Abstract
Background and purpose:
To determine whether there is an association between vascular NADPH oxidase (NOX), superoxide, the small GTPase Rac1 and PDE type 5 (PDE5) in human vascular smooth muscle cell (hVSMCs).
Experimental approach:
hVSMCs were incubated with xanthine–xanthine oxidase (X-XO; a superoxide generating system) or the thromboxane A2 analogue, U46619 (±superoxide dismutase (SOD) or apocynin) for 16 h. The expression of PDE5 and NOX-1 was assessed using Western blotting and superoxide measured. The role of Rac1 in superoxide generation was assessed by overexpressing either the dominant-negative or constitutively active Rac isoforms. The effects of iloprost, DETA-NONOate and the Rho-kinase inhibitor, Y27632, on PDE5 and NOX-1 expression were also studied.
Key results:
Following 16 h incubation, U46619 and X-XO promoted the expression of PDE5 and NOX-1, an effect blocked by SOD or apocynin when co-incubated over the same time course. X-XO and U46619 both promoted the formation of superoxide. Overexpression of dominant-negative Rac1 or addition of iloprost, DETA-NONOate or Y27632 completely blocked both superoxide release and PDE5 protein expression and activity.
Conclusions and implications:
These data demonstrate that superoxide derived from NOX upregulates the expression of PDE5 in human VSMCs. As PDE5 hydrolyses cyclic GMP, this effect may blunt the vasculoprotective actions of NO.
Keywords: superoxide, type 5 PDE, NADPH oxidase, vascular smooth muscle cell, Rho-kinase, nitric oxide
Introduction
Nitric oxide (NO) is an endogenous vascular factor that protects against vasculopathy (Jeremy et al., 1999). These protective effects include the inhibition of vascular smooth muscle cell (VSMC) replication and migration, vasodilation, inhibition of adhesion molecule expression and prevention of thrombosis (Jeremy et al., 1999; Friebe and Koesling, 2003). The biological actions of NO are mediated principally by the activation of GC, which generates cGMP (Jeremy et al., 1999). In turn, cGMP activates PKG which then phosphorylates other proteins that elicit vasculoprotection (Jeremy et al., 1999). The biological effects of the NO–PKG system are reduced by type 5 PDEs (PDE5), which hydrolyze cGMP to inactive GMP (Maurice et al., 2003; Rybalkin et al., 2003). It follows that an increase in PDE5 activity, by decreasing intracellular cGMP formation, would diminish the protective attributes of NO and therefore promote vasculopathy. Little is known of the factors that may modulate PDE5 expression in vascular tissue, however.
A major vascular factor that alters the bioactivity of NO is superoxide (O2•−). Increased formation of O2•− is now considered central to the aetiology of cardiovascular diseases as O2•− reacts with NO leading to reduced ‘NO drive' (Cai and Harrison, 2000; Jeremy et al., 2004; Li and Shah, 2004). A principal intravascular source of O2•− is nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Cai et al., 2003; Muzaffar et al., 2003, 2004a, 2004b, 2005a, 2005b). Many vasculopathic factors increase the activity and expression of NADPH oxidase. These include thromboxane A2, cytokines, angiotensin II, hypoxia and O2•− itself (Muzaffar et al., 2004a, 2004b, 2005a, 2005b, 2005c, 2006). Conversely, NO and NO donors inhibit the activity and expression of NADPH oxidase (and therefore O2•−) in VSMCs through a cGMP-PKG-mediated mechanism (Muzaffar et al., 2003, 2004a). This effect of NO is augmented by sildenafil, a PDE5 inhibitor (Muzaffar et al., 2005b). PDE5 status may therefore be crucial to these antioxidative actions of NO.
A key effect of O2•− is that it activates signal-transduction systems and kinases, including Rho/Rho-kinase, that control gene expression (Jin et al., 2004; Li and Shah, 2004), It is not unreasonable to suggest, therefore, that O2•− may increase the expression of PDE5, which would constitute a self-amplifying positive feedback mechanism that may augment vasculopathy by reducing the antioxidative and other protective actions of NO.
This study was therefore undertaken to determine whether O2•− derived from NADPH oxidase influenced PDE5 expression and activity in isolated human VSMCs(hVSMCs). NADPH oxidase was induced by the thromboxane A2 agonist, U46619. The concomitant expression of NOX-1 and NOX-4 (catalytic subunits of NADPH oxidase found in VSMCs) was studied in parallel. To study the direct effects of O2•−, cells were incubated with xanthine–xanthine oxidase (X-XO), and PDE5 and NOX-1 expressions were assessed. As O2•− activates Rho-kinase (Jin et al., 2004), the possible role of this kinase in mediating the upregulation of PDE5 was also studied using the specific inhibitor, Y27632. Phosphorylation of the subunit of myosin phosphatase that targets myosin light-chain (MYPT1) was also studied, as this is an index of Rho-kinase activity (Ito et al., 2004). Effects on the activation of the Rho-like small GTPase, Rac1, were also studied using adenoviruses, as Rac1 is a key component of NOX activation (Hordijk, 2006). Finally, the effects of iloprost, a prostacyclin mimetic, and an NO donor, NONOate, were studied as it had been established that prostacylin and NO inhibited NOX expression and activity (Muzaffar et al., 2004a, 2004b). It follows, therefore, that iloprost and NONOate should block the expression of PDE5, which in turn would expand the therapeutic attributes and possible novel applications of these important drug classes.
Methods
The investigation conforms to the principles outlined in the Declaration of Helsinki for the use of human tissue or subjects.
