Abstract
Background and purpose:
α1-Adrenoceptors in the rabbit prostate have been studied because of their controversial pharmacological profiles in functional and radioligand binding studies. The purpose of the present study is to determine the native profiles of α1-adrenoceptor phenotypes and to clarify their relationship.
Experimental approach:
Binding experiments with [3H]-silodosin and [3H]-prazosin were performed using intact tissue segments and crude membrane preparations of rabbit prostate and the results were compared with α1-adrenoceptor-mediated prostate contraction.
Key results:
[3H]-Silodosin at subnanomolar concentrations bound specifically to intact tissue segments of rabbit prostate. However, [3H]-prazosin at the same range of concentrations failed to bind to α1-adrenoceptors of intact segments. Binding sites of [3H]-silodosin in intact segments were composed of α1L phenotype with low affinities for prazosin (pKi=7.1), 5-methyurapidil and N-[2-(2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-α,α-dimethyl-1H-indole-3-ethamine hydrochloride (RS-17053), and α1A-like phenotype with moderate affinity for prazosin (pKi=8.8) but high affinity for 5-methyurapidil and RS-17053. In contrast, both radioligands bound to a single population of α1-adrenoceptors in the membrane preparations at the same density with a subnanomolar affinity, showing a typical profile of ‘classical' α1A-adrenoceptors (pKi for prazosin=9.8). The pharmacological profile of α1-adrenoceptor-mediated prostate contraction was in accord with the α1L phenotype observed by intact segment binding approach.
Conclusions and implications:
Three distinct phenotypes (α1L and α1A-like phenotypes in the intact segments and a classical α1A phenotype in the membranes) with different affinities for prazosin were detected in rabbit prostate. It appears that the three phenotypes are phenotypic subtypes of α1A-adrenoceptors, but are not genetically different subtypes.
Keywords: prostate, receptor phenotype, adrenergic, α1Aand α1L, radioligand-binding assay
Introduction
At present, three distinct subtypes of α1-adrenoceptors (α1A, α1B and α1D; Alexander et al 2008) have been cloned and are known to be widely distributed in mammals including humans (Lomasney et al., 1991; Hieble et al., 1995; Zhong and Minneman, 1999; Michelotti et al., 2000). Pharmacological features of the three classical α1-adrenoceptors are high (subnanomolar) affinity for prazosin, a prototypic, selective α1-adrenoceptor antagonist, although several compounds show distinct subtype-selectivity; for example, silodosin, 5-methylurapidil and N-[2-(2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-α,α-dimethyl-1H-indole-3-ethamine hydrochloride (RS-17053) are selective for α1A-adrenoceptors, and 8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4,5]decane-7,9-dione dihydrochloride (BMY-7378) is selective for α1D-adrenoceptors (Lomasney et al., 1991; Hieble et al., 1995; Ford et al., 1996; Murata et al., 1999; Piao et al., 2000).
Contraction of prostatic and urethral smooth muscle is mediated by α1-adrenoceptors, and these receptors are a primary target of α1-adrenoceptor antagonist therapy of urinary outlet obstruction in patients with benign prostatic hyperplasia (Lepor and Shapiro, 1994; Cooper et al., 1999; Chapple, 2001; Andersson, 2002). However, in vitro functional studies with prostate and some blood vessels have revealed that α1-adrenoceptor-mediated contractions are relatively insensitive to prazosin, suggesting the presence of a unique α1-adrenoceptor type, different from the classical α1-adrenoceptors (Ford et al., 1996; Testa et al., 1997; Van der Graaf et al., 1997; Takeda et al., 1999; Argyle and McGrath, 2000; Morishima et al., 2007b). It has been proposed that the α1-adrenoceptors mediating prostate and vascular contractions are of a different subtype from the classical ones; this subtype has been named α1L because of its lower affinity for prazosin (Flavahan and Vanhoutte, 1986; Muramatsu et al., 1990). However, a distinct gene corresponding to the putative α1L subtype has not yet been identified; rather, it has been suggested that the α1L subtype may be a functional phenotype of the α1A-adrenoceptor, because the functional studies with recombinant α1A-adrenoceptor have revealed a relatively low affinity for prazosin (Ford et al., 1997; Daniels et al., 1999). In contrast, binding studies with recombinant α1A-adrenoceptor and with membrane preparations of prostate have revealed a high (subnanomolar) affinity for prazosin, failing to detect the α1L profile (Testa et al., 1993; Daniels et al., 1999; Piao et al., 2000; Ramsay et al., 2004). Recently, we demonstrated that α1A and α1L subtypes coexist as distinct entities when radioligand binding studies were performed using the intact segments of several tissues including human prostate but that the α1L subtype converted its profile to α1A-adrenoceptor after homogenization (Hiraizumi-Hiraoka et al., 2004; Morishima et al., 2007b). More recently, we found that both α1A and α1L subtypes were abolished in α1A-adrenoceptor gene knockout mice (Morishima et al., 2007a). These results suggest that for the same α1A-adrenoceptor gene product, different pharmacological phenotypes may be expressed in some native tissues and that this phenotypic diversity may solve the controversy in α1A/L pharmacology (Muramatsu et al., 2005; Nelson and Challiss, 2007).
