Abstract
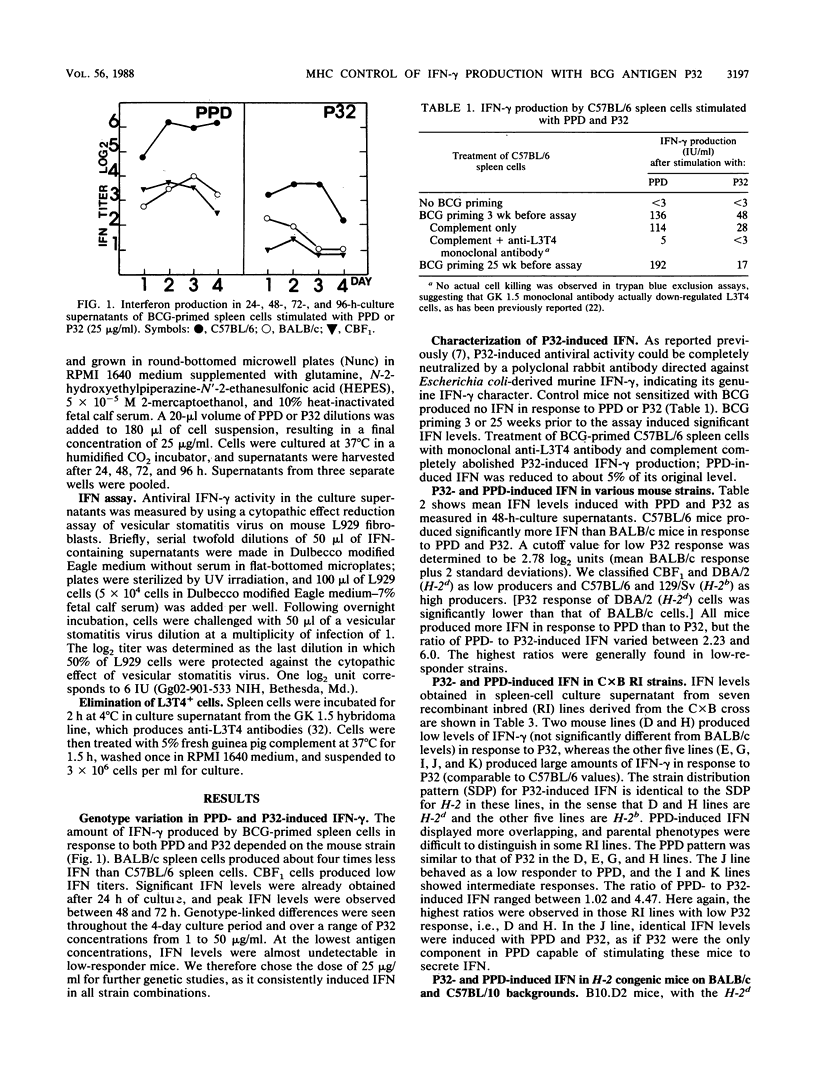
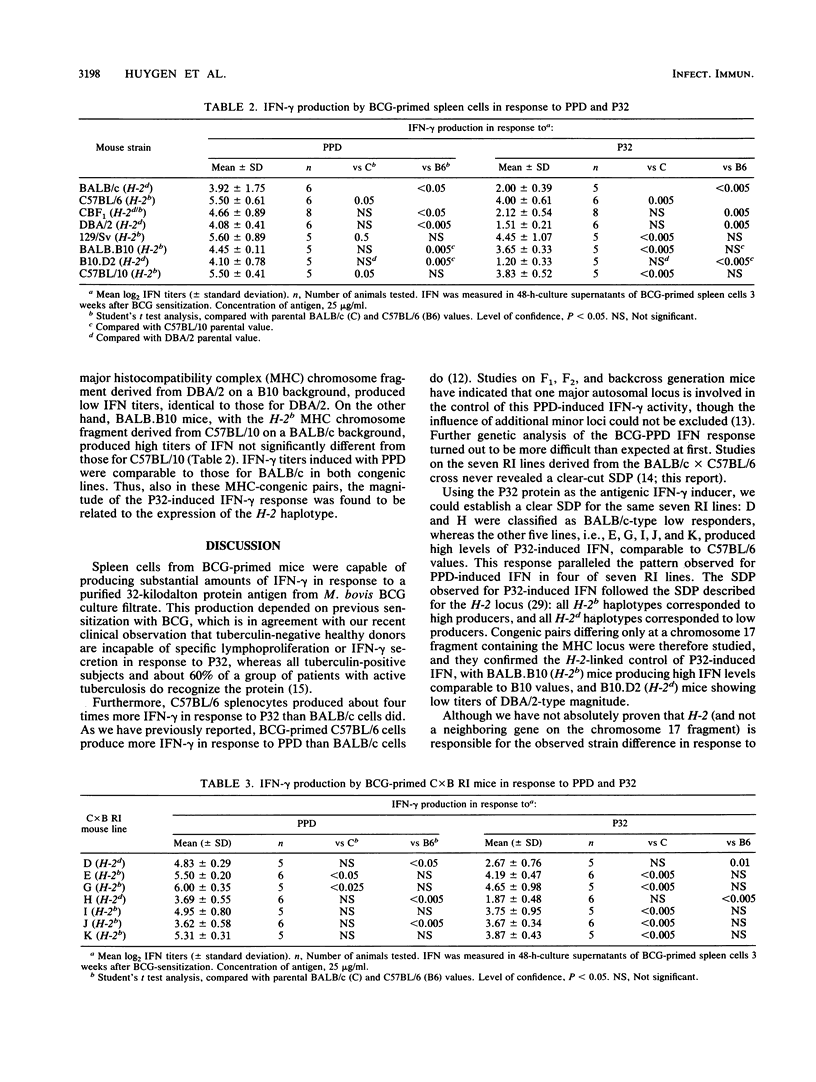
A 32-kilodalton protein antigen (P32) was previously purified to homogeneity from culture filtrate of Mycobacterium bovis BCG (J. De Bruyn, K. Huygen, R. Bosmans, M. Fauville, R. Lippens, J. P. Van Vooren, P. Falmagne, H. G. Wiker, M. Harboe, and M. Turneer, Microb. Pathog. 2:351-366, 1987). Spleen cells from BCG-sensitized mice produce significant amounts of gamma interferon (IFN-gamma) in response to this P32 protein. The amount of secreted IFN-gamma is influenced by mouse genotype, with C57BL/6 (H-2b), C57BL/10 (H-2b), and 129/Sv (H-2b) mice producing about four times more than BALB/c (H-2d), CBF1 (H-2d/b), and DBA/2 (H-2d) mice do. Analysis of seven recombinant inbred strains derived from the BALB/c x C57BL/6 cross and of congenic mice differing in major histocompatibility complex-coding chromosome 17 fragments indicates a probable H-2-linked control of this IFN-gamma induction, with H-2b cells producing high titers and H-2d cells producing low titers in response to the P32 antigen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell J., Freeman J., Bradley D. Influence of H-2 complex on acquired resistance to Leishmania donovani infection in mice. Nature. 1980 Jan 3;283(5742):72–74. doi: 10.1038/283072a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Closs O., Løvik M., Wigzell H., Taylor B. A. H-2-linked gene(s) influence the granulomatous reaction to viable Mycobacterium lepraemurium in the mouse. Scand J Immunol. 1983 Jul;18(1):59–63. doi: 10.1111/j.1365-3083.1983.tb00836.x. [DOI] [PubMed] [Google Scholar]
- De Bruyn J., Bosmans R., Turneer M., Weckx M., Nyabenda J., Van Vooren J. P., Falmagne P., Wiker H. G., Harboe M. Purification, partial characterization, and identification of a skin-reactive protein antigen of Mycobacterium bovis BCG. Infect Immun. 1987 Jan;55(1):245–252. doi: 10.1128/iai.55.1.245-252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyn J., Huygen K., Bosmans R., Fauville M., Lippens R., Van Vooren J. P., Falmagne P., Weckx M., Wiker H. G., Harboe M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987 May;2(5):351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- Harboe M., Mshana R. N., Closs O., Kronvall G., Axelsen N. H. Cross-reactions between mycobacteria. II. Crossed immunoelectrophoretic analysis of soluble antigens of BCG and comparison with other mycobacteria. Scand J Immunol. 1979;9(2):115–124. doi: 10.1111/j.1365-3083.1979.tb02713.x. [DOI] [PubMed] [Google Scholar]