Culture and incubation of VSMCs
Saphenous veins were obtained from patients undergoing coronary artery bypass graft surgery for which ethical approval and patient consent had been obtained. Veins were placed in medium RPMI 1640 containing 2% amphotericin (Gibco BRL, Paisley, Scotland) and 0.4% heparin (Sigma Chemical Co., Poole Dorset, UK). Human VSMCs were then grown in Dulbecco's minimum essential medium-Glutamax without sodium pyruvate (DMEM; Gibco BRL), containing 100 U mL−1 penicillin (Sigma), 100 μg mL−1 streptomycin (Sigma) and 10% foetal calf serum (Gibco BRL). After passage 4, hVSMCs were seeded in a 6-well or 24-well plates at a density of 6 × 104 cells per well and cultured for 2 days in DMEM/foetal calf serum. Cells were then rendered quiescent for 3 days in serum-free medium (Gibco BRL) before commencing experiment. Under these conditions, there was no loss of cell numbers over this time course. Cells were incubated with the thromboxane A2 analogue, U46619 (100 nM) or X-XO system (100 μM-1 mU mL−1) for 16 h in the continual presence of one of the following: superoxide dismutase (SOD; 500 U mL−1); apocynin (10 μM); allopurinol (100 μM); L-NAME (100 μM); rotenone (10 μM); Rho-kinase inhibitor, Y27632; iloprost (100 ng mL−1) or NONOate (10 μM). Release of O2•− was measured by ferricytochrome c assay, and protein expression was measured by the Western analysis.
Measurement of O2•−
The measurement of O2•− formation and release by cultured cells was performed by the detection of ferricytochrome c reduction, as previously described (Muzaffar et al., 2003, 2004a, 2004b, 2005c). Following incubation, cells were washed three times with phosphate-buffered saline (PBS) and equilibrated in DMEM without phenol red for 10 min at 37 °C in a 95% air-5% CO2 incubator (Heraeus, Hera Cell, Kandro Laboratory Products, Langenselbold, Germany). A volume of 20 μM horseradish cytochrome c with or without 500 U mL−1 copper-zinc SOD was added to the cells and incubated at 37 °C in a 95% air-5% CO2 incubator for an hour. The final volume of the reaction mixture was 0.5 mL per well. After 1 h, the reaction medium was removed and maximum rate of reduction of cytochrome c was determined at 550 nM on a temperature controlled Anthos Lucy 1 spectrometer (Lab-tech International, Ringmer, East Sussex, UK) and converted to micromoles of O2•−, using ΔE550 nm=21.1 mM cm−1 min−1 as the extinction coefficient for (reduced-oxidized) cytochrome c. The reduction of cytochrome c that could be inhibited with SOD reflected actual formation of O2•−. Cells were rinsed in PBS, lysed with 0.1% v/v Triton-X 100 and total protein content was measured using the BCA-protein assay kit.
Western blotting
For Western analysis of PDE5 and NOX-1, hVSMC were washed and lysed with Tris buffer (100 mM, pH 6.8) containing 10% glycerol and 1% sodium dodecyl sulphate (Muzaffar et al., 2003, 2004a, 2004b, 2005c). Extracts were boiled at a 1:1 ratio with the loading buffer containing Tris (125 mM, pH 6.8); 4% w/v sodium dodecyl sulphate; 10% v/v glycerol; 4% v/v 2-mercaptoethanol and 2 mg mL−1 bromophenol blue. Total cell lysates of equal protein (30–50 μg) were loaded onto 10% Tris-glycine sodium dodecyl sulphate gels and separated by electrophoresis. After transfer to nitrocellulose, the blots were primed overnight with either NOX-1 or NOX-4 antibody (both 1:500) or PDE5 antibody (1:1000) or Thr696 phospho-MYPT1 (1:1000). The blots were then incubated with the corresponding secondary antibodies conjugated to horseradish peroxidase for an hour and developed by enhanced chemiluminescence (Amersham International, Little chalfond, Buckinghamshire, UK). Rainbow markers (10–250 kDa; Amersham) were used for molecular weight determination. Membranes were either reprobed with anti-GAPDH monoclonal antibody (Chemicon International, Temecula, CA, USA) or α-actin antibody as an internal control for equal protein loading.
Quantitative and semiquantitative reverse transcription–PCR
Total RNA was extracted from hVSMCs using the RNeasy RNA extRaction kit (Qiagen, Crawley, West Sussex, UK). RNA quality was determined using an Agilent Bioanalyzer 2100 (Agilent Technologies UK Ltd, Stockport, Cheshire, UK) and samples with rRNA 28S/18S ratios more or equal to 1.8 were used. First-strand cDNA was synthesized by random priming using the Quantitect Reverse Transcription kit (Qiagen). The primer sets used to amplify various NOX isoforms and PDE5 were as follows: NOX-1 (forward: 5′-GTTTTGGAATTGCAGATGAACAAG-3′, reverse: 5′-CATACTGGAAAACATCCTCACTGG-3′; expected fragment size=268 bp), NOX-4 (forward: 5′-CCTCAGCATCTGTTCTTAACCTCA-3′, reverse: 5′-GCTGCATTCAGTTCAACAAAGTCT-3′; 256 bp), NOX-5 (forward: 5′-AAGACACTATCTGGCTGCACATTC-3′, reverse: 5′-GGCAAGTATGCTTTCTTTTCTGGT-3′; 342 bp) and PDE5 (forward: 5′-AAAAGGACTTTGCTGCTTATTTGG-3′, reverse: 5′-AAAAGAATCGGAGCAATCTTCATC-3′; 242 bp). PCR amplification was performed with Taq polymerase for 35 cycles at 94 °C for 30 s, 58 °C for 30 s and 72 °C for 45 s. Products were confirmed by electrophoresis on a 1.5% agarose gel. Quantitative PCR was performed using a LightCycler (Roche Products Ltd., Hertfordshire, UK) with QuantiTect SYBR Green PCR system (Qiagen) and primers for PDE5 (as above) and 18S ribosomal RNA (forward: 5′-CGCGGTTCTATTTTGTTGGT-3′, reverse: 5′-CTTCAAACCTCCGACTTTCG-3′). The relative expression level of PDE5 in stimulated hVSMCs was normalized to that of 18S and plotted as percentage change compared with untreated cells. 2−ΔΔCT method was used to determine the relative change in PDE5 gene expression.
Rho-kinase activity assay
Myosin light-chain phosphatase target subunit (MYPT1) is phosphorylated by Rho-kinase on Thr-696 (Ito et al., 2004). Rho-kinase activity in hVSMC samples was therefore assessed quantified by the extent of MYPT1 phosphorylation with Western blot.