With regard to α1-adrenoceptors in the prostate, most studies have been performed in rabbits and humans. Because rabbit prostate also expresses α1A-adrenoceptor at the mRNA level (Piao et al., 2000), we speculated that rabbit prostate would be an ideal tissue to evaluate different phenotypes derived from the α1A-adrenoceptor gene without contamination by other α1-adrenoceptors (α1B and α1D). The purpose of this study is to investigate pharmacological phenotypes of α1-adrenoceptor populations in rabbit prostate and then to explore their relationship. To detect all kinds of phenotypes, binding experiments were conducted in the intact segments and membrane preparations of rabbit prostate using two radioligands: [3H]-silodosin, which has very high apparent affinities for both α1A- and α1L-adrenoceptor subtypes, and [3H]-prazosin, which shows high affinity for α1A-, α1B-, and α1D-adrenoceptor subtypes.
Materials and methods
Animals and tissue isolation
This study was performed according to the Guidelines for Animal Experiments, University of Fukui (which is accredited by the Ministry of Education, Culture, Sports, Science and Technology, Japan). Male pigmented rabbits (1.5–2.5 kg) were anesthetized with sodium pentobarbital (100 mg kg−1) and killed. The prostate was isolated and cleaned in a modified Krebs–Henseleit solution (120.7 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 2.0 mM CaCl2, 1.2 mM NaH2PO4, 25.5 mM NaHCO3, and 11.5 mM D-glucose, pH 7.4) aerated with 95% O2 and 5% CO2.
Tissue segment binding experiments with [3H]-silodosin and [3H]-prazosin
Tissue segment binding was performed as described previously (Muramatsu et al., 2005). Briefly, the prostate isolated from each rabbit was cut into 25–30 pieces (approximately 1.5 × 3 × 3 mm) and used in either a saturation or competition experiment. The segments were incubated with [3H]-silodosin or [3H]-prazosin for 15–16 h at 4 °C in Krebs incubation buffer. Incubation volume was 1 ml, which was enough not to cause radioligand depletion during incubation. The composition of Krebs incubation buffer was essentially the same as a modified Krebs–Henseleit solution, except that the NaHCO3 concentration was reduced to 10.5 mM to adjust the pH to 7.4 in air. [3H]-silodosin or [3H]-prazosin (50–1000 pM) was used in saturation experiments and 300 pM [3H]-silodosin or 500 pM [3H]-prazosin was used for competition experiments. After incubation, the tissue segments were washed with incubation buffer at 4 °C for 1 min and then solubilized in 0.3 M NaOH solution. The non-specific binding was determined in the presence of 30 μM phentolamine. Radioactivity and protein concentrations were determined as described previously (Morishima et al., 2008).
Membrane binding experiments with [3H]-silodosin and [3H]-prazosin
Prostates isolated from 3–5 rabbits were pooled and used in one or two saturation or competition experiments. The prostate was homogenized in Krebs incubation buffer containing proteinase inhibitors (Complete, EDTA-free tablet, Roche, Penzberg, Germany). After centrifugation, the crude membrane preparations were used in binding experiments (Morishima et al., 2007b, 2008).
Binding saturation and competition experiments with [3H]-silodosin or [3H]-prazosin (50–1000 or 2000 pM) were carried out for 4 h at 4 °C. Reactions were terminated by rapid filtration using a Brandel cell harvester onto Whatman GF/C filters and the trapped radioactivity was measured. Non-specific binding was defined as the binding in the presence of 30 μM phentolamine.
Functional study
A prostate tissue strip was set up in an organ bath containing modified Krebs–Henseleit solution aerated with 95% O2 and 5% CO2 at 37 °C and the isometric tension change was measured (Morishima et al., 2007b). Desipramine (0.1 μM), deoxycorticosterone (5 μM) and propranolol (1 μM) were added to the bathing solution to block neural and extraneural uptake of noradrenaline and to block β-adrenoceptors. Antagonists were added to the bath 45 min before and during the evaluation of cumulative concentration–response curves for noradrenaline.
Data analysis
As described previously (Muramatsu et al., 2005; Morishima et al., 2008), binding data were analysed with Graph Pad PRISM software (Ver. 3, Graph Pad Software, San Diego, CA, USA).
The abundance of α1-adrenoceptor was expressed as the maximal binding capacity per mg of total tissue protein (Bmax: fmol mg−1 of total tissue protein); that is, the binding amount in the crude membranes was divided by the total tissue protein (homogenate protein). In saturation binding studies, data were fitted by a one-site saturation binding isotherm. In competition studies, the data were first fitted to a one- and then a two-site model, and if the residual sums of squares were significantly less for a two-site fit of the data than for a one-site fit (P-value <0.05 as determined by F-test), then a two-site model was accepted.