- Hla K. M., Feussner J. R. Screening for pseudohypertension. A quantitative, noninvasive approach. Arch Intern Med. 1988 Mar;148(3):673–676. [PubMed] [Google Scholar]
- Huygen K., Palfliet K. In vitro production of gamma interferon is dependent on the mouse genotype. J Interferon Res. 1983;3(1):129–137. doi: 10.1089/jir.1983.3.129. [DOI] [PubMed] [Google Scholar]
- Huygen K., Palfliet K. Strain variation in interferon gamma production of BCG-sensitized mice challenged with PPD II. Importance of one major autosomal locus and additional sexual influences. Cell Immunol. 1984 Apr 15;85(1):75–81. doi: 10.1016/0008-8749(84)90279-x. [DOI] [PubMed] [Google Scholar]
- Huygen K., Palfliet K. Strain variation in interferon gamma production of BCG-sensitized mice challenged with PPD. I. CBA/Ca mice are low producers in vivo, but high producers in vitro. Cell Immunol. 1983 Sep;80(2):329–334. doi: 10.1016/0008-8749(83)90121-1. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Sharp K. Control by H-2 genes of murine antibody responses to protein antigens of Mycobacterium tuberculosis. Immunology. 1986 Nov;59(3):329–332. [PMC free article] [PubMed] [Google Scholar]
- Klein J., Figueroa F., David C. S. H-2 haplotypes, genes and antigens: second listing. II. The H-2 complex. Immunogenetics. 1983;17(6):553–596. doi: 10.1007/BF00366126. [DOI] [PubMed] [Google Scholar]
- Neta R., Salvin S. B. In vivo release of lymphokines in different strains of mice. Cell Immunol. 1980 Apr;51(1):173–178. doi: 10.1016/0008-8749(80)90247-6. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Kaplan G., Levy E., Sarno E. N., Kushner P., Granelli-Piperno A., Vieira L., Colomer Gould V., Levis W., Steinman R. Defective gamma interferon production in leprosy. Reversal with antigen and interleukin 2. J Exp Med. 1983 Dec 1;158(6):2165–2170. doi: 10.1084/jem.158.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J. E., Blackwell J. M., O'Brien A. D., Bradley D. J., Glynn A. A. Are the Lsh and Ity disease resistance genes at one locus on mouse chromosome 1? Nature. 1982 Jun 10;297(5866):510–511. doi: 10.1038/297510a0. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Burakoff S. J., Greenstein J. L. The role of the L3T4 molecule in mitogen and antigen-activated signal transduction. Cell. 1987 Jun 19;49(6):845–853. doi: 10.1016/0092-8674(87)90622-2. [DOI] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Shigekane V. M., Fisher W. L., Locksley R. M. Cellular and humoral immunity to Leishmania major in genetically susceptible mice after in vivo depletion of L3T4+ T cells. J Immunol. 1987 Aug 15;139(4):1303–1309. [PubMed] [Google Scholar]
- Sadick M. D., Locksley R. M., Tubbs C., Raff H. V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-gamma in response to Leishmania antigens in vitro. J Immunol. 1986 Jan;136(2):655–661. [PubMed] [Google Scholar]
- Singh S. P., Mehra N. K., Dingley H. B., Pande J. N., Vaidya M. C. Human leukocyte antigen (HLA)-linked control of susceptibility to pulmonary tuberculosis and association with HLA-DR types. J Infect Dis. 1983 Oct;148(4):676–681. doi: 10.1093/infdis/148.4.676. [DOI] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Stach J. L., Gros P., Forget A., Skamene E. Phenotypic expression of genetically-controlled natural resistance to Mycobacterium bovis (BCG). J Immunol. 1984 Feb;132(2):888–892. [PubMed] [Google Scholar]
- Turneer M., Van Vooren J. P., De Bruyn J., Serruys E., Dierckx P., Yernault J. C. Humoral immune response in human tuberculosis: immunoglobulins G, A, and M directed against the purified P32 protein antigen of Mycobacterium bovis bacillus Calmette-Guérin. J Clin Microbiol. 1988 Sep;26(9):1714–1719. doi: 10.1128/jcm.26.9.1714-1719.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- Zawatzky R., Hilfenhaus J., Marcucci F., Kirchner H. Experimental infection of inbred mice with herpes simplex virus type 1. I. Investigation of humoral and cellular immunity and of interferon induction. J Gen Virol. 1981 Mar;53(Pt 1):31–38. doi: 10.1099/0022-1317-53-1-31. [DOI] [PubMed] [Google Scholar]
- de Gee A. L., Sonnenfeld G., Mansfield J. M. Genetics of resistance to the African trypanosomes. V. Qualitative and quantitative differences in interferon production among susceptible and resistant mouse strains. J Immunol. 1985 Apr;134(4):2723–2726. [PubMed] [Google Scholar]
- de Maeyer E., de Maeyer-Guignard J. Gene with quantitative effect on circulating interferon induced by Newcastle disease virus. J Virol. 1969 May;3(5):506–512. doi: 10.1128/jvi.3.5.506-512.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Gonzalez N. M., de Vries R. R., Convit J., van Rood J. J. HLA-linked control of predisposition to lepromatous leprosy. J Infect Dis. 1985 Jan;151(1):9–14. doi: 10.1093/infdis/151.1.9. [DOI] [PubMed] [Google Scholar]