Recombinant adenoviruses and infection of VSMCs
To determine the role of Rac1 in mediating PDE5 and NOX-1 expression, an adenovirus driven by cytomegalovirus promoter containing an empty expression cassette (Ad:Control) was used as described previously (Bond et al., 2004). Rac1 mutants (constitutively active G12V and dominant-negative T17N) were generated by PCR and cloned into pDC515 shuttle vector (Microbix, Toronto, Ontario, Canada). Replication-deficient adenoviruses were generated by recombination of co-transfected shuttle and genomic plasmids in HEK293 cells. Virus stocks were plaque-purified, amplified, CsCl-banded and titrated by plaque assay. Asynchronous hVSMCs were infected with adenovirus at multiplicity of infection of 50 in DMEM/10% foetal calf serum. Twenty-four hours post–infection, cells were growth-arrested for 3 days and then treated with either vehicle alone or U46619 (100 nM) for 16 h. Superoxide release was measured by ferricytochrome c assay and protein expression was measured by the Western analysis.
Transfection with small interfering RNAs
Predesigned small interfering RNA (siRNA) targeting human NOX-1 and NOX-4 and scrambled siRNA (as a negative control) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Growth-arrested hVSMCs were trypsinized, pelleted and resuspended in nucleofector solution, provided in the Basic nucleofector kit for primary smooth muscle cells (Amaxa biosystems, Cologne, Germany), at a density of 0.8 × 106 cells (100 μL)−1. The desired siRNA was then introduced into each reaction mixture at a final concentration of 80 pmoles (1 μg) and transfection was carried out using Nucleofector II device no. 300661 (Amaxa biosystems), according to the manufacturer's instructions. Following transfection, cells were plated out at a density of 5 × 104 cells per well in a 24-well plate either in the presence or absence of U46619 (100 nM). The cells were subjected to analysis 16 h after transfection.
PDE5 activity assay
Following treatment, hVSMCs were rinsed in cold PBS and lysed in 200 μL per well (six-well plate) of lysis buffer (20 mM Tris pH 7.4, 140 mM of NaCl, 0.75 mM MgCl2, 1 mM EGTA, 1% Triton X-100 and 20% glycerol) containing protease inhibitors and phosphatase inhibitors. Insoluble material was removed by centrifugation at 16 000 g for 5 min at 4 °C. The total protein concentration was measured using the BCA-protein assay kit and cell lysates were stored at −80 °C until analysis. PDE activity was determined using a modification of the assay method of Thompson and Appleman (1971). The assay consists of a two-step isotopic procedure. In the first step, cyclic [3H]GMP is hydrolyzed to 5′-[3H]GMP by PDE. In the second step, 5′-[3H]GMP is further hydrolyzed to [3H]guanosine by the snake venom nucleotidase. Before commencing the assay, 100 μL cell lysate was incubated at 37 °C for 10 min in the absence and presence of 100 nM sildenafil to define PDE5 activity. The assay was initiated by adding 50 μL test-mix (0.2 μCi [3H]cGMP, 1 μM cGMP, 10 mM Tris pH 7.4, 5 mM MgCl2, 100 μM EGTA). The reaction was carried out at 37 °C for 30 min and terminated by immediate boiling for 2 min followed by cooling on ice. Next, 25 μL (1 mg mL−1) of snake venom (Crotalus atrox) was added to all samples and incubated at 37 °C for 30 min. A 400 μL of anion exchange resin slurry (1 g AG 1–8X resin/1.1 mL ethanol/1.1 mL ddH2O) was added to each reaction, vortexed and kept at room temperature for 20 min. The resin binds to all charged nucleotides and leaves [3H]-guanosine as the only labelled compound to be counted. The samples were centrifuged at 16 000 g for 2 min to precipitate the resin. A 150 μL aliquot of the supernatant was then added to 5 mL of a scintillation cocktail and the radioactivity was measured by liquid scintillation counting. PDE5 activity was determined by subtracting the non-PDE5 (in the presence of 100 nM sildenafil) from the total PDE activity (in the absence of sildenafil).
Data analysis
The data were tested for normality by inspecting histograms and by applying the Kolmogorov–Smirnov test (automatically applied by Sigma Stat as part of the procedure for producing ANOVA results). In all cases, the data did not deviate sufficiently from normality to warrant nonparametric statistics. The data were expressed as mean±s.e.mean. Both one-way and two-way ANOVA were used to determine statistical significance. Two-way ANOVA tests were used where two conditions existed and one-way ANOVA was used when comparing effects of drug treatments with untreated controls.
Drugs
9, 11-Dideoxy-9α, 11α-methanoepoxyprostaglandin F2α (U46619) and Rho-kinase inhibitor, (+)-(R)-trans-4-(1-aminoethyl-N-4-pyridyl) cyclohexanecarboxamide dihydrochloride (Y27632), were purchased from Calbiochem (Nottingham, UK). Monoclonal antibody to Rac1 and polyclonal antibody to Thr969phospho-MYPT1 were obtained from Upstate (AQ15USA) (Watford, Hertfordshire, UK); antibodies against NOX-1 and NOX-4 were purchased from Santa Cruz Biotechnology; anti-PDE5 antibody was obtained from Cell Signaling Technology (Danvers, MA, USA). Cyclic [3H]GMP was purchased from Amersham Biosciences (Little Chalfond, Buckinghamshire, UK). Iloprost was purchased from Schering (Berlin, Germany). All tissue and cell culture reagents were purchased from Gibco BRL. All the other drugs and anti-α-actin were purchased from Sigma Chemical Co. (Poole, Dorset, UK) unless otherwise stated.
