Abstract
Background and purpose:
Drug efflux tranporters (P-glycoprotein (P-gp), multidrug resistance-associated protein (MRP)) limit the cellular uptake of human immunodeficiency virus protease inhibitors but the contribution of influx transporters in cells that (over)express P-gp or MRP is less clear. Here, we studied the expression of one influx transporter system, human organic anion-transporting polypeptide (hOATP), in some T-cell lines (CEM, CEMVBL, CEME1000) and in peripheral blood mononuclear cells (PBMCs) and examined the effects of manipulation of influx/efflux transporters on the uptake of saquinavir and lopinavir.
Experimental approach:
The expression of hOATPs was studied by PCR. We used hOATP substrate or inhibitor (estrone-3-sulphate (E-3-S) or montelukast, respectively) and inhibitors of P-gp (XR9576) and MRP (MK571 and frusemide) to study functional interactions between influx and efflux transporters in the uptake of saquinavir and lopinavir. Lipophilicity of the drugs was measured by octanol/saline partition coefficient.
Key results:
CEM cells, their variants and PBMCs express various hOATP isoforms, with OATP3A1 detected in all of the cells. MK571, XR9576 and frusemide increased the uptake of saquinavir and lopinavir. E-3-S and montelukast reduced the uptake of saquinavir and lopinavir in some, but not all, of the cells. Pretreatment of the cells with MK571, XR9576 or frusemide, followed by E-3-S co-incubation reduced the cellular accumulation of saquinavir and lopinavir. Lopinavir is much more lipophilic than saquinavir.
Conclusions and implications:
Human OATPs, MRP, P-gp and lipophilicity determine the cellular uptake and retention of saquinavir and lopinavir. These data may have important implications for drug–drug interactions, drug safety and efficacy.
Keywords: saquinavir, lopinavir, estrone-3-sulphate, influx, efflux, montelukast, lipophilicity
Introduction
Despite the fact that the therapeutic effects of some anti-retrovirals correlate with their plasma concentrations (Aarnoutse et al., 2003; Gerber and Acosta, 2003; la Porte et al., 2006), there are some human immunodeficiency virus (HIV)-infected patients who experience treatment failure although having seemingly adequate plasma drug concentrations. In contrast, there are others who show suppression of virus in the presence of inadequate plasma drug concentrations. These problems may be transporter-associated at the target site, as variability in transporter expression may explain variable plasma and cellular pharmacokinetics in vivo (Kim, 2005).
The target for some antiretrovirals, including HIV-1 protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors, is substantially within HIV-infected cells. Thus, for these drugs to inhibit the replicating virus, they need to be present in adequate concentrations within cells that support viral replication. It is now recognized that PIs are substrates of membrane transporters such as P-glycoprotein (P-gp) and multidrug resistance-associated proteins (MRPs). These transporter proteins may affect drug disposition, efficacy, drug–drug interactions, drug–endogenous substance interactions and toxicity. Several studies have demonstrated in cultured and, more importantly, in primary human lymphocytes that saquinavir is a substrate of P-gp, MRP1, MRP2 and the organic anion-transporting polypeptide (OATP) (Kim et al., 1998; Jones et al., 2001; Huisman et al., 2002; Williams et al., 2002; Su et al., 2004; Janneh et al., 2005). In contrast, there are less data on the function of P-gp or MRP in the intracellular accumulation of lopinavir (Woodahl et al., 2005; Agarwal et al., 2007; Janneh et al., 2007). Clearly, the intracellular accumulation of any drug is a composite of efflux and influx processes, together with other factors including the physicochemical properties (passive diffusion, ion trapping and lipophilicity). Influx transporters such as the OATP, the organic anion transporter and the organic cation transporter families have been found to mediate the transport of diverse organic ions and some drugs (Ford et al., 2004; Kim, 2005 and references therein). Human OATPs mediate the uptake of a number of organic compounds, such as conjugated metabolites of steroid hormones, bile acids and bilirubin, and numerous drugs like pravastatin and benzylpenicillin (Kullak-Ublick et al., 2001; Meier and Stieger, 2002; Kim, 2003; Campbell et al., 2004). Xenopus laevis oocytes and studies in Hep G2 cells showed the involvement of OATP1A2 in the uptake of saquinavir (Su et al., 2004). However, the impact of hOATPs expression on the cellular uptake of saquinavir and other PIs in cultured CD4T cells (that is, CEM cells and related cells such as CEMVBL (which overexpresses P-gp) and CEME1000 (overexpressing MRP1), and peripheral blood mononuclear cells (PBMCs; which act as important sites for viral replication in vivo) is unclear.
Although the contribution of P-gp and MRP to the transport of PIs has been widely studied, research into the relevance of OATP in the transport of PIs is only just beginning. In particular, the contribution of hOATPs in the transport of PIs in CEM, its variant cells (P-gp- and MRP1-overexpressing) and PBMCs (Pgp-, MRP1-, MRP2-, BCRP- (breast cancer resistance protein) and human organic anion-transporting polypeptide (hOATP)-expressing; Tamai et al., 2000; Janneh et al., 2005, 2007) has received little attention. This may be due to the difficulty in discerning the contribution of influx transporters in the uptake of HIV PIs in these cells, given the plethora of efflux transporters that are (over)expressed. The aim of this study is to investigate, using different inhibitors, the function of influx transporters on the uptake of saquinavir and lopinavir in CEM, its variant cells and more importantly in PBMCs, which have not been manipulated to overexpress transporters. In order to minimize methodological anomalies, and to characterize definitively the activities of drug influx transporters in these cells, incubations containing substrates or inhibitors of influx transporters and inhibitors of drug efflux transporters (singly and in combination) were examined. Overall, we conclude that P-gp, MRP and hOATPs exert an effect in concert to control the vectorial transport of saquinavir and lopinavir in the cells.