In functional studies, antagonist affinity estimates (pKB values) were obtained by plotting the data according to Arunlakshana and Schild (1959). When the straight lines yielded a slope with unity, the pA2 value estimated was represented as the pKB value. When a single concentration of antagonist was tested, the pKB value was also determined for a single concentration of antagonist by the concentration ratio method (Furchgott, 1972).
Data are shown as the mean±s.e.mean with the number of experiments. Values were compared by Student's t-test and P-values <0.05 were considered significant.
Drugs
The chemicals used were as follows: [3H]-silodosin (1.92 TBq mmol−1), silodosin (formerly known as KMD-3213), tamsulosin (from Kissei Pharmaceutical Co. Ltd., Matsumoto, Japan); [3H]-prazosin (7-methoxy-[3H]-prazosin, 2.74 TBq mmol−1; Amersham, Buckinghamshire, UK), and bunazosin hydrochloride (Santen Co. Ltd, Osaka, Japan). Other drugs were purchased commercially.
Results
[3H]-silodosin binding in intact segments and membrane preparations of rabbit prostate
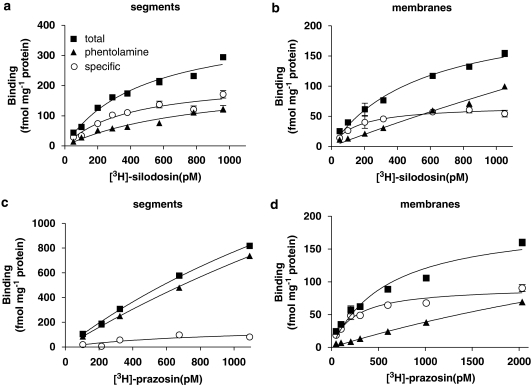
[3H]-silodosin (50–1000 pM) bound to intact segments of rabbit prostate in a concentration-dependent manner (Figure 1a). The specific binding was more than 60% of total binding at 1000 pM [3H]-silodosin and the Hill coefficient was close to unity (0.93). Therefore, it was concluded that [3H]-silodosin bound to a single class of sites. The dissociation constant (KD) and maximal binding capacity (Bmax) were 380±30 pM and 210±25 fmol mg−1 total tissue protein, respectively (n=5). [3H]-silodosin also bound to the crude membrane preparations of rabbit prostate, but the density was low (Bmax=77±8 fmol mg−1 total tissue protein, KD=210±10 pM, n=5) (Figure 1b).
Figure 1.
Binding of [3H] silodosin (a, b) and [3H]-prazosin (c, d) to rabbit prostate. Saturation binding curves in intact segments (a, c) and crude membranes (b, d) of rabbit prostate. The ordinate scale represents binding (fmol mg−1 total tissue protein). The specific binding was determined by subtracting the amount bound in the presence of 30 μM phentolamine (non-specific binding) from the total amount bound. Each point represents the mean of duplicate determinations. Each figure is representative of similar results obtained in four separate experiments.
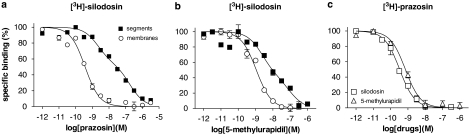
The pharmacological profiles of [3H]-silodosin-binding sites were examined in competition binding studies using several antagonists. The competition curve for prazosin in the tissue segments was shallow, better fitting a two-site model by computer analysis (Figure 2a). The pKi values for prazosin at high- and low-affinity sites were 8.8 and 7.1, respectively, and the proportion of high affinity sites was 42% (Table 1). Two similar affinity sites were estimated in the competition curves for bunazosin, RS-17053 and 5-methylurapidil (Figure 2b), but not for silodosin, tamsulosin or BMY-7378. On the other hand, in the crude membrane preparations, the tested compounds showed monophasic competition curves (Figures 2a and b; Table 1), supporting a single component of [3H]-silodosin binding sites in the membrane preparations.
Figure 2.
Competition curves for prazosin and 5-methylurapidil at [3H]-silodosin-binding sites (a, b) and for silodosin and 5-methylurapidil at [3H]-prazosin-binding sites (c) in rabbit prostate. In (a) and (b), 300 pM [3H]-silodosin binding to intact segments and membranes was in competition with prazosin (a) and 5-methylurapidil (b). In (c), 500 pM [3H]-prazosin binding to membranes was in competition with silodosin and 5-methylurapidil. Each figure is representative of similar results obtained in four separate experiments.
Table 1.
