 257±16*; ODQ (1 μM), 96±3; ODQ (10 μM), 103±8; ODQ (100 μM), 182±16*; NS2028 (100 μM), 99±4 (means±s.d., n=3; *P<0.05).
Figure 3.
ODQ-enhanced caspase-3 activity. Confluent LNCaP (a), PC-3 (b) and DU-145 (c) cells were treated with the indicated concentration of ODQ. After 48 h, both floating and adherent cells were collected and assayed for caspase-3 activity, as described in Materials and methods. As a positive control for the induction of apoptosis, cells were treated with 100 μg mL−1 of the protein synthesis inhibitor cycloheximide. Values are means±s.e.m., n=4 wells; *P<0.05 from control.
Figure 4.
ODQ-enhanced cell death in LNCaP cells. Cells were treated with the indicated ODQ concentration or cycloheximide (100 μg mL−1) as positive control. After 48 h, the treatment cells were washed twice with ice-cold phosphate-buffered saline, harvested and lysed. (a) Cytosolic DNA was isolated, electrophoresed on an agarose gel and stained with ethidium bromide. This experiment was performed twice with similar results. (b) Oligonucleosomes were measured in the cytosol of cells using a commercially available kit. Values are means±s.e.m., n=4 wells; *P<0.05 from control.
To test whether ODQ also promotes cell death/apoptosis in normal prostate cells, primary prostate epithelial cells were used. These cells readily express sGC, as indicated by the ODQ-sensitive increase in cGMP accumulation observed after exposure to SNP (Figure 5a). Interestingly, incubation of normal epithelial cells with sGC inhibitors (ODQ and NS2028; Figures 5b and c) did not activate caspase-3, suggesting that the apoptotic effects of ODQ were restricted to tumour cells.
Figure 5.
Caspase-3 activity in normal human prostate epithelial cells (HPrECs) was not increased following ODQ treatment. (a) Primary human epithelial prostate cells were pretreated with the vehicle (dimethylsulphoxide; DMSO) or ODQ (10 μM) for 30 min. Cells were then stimulated with 100 μM SNP for 15 min, in the presence of the phosphodiesterase inhibitor isobutylmethylxanthine (IBMX; 1 mM), before extraction of cGMP with HCl. Values are means±s.e.m., n=4 wells; *P<0.05 from vehicle. Confluent HPrECs were treated with the indicated concentration of ODQ (b) or 4H-8-bromo-1,2,4-oxadiazolo(3,4-d)benz(b)(1,4)oxazin-1-one (c). After 48 h, both floating and adherent cells were collected and assayed for caspase-3 activity, as described in Materials and methods. As a positive control for the induction of apoptosis, cells were treated with 100 μg mL−1 of the protein synthesis inhibitor cycloheximide. Values are means±s.e.m., n=3 wells; *P<0.05 from control.
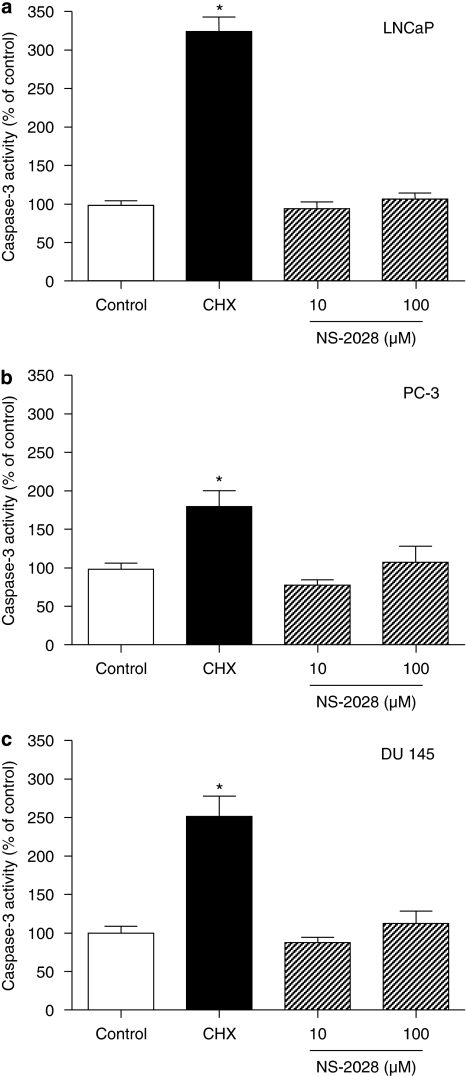
NS-2028 has no effect on caspase-3 activity in prostate cancer cells
4H-8-bromo-1,2,4-oxadiazolo(3,4-d)benz(b)(1,4)oxazin-1-one is an sGC inhibitor structurally related to ODQ (Olesen et al., 1998). To examine whether this sGC inhibitor has similar effects with regard to cell death in prostate cancer cells, we treated LNCaP, DU-145 and PC3 cells with NS-2028. Surprisingly, concentrations of NS-2028 up to 100 μM did not affect caspase-3 activity (Figure 6). Proving its effectiveness as an sGC inhibitor, a concentration of 10 μM NS-2028 reduced NO-stimulated cGMP accumulation by 95% (data not shown). The data provided above further support the hypothesis that the cell death-triggering effect of ODQ in prostate cancer cell lines is sGC independent.