Materials and methods
Cell culture
The parental cell line was CEM (a CD4+ T-cell line). CEMVBL (VBL) cells have increased expression of the efflux transporter P-gp and were selected with vinblastine (Beck et al., 1979). CEME1000 (E1000) cells were selected with epirubicin and have increased expression of MRP1 (Davey et al., 1996). These cells were kind gifts from Prof R. Davey (Sydney, New South Wales, Australia). The cells were maintained at 37 °C and 5% CO2 in RPMI 1640 media supplemented with 10% foetal calf serum.
Isolation of PBMCs
Blood buffy coats were obtained from the Regional Blood Transfusion Centre (Liverpool, UK). Lymphoprep (Sigma Chemical Co, Poole, UK) was used to isolate PBMCs from the blood buffy coats according to procedures outlined by the manufacturer.
Detection of hOATP transporter expression by PCR Total cellular RNA was isolated from the cell lines, PBMCs and human liver (used as positive control) using TRI Reagent solution (Sigma, St Louis, Missouri USA) as per the manufacturer's instructions and total RNA concentration of each sample was measured. Equal amounts of each isolated RNA were reverse-transcribed using TaqMan Reverse transcription reagents (Applied Biosystems, Roche Molecular Systems Inc., Branchburg, NJ, USA) in a total volume of 100 μL containing 1 μg mRNA, 2.5 μM random hexamers, 2 μM dNTPs, 5.5 μM MgCl2, 1 × TaqMan RT buffer, 40 U RNase inhibitor and 125 U reverse transcriptase.
Polymerase chain reaction was performed with the GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). PCR was performed in a total volume of 50 μL using the expand high-fidelity PCR system (Roche) containing 5 μL cDNA, 800 μM dNTPs, 1 × expand high-fidelity buffer including 1.5 mM MgCl2, 3.5 U expand high-fidelity enzyme mixture, 0.2 pmol of each forward and reverse primers with the exception of OATP2B1 primers in which 2 pmol of each forward and reverse primers were used. The detection of specific mRNAs for OATP1A2 to OATP4A1 was achieved using the following forward and reverse primers as shown in Table 1. The primers used for the detection of OATP1A2 were as described previously (Schiffer et al., 2003).
Table 1.
Primers used for reverse transcriptase-PCR detection of hOATP isoforms. Figures in brackets represent accession numbers
| OATPs (reference sequencea) | Forward primer | Reverse primer |
|---|---|---|
| OATP1A2 (NM_005075) | AAGAAGAGGTCAAGAAGGAAAAAT | GGAGCATCAAGGAACAGTCAGGTC |
| OATP1B1 (AB026257) | TCTCGATGGGTTGGAGCTTGG | AGGCTGACCATACTGTTGCTC |
| OATP1B3 (HSA251506) | CATTCCTAGGGTTGCAACTGG | TTGTTCCCACAGACTGGTTCC |
| OATP2B1 (AB026256) | TGCTTGTCATCCATGGCTG | CTGTTGTCCAGAGCATCCTG |
| OATP3A1 (AB031050) | TGATCCCGAAGGTCAC | CGGTTGCGTTCTCAG |
| OATP4A1 (AB031051) | TGAGTGCCTCAGAAGCTGC | AGTGCATTTCCCTGCAGTG |
Accession numbers are for reference sequences from the ncbi nucleotide database (http://www.ncbi.nlm.nih.gov/entrez).
The PCR conditions consisted of an initial denaturation at 95 °C for 20 s followed by a further denaturation at 95 °C for 2 s. The annealing temperature was at 68–54 °C for 30 s with a −2 °C decrease per two cycles, followed by an extension step at 72 °C for 60 s. This was then followed by 32 cycles of 95 °C for 2 s, 50 °C for 30 s and 72 °C for 90 s. PCR products were separated on a 2% agarose gel (1 × Tris/Borate/EDTA(TBE)) and stained with ethidium bromide.
Measurement of drug transport
Radiolabelled drug uptake was measured as described previously (Janneh et al., 2005) with the following modifications: for the cultured CEM cells and PBMCs, a cell density of 2.5 × 106 and 5 × 106 cells mL−1, respectively, were incubated in RPMI media with 10% foetal calf serum containing [3H]-saquinavir (0.1 μCi, 1 μM) and [14C]-lopinavir (0.1 μCi, 0.5 μM). Cells were incubated in the absence or presence of varying concentrations of inhibitors of (i) P-gp (XR9576, 0.1 μM) (Roe et al., 1999); (ii) MRP and OATP (MK571, 50 μM) (Jones et al., 2001; Janneh et al., 2005; Letschert et al., 2006); (iii) OATP (montelukast, 0–100 μM) (Letschert et al., 2006); and (iv) OATP (estrone-3-sulphate (E-3-S, 0–100 μM; substrate of OATP (http://bigfoot.med.unc.edu/watkinsLab/website/intesinfo.htm)) for 15 min and the cell-associated and free drug levels in the media were determined (Janneh et al., 2005).