Binding affinities for various α1-adrenoceptor antagonists estimated at [3H]-silodosin and [3H]-prazosin-binding sites in rabbit prostate
| Drug |
[3H]-silodosin |
[3H]-prazosin | ||
|---|---|---|---|---|
|
Segments |
Membranes | Membranes | ||
| pKi high (% high) | pKi low | pKi | pKi | |
| Prazosin | 8.8±0.1 | 7.1±0.2 | 9.9±0.2 | 9.8±0.2 |
| (42±4%) | ||||
| Bunazosin | 9.1±0.1 | 7.8±0.2 | ||
| (34±7%) | ||||
| Silodosin | 9.5±0.1 | 9.8±0.1 | 10.1±0.1 | |
| Tamsulosin | 9.7±0.1 | |||
| RS-17053 | 9.6±0.6 | 6.8±0.3 | ||
| (27±8%) | ||||
| 5-Methylurapidil | 9.6±0.2 | 7.6±0.1 | 9.5±0.3 | 9.8±0.2 |
| (45±5%) | ||||
| BMY-7378 | 5.2±0.2 | |||
Values from four or five experiments.
pKi high and pKi low: negative logarithm of equilibrium dissociation constants at high- and low-affinity sites for tested drugs.
% high: proportion of high-affinity sites.
[3H]-prazosin binding in intact segments and membrane preparations of rabbit prostate
[3H]-prazosin (50–1000 pM) also bound to intact segments of rabbit prostate. However, the proportion of specific binding was extremely low (less than 10% of total binding at 1000 pM [3H]-prazosin, Figure 1c); thus, it was impossible to analyse the specific binding statistically. Nevertheless, in the crude membrane preparations, [3H]-prazosin (50–2000 pM) generated significant binding with a high affinity (Figure 1d) (KD=204±12 pM, Bmax=83±8 fmol mg−1 total tissue protein, n=5). The binding sites in crude membranes were competitively bound by prazosin, silodosin and 5-methylurapidil with high monophasic affinity (Figure 2c for silodosin and 5-methylurapidil, Table 1). Other drugs were not examined because of the limited amount of crude membranes.
Functional affinities for various α1-adrenoceptor antagonists
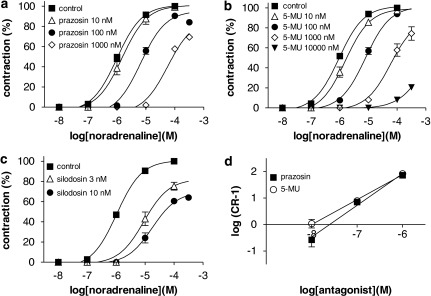
Cumulative application of noradrenaline produced concentration-dependent contractions in the strips of rabbit prostate (pEC50=6.0±0.1, n=12). This concentration–response curve was shifted to the right by relatively high concentrations of prazosin (0.1–1 μM, Figure 3a), 5-methylurapidil (0.1–1 μM, Figure 3b) or RS-17053 (1 μM). Thus, low pKB values were estimated from the Schild plot analysis and concentration ratio method (Figure 3d, Table 2). In contrast, silodosin (Figure 3c) and tamsulosin at 3–10 nM produced an unsurmountable inhibition in the concentration–response curves for noradrenaline. BMY-7378 (1 μM) had no effect on the contraction.
Figure 3.
Concentration–response curves for noradrenaline in rabbit prostate. (a–c) Effects of prazosin, 5-methylurapidil (5-MU), and silodosin, respectively, on the concentration–response curves for noradrenaline. (d) Schild plot analysis for prazosin and 5-methylurapidil; their slopes were 1.15±0.15 and 0.94±0.14, respectively. Data show mean±s.e.mean of 4–5 experiments.
Table 2.
Functional affinities for various α1-adrenoceptor antagonists estimated in contractile responses to noradrenaline in rabbit prostate
| Drug | pKB | Slope (Schild analysis) |
|---|---|---|
| Prazosin | 7.6±0.2 | 1.15 |
| Bunazosin | 7.9±0.2 | 1.03 |
| Silodosin | 9.6±0.1a | |
| Tamsulosin | 9.6±0.1a | |
| RS-17053 | 6.7±0.3a | |
| 5-Methylurapidil | 8.0±0.1 | 0.94 |
| BMY-7378 | NI |
Mean±s.e. from four or five experiments.
NI: no inhibition at 1 μM BMY-7378.
Estimated at 3 nM silodosin or tamsulosin and 1 μM RS-17053 by the concentration ratio method (Furchgott, 1972).