Results
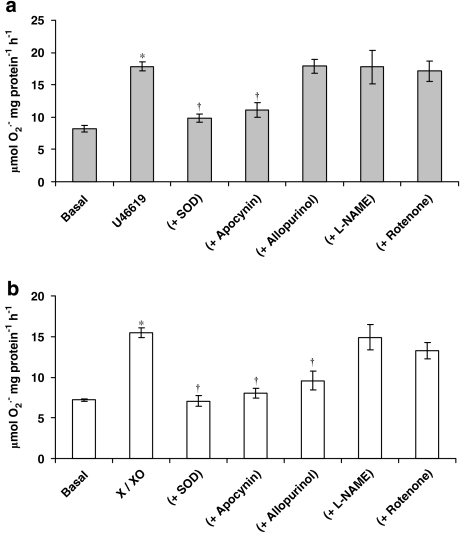
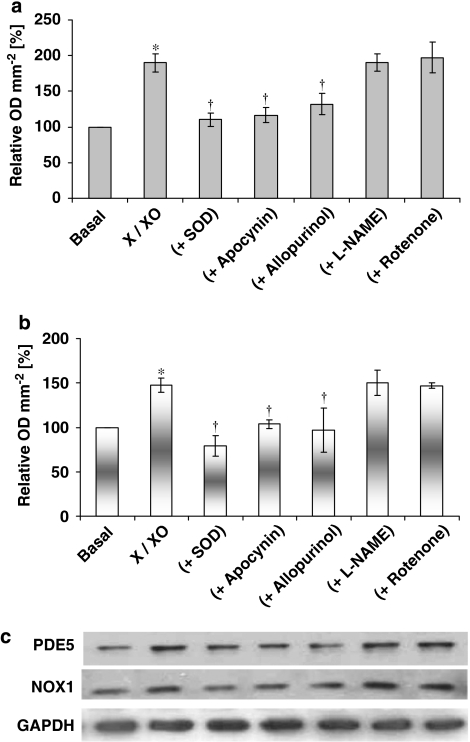
Incubation of hVSMCs with the thromboxane A2 analogue, U46619, elicited an increase in O2•− formation (Figure 1a). Incubation of hVSMCs for 16 h with an O2•− generating system (10 μM xanthine+1 mU mL−1 xanthine oxidase) followed by extensive washing of the cells with PBS, also elicited an increase in the formation of O2•− (Figure 1b). SOD and apocynin, an inhibitor of NADPH oxidase activity (Stolk et al., 1994), when co-incubated with U46619 or X-XO system for 16 h, again followed by extensive washing of the cells with PBS reduced the increased O2•− formation in hVSMCs (Figures 1a and b). By contrast, rotenone (10 μM), allopurinol (100 μM) and L-NAME (100 μM) were without effect when co-incubated with the thromboxane A2 analogue, U46619 (Figure 1a). The inhibitory effect of allopurinol on X-XO-induced O2•− formation is expected, as this compound is known to be an inhibitor of xanthine oxidase (Figure 1b). Incubation with apocynin alone had no effect on basal O2•− formation (data not shown).
Figure 1.
Effect of: (a) U46619 (100 nM) and (b) xanthine–xanthine oxidase (X-XO; 10 μM-1 mU mL−1) on superoxide (O2•−) formation in the absence or presence of superoxide dismutase (SOD) (500 U mL−1), apocynin (10 μM), allopurinol (100 μM), L-NAME (100 μM) and rotenone (10 μM) by human isolated vascular smooth muscle cells following a 16 h incubation. Each point represents the mean (±s.e.means), n=6. *P<0.05, significantly greater than control; †P<0.01, significantly reduced compared with relevant stimulus.
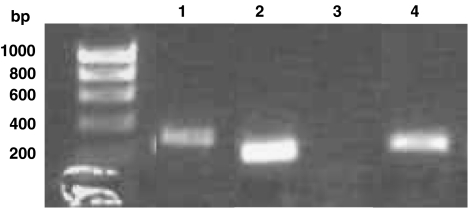
The mRNAs for PDE5, NOX-1 and NOX-4, but not NOX-5, were all detected in human saphenous vein VSMCs by reverse transcription–PCR (Figure 2).
Figure 2.
mRNA expression of PDE5 and NOX isoforms in human vascular smooth muscle cells. Lane 1, NOX-1; lane 2, NOX-4; lane 3, NOX-5; lane 4, PDE5. Similar results were obtained with six independent batches of cells.
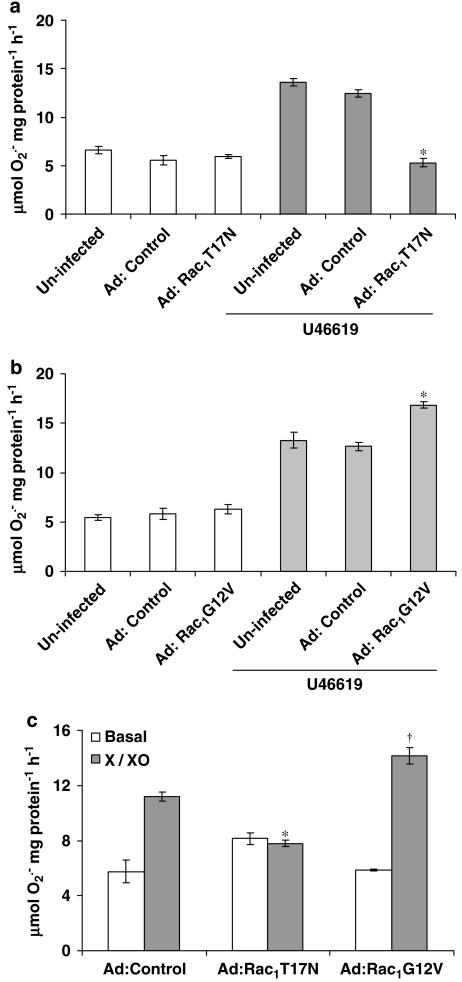
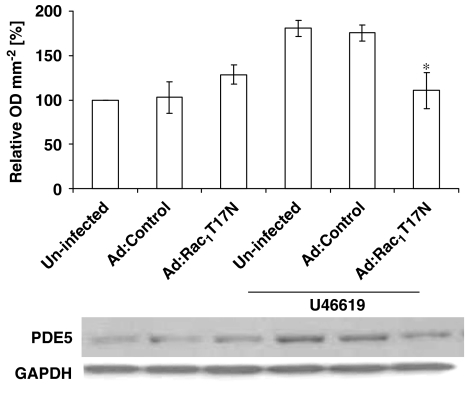
Overexpression of dominant-negative Rac1 (Ad:Rac1T17N) significantly inhibited O2•− release from hVSMCs incubated with U46619 or X-XO compared with control virus (Ad:Control) infected cells demonstrating that Rac1 is essential for full NADPH oxidase activity in human saphenous vein VSMCs (Figures 3a and c). This was confirmed by the expression of constitutively active Rac1 (Ad:Rac1G12V), which further enhanced U46619-stimulated O2•− release from HVSMCs (Figures 3b and c).