Intracellular drug accumulation is a net effect of transport across cell membranes in opposing directions, and drug influx into cells may be concealed by a more dominant efflux process (particularly in cultured cell lines). Thus, in separate experiments, the effects of E-3-S on the transport and accumulation of the radiolabelled drugs was investigated after inhibition of P-gp-, MRP1- or MRP2-mediated drug efflux in the cells. Here, cells were pre-incubated for 5 min either with XR9576 (0.1 μM), MK571 (50 μM) or frusemide (50 μM; inhibits MRP1/2 (Janneh et al., 2005)). Thereafter, the effects of E-3-S (at 30 and 100 μM) co-incubation on the uptake and accumulation of the radiolabelled drugs were studied as described previously (Janneh et al., 2005 and references therein). Data were expressed as cellular accumulation ratio (CAR), which is the ratio of the amount of radiolabelled drug in the cell pellets to the amount in a similar volume of media after incubation, taking the cell volume (1 pL for CEM cells (Jones et al., 2001) and 0.4 pL for PBMCs (Chapman et al., 1981; Janneh et al., 2005, 2007)) into account.
Octanol/saline partition coefficient
The cellular association of a drug within target cells is dependent upon a number of factors including active influx/efflux; passive diffusion; ion trapping; and the lipophilicity. The lipophilicity of ([14C]- lopinavir and [3H]- saquinavir) were measured as described previously (Gibbs and Thomas, 2002) to establish if simple physiochemical characteristics were major determinants of intracellular accumulation. [14C]-mannitol was used as the positive control. Briefly, an equal volume of octanol and phosphate-buffered saline were first vigorously vortexed for 5 min to presaturate the octanol. The suspension was then allowed to settle for 10 min before the upper saturated octanol layer was carefully removed and stored in a separate tube. Then 0.60, 0.07 and 0.16 μCi of [14C]- lopinavir, [3H]- saquinavir and [14C]-mannitol, respectively, were diluted with 3 ml of phosphate buffered saline before equal volumes (540 μl) of each compound were added to equal volumes of octanol that was previously presaturated with phosphate buffered saline. The samples were vortexed for 5 min before being centrifuged (1000 g, 5 min). The concentrations of radiolabelled drug in a volume of the octanol, upper layer, and in a similar volume of saline, lower layer, were measured using scintillation counting. The octanol–saline partition coefficient was determined as a ratio of radiolabelled drug in the octanol phase to radiolabelled drug concentration in the saline phase.
Statistical analysis
Data are expressed as mean±s.d.; the Shapiro–Wilk test was used to assess the distribution of the data. Statistically significant differences between controls and test samples were then assessed either by one-way ANOVA followed by Bonferroni's correction or Kruskal–Wallis test using StatsDirect statistical software, version 2.6.3, 2007 (Cheshire, UK). Significance between controls and test samples was assumed if P<0.05.
Reagents
[3H]-Saquinavir (specific activity, 26 μCi mg−1), [14C]-lopinavir (specific activity, 52.1 mCi mmol−1) and XR9576 (tariquidar) were donated by Roche Pharmaceuticals (Welwyn Garden City, UK), Abbott Laboratories (North Chicago, IL, USA) and Xenova Group Plc (Berkshire, UK), respectively. MK571 and montelukast were obtained from Merck Frosst (Quebec, Kirkland, Canada). All other chemicals, unless otherwise stated, were purchased from Sigma Chemical Co. (Poole, UK).
Results
CEM, CEMVBL, CEME1000 and PBMCs express different hOATP isoforms
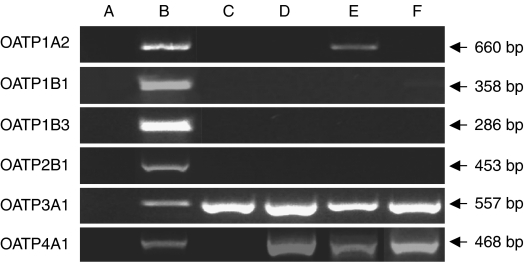
Using human liver as positive control, PBMCs, CEM, CEMVBL and CEME1000 cells were screened for the presence of six different hOATP isoforms (OATP1A2, OATP1B1, OATP1B3, OATP2B1, OATP3A1 and OATP4A1) and the results are shown in Figure 1. No hOATP isoform was detected in the negative control (water, lane A), but all of the six hOATP isoforms tested were detected in human liver (lane B). OATP1A2 was detected only in the CEMVBL cells (lane E), but not in PBMCs, CEM and CEME1000 cells. No OATP1B1, OATP1B3 and OATP2B1 were detected in any of the CEM or PBMC samples. However, OATP3A1 was detected in all of the cells. Although we detected no OATP4A1 in the PBMC samples (lane C), it was detected in the CEM, CEMVBL and CEME1000 cells.
Figure 1.
Polymerase chain reaction products to show that CEM, its variant cells and peripheral blood mononuclear cells (PBMCs) express various organic anion-transporting polypeptide (OATP) isoforms. PCR products were separated on 2% agarose gels (1 × TBE) and stained with ethidium bromide (see Materials and methods section for details). Lane A, negative control (water); lane B, positive control (human liver); lane C, PBMCs; lane D, CEM; lane E, CEMVBL; and lane F, CEME1000. Arrows indicate base pair (bp) sizes for each OATP.