Discussion
In the intact segments (strips) and membrane preparations of rabbit prostate, pharmacologically different profiles of α1-adrenoceptors have been identified by radioligand binding and functional approaches. In this study, [3H]-silodosin selectively bound to the α1-adrenoceptors in the intact tissue segments, and the binding sites were composed of two different components with distinct affinities not only for prazosin, but also for other α1A-adrenoceptor-selective antagonists (5-methylurapidil and RS-17053) (Ford et al., 1996; Morishima et al., 2008). However, the subtype-non-selective antagonist tamsulosin did not discriminate between components, and BMY-7378, an α1D-adrenoceptor antagonist, showed a low affinity. According to the criteria of subclassification of α1-adrenoceptors (Hieble et al., 1995; Ford et al., 1996; Morishima et al., 2007b; Alexander et al., 2008) and the selectivity of [3H]-silodosin to α1A- and α1L-adrenoceptors (Morishima et al., 2008), it is likely that the [3H]-silodosin-binding sites in the intact segments consist of α1L subtype along with another subtype, designated here as ‘α1A-like' subtype because of its moderate affinity for prazosin (pKi=8.8) and high affinities for other α1A antagonists (silodosin, 5-methylurapidil and RS-17053). Such an α1L profile, but not an α1A-like profile, was also identified in the contractile response to noradrenaline, in which the intact strips of rabbit prostate were used. These results were consistent with those recently obtained in intact segments of human prostate (Morishima et al., 2007b).
In addition, this study further demonstrated that [3H]-prazosin at subnanomolar concentrations did not bind significantly to the α1-adrenoceptors in the intact segments of rabbit prostate (Figure 1c). This low capacity of specific binding seemed to be due to the low affinity for prazosin to the α1-adrenoceptors in intact segments. This point was confirmed from the competition of prazosin at the [3H]-silodosin-binding sites in the intact segments, from which two low-affinity constants were estimated for prazosin (pKi=8.8 and 7.1) (Figure 2a, Table 1). An extremely high proportion of non-specific binding of [3H]-prazosin (Figure 1c) made it impossible to estimate the specific binding and to use higher concentrations of [3H]-prazosin. Thus, it seemed that two phenotypes consisting of α1L subtypes and apparent α1A-like subtypes coexisted in the intact segments of rabbit prostate, but that their affinities for prazosin were too low to define specific binding adequately with this ligand.
In contrast to the intact tissue segments, the crude membrane preparations of rabbit prostate bound not only [3H]-silodosin but also [3H]-prazosin with high affinities (KD=210 and 204 pM, respectively) and with the same densities (Bmax=77 and 83 fmol mg−1 total tissue protein for [3H]-silodosin and [3H]-prazosin, respectively). The binding sites of both radioligands were composed of a single class of α1-adrenoceptor, and the pharmacological profile corresponded to the characteristic profile of classical α1A-adrenoceptors (pKi for prazosin=approximately 9.8) (Daniels et al., 1999; Ramsay et al., 2004). This suggested that two distinct α1-adrenoceptor phenotypes occurring in intact segments became a single phenotype with a high affinity for prazosin upon homogenization, resulting in significant specific binding at subnanomolar concentrations of [3H]-prazosin. Thus, only one phenotype corresponding to classical α1A-adrenoceptors was detected in the membrane preparations of rabbit prostate.
However, the densities of α1-adrenoceptors detected in the membrane preparations were substantially lower than the Bmax (210 fmol mg−1 total tissue protein) for [3H]-silodosin binding in the intact segments. This represents a significant loss of α1-adrenoceptors in the crude membranes. Such a loss of receptor after homogenization has also been reported in the α1-adrenoceptors of blood vessels (Faber et al., 2001; Hiraizumi-Hiraoka et al., 2004; Tanaka et al., 2004) and prostate (Morishima et al., 2007b), the β-adrenoceptors of heart (Horinouchi et al. 2006) and the muscarinic acetylcholine receptors of urinary bladder (Anisuzzaman et al., 2008). However, no loss has been observed in the α1-adrenoceptors and muscarinic acetylcholine receptors of cerebral cortex (Muramatsu et al., 2005; Morishima et al., 2008). Therefore, it is likely that the low yield of receptors after homogenization is not related to either the kind of receptors or the changes in phenotypes mentioned above. Rather, it may be dependent on the tissues, as such losses are more evident in fibrous tissues (Muramatsu et al., 2005).
Therefore, this study has identified three distinct pharmacological phenotypes of α1-adrenoceptors in the rabbit prostate: α1L phenotype with low affinity for prazosin (pKi=7.1) in the intact segments, α1A-like phenotype with relatively low affinity for prazosin (pKi=8.8) in the intact segments and typical α1A phenotype having the same profile as classical α1A-adrenoceptor (pKi for prazosin=9.8) in the membranes. Ford's group have reported that recombinant α1A-adrenoceptors behave functionally as the α1L phenotype (Ford et al., 1997; Daniels et al., 1999). Our recent studies, including this one, have revealed that the α1L phenotype converts into the classical α1A-adrenoceptor after homogenization (Hiraizumi-Hiraoka et al., 2004; Morishima et al., 2007b, 2008). At the mRNA level, the α1A-adrenoceptor was demonstrated to be the dominant α1-adrenoceptor in the rabbit prostate (Piao et al., 2000), and α1B- and α1D-adrenoceptors were not detected in this and previous binding studies (Testa et al., 1993). Recently, we have found that the α1L-adrenoceptor or its phenotype occurs in the wild-type mouse but is selectively abolished in α1A-adrenoceptor gene knockout mice (Morishima et al., 2007a). These lines of evidence suggest that the three distinct phenotypes identified in the rabbit prostate and under independent assay conditions are not genetically different subtypes; rather they are more likely to have originated from a single α1A-adrenoceptor gene product.