Figure 3.
Effect of infection of human vascular smooth muscle cells with an empty virus (Ad:Control), a dominant-negative Rac mutant isoform (Ad:Rac1T17N) or a constitutively active Rac mutant isoform (Ad:Rac1G12V) on O2•− formation following a 16 h incubation with U46619 (a, b) or xanthine–xanthine oxidase (X-XO) (c). Each point represents the mean (±s.e.mean), n=6. *,†P<0.01, significantly altered compared with stimulated cells expressing an empty virus.
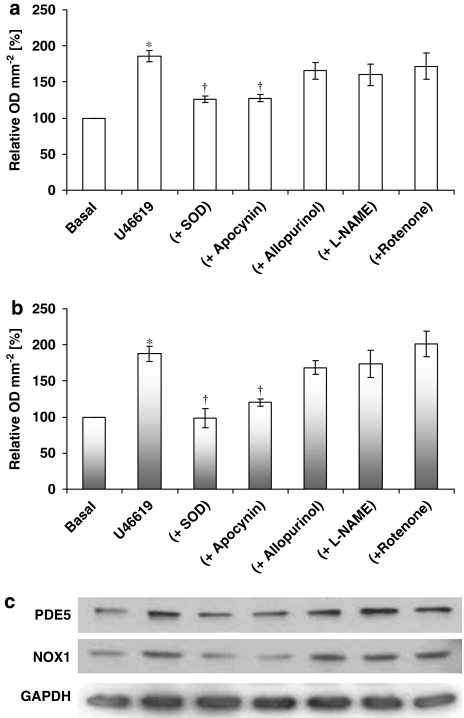
U46619 or X-XO induced both NOX-1 and PDE5 expression in HVSMCs after 16 h incubation, an effect blocked by the continual presence of SOD or apocynin (Figures 4 and 5). Furthermore, expression of dominant-negative Rac1 significantly inhibited U46619-stimulated PDE5 protein expression, indicating the role of Rac1 signalling in the regulation of PDE5 (Figure 6).
Figure 4.
Effect of U46619 (100 nM) and co-incubation of U46619 with the inhibitors of different sources of O2•− on (a) PDE5 expression and (b) NOX-1 expression by human isolated human vascular smooth muscle cells following a 16 h incubation. In (a and b), densitometric analyses of six blots (expressed as relative OD mm−2; mean±s.e.mean) In (c), representative Western blots. GAPDH expression was used as a loading control. *P<0.001, significantly greater than control; †P<0.01, significantly less than U46619 alone.
Figure 5.
The effect of xanthine–xanthine oxidase (X-XO; 10 μM-1 mU mL−1) and co-incubation of X-XO with the inhibitors of different sources of O2•− by on (a) PDE5 expression and (b) NOX-1 expression by human isolated vascular smooth muscle cells (hVSMCs) following a 16 h incubation. In (a and b), densitometric analyses of six blots (expressed as relative OD mm−2; mean±s.e.mean). In (c), representative Western blots. Each point represents the mean (±s.e.mean), n=6. *P<0.001, significantly greater than control; †P<0.01, significantly less than X-XO alone.
Figure 6.
Effect of infection of human isolated vascular smooth muscle cells with an empty virus (Ad:Control) or virus overexpressing dominant-negative Rac (Ad:Rac1T17N) on PDE5 protein expression following a 16 h incubation with 10 nM U46619. Densitometric analyses of six blots (expressed as relative OD mm−2; mean±s.e.mean). *P<0.01.
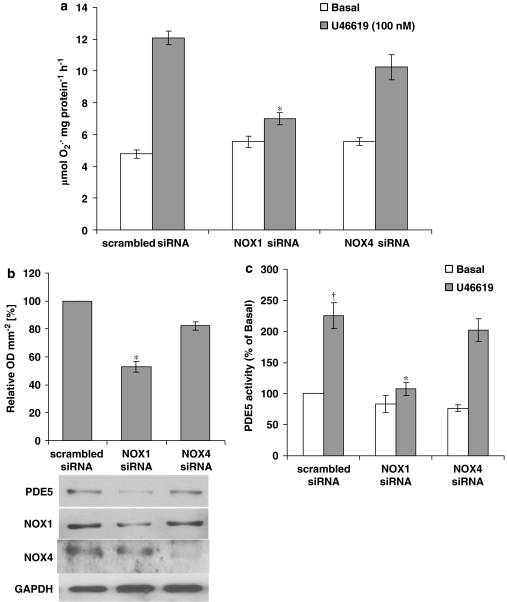
Human VSMCs transfected with NOX-1 siRNA blocked O2•− formation and induction of PDE5 protein expression and activity in response to U46619 (Figure 7), whereas NOX-4 siRNA had no effect on O2•− release and PDE5 activity and expression (Figure 7). The representative blots in Figure 7b show that NOX-1 siRNA transfection of hVSMCs does not suppress NOX-4 expression and vice versa.
Figure 7.
Inhibition of O2•− formation and PDE5 protein expression and activity in U46619-stimulated human isolated vascular smooth muscle cells (hVSMCs) by small interfering RNA (siRNA) directed against NOX-1. (a) O2•− generation measured by ferricytochrome c assay in scrambled, NOX-1 and NOX-4 siRNA transfected hVSMCs. (b) The upper panel: densitometric analysis of U46619-stimulated PDE5 protein expression quantified by Western blotting in scrambled, siNOX-1 or siNOX-4 transfected hVSMCs; data shown as relative OD mm−2; mean±s.e.mean). The lower panels: representative blots. (c) PDE5 activity measured by [3H]-cGMP hydrolysis assay in scrambled, NOX-1 and NOX-4 siRNA transfected hVSMCs. *P<0.01, significantly reduced compared with corresponding scrambled siRNA; †P<0.01, significantly increased compared with basal.