The pharmacology of drug influx in cells (over)expressing P-gp, MRP and OATP
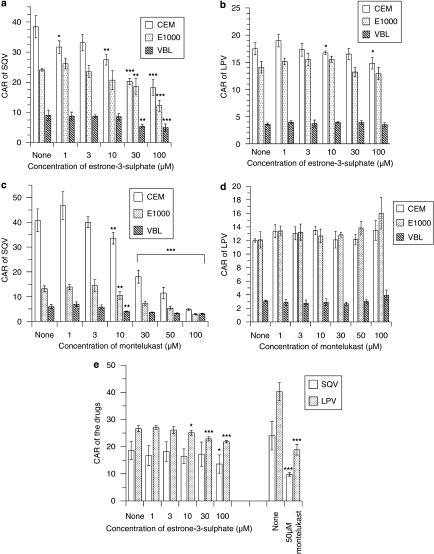
Estrone-3-sulphate and montelukast showed variable effects on the uptake of saquinavir and lopinavir. E-3-S demonstrated a concentration-dependent reduction in the uptake of saquinavir but not lopinavir (Figures 2a and b). As can be seen in Figure 2a, E-3-S significantly reduced (P⩽0.01) the CAR of saquinavir in the cells, with the following inhibition profiles: CEM>CEME1000>CEMVBL. At 100 μM E-3-S, the CAR of saquinavir was reduced in CEM, CEME1000 and CEMVBL cells by 52, 49 and 45%, respectively. In contrast, E-3-S only significantly (P<0.05) reduced the uptake of lopinavir in CEM cells at 10 and 100 μM by 4 and 15%, respectively (Figure 2b). Similarly, montelukast significantly (P⩽0.01) reduced the uptake of saquinavir, but not lopinavir, in a concentration-dependent manner (Figures 2c and d). Montelukast (at 100 μM) produced a more marked effect on the uptake of saquinavir in CEM cells than its variant cells. It reduced the CAR of saquinavir in CEM, CEME1000 and CEMVBL cells by 88, 78 and 49%, respectively. There was variable accumulation of saquinavir in the PBMC samples (Figure 2e). In contrast to the observations in cultured cells, E-3-S (at 100 μM) significantly (P<0.05) reduced only the CAR of saquinavir in the PBMCs, whereas it (at 10–100 μM) significantly (P<0.05) decreased the CAR of lopinavir in these cells (Figure 2e). Montelukast (at 50 μM) significantly (P<0.001) reduced the uptake of saquinavir and lopinavir in the PBMCs (Figure 2e).
Figure 2.
Effects of the organic anion-transporting polypeptide (OATP) substrate, estrone-3-sulphate (E-3-S) and OATP inhibitor montelukast on the accumulation (CAR) of saquinavir (SQV) and lopinavir (LPV) in CEM, CEMVBL, CEME1000 cells and PBMCs. Concentration-dependent effects of (a) E-3-S on the CAR of saquinavir, (b) E-3-S on the CAR of lopinavir, (c) montelukast on the CAR of saquinavir and (d) montelukast on the CAR of lopinavir in CEM and its variant cells. Concentration-dependent effects of E-3-S and montelukast (at 50 μM) on the CAR of (e) saquinavir and lopinavir in PBMCs. The data on montelukast (at 50 μM) were generated from four other PBMC samples different from those used to assay the effects of E-3-S in the same figure. Note that the volume of each CEM cell is assumed as 1 pL. CAR, cellular accumulation ratio, is the amount of radiolabelled drug associated with the cell pellets relative to the same volume of supernatant. Each bar represents the mean±s.d. (n=4). *P<0.05, **P<0.01, ***P<0.0001 compared with controls. E-3-S, estrone-3-sulphate; PBMC, peripheral blood mononuclear cells; CAR, cellular accumulation ratio.
The intracellular accumulation of saquinavir and lopinavir are amenable to manipulation by inhibitors of MRP and P-gp
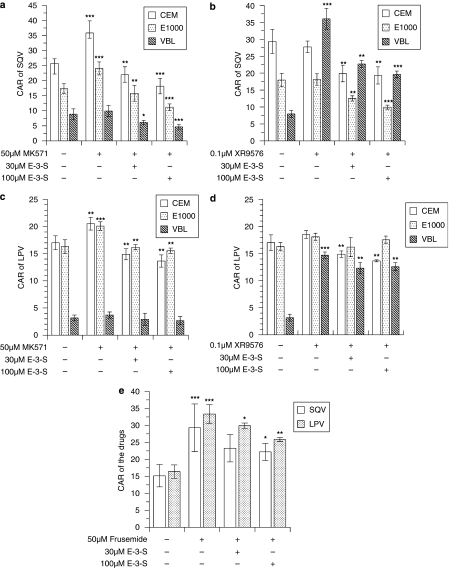
We observed differential uptake of saquinavir and lopinavir in the CEM cells, with the following rank order of accumulation: CEM>CEME1000>CEMVBL (Figure 3). Treatment of the cells with MK571, compared with the controls, significantly (P⩽0.01) increased the baseline CAR of saquinavir in the CEM and CEME1000 cells, but not in the P-gp-overexpressing CEMVBL cells (Figure 3a). The CAR of saquinavir and lopinavir were significantly (P<0.001) increased by XR9576 in the P-gp-overexpressing CEMVBL cells, but not in the CEM and CEME1000 cells (Figures 3b and d). MK571 significantly (P⩽0.01) increased the CAR of lopinavir in the CEM and CEME1000 cells, but not in CEMVBL cells (Figure 3c).
Figure 3.