Figure 6.
4H-8-bromo-1,2,4-oxadiazolo(3,4-d)benz(b)(1,4)oxazin-1-one (NS-2028) did not alter caspase-3 activity. Confluent LNCaP (a), PC-3 (b) and DU-145 (c) cells were treated with the indicated concentration of NS-2028. After 48 h, both floating and adherent cells were collected and assayed for caspase-3 activity, as described in Materials and methods. As a positive control for the induction of apoptosis, cells were treated with 100 μg mL−1 of the protein synthesis inhibitor cycloheximide. Values are means±s.e.m., n=4 wells; *P<0.05 from control.
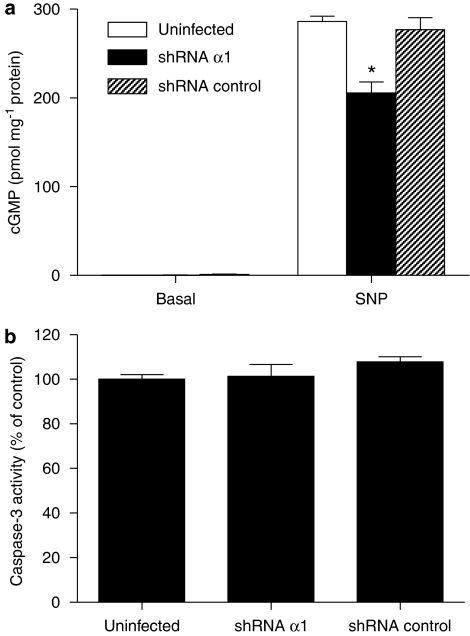
To confirm that sGC is not involved in the cell death-enhancing properties of ODQ, we also used a gene knockdown approach. An shRNA for the α1 sGC subunit was generated and introduced into adenoviral vectors, and LNCaP cells were infected at four multiplicities of infection for 48 h. The specificity of the shRNA for the α1 was tested in COS-7 cells overexpressing sGC; in these experiments, the α1 shRNA specifically blocked the expression of the α1 subunit, whereas the control sequence used had no effect on α1 levels (data not shown). LNCaP cells in which the shRNA for the α1 subunit was overexpressed exhibited reduced cGMP-forming ability (Figure 7a). In spite of reduced expression of catalytically active sGC, the cells did not express enhanced caspase-3 activity (Figure 7b), further confirming the notion that inhibition of sGC activity is not the mechanism through which ODQ promotes cellular destruction in LNCaP cells.
Figure 7.
Reduced expression of sGC did not induce apoptosis in LNCaP cells. (a) Cells were infected with short hairpin RNA (shRNA)-expressing adenoviruses and exposed to vehicle (basal) or 100 μM SNP for 15 min, in the presence of the phosphodiesterase inhibitor isobutylmethylxanthine (IBMX; 1 mM), before extraction of cGMP with HCl. Values are means±s.e.m., n=4 wells ; *P<0.05 from shRNA control group. In parallel cultures (b), caspase-3 activity as an index of apoptosis was measured.