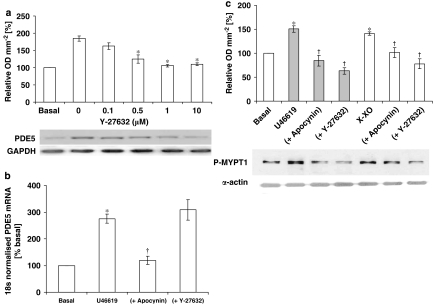
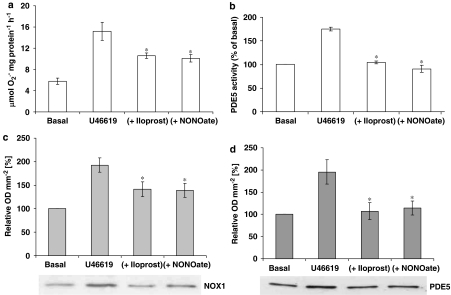
The Rho-kinase inhibitor blocked U46619-stimulated PDE5 protein expression (Figure 8a), indicating that Rho-kinase may be one of the upstream signalling components of PDE5. Quantitative reverse transcription–PCR of U46619-stimulated hVSMCs RNA extracts revealed that mRNA expression for PDE5 was increased 2.75±0.18-fold over basal levels (Figure 8b). Apocynin prevented the increased expression of PDE5 mRNA in hVSMCs, whereas the Rho-kinase inhibitor had no affect (Figure 8b). U46619 and X-XO both increased MYPT1 phosphorylation by 1.5±0.06- and 1.41±0.03-fold, respectively (Figure 8c). Apocynin or Rho-kinase inhibitor (Y27632) blocked the stimulated MYPT1 phosphorylation (Figure 8c). No changes were detected in GAPDH expression in all studies. Incubation of cells with iloprost (100 ng mL−1) or NONOate (1 μM) over the 16 h incubation period blocked the increased O2•− formation (Figure 9a) and PDE5 activity (Figure 9b) in hVSMCs when co-incubated with U46619, as well as NOX-1 expression (Figure 9c) and PDE5 expression (Figure 9).
Figure 8.
(a) Effect of the Rho-kinase inhibitor (Y27632) on PDE5 protein expression following 16-h incubation with 10 nM U46619. Each point represents the mean (±s.e.mean), n=6. *P<0.01. Representative blots of PDE5 and GAPDH are shown beneath. (b) Expression of mRNA for PDE5 measured by quantatitive reverse transcription–PCR in human isolated vascular smooth muscle cells (hVSMCs) incubated with U46619 (100 nM), ±apocynin (10 μM) or Rho-kinase inhibitor (Y27632; Y27632; 1 μM). Each point represents the mean (±s.e.mean), n=8. *P<0.01, significantly increased compared with basal; †P<0.05, significantly reduced compared with U46619-alone treated cells. (c) Effect of U46619 and X-XO (with and without apocynin (10 μM) or Rho-kinase inhibitor, Y27632, (1 μM)) on the phosphorylation of the myosin light-chain phosphatase target subunit (MYPT1). The upper panel shows the result of the densitometric analyses of six blots (expressed as relative OD mm−2) and the lower panel shows the representative blots. *P<0.01, significantly increased compared with basal; †P<0.05, significantly reduced compared with respective stimulus.
Figure 9.
Effect of the continual presence of iloprost (100 ng mL−1) or NONOate (10 μM) on U46619-stimulated (a) O2•− release or (b) PDE5 activity or (c) NOX-1 protein expression or (d) PDE5 protein expression in isolated human vascular smooth muscle cells. For (a, b) data shown are the mean (±s.e.mean), n=6. The upper panels in both (c) and (d) are the results of the densitometric analyses of six blots each (expressed as relative OD mm−2) and the lower panels show representative blots. *P<0.01, significantly reduced compared with U46619-alone treated cells.
Discussion
This study demonstrated firstly that U46619 promoted O2•− formation in hVSMCs following a 16 h incubation. This effect was inhibited by apocynin, a NOX inhibitor (Fung et al., 2005), and SOD (dismutates and removes O2•−) but not by rotenone (inhibitor of the mitochondrial respiratory chain), allopurinol (inhibitor of xanthine oxidase) or L-NAME (inhibitor of NOS) following this 16 h incubation (Muzaffar et al., 2006, 2008). These data confirm that the principal source of increased O2•− formation in hVSMCs, in response to U46619, is NADPH oxidase.
NOX-1 and NOX-4 but not NOX-5 are expressed in unstimulated hVSMCs. Furthermore, transfection of cells with NOX-1 siRNA blocked O2•− formation in response to U46619, whereas NOX-4 siRNA had no effect, indicating that the present effects are related to NOX-1 rather than NOX-4. Furthermore, incubation with U46619 promoted the expression of NOX-1, a plasma membrane-associated subunit of the NOX complex (Griendling et al., 2000; Hordijk, 2006). In turn, 16 h incubation with the O2•− generating system (X-XO) upregulated NOX-1, an effect blocked by apocynin and SOD. This indicates that O2•− augments the formation of O2•− by positive feedback upregulation of NOX-1 expression and activity, in agreement with a previous report (Muzaffar et al., 2006). Notably, incubation for 16 h with apocynin alone (that is, in the absence of X-XO or U46619) had no effect on O2•− formation, confirming that the present effects were due to inducible rather than constitutive NOX.
Second, as Rac1 plays a key role in mediating the activation of NOX (Hordijk, 2006), the role of Rac1 was investigated following infection of hVSMCs with appropriate adenoviruses. Overexpression of Rac1 with a dominant-negative adenovirus significantly inhibited O2•− release by hVSMCs incubated with U46619 compared with control virus, whereas overexpression of constitutively active Rac1 further enhanced U46619-stimulated O2•− release from hVSMCs. Taken together, these data demonstrate and confirm that Rac1 was essential for NOX activity in hVSMCs, as has been demonstrated for VSMCs from other species.