The impact of E-3-S in reversing the effects of efflux modulators (XR9576, MK571 and frusemide) on the cellular accumulation of saquinavir (SQV) and lopinavir (LPV) in CEM, CEMVBL, CEME1000 cell lines and in PBMCs. Untreated cells are presented for comparative purposes only. (a) Effects of E-3-S (at 30 and 100 μM) co-incubation on the CAR of saquinavir after pretreatment of CEM and its variant cells with 50 μM MK571. (b) Effects of E-3-S (at 30 and 100 μM) co-incubation on the CAR of saquinavir after pretreatment of CEM and its variant cells with 0.1 μM XR9576 and (c) effects of pretreatment of the cells with 50 μM MK571 followed by E-3-S (at 30 and 100 μM) on the CAR of lopinavir in CEM and its variant cells. (d) Effects of E-3-S co-incubation after pretreatment of the cells with 0.1 μM XR9576 in CEM and its variant cells. (e) Effects of E-3-S (at 30 and 100 μM) co-incubation on the uptake of saquinavir and lopinavir in PBMCs pretreated with 50 μM frusemide. Significance between cells incubated with inhibitor and cells coincubated with E-3-S after pretreatment with inhibitor was tested by Shapiro–Wilk followed by Kruskal–Wallis tests. Each bar represents the mean±s.d. (n=3–4, with four incubations per treatment). *P<0.05, **P<0.01, ***P<0.0001 compared with inhibitor-treated controls. E-3-S, estrone-3-sulphate; PBMC, peripheral blood mononuclear cells; CAR, cellular accumulation ratio.
To study the influx of the drugs in these cells which (over)express drug efflux transporters and also express some influx transporters, we blocked the efflux transporters by first pretreating the cells with the inhibitors of drug efflux transporters followed by co-treatment with E-3-S. To inhibit P-gp and MRP1 activities that caused reduced intracellular concentrations of saquinavir and lopinavir (Figures 2 and 3), the cells were pretreated either with XR9576 (to inhibit P-gp), MK571 or frusemide (to inhibit MRP) before further investigating the effects of E-3-S on the uptake of saquinavir or lopinavir.
There was a significant (P<0.01) reduction in the CAR of saquinavir in the CEM and its variant cells that were pretreated with 50 μM MK571 and subsequently treated with 30 μM E-3-S (Figure 3a). Similarly, compared with the 50 μM MK571-pretreated control, E-3-S (at 100 μM) treatment of MK571-pretreated cells significantly reduced (P⩽0.01) the CAR of saquinavir in CEM, CEME1000 and CEMVBL cells by 49, 54 and 47%, respectively (Figure 3a).
Compared with 0.1 μM XR9576-pretreated controls, co-incubation with E-3-S (at 30 and 100 μM) significantly reduced (P⩽0.01) the CAR of saquinavir in CEM, CEME1000 and CEMVBL cells pretreated with 0.1 μM XR9576 by 30% (27.76±1.69 to 19.31±2.53), 45% (18.12±1.70 to 9.96±0.60) and 46% (36.03±3.07 to 19.60±1.03), respectively (Figure 3b).
CEM and CEME1000 cells, but not CEMVBL cells, which had been previously pretreated with 50 μM MK571 followed by further treatment with either E-3-S (at 30 or 100 μM) showed significantly (P⩽0.01) reduced uptake of lopinavir (Figure 3c). We observed that co-incubation of E-3-S (at 100 μM) with 50 μM MK571 (at 50 μM) caused a reduction in the CAR of lopinavir in CEM and CEME1000 cells by 33 and 23%, respectively. Co-incubation of E-3-S with XR9576-pretreated cells significantly (P⩽0.01) reduced CAR of lopinavir in the CEM and CEMVBL cells (by 26 and 14%, respectively), but not in the CEME1000 cells (Figure 3d). We observed that incubation of PBMCs with 50 μM frusemide (to inhibit MRP1/2) significantly (P<0.001) increased the CAR of saquinavir and lopinavir (Figure 3e). The CAR of lopinavir in PBMCs pretreated with frusemide was significantly reduced by both the concentrations (30 and 100 μM) tested of E-3-S. In contrast, the CAR of saquinavir was only significantly reduced by E-3-S (at 100 μM) under similar conditions (Figure 3e).
The octanol/saline partition coefficients for [14C]-lopinavir, [3H]-saquinavir and [14C]-mannitol (used as control) were 214±49, 18±3 and 0.002±0.001, respectively. The octanol/saline partition coefficient determined for [14C]-mannitol (0.002±0.0001) was similar to previously published values of 0.002±0.0004 (Gibbs and Thomas, 2002).
Discussion
Low and variable bioavailability and poor penetration of HIV sanctuary sites such as the brain and testis may limit the intracellular concentration and efficacy of many antiretroviral drugs (Kim et al., 1998; Owen et al., 2005). There is strong evidence that membrane-bound drug transport proteins have an important function in all aspects of drug disposition, including cellular accumulation of HIV PIs (Choo et al., 2000; Jones et al., 2001; Huisman et al., 2002; Janneh et al., 2005, 2007; Kim, 2005). Indeed, there is a large body of evidence showing that saquinavir and lopinavir are substrates of P-gp, MRP1 and MRP2 (Kim et al., 1998; Huisman et al., 2001; Jones et al., 2001; Williams et al., 2002; Janneh et al., 2005, 2007; Woodahl et al., 2005; Agarwal et al., 2007), which could have possible implications for accelerated antiretroviral resistance.