Effects of ODQ on migration and proliferation
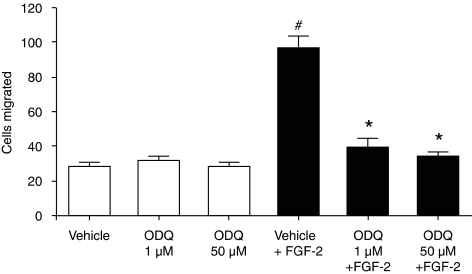
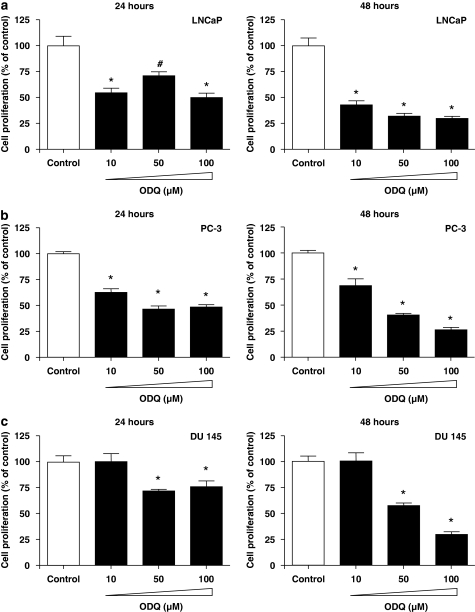
To determine if ODQ apart from the inhibition of cell survival exerts other anti-tumour actions in prostate cancer cells, migration and proliferation was measured in the presence and absence of this agent. Incubation with ODQ did not block basal LNCaP migration, but abolished the FGF-2-stimulated responses. The effect of ODQ was already maximal at 1 μM (Figure 8). However, we believe that the action of ODQ on LNCaP migration is sGC independent, as FGF-2 did not stimulate the production of cGMP in these cells (10.3±4.0 vs 8.2±1.7 pmol per mg protein for control and FGF-2-treated cells; n=4). We finally, measured the ability of ODQ to modulate cell growth in prostate cancer cells. ODQ exerted an anti-proliferative effect in all three cell lines, irrespective of the presence of sGC (Figure 9).
Figure 8.
ODQ-inhibited migration of LNCaP cells. Cells were serum-starved overnight. They were then pretreated with vehicle or the indicated concentration of ODQ for 2 h, trypsinized and placed in transwells. LNCaP cells were allowed to migrate for 4 h in the absence or presence of fibroblast growth factor-2 (10 ng mL−1). Data are expressed as mean±s.e.m.; n=5; *P<0.05 from FGF-2; #P<0.05 from vehicle.
Figure 9.
Effect of ODQ on prostate cancer cell proliferation. LNCaP (a), PC-3 (b) and DU-145 (c) cells were seeded in 24-well plates, treated with the indicated concentration of ODQ or vehicle (dimethylsulphoxide; DMSO) and allowed to proliferate for 24–48 h. Cell number was measured using the CyQUANT assay. Data are expressed as means±s.e.m.; n=10; *P<0.05 from control.
Discussion
Soluble guanylyl cyclase has been a therapeutic target for cardiovascular disease for more than a century. In addition to the NO donor compounds that are available, a new generation of sGC-activating agents has been recently described. These agents stimulate cGMP production without releasing NO and enhance sGC activity without requiring the presence of the haem prosthetic group (Evgenov et al., 2006). One the other hand, only two chemically related agents exist that have the capacity to inhibit sGC, ODQ and NS-2028 (Garthwaite et al., 1995; Olesen et al., 1998). In this study, we provide evidence that the sGC inhibitor ODQ exerts anti-tumour effects on prostate cancer cells by promoting cell death and inhibiting cell growth and migration in a manner that is independent of inhibition of sGC enzymatic activity.
To determine whether sGC is expressed in prostate cancer cell lines, western blot analysis was performed using subunit-specific antibodies. In line with previous findings (Chen et al., 2002), we observed the presence of immunoreactive bands for both the α1 and β1 subunits in LNCaP lysates, suggesting that these cells express the most common form of the heterodimer. In addition, PC-3 cells exhibited lower levels for both subunits, whereas DU-145 cells were devoid of sGC. In agreement with the protein expression data, we found that LNCaP and PC-3 cells responded to exogenously administered NO by increasing their intracellular cGMP levels, with LNCaP accumulating more cGMP than PC-3 cells; no catalytically active sGC could be found in DU-145 cells. It should be noted that the α1 subunit of sGC is increased in patients with prostate cancer in a manner that correlates with the stage of cancer, with α1 being found in both epithelial and stromal cells (Cai et al., 2007). In addition, androgens have been shown to enhance sGC expression (Cai et al., 2007; Oka et al., 2007).