This study then demonstrated that U46619 upregulated the expression of PDE5 protein and mRNA levels in hVSMCs following 16 h incubation. This effect on PDE5 protein and mRNA levels was negated by co-incubation with the specific NOX inhibitor, apocynin, and by SOD indicating that O2•− derived from NOX mediates this effect. This conclusion was supported by the observation that the X-XO mixture, which generates O2•− in vitro, also upregulated PDE5 expression, an effect again blocked by both apocynin and SOD. This is consistent with a previous study in which it was demonstrated that O2•− upregulated NOX expression in pig pulmonary arterial VSMCs (Muzaffar et al., 2006). Furthermore, infection of hVSMCs with dominant-negative adenovirus for active Rac1 blocked the increase in PDE5 expression in response to U46619. Transfection of cells with NOX-1 siRNA blocked both the increase in O2•− and the expression of PDE5, whereas transfection with NOX-4 siRNA was without effect. Taken together, therefore, these data indicate that upregulation of the Rac1-NOX1 system increased PDE5 expression, an effect mediated by O2•− derived from NADPH oxidase.
The next step was to determine how O2•− might upregulate PDE5 expression. As O2•− activates Rho-kinase, as has been demonstrated in the rat aorta (Jin et al., 2004), the possible role of this kinase in mediating the upregulation of PDE5 was studied initially using the specific inhibitor, Y27632. Y27632 completely inhibited PDE5 expression, demonstrating that Rho-kinase mediates the upregulation of PDE5. U46619 also increased the phosphorylation of MYPT1, a target protein that is phosphorylated by Rho-kinase and is a standard assay for assessing Rho-kinase activity (Ito et al., 2004). Furthermore, the increased phosphorylation of MYPT1 in response to both U44619 and O2•− was blocked by apocynin, SOD and by the Rho-kinase inhibitor, Y27632. Apocycin, but not Rho-kinase inhibition, prevented the expression of PDE5 mRNA, indicating that O2•− derived from NOX-1 affected PDE5 expression at a transcriptional level, whereas Rho-kinase did so at a post-transcriptional level. Many studies have now demonstrated that Rho-kinase modulates both transcriptional and/or post-translational modification of key proteins that regulate diverse cellular functions, including smooth muscle contraction, actin cytoskeleton organization, cell adhesion and motility, and gene expression (Brown et al., 2006). Among its many roles, Rho-kinase has been shown to activate the transcription factor, AP-1, and fibronectin induction in mesangial cells (Peng et al., 2008) and to downregulate eNOS expression (Takemoto et al., 2002) and activity by the PI-3 kinase/PK Akt system (Ming et al., 2002; Wolfrum et al., 2004). Other downstream targets include the insulin receptor substrate-1 (resulting in uncoupling of insulin receptor from PI-3-kinase and potentially GLUT4 activation) (Farah et al., 1998) and various cytoskeleton organization regulating proteins, such as F-actin-binding proteins (Izawa et al., 2000) and intermediate filament proteins (Goto et al., 1998).
Both iloprost and NO blocked the upregulation of PDE5 in response to U46619 in hVSMCs. This is as one would expect, as it has been demonstrated that both iloprost and NO donors block upregulation of NOX expression and concomitant O2•− formation (Muzaffar et al., 2004a, 2004b). These inhibitory effects are mediated by activation of cAMP-PKA and cGMP-PKG systems by prostacylin and NO respectively (Muzaffar et al., 2004a, 2004b). Thus, through an inhibition of NOX, iloprost and NO inhibit the upregulation of PDE5. Therapeutically, iloprost and NO donors are used in clinical practice to treat a large number of conditions that include peripheral arterial disease, hypertension, angina pectoris, peripheral vascular disease and erectile dysfunction (Jeremy et al., 1999; Muzaffar et al., 2005a). It is possible, therefore, that the therapeutic action of iloprost and NO donors involves downregulation of both NOX and PDE5. Furthermore, as iloprost and NONOate are mimetics of endogenous prostacylin and NO, both of which are vasculoprotective, this indicates that these factors may endogenously suppress the expression of both NOX and PDE5, which would reduce vasculopathy.
To summarize, the upregulation and activation of Rac1 and NOX and the concomitant formation of O2•− results in an increase in PDE5 expression in human VSMCs. The activation of Rho-kinase by O2•− derived from NOX mediates this effect. As PDE5 hydrolyses cGMP (generated by the activation of GC by NO), this effect would reduce the vasculoprotective actions of NO. The importance of PDE5 in the aetiology of vasculopathies is exemplified by the proven and postulated therapeutic use of sildenafil and other PDE5 inhibitors to treat an array of cardiovascular diseases and syndromes. These include erectile dysfunction, pulmonary hypertension, angina pectoris, myocardial infarction, ischaemia reperfusion injury, vein graft disease and heart failure (Fung et al., 2005). In turn, the overproduction of O2•− and an upregulation of NOX have been demonstrated in these clinical conditions (Muzaffar et al., 2005a). PDE5 inhibitors also influence a wide range of basic vascular functions, which include the inhibition of VSMCs, augmentation of angiogenesis and relaxation of vascular smooth muscle (Jeremy et al., 1999). These effects are expected as the inhibition of PDE5 augments the bio-impact of the NO–PKG pathway, through augmentation of cGMP formation, in response to NO. PDE5 inhibition has also been shown to augment the inhibitory effect of NO on NOX expression and O2•− formation through a potentiation of the cGMP–PKG pathway (Muzaffar et al., 2005b).
Despite the proven importance of the NO–PKG signalling axis in providing vasculoprotection (Jeremy et al., 1999, 2004, 2007), little attention has been paid to the possible impact of PDE5 status on the progression of vascular diseases. This study predicts that where there is an increase in intravascular NOX expression and/or a concomitant increase in O2•− formation, then there will be a knock-on effect of PDE5 upregulation, which would further diminish the protective role of the NO–PKG system. Such a possibility warrants further investigation.
Acknowledgments
This research was funded by a grant from the British Heart Foundation (SS/06/056/20884).