Here, we investigate the functional activity of drug influx and efflux transporters in the vectorial transport of saquinavir and lopinavir in cultured CEM, its variant cells and in primary human lymphocytes. Although drug transport has been studied previously in the CEM cells (Davey et al., 1995; Jones et al., 2001), it is unclear if (i) the CEM and its variant cells express drug influx transporters and (ii) whether, given the relatively large levels of MRP1 and P-gp expressed by these cells (data not shown), the intracellular accumulation of saquinavir and lopinavir can be modulated by manipulation of influx transporters. Although it is beyond the scope of this work to characterize all of the known hOATPs that may influence the intracellular accumulation of saquinavir and lopinavir, nevertheless we attempted to investigate the expression (at mRNA) of some six hOATP isoforms in CEM, its variants and in PBMCs. Here, we provide the first evidence that CEM and its variant cells express some hOATPs and confirm the observation by Tamai et al. (2000) of the expression of OATP3A1 in PBMCs. Of the six hOATP isoforms studied, only OATP3A1 was detected in all cell types tested. Interestingly, OATP1A2 was detected only in the CEMVBL cells, and we postulate that the manipulation of CEM cells (to give rise to CEMVBL) may also have resulted in the expression of OATP1A2.
Moreover, using relatively specific substrates or inhibitors of hOATP such as E-3-S or montelukast to investigate the uptake of saquinavir and lopinavir, we were able to demonstrate the function of the identified hOATPs. Our data demonstrate that the uptake of saquinavir and lopinavir can be modulated by inhibitors/substrates of hOATPs. E-3-S was more potent at reducing the uptake of saquinavir than lopinavir in the CEM and its variant cells (Figures 2a versus b), suggesting potential interference by efflux transporters in the uptake and accumulation of lopinavir. As E-3-S did not similarly reduce the uptake of lopinavir (Figure 2b), the observed effects of E-3-S on the uptake of saquinavir cannot be due to the cytotoxic effects of E-3-S, but rather due to the differential inhibition of the influx of saquinavir and lopinavir by E-3-S. Indeed, we observed that both E-3-S and montelukast (at 1–100 μM) demonstrated no cytotoxic effects over the 10-min incubation period used in the drug transport studies (data not shown). Montelukast was recently shown to inhibit the transport of OATP substrates such as [3H]-bromosulphophthalein and [3H]-amanitin in transformed MDCKII cells and human hepatocytes, with OATP1B3 being more susceptible to inhibition than OATP2B1 and OATP1B1 (Letschert et al., 2006). Here, we investigated the effects of montelukast on the uptake of saquinavir and lopinavir and observed identical inhibitory profiles to that of E-3-S in the cultured CEM cell line and its variants, with little or no effect on the uptake of lopinavir, suggesting that the uptake of saquinavir is more amenable to manipulation by E-3-S and monteluklast than lopinavir in the CEM and its variant cells. However, the CAR of saquinavir and lopinavir were reduced by both E-3-S and montelukast in the PBMCs (Figure 2e). These data suggest that E-3-S and montelukast have differential effects on the uptake of saquinavir and lopinavir in the CEM, its variant cells and in PBMCs that may be due to some variability in the expression of other unidentified hOATP isoforms (other than OATP4A1 and OATP1A2, for example, OATP2A1, OATP5A1, OATP6A1, OATP1C1 and OATP4C1 and other known influx transporters like organic cation transporter-1) between the cells (Figure 1). Furthermore, our data suggest that the actions of montelukast may not be limited to inhibition of OATP1B3, OATP2B1 and OATP1B1 (Letschert et al., 2006), as these OATP isoforms were not identified in the CEM cells (Figure 1). Thus, the inhibitory effects of montelukast on other OATP isoforms such as OATP1A2, OATP3A1, OATP4A1 and so on may account for the reduced uptake of saquinavir (and of lopinavir) in this study. It is worth noting that montelukast is extensively metabolized by CYP3A4 and CYP2C9, with the former also involved in the metabolism of saquinavir and lopinavir. Thus, in addition to potentially reducing the intracellular accumulation of saquinavir and lopinavir, montelukast may compete for metabolism with saquinavir and lopinavir, potentially leading to some significant drug–drug interactions.
To study the contribution of influx transporters to the uptake of saquinavir and lopinavir in the CEM, its variant cells and PBMCs, we inhibited MRP or P-gp activities in these cells by previous treatment with MK571, XR957 or frusemide, followed by co-incubation with E-3-S. These manipulations abolished the activities of MRP and P-gp, causing increased intracellular accumulation of saquinavir and lopinavir, allowing further functional study of the contribution of drug influx proteins in the uptake of saquinavir and lopinavir in these cells. As controls, MK571, XR9576 and frusemide increased the CAR of saquinavir and lopinavir in CEME1000, CEMVBL and PBMCs, respectively, confirming previous observations that these drugs are substrates of MRP and Pgp (Kim et al., 1998; Huisman et al., 2001; Jones et al., 2001; Williams et al., 2002; Janneh et al., 2005, 2007; Agarwal et al., 2007). Although we observed differential accumulation and lipophilicity of the drugs, with lopinavir being much more lipophilic than saquinavir, there was no direct relationship between the measured CAR of the drugs to their lipophilicity, suggesting that other factors, such as protein binding and ion trapping may impact on the uptake and retention of the drugs.
Interestingly, E-3-S (at 30 and 100 μM) co-incubation with 50 μM MK571- or 0.1 μM XR9576-pretreated cells significantly reduced the uptake of saquinavir and lopinavir. Although a moderate reduction was observed in the CEM cells pretreated with 0.1 μM XR9576, followed by 100 μM E-3-S, compared with the CEM cells treated with 100 μM E-3-S alone, the reductions seen in CEME1000 and CEMVBL cells were comparable with that observed in the cells treated with E-3-S alone.