Guanosine 3′,5′ cyclic monophosphate has been shown to modulate cell survival in a variety of cell types. Having confirmed the presence of catalytically active sGC in LNCaP cells, we proceeded to test the effects of sGC inhibition on apoptosis in this cell line. To this end, we measured caspase-3 as an index of apoptosis. Incubation of cells with ODQ increased caspase-3 activity only at high concentrations (50 and 100 μM), whereas the majority of sGC activity could be inhibited at 0.1–1 μM in these cells. Moreover, ODQ was able to induce caspase-3 activity in DU-145, a cell line in which sGC is not expressed. These data taken together suggest that ODQ promotes cell death independently of its ability to inhibit cGMP formation. This conclusion is further supported by the observation that cell-permeable analogues of cGMP did not reverse the effects of ODQ (data not shown). It should be emphasized that normal prostate epithelial cells incubated with high concentrations of ODQ did not exhibit an increase in caspase-3 activity, indicating that only tumour cells were susceptible to ODQ-induced cell death. Although many biochemical markers have been used to monitor apoptosis, the ultimate proof that cells are undergoing programmed cell death is DNA fragmentation. To determine if ODQ treatment of unstressed cells leads to breaks in DNA strands, cells growing in complete medium were incubated with increasing concentrations of this sGC inhibitor. Indeed, DNA fragmentation and nucleosome formation was evident when concentrations of ODQ exceeding 10 μM were used. However, these high ODQ concentrations also increase lactate dehydrogenase release, indicating that disruption of cell membrane integrity occurs after incubation with ODQ, and this might be indicative of post-apoptotic necrosis. In spite of the fact that ODQ has an IC50 in the low nM range (Garthwaite et al., 1995) and that high concentrations of ODQ (100 μM) have been shown to inhibit other haem-containing proteins, including NO synthases and cytochrome P450 enzymes (Feelisch et al., 1999), concentrations of this sGC inhibitor above 10 μM are often used in the literature (Chen et al., 2002; Chan and Fiscus, 2003; Fraser et al., 2005). Results obtained using such high ODQ concentrations should be interpreted with caution, as the effects seen might not always be related to sGC inhibition.
Only two sGC inhibitors are currently available, namely ODQ and the structurally related analogue NS-2028 (Garthwaite et al., 1995; Olesen et al., 1998). LY-53853 and methylene blue that were originally thought to be sGC inhibitors are now known to inhibit sGC activity indirectly by generating superoxide anions that scavenge NO (Cherry et al., 1990; Marczin et al., 1992; Kontos and Wei, 1993). To determine if NS-2028, similar to ODQ, has the ability to inhibit cell survival, cells were treated with this agent. Surprisingly, concentrations of NS-2028 up to 100 μM failed to promote apoptosis in prostate cancer cell lines, suggesting that the apoptosis-inducing properties are unique to ODQ and re-enforcing the notion that ODQ promotes cell death, in a manner that is independent of cGMP generation. This conclusion is further supported by the observation that reducing the amount of catalytically active sGC using shRNA for the α1 subunit did not promote apoptosis. It is worth noting that NS-2028 differs from ODQ only in the presence of a bromide atom on the benzene ring and in oxygen for nitrogen substitution in the six-membered heterocyclic ring; we thus speculate that the apoptotic action of ODQ that is unrelated to sGC inhibition results from one of the above-mentioned structural differences.
Migration of tumour cells is a property that contributes to the development of metastasis. To test the ability of ODQ to inhibit cell motility, LNCaP cells were treated with FGF-2 in the presence or absence of ODQ, and their ability to migrate was determined. In agreement with previous results, FGF-2 stimulated migration of LNCaP cells (Hatziapostolou et al., 2006), whereas incubation with ODQ blocked this effect. However, FGF-2 did not increase the production of cGMP, further suggesting that the inhibitory action of ODQ is independent of cGMP generation. This is in line with the finding that FGF-2-driven angiogenic responses in endothelial cells are also independent of the NO/cGMP pathway (Ziche et al., 1997).
Guanosine 3′,5′ cyclic monophosphate has been shown to modulate cell growth. For example, in endothelial cells, cGMP stimulates ERK1/2 activity, exerting a mitogenic effect (Parenti et al., 1998; Pyriochou et al., 2006), whereas in smooth muscle cells, it is believed to attenuate growth (Lincoln et al., 2006; Vermeersch et al., 2007). To study the function of ODQ in prostate cancer cell proliferation, LNCaP, PC-3 and DU-145 cells were treated with varying concentrations of this agent and cell number was determined. As observed with the apoptotic effect, ODQ reduced growth even in cells not expressing sGC, that is, DU-145 cells. In LNCaP and PC-3 cells, the inhibitory action of ODQ was only evident at higher concentrations, suggesting that in this case too, the effects of ODQ were unrelated to cGMP generation. Previous observations showing that NO does not affect the growth of LNCaP cells further support our hypothesis for a sGC-independent action of ODQ in these cells (Cai et al., 2007).
