Abstract
Background and purpose:
Levosimendan is used in the treatment of decompensated heart failure. It increases the contractility of the myocardium by sensitizing troponin C to calcium. In addition, levosimendan has been reported to have beneficial effects in experimental models of septic shock. Because heart failure and sepsis have been associated with excessive nitric oxide (NO) production through inducible NOS (iNOS), we investigated the effects of the simendans on NO production and iNOS expression and on generation of pro-inflammatory cytokines.
Experimental approach:
Macrophages and fibroblasts were exposed to inflammatory stimuli to induce iNOS expression. Proteins were measured by western blot and mRNA expression was determined by quantitative RT-PCR. Promoter activity and nuclear factor-κB (NF-κB) and the γ-activated site (GAS; binding site for signal transducer and activator of transcription 1; STAT1)-mediated transcription were investigated using luciferase reporter constructs. Cytokines tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were measured by ELISA.
Key results:
Levosimendan and dextrosimendan decreased NO production in a dose-dependent manner in cells exposed to inflammatory stimuli. The simendans decreased iNOS protein and mRNA expression but did not affect iNOS mRNA decay. These compounds decreased iNOS promoter activity and inhibited NF-κB-mediated transcription but not that mediated by STAT1/GAS. The simendans reduced IL-6 production slightly but they had no effect on TNF-α synthesis.
Conclusions and implications:
The simendans downregulated NF-κB-dependent transcription and decreased iNOS promoter activity, iNOS expression and NO production. These mechanisms may contribute to their beneficial clinical effects.
Keywords: inflammation, nitric oxide, iNOS, levosimendan, dextrosimendan
Introduction
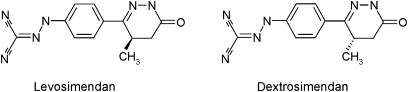
Levosimendan, the levorotatory enantiomer of [[4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl]hydrazono]propanedinitrile, is a calcium sensitizer that has been developed for the treatment of decompensated heart failure (Mathew and Katz, 1998). Dextrosimendan is a stereoisomer of levosimendan. It is not used clinically because it is a less effective calcium sensitizer than levosimendan (Kaheinen et al., 2006). In addition to sensitizing troponin C to calcium, levosimendan opens ATP-sensitive potassium channels (KATP), causing vasodilatation (Yokoshiki et al., 1997). Clinical evidence suggests that levosimendan also has anti-inflammatory properties. It has been shown to decrease plasma levels of tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6) and soluble Fas ligand in patients with heart failure (Parissis et al., 2004; Kyrzopoulos et al., 2005; Trikas et al., 2006). Simendans were shown to reduce swelling in carrageenan-induced paw oedema in rats (Haikala et al., 2002) and to have beneficial effects in experimental models of septic shock (Oldner et al., 2001; Faivre et al., 2005). Simendans have also been shown to induce apoptosis in human primary eosinophils, and dextrosimendan reduced lung eosinophilia in ovalbumin-sensitized mice (Kankaanranta et al., 2007).
Nitric oxide (NO) is a signalling molecule that regulates cellular functions in both physiological and pathophysiological conditions in the human body. In inflammation, NO modulates immune responses and the inflammatory process (Moilanen et al., 1999; Korhonen et al., 2005). Overproduction of NO is associated with the pathophysiology of various inflammatory diseases such as asthma (Ricciardolo et al., 2004) and arthritis (Cuzzocrea, 2006; Vuolteenaho et al., 2007). NO is one of the inflammatory mediators involved in the excessive inflammatory response in sepsis (Kirkebøen and Strand, 1999; Cobb, 2001). NO is synthesized from L-arginine by NOS enzymes. In mammalian cells, there are three isoforms of the enzyme: neuronal nNOS and endothelial eNOS are constitutively expressed, and the third isoform, inducible NOS (iNOS), is induced in response to cytokines and bacterial products in inflammatory and tissue cells (Alderton et al., 2001; Kleinert et al., 2004; Korhonen et al., 2005). NO production through the iNOS pathway is regulated mainly at the level of iNOS expression. iNOS is induced in response to inflammatory stimuli such as pro-inflammatory cytokines (for example, IL-1, TNF-α and interferon-γ (IFN-γ)) and bacterial lipopolysaccharide (LPS). Induction of iNOS expression by LPS and cytokines is because of the transcriptional activation of iNOS gene and stabilization of newly synthesized iNOS mRNA (Kleinert et al., 2004; Korhonen et al., 2005). Nuclear factor-κB (NF-κB) and signal transducer and activator of transcription 1 (STAT1) are important transcription factors in iNOS expression (Xie et al., 1994; Meraz et al., 1996). Once expressed, iNOS produces high amounts of NO for long periods of time. Compounds that inhibit iNOS expression or iNOS activity have anti-inflammatory properties, on the basis of their effects in various forms of experimentally induced inflammation (Tinker and Wallace, 2006). In heart failure, excessive NO