Overall, although these manipulations (that is, pretreatments followed by co-incubation with E-3-S) did not cause any marked reductions in the transport of the drugs over that measured previously (Figure 2), they provide an alternative strategy by which the influx of some drugs can be studied in cells (over)expressing drug efflux transporters. Indeed, our studies allowed the characterization of the uptake of lopinavir in CEME1000 and CEMVBL cells, with a more marked and significantly reduced CAR of lopinavir measured in pretreated (MK571- or XR9576-pretreatment followed by E-3-S co-incubation) cells compared with cells treated with varying concentrations of E-3-S alone (Figures 3c and d versus Figure 2b). Furthermore, these manipulations have showed that neither MK571 nor XR9756 antagonized the activity of E-3-S or vice versa. It has recently been shown that MK571 also inhibits hOATP (Letschert et al., 2006). However, given that no reduction in the uptake of lopinavir or saquinavir were measured under any of the conditions that used MK571 is sufficient indication that hOATP was not inhibited at any of the MK571 concentrations used. We also demonstrated that E-3-S reduced the uptake of saquinavir and lopinavir in PBMCs pretreated with frusemide. Our data not only confirm previous observations that hOATPs are involved in the uptake of saquinavir (Su et al., 2004), but that these influx transporters may also contribute to the uptake of lopinavir in these cells. Studies have also shown inhibition of OATP-mediated uptake of fexofenadine and oestradiol-17β-glucuronide by some PIs (Dresser et al., 2002; Tirona et al., 2003; http://bigfoot.med.unc.edu/watkinsLab/website/intesinfo.htm), with the drugs having potential effects on the transcripts of OATPs (Dixit et al., 2007). Altogether, these data suggest that PIs may be substrates and inhibitors of hOATPs and may also compete for influx with a large number of substrate drugs.
In conclusion, simple diffusion across the plasma membrane as well as an interplay between efflux (P-gp/MRP) and influx (hOATP) transporters determine the vectorial transport of saquinavir and lopinavir in CEM, its variants and in PBMCs. Knowledge of the relative expression of efflux and influx transporters in target cells may be of crucial importance in understanding drug efficacy, drug–drug interactions, drug–endogenous substance interactions and toxicity and warrants further investigation.
Acknowledgments
The British Society for Antimicrobial Chemotherapy (BSAC) supported this work. The BSAC Academic Initiative Grant supported OJ. SAW is supported by the Wellcome Trust and by the BBSRC.
Abbreviations
- CAR
cellular accumulation ratio
- E-3-S
estrone-3-sulphate
- HIV
human immunodeficiency virus
- hOATP
human organic anion-transporting polypeptide
- MRP
multidrug resistance-associated protein
- OAT
organic anion transporter
- PBMC
peripheral blood mononuclear cell
- P-gp
P-glycoprotein
- PI
protease inhibitor
Conflict of interest
DJB and SHK have received grant/research support from Abbott, Bristol-Myers Squibb, GlaxoSmithKline and Roche Pharmaceuticals. They have served as consultants for Gilead Sciences, GlaxoSmithKline and Vertex.
References
- Aarnoutse RE, Schapiro JM, Boucher CA, Hekster YA, Burger DM. Therapeutic drug monitoring: an aid to optimising response to antiretroviral drugs. Drugs. 2003;63:741–753. doi: 10.2165/00003495-200363080-00002. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Pal D, Mitra AK. Both P-gp and MRP2 mediate transport of Lopinavir, a protease inhibitor. Int J Pharm. 2007;339:139–147. doi: 10.1016/j.ijpharm.2007.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck W, Mueller T, Tanzer L. Altered surface membrane glycoproteins in vinka alkaloid-resistant human leukemic lymphoblasts. Cancer Res. 1979;39:2070–2076. [PubMed] [Google Scholar]
- Campbell SD, de Morais SM, Xu JJ. Inhibition of human organic anion transporting polypeptide OATP 1B1 as a mechanism of drug-induced hyperbilirubinemia. Chem Biol Interact. 2004;150:179–187. doi: 10.1016/j.cbi.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Chapman EH, Kurec AS, Davey FR. Cell volumes of normal and malignant mononuclear cells. J Clin Pathol. 1981;34:1083–1090. doi: 10.1136/jcp.34.10.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo EF, Leake B, Wandel C, Imamura H, Wood AJ, Wilkinson GR, et al. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab Dispos. 2000;28:655–660. [PubMed] [Google Scholar]
- Davey MW, Hargrave RW, Davey RA. Comparison of drug accumulation in P-glycoprotein-expressing and MRP-expressing human leukaemia cells. Leuk Res. 1996;20:657–664. doi: 10.1016/0145-2126(96)00023-9. [DOI] [PubMed] [Google Scholar]
- Davey RA, Longhurst TJ, Davey MW, Belov L, Harvie RM, Hancox D, et al. Drug resistance mechanisms and MRP expression in response to epirubicin treatment in a human leukaemia cell line. Leuk Res. 1995;19:275–282. doi: 10.1016/0145-2126(94)00159-8. [DOI] [PubMed] [Google Scholar]
- Dixit V, Hariparsad N, Li F, Desai P, Thummel KE, Unadkat JD. Cytochrome P450 enzymes and transporters induced by anti-human immunodeficiency virus protease inhibitors in human hepatocytes: implications for predicting clinical drug interactions. Drug Metab Dispos. 2007;35:1853–1859. doi: 10.1124/dmd.107.016089. [DOI] [PubMed] [Google Scholar]
- Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- Ford J, Khoo SH, Back DJ. The intracellular pharmacology of antiretroviral protease inhibitors. J Antimicrob Chemother. 2004;54:982–990. doi: 10.1093/jac/dkh487. [DOI] [PubMed] [Google Scholar]
- Gerber JG, Acosta EP. Therapeutic drug monitoring in the treatment of HIV-infection. J Clin Virol. 2003;27:117–128. doi: 10.1016/s1386-6532(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Gibbs JE, Thomas SA. The distribution of the anti-HIV drug, 2′3′-dideoxycytidine (ddC), across the blood–brain and blood–cerebrospinal fluid barriers and the influence of organic anion transport inhibitors. J Neurochem. 2002;80:392–404. doi: 10.1046/j.0022-3042.2001.00711.x. [DOI] [PubMed] [Google Scholar]
- Huisman MT, Smit JW, Crommentuyn KM, Zelcer N, Wiltshire HR, Beijnen JH, et al. Multidrug resistance protein 2 (MRP2) transports HIV protease inhibitors, and transport can be enhanced by other drugs. AIDS. 2002;16:2295–2301. doi: 10.1097/00002030-200211220-00009. [DOI] [PubMed] [Google Scholar]
- Huisman MT, Smit JW, Wiltshire HR, Hoetelmans RM, Beijnen JH, Schinkel AH. P-glycoprotein limits oral availability, brain, and foetal penetration of saquinavir even with high doses of ritonavir. Mol Pharmacol. 2001;59:806–813. doi: 10.1124/mol.59.4.806. [DOI] [PubMed] [Google Scholar]
- Janneh O, Jones E, Chandler B, Owen A, Khoo SH. Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4T cells and primary human lymphocytes. J Antimicrob Chemother. 2007;60:987–993. doi: 10.1093/jac/dkm353. [DOI] [PubMed] [Google Scholar]
- Janneh O, Owen A, Chandler B, Hartkoorn RC, Hart CA, Bray PG, et al. Modulation of the intracellular accumulation of saquinavir in peripheral blood mononuclear cells by inhibitors of MRP1, MRP2, P-gp and BCRP. AIDS. 2005;19:2097–2102. doi: 10.1097/01.aids.0000194793.36175.40. [DOI] [PubMed] [Google Scholar]
- Jones K, Bray PG, Khoo SH, Davey RA, Meaden ER, Ward SA, et al. P-Glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: potential for accelerated viral drug resistance. AIDS. 2001;15:1353–1358. doi: 10.1097/00002030-200107270-00004. [DOI] [PubMed] [Google Scholar]
- Kim AE, Dintaman JM, Waddell DS, Silverman JA. Saquinavir, an HIV protease inhibitor, is transported by P-glycoprotein. J Pharmacol Exp Ther. 1998;286:1439–1445. [PubMed] [Google Scholar]
- Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest. 2003;33 Suppl 2:1–5. doi: 10.1046/j.1365-2362.33.s2.5.x. [DOI] [PubMed] [Google Scholar]
- Kim RB. Transporters and drug discovery: why, when, and now. Mol Pharm. 2005;3:26–32. doi: 10.1021/mp050084o. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, et al. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120:525–533. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- la Porte CJL, Back DJ, Blaschke T, Boucher CAB, Flexner CV, Gerber JG, et al. Updated guidelines to perform therapeutic drug monitoring for antiviral agents. Antivir Ther. 2006;3:4–14. [Google Scholar]
- Letschert K, Faulstich H, Keller D, Keppler D. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci. 2006;91:140–149. doi: 10.1093/toxsci/kfj141. [DOI] [PubMed] [Google Scholar]
- Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- Owen A, Janneh O, Hartkoorn RC, Chandler B, Bray PG, Martin P, et al. In vitro synergy and enhanced murine brain penetration of saquinavir coadministered with mefloquine. J Pharmacol Exp Ther. 2005;314:1202–1209. doi: 10.1124/jpet.105.086272. [DOI] [PubMed] [Google Scholar]
- Roe M, Folkes A, Ashworth P, Brumwell J, Chima L, Hunjan S, et al. Reversal of P-glycoprotein mediated multidrug resistance by novel anthranilamide derivatives. Bioorg Med Chem Lett. 1999;9:595–600. doi: 10.1016/s0960-894x(99)00030-x. [DOI] [PubMed] [Google Scholar]
- Schiffer R, Neis M, Holler D, Rodriguez F, Geier A, Gartung C, et al. Active influx transport is mediated by members of the organic anion transporting polypeptide family in human epidermal keratinocytes. J Invest Dermatol. 2003;120:285–291. doi: 10.1046/j.1523-1747.2003.12031.x. [DOI] [PubMed] [Google Scholar]
- Su Y, Zhang X, Sinko PJ. Human organic anion-transporting polypeptide OATP-A (SLC21A3) acts in concert with P-glycoprotein and multidrug resistance protein 2 in the vectorial transport of Saquinavir in Hep G2 cells. Mol Pharm. 2004;1:49–56. doi: 10.1021/mp0340136. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, et al. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–260. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Wolkoff AW, Kim RB. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J Pharmacol Exp Ther. 2003;304:223–228. doi: 10.1124/jpet.102.043026. [DOI] [PubMed] [Google Scholar]
- Williams GC, Liu A, Knipp G, Sinko PJ. Direct evidence that saquinavir is transported by multidrug resistance-associated protein (MRP1) and canalicular multispecific organic anion transporter (MRP2) Antimicrob Agents Chemother. 2002;46:3456–3462. doi: 10.1128/AAC.46.11.3456-3462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodahl EL, Yang Z, Bui T, Shen DD, Ho RJ. MDR1 G1199A polymorphism alters permeability of HIV protease inhibitors across P-glycoprotein-expressing epithelial cells. AIDS. 2005;19:1617–1625. doi: 10.1097/01.aids.0000183626.74299.77. [DOI] [PubMed] [Google Scholar]