Recently, it has been suggested that antagonist affinity may not necessarily remain constant between tissues expressing the same receptors, especially under different assay conditions (Kenakin et al., 1995; Muramatsu et al., 2005; Nelson and Challiss, 2007). Among the three different phenotypes observed in this study, two distinct α1A phenotypes may be simply accounted for by a change in receptor environment between intact segments and membranes (Muramatsu et al., 2005). However, another α1L phenotype was identified as an entity, completely distinct from the α1A-like phenotype in the same tissue segments and under the same assay conditions. Therefore, if both α1L and α1A-like phenotypes originate from a single gene, additional mechanisms or factors that would display the α1L profile may be involved in the expression. We are now exploring these underlying mechanisms.
How many phenotypes exist or are detected in native tissues? The binding and functional affinities (pKi/pKB) of α1L phenotype for prazosin were 7.1/7.6∼8.1 in the rabbit prostate (this study; Testa et al., 1997; van der Graaf et al., 1997), which were slightly lower than the affinities (8.3/8.4∼8.7) estimated in the human prostate (Ford et al., 1996; Morishima et al., 2007b) and those (8.3/7.9) in the rabbit ear artery (Hiraizumi-Hiraoka et al., 2004); however, the affinities for prazosin were higher than the affinities (6.5/6.7) estimated in the rabbit iris dilator (I Muramatsu et al., unpublished data). On the other hand, the affinities for some antagonists such as RS-17053 are relatively consistent with α1L phenotypes of the rabbit and human prostates and the rabbit ear artery. These results suggest that there may be wide variation in the pharmacological profile among α1L phenotypes (and probably other α1-adrenoceptor phenotypes or subtypes) identified in many native tissues and species, and that the different pharmacological profiles may be distinguished by a subset of ligands. At this point, it is interesting to remember that a relatively wide range of functional affinities estimated for several antagonists was originally categorized into one subtype (group) in primary α1-adrenoceptor subclassification (Drew, 1985; Flavahan and Vanhoutte, 1986; Muramatsu et al., 1990).
There has been significant controversy regarding the identity of α1-adrenoceptors mediating prostatic contraction. This appears to be mainly due to a discrepancy between α1-adrenoceptor profiles obtained by the bioassay approach (α1L phenotype) and the membrane binding approach (classical α1A-adrenoceptor). However, this study clearly shows that the classical α1A phenotype does not occur in the intact prostatic segments and that there is a good concordance between the functional α1L phenotype and binding α1L phenotype, both of which were obtained from intact strips or segments. These results strongly suggest that the α1L phenotype is a functional α1-adrenoceptor involved in prostatic contraction and is a major target of α1-adrenoceptor antagonist therapy of urinary outlet obstruction in patients with benign prostatic hyperplasia (Lepor and Shapiro, 1994; Cooper et al., 1999; Chapple, 2001; Andersson, 2002).
In conclusion, three pharmacologically distinct phenotypes of α1-adrenoceptor were detected in the rabbit prostate, and all of the phenotypes appeared to be derived from a single α1A-adrenoceptor gene. Among them, only the α1L phenotype occurring in intact tissue segments is involved in prostatic contraction.
Note added in proof: Another paper in press has also dealt with this question: Gray KT, Short JL, Ventura S (2008). The α1A-adrenoceptor gene is required for the α1L-adrenoceptor-mediated response in isolated preparations of the mouse prostate. Br J Pharmacol [e-pub ahead of print: advance online 16 June 2008; doi:10.1038/bjp.2008.245].
Acknowledgments
This study is supported by Grant-in-Aid for Scientific Research and the twenty-first COE Research Program (Medical Science) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by a grant from Smoking Research Foundation of Japan.