Abbreviations
- MYPT1
myosin light-chain phosphatase target subunit 1
- NADPH
nicotinamide adenine dinucleotide phosphate
- PDE5
PDE type 5
- SOD
superoxide dismutase
- VSMCs
vascular smooth muscle cells
- X-XO
xanthine–xanthine oxidase
Conflict of interest
The authors state no conflict of interest.
References
- Bond M, Sala-Newby GB, Newby AC. Focal adhesion kinase (FAK)-dependent regulation of S-phase kinase-associated protein-2 (Skp-2) stability. A novel mechanism regulating smooth muscle cell proliferation. J Biol Chem. 2004;279:37304–37310. doi: 10.1074/jbc.M404307200. [DOI] [PubMed] [Google Scholar]
- Brown JH, Del Re DP, Sussman MA. The rac and rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases. The role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Farah S, Agazie Y, Ohan N, Ngsee JK, Liu Xj. A Rho-associated protein kinase, ROKalpha, binds insulin receptor substrate-1 and modulates insulin signaling. J Biol Chem. 1998;273:4740–4746. doi: 10.1074/jbc.273.8.4740. [DOI] [PubMed] [Google Scholar]
- Friebe A, Koesling D. Regulation of nitric oxide sensitive guanylyl cylcase. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- Fung E, Fiscus RR, Yim AP, Angelini GD, Arifi AA. The potential use of type-5 phosphodiesterase inhibitors in coronary artery bypass graft surgery. Chest. 2005;128:3065–3073. doi: 10.1378/chest.128.4.3065. [DOI] [PubMed] [Google Scholar]
- Goto H, Kosako H, Tanabe K, Yanagida M, Sakurai M, Amano M, et al. Phosphorylation of vimentin by rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J Biol Chem. 1998;273:11728–11736. doi: 10.1074/jbc.273.19.11728. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- Hordijk Pl. Regulation of NADPH oxidases: the role of rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- Ito M, Nakano T, Erodi F, Hawthorne DJ. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- Izawa T, Fukata Y, Kimura T, Iwamatsu A, Dohi K, Kaibuchi K. Elongation factor-1 alpha is a novel substrate of Rho-associated kinase. Biochem Biophys Res Commun. 2000;278:72–78. doi: 10.1006/bbrc.2000.3772. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Jonesa RA, Kouparris A, Hotston M, Persad R, Angelini GD, et al. Oxidative stress and erectile dysfunction: possible role of NADPH oxidase. Int J Impotence Res. 2007;19:265–280. doi: 10.1038/sj.ijir.3901523. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Rowe D, Emsley AM, Newby AC. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc Res. 1999;43:580–594. doi: 10.1016/s0008-6363(99)00171-6. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Shukla N, Muzaffar S, Angelini GD. Reactive oxygen species, vascular disease and cardiovascular surgery. Curr Vasc Pharmacol. 2004;2:229–236. doi: 10.2174/1570161043385691. [DOI] [PubMed] [Google Scholar]
- Jin L, Ying Z, Webb RC. Activation of rho/rho-kinase signalling pathway by reactive oxygen species in rat aorta. Am J Physiol. 2004;287:H1495–H1500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- Maurice DH, Palmer D, Tilley DG, Dunkerley HA, Netherton SJ, Raymond DR, et al. Cyclic nucleotide phosphodiesterase activity, expression and targeting of the cardiovascular system. Mol Pharmacol. 2003;64:533–540. doi: 10.1124/mol.64.3.533. [DOI] [PubMed] [Google Scholar]
- Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, et al. Rho GTPase/Rho-kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase β/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzaffar S, Jeremy JY, Angelini GD, Stuart-Smith K, Shukla N. The role of the endothelium and nitric oxide synthases in modulating superoxide formation induced by endotoxin and cytokines in porcine pulmonary arteries. Thorax. 2003;58:598–604. doi: 10.1136/thorax.58.7.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Nitroaspirins and SIN-1, but not aspirin, inhibit the expression of endotoxin- and cytokine- induced nadph oxidase in vascular smooth muscle cells from pig pulmonary arteries. Circulation. 2004a;110:1140–1147. doi: 10.1161/01.CIR.0000139851.50067.E4. [DOI] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Nicotinamide adenine dinucleotide phosphate oxidase: a promiscuous therapeutic target for cardiovascular drugs. Trends Cardiovasc Med. 2005a;15:278–282. doi: 10.1016/j.tcm.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Sildenafil citrate and sildenafil nitrate inhibit superoxide formation and gp91phox expression in porcine pulmonary endothelial cells through a cyclic GMP dependent mechanism. Br J Pharmacol. 2005b;146:109–117. doi: 10.1038/sj.bjp.0706305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Hypoxia and the expression of gp91phox and endothelial nitric oxide synthase in the pulmonary artery. Thorax. 2005c;60:305–313. doi: 10.1136/thx.2003.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Superoxide auto-augments superoxide formation in isolated porcine pulmonary artery endothelial cells through upregulation of NADPH oxidase expression: inhibition with iloprost. Eur J Pharmacol. 2006;538:108–114. doi: 10.1016/j.ejphar.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Bond M, Sala-Newby G, Angelini GD, Newby AC, et al. Acute inhibition of superoxide formation and Rac1 activation by nitric oxide and iloprost in human vascular smooth muscle cells in response to the thromboxane A2 analogue, U46619. Prostaglandins Leukot Essent Fatty Acids. 2008;78:247–255. doi: 10.1016/j.plefa.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Lobo C, Angelini GD, Jeremy JY. Iloprost inhibits superoxide formation and NADPH oxidase expression induced by the thromboxane A2 analogue, U46619, and isoprostane F2α in cultured porcine pulmonary artery vascular smooth muscle cells. Br J Pharmacol. 2004b;141:488–496. doi: 10.1038/sj.bjp.0705626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Wu D, Gao B, Ingram AJ, Zhang B, Chorneyko K, et al. RhoA/rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes. 2008;57:1683–1692. doi: 10.2337/db07-1149. [DOI] [PubMed] [Google Scholar]
- Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- Thompson WJ, Appleman MM. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971;10:311–316. [PubMed] [Google Scholar]
- Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, et al. Inhibition of rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]