Although we have not identified the target molecule that ODQ acts upon to exert its effects in prostate cancer cells, our results demonstrate that its action is tumour specific, as it does not promote apoptosis in normal prostate epithelial cells and is unrelated to inhibition of cGMP production. Our results also suggest that to avoid possible off-target effects of ODQ, concentrations in the sub-μM range should be employed. As we have recently shown that sGC inhibition blocks angiogenesis (Pyriochou et al., 2006), it would be tempting to speculate that ODQ might have a dual action in prostate cancer: it could reduce tumour vascularity through inhibition of cGMP generation, whereas exerting anti-proliferative and pro-apoptotic actions on prostate cancer cells through cGMP-independent pathways.
Acknowledgments
This study was supported by funds from the Thorax Foundation, the Greek Ministry of Education and the Greek Secretariat of Research and Technology.
Abbreviations
- FGF
fibroblast growth factor
- HPrEC
human prostate epithelial cell
- IBMX
isobutylmethylxanthine
- NS-2028
4H-8-bromo-1,2,4-oxadiazolo(3,4-d)benz(b)(1,4)oxazin-1-one
- NO
nitric oxide
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- sGC
soluble guanylyl cyclase
- shRNA
short hairpin RNA
- SNP
sodium nitroprusside
References
- Cai C, Chen S-Y, Zheng Z, Omwancha J, Lin M-F, Balk SP, et al. Androgen regulation of soluble guanylyl cyclase[alpha]1 mediates prostate cancer cell proliferation. Oncogene. 2007;26:1606–1615. doi: 10.1038/sj.onc.1209956. [DOI] [PubMed] [Google Scholar]
- Chan SL, Fiscus RR. Guanylyl cyclase inhibitors NS2028 and ODQ and protein kinase G (PKG) inhibitor KT5823 trigger apoptotic DNA fragmentation in immortalized uterine epithelial cells: anti-apoptotic effects of basal cGMP/PKG. Mol Hum Reprod. 2003;9:775–783. doi: 10.1093/molehr/gag094. [DOI] [PubMed] [Google Scholar]
- Chen Z, Vetter M, Che D, Liu S, Tsai M, Chang C. The bradykinin/soluble guanylate cyclase signaling pathway is impaired in androgen-independent prostate cancer cells. Cancer Lett. 2002;177:181–187. doi: 10.1016/s0304-3835(01)00788-1. [DOI] [PubMed] [Google Scholar]
- Cherry P, Omar H, Farrell K, Stuart J, Wolin M. Superoxide anion inhibits cGMP-associated bovine pulmonary arterial relaxation. Am J Physiol. 1990;259:H1056–H1062. doi: 10.1152/ajpheart.1990.259.4.H1056. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher A. Nitric oxide—an endothelial cell survival factor. Cell Death Differ. 1999;6:964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- Ehsan A, Sommer F, Schmidt A, Klotz T, Koslowski J, Niggemann S, et al. Nitric oxide pathways in human bladder carcinoma. The distribution of nitric oxide synthases, soluble guanylyl cyclase, cyclic guanosine monophosphate, and nitrotyrosine. Cancer. 2002;95:2293–2301. doi: 10.1002/cncr.10942. [DOI] [PubMed] [Google Scholar]
- Evgenov O, Pacher P, Schmidt P, Hasko G, Schmidt H, Stasch J. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelisch M, Kotsonis P, Siebe J, Clement B, Schmidt HHHW. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide donor bioactivation. Mol Pharmacol. 1999;56:243–253. doi: 10.1124/mol.56.2.243. [DOI] [PubMed] [Google Scholar]
- Fiscus R. Involvement of cyclic GMP and protein kinase G in the regulation of apoptosis and survival in neural cells. Neurosignals. 2002;11:175–190. doi: 10.1159/000065431. [DOI] [PubMed] [Google Scholar]
- Fraser M, Chan SL, Chan SSL, Fiscus RR, Tsang BK. Regulation of p53 and suppression of apoptosis by the soluble guanylyl cyclase/cGMP pathway in human ovarian cancer cells. Oncogene. 2005;25:2203–2212. doi: 10.1038/sj.onc.1209251. [DOI] [PubMed] [Google Scholar]
- Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton C, Nielsen E, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- Hatziapostolou M, Polytarchou C, Katsoris P, Courty J, Papadimitriou E. Heparin Affin regulatory peptide/pleiotrophin mediates fibroblast growth factor 2 stimulatory effects on human prostate cancer cells. J Biol Chem. 2006;281:32217–32226. doi: 10.1074/jbc.M607104200. [DOI] [PubMed] [Google Scholar]