Abbreviations
- BMY 7378, 8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4,5]decane-7,9-dione dihydrochloride; Bmax
maximal binding capacity
- RS-17053
N-[2-(2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-α,α-dimethyl-1H-indole-3-ethamine hydrochloride
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br. J. Pharmacol. 2008;153 Suppl. 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KE. Alpha-adrenoceptors and benign prostatic hyperplasia: basic principles for treatment with alpha-adrenoceptor antagonists. World J Urol. 2002;19:390–396. [PubMed] [Google Scholar]
- Anisuzzaman ASM, Morishima S, Suzuki F, Tanaka T, Yoshiki H, Sathi ZS, et al. Assessment of muscarinic receptor subtypes in human and rat lower urinary tract by tissue segment binding assay. J Pharmacol Sci. 2008;106:271–279. doi: 10.1254/jphs.fp0071435. [DOI] [PubMed] [Google Scholar]
- Argyle SA, McGrath JC. An alpha(1A)/alpha(1L)-adrenoceptor mediates contraction of canine subcutaneous resistance arteries. J Pharmacol Exp Ther. 2000;295:627–633. [PubMed] [Google Scholar]
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol. 1959;114:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple CR. Alpha adrenoceptor antagonists in the year 2000: is there anything new. Curr Opin Urol. 2001;11:9–16. doi: 10.1097/00042307-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Cooper KL, McKiernan JM, Kaplan SA. Alpha-adrenoceptor antagonists in the treatment of benign prostatic hyperplasia. Drugs. 1999;57:9–17. doi: 10.2165/00003495-199957010-00002. [DOI] [PubMed] [Google Scholar]
- Daniels DV, Gever JR, Jasper JR, Kava MS, Lesnick JD, Meloy TD, et al. Human cloned alpha1A-adrenoceptor isoforms display alpha1L-adrenoceptor pharmacology in functional studies. Eur J Pharmacol. 1999;370:337–343. doi: 10.1016/s0014-2999(99)00154-5. [DOI] [PubMed] [Google Scholar]
- Drew GM. What do antagonists tell us about α-adrenoceptors. Clin Sci. 1985;68 Suppl. 10:15s–19s. doi: 10.1042/cs068s015. [DOI] [PubMed] [Google Scholar]
- Faber JE, Yang N, Xin XH. Expression of alpha-adrenoceptor subtypes by smooth muscle cells adventitial fibroblast in rat aorta in cell culture. J Pharmacol Exp Ther. 2001;298:441–452. [PubMed] [Google Scholar]
- Flavahan NA, Vanhoutte PM. Alpha-1 and alpha-2 adrenoceptor: response coupling in canine saphenous and femoral veins. Trends Pharmacol Sci. 1986;7:347–349. [PubMed] [Google Scholar]
- Ford AP, Arredondo NF, Blue DR, Jr, Bonhaus DW, Jasper J, Kava MS, et al. RS-17053 (N-[2-(2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-alpha, alpha-dimethyl-1H-indole-3-ethanamine hydrochloride), a selective alpha 1A-adrenoceptor antagonist, displays low affinity for functional alpha 1-adrenoceptors in human prostate: implications for adrenoceptor classification. Mol Pharmacol. 1996;49:209–215. [PubMed] [Google Scholar]
- Ford AP, Daniels DV, Chang DJ, Gever JR, Jasper JR, Lesnick JD, et al. Pharmacological pleiotropism of the human recombinant alpha1A-adrenoceptor: implications for alpha1-adrenoceptor classification. Br J Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF.The classification on adrenoceptors (adrenergic receptors): an evaluation from the standpoint of receptor theory Handbuch der Experimentellen Pharmacology 1972Springer: New York; 283–335.In: Blaschko H, Muscholl E (eds).vol 3 [Google Scholar]
- Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, et al. International Union of Pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- Hiraizumi-Hiraoka Y, Tanaka T, Yamamoto H, Suzuki F, Muramatsu I. Identification of alpha-1L adrenoceptor in rabbit ear artery. J Pharmacol Exp Ther. 2004;310:995–1002. doi: 10.1124/jpet.104.066985. [DOI] [PubMed] [Google Scholar]
- Horinouchi T, Morishima S, Tanaka T, Suzuki F, Tanaka Y, Koike K, et al. Pharmacological evaluation of plasma membrane β-adrenoceptors in rat hearts using the tissue segment binding method. Life Sci. 2006;79:941–948. doi: 10.1016/j.lfs.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Morgan P, Lutz M. On the importance of the ‘antagonist assumption' to how receptors express themselves. Biochem Pharmacol. 1995;50:17–26. doi: 10.1016/0006-2952(95)00137-o. [DOI] [PubMed] [Google Scholar]
- Lepor H, Shapiro E. Prostatic alpha adrenoceptors. Prog Clin Biol Res. 1994;386:271–277. [PubMed] [Google Scholar]
- Lomasney JW, Cotecchia S, Lefkowitz RJ, Caron MG. Molecular biology of alpha-adrenergic receptors: implications for receptor classification and for structure–function relationships. Biochim Biophys Acta. 1991;1095:127–139. doi: 10.1016/0167-4889(91)90075-9. [DOI] [PubMed] [Google Scholar]
- Michelotti GA, Price DT, Schwinn DA. Alpha 1-adrenergic receptor regulation: basic science and clinical implications. Pharmacol Ther. 2000;88:281–309. doi: 10.1016/s0163-7258(00)00092-9. [DOI] [PubMed] [Google Scholar]
- Morishima S, Suzuki F, Anisuzzaman ASM, Tanaka T, Akino H, Yokoyama O, et al. Molecular and functional characterization of alpha-1L adrenoceptor 2007a. In 5th International Symposium on Receptor Mechanisms, Signal Transduction and Drug Effects (Shizuoka, Japan) abstract p48–p49
- Morishima S, Suzuki F, Yoshiki H, Md Anisuzzaman AMS, Sathi ZS, Tanaka T, et al. Identification of the alpha(1L)-adrenoceptor in rat cerebral cortex and possible relationship between alpha(1L)- and alpha(1A)-adrenoceptors. Br J Pharmacol. 2008;153:1485–1494. doi: 10.1038/sj.bjp.0707679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima S, Tanaka T, Yamamoto H, Suzuki F, Akino H, Yokoyama O, et al. Identification of alpha-1L and alpha-1A adrenoceptors in human prostate by tissue segment binding. J Urol. 2007b;177:377–381. doi: 10.1016/j.juro.2006.08.080. [DOI] [PubMed] [Google Scholar]
- Muramatsu I, Ohmura T, Kigoshi S, Hashimoto S, Oshita M. Pharmacological subclassification of alpha 1-adrenoceptors in vascular smooth muscle. Br J Pharmacol. 1990;99:197–201. doi: 10.1111/j.1476-5381.1990.tb14678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu I, Tanaka T, Suzuki F, Li Z, Hiraizumi-Hiraoka Y, Anisuzzaman AS, et al. Quantifying receptor properties: the tissue segment binding method—a powerful tool for the pharmacome analysis of native receptors. J Pharmacol Sci. 2005;98:331–339. doi: 10.1254/jphs.cpj05001x. [DOI] [PubMed] [Google Scholar]
- Murata S, Taniguchi T, Muramatsu I. Pharmacological analysis of the novel, selective alpha1-adrenoceptor antagonist, KMD-3213, and its suitability as a tritiated radioligand. Br J Pharmacol. 1999;127:19–26. doi: 10.1038/sj.bjp.0702489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CP, Challiss RA. ‘Phenotypic' pharmacology: the influence of cellular environment on G protein-coupled receptor antagonist and inverse agonist pharmacology. Biochem Pharmacol. 2007;73:737–751. doi: 10.1016/j.bcp.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Piao H, Taniguchi T, Nakamura S, Zhu J, Suzuki F, Mikami D, et al. Cloning of rabbit alpha(1b)-adrenoceptor and pharmacological comparison of alpha(1a)-, alpha(1b)- and alpha(1d)-adrenoceptors in rabbit. Eur J Pharmacol. 2000;396:9–17. doi: 10.1016/s0014-2999(00)00171-0. [DOI] [PubMed] [Google Scholar]
- Ramsay D, Carr IC, Pediani J, Lopez-Gimenez JF, Thurlow R, Fidock M, et al. High-affinity interactions between human alpha1A-adrenoceptor C-terminal splice variants produce homo- and heterodimers but do not generate the alpha1L-adrenoceptor. Mol Pharmacol. 2004;66:228–239. doi: 10.1124/mol.66.2.228. [DOI] [PubMed] [Google Scholar]
- Takeda M, Hatano A, Arai K, Obara K, Tsutsui T, Takahashi K. Alpha1- and alpha2-adrenoceptors in BPH. Eur Urol. 1999;36 Suppl 1:31–34. doi: 10.1159/000052315. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Zhang L, Suzuku F, Muramatsu I. Alpha-1 adrenoceptors: evaluation of receptor subtype binding kinetics in intact arterial tissues and comparison with membrane binding. Br J Pharmacol. 2004;141:468–476. doi: 10.1038/sj.bjp.0705627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa R, Guarneri L, Angelico P, Poggesi E, Taddei C, Sironi G, et al. Pharmacological characterization of the uroselective alpha-1 antagonist Rec 15/2739 (SB 216469): role of the alpha-1L adrenoceptor in tissue selectivity, part II. J Pharmacol Exp Ther. 1997;281:1284–1293. [PubMed] [Google Scholar]
- Testa R, Guarneri L, Ibba M, Strada G, Poggesi E, Taddei C, et al. Characterization of alpha 1-adrenoceptor subtypes in prostate and prostatic urethra of rat, rabbit, dog and man. Eur J Pharmacol. 1993;249:307–315. doi: 10.1016/0014-2999(93)90527-o. [DOI] [PubMed] [Google Scholar]
- Van der Graaf PH, Deplanne V, Duquenne C, Angel I. Analysis of alpha1-adrenoceptors in rabbit lower urinary tract and mesenteric artery. Eur J Pharmacol. 1997;327:25–32. doi: 10.1016/s0014-2999(97)89674-4. [DOI] [PubMed] [Google Scholar]
- Zhong H, Minneman KP. Alpha1-adrenoceptor subtypes. Eur J Pharmacol. 1999;375:261–276. doi: 10.1016/s0014-2999(99)00222-8. [DOI] [PubMed] [Google Scholar]