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- Kontos H, Wei E. Hydroxyl radical-dependent inactivation of guanylate cyclase in cerebral arterioles by methylene blue and by LY83583. Stroke. 1993;24:427–434. doi: 10.1161/01.str.24.3.427. [DOI] [PubMed] [Google Scholar]
- Lala P, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–156. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- Lincoln T, Wu X, Sellak H, Dey N, Choi C. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Front Biosci. 2006;11:356–367. doi: 10.2741/1803. [DOI] [PubMed] [Google Scholar]
- Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- Marczin N, Ryan US, Catravas JD. Methylene blue inhibits nitrovasodilator- and endothelium-derived relaxing factor-induced cyclic GMP accumulation in cultured pulmonary arterial smooth muscle cells via generation of superoxide anion. J Pharmacol Exp Ther. 1992;263:170–179. [PubMed] [Google Scholar]
- McCarty M. Targeting multiple signaling pathways as a strategy for managing prostate cancer: multifocal signal modulation therapy. Integr Cancer Ther. 2004;3:349–380. doi: 10.1177/1534735404270757. [DOI] [PubMed] [Google Scholar]
- Mitsiades C, Koutsilieris M. Molecular biology and cellular physiology of refractoriness to androgen ablation therapy in advanced prostate cancer. Expert Opin Investig Drugs. 2001;10:1099–1115. doi: 10.1517/13543784.10.6.1099. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- Oka M, Karoor V, Homma N, Nagaoka T, Sakao E, Golembeski S, et al. Dehydroepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc Res. 2007;74:377–387. doi: 10.1016/j.cardiores.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen S-P, Drejer J, Axelsson O, Moldt P, Bang L, Nielsen-Kudsk JE, et al. Characterization of NS 2028 as a specific inhibitor of soluble guanylyl cyclase. Br J Pharmacol. 1998;123:299–309. doi: 10.1038/sj.bjp.0701603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos A, Cziraki A, Rubin J, Stone C, Catravas J. cGMP accumulation and gene expression of soluble guanylate cyclase in human vascular tissue. J Cell Physiol. 1996;167:213–221. doi: 10.1002/(SICI)1097-4652(199605)167:2<213::AID-JCP4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Parenti A, Morbidelli L, Cui X-L, Douglas JG, Hood JD, Granger HJ, et al. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- Pomerantz M, Kantoff P. Advances in the treatment of prostate cancer. Annu Rev Med. 2007;58:205–220. doi: 10.1146/annurev.med.58.101505.115650. [DOI] [PubMed] [Google Scholar]
- Postovit L-M, Adams MA, Lash GE, Heaton JP, Graham CH. Oxygen-mediated regulation of tumor cell invasiveness. Involvement of a nitric oxide signaling pathway. J Biol Chem. 2002;277:35730–35737. doi: 10.1074/jbc.M204529200. [DOI] [PubMed] [Google Scholar]
- Pyriochou A, Beis D, Koika V, Potytarchou C, Papadimitriou E, Zhou Z, et al. Soluble guanylyl cyclase activation promotes angiogenesis. J Pharmacol Exp Ther. 2006;319:663–671. doi: 10.1124/jpet.106.108878. [DOI] [PubMed] [Google Scholar]
- Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157:1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa WC. Nitric oxide synthase inhibition and cancer. Lancet Oncol. 2007;8:88–89. doi: 10.1016/S1470-2045(07)70006-2. [DOI] [PubMed] [Google Scholar]
- Vermeersch P, Buys E, Pokreisz P, Marsboom G, Ichinose F, Sips P, et al. Soluble guanylate cyclase-{alpha}1 deficiency selectively inhibits the pulmonary vasodilator response to nitric oxide and increases the pulmonary vascular remodeling response to chronic hypoxia. Circulation. 2007;116:936–943. doi: 10.1161/CIRCULATIONAHA.106.677245. [DOI] [PubMed] [Google Scholar]
- Ying L, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007;67:1407–1410. doi: 10.1158/0008-5472.CAN-06-2149. [DOI] [PubMed] [Google Scholar]
- Ziche M, Morbidelli L, Choudhuri R, Zhang H-T, Donnini S, Granger HJ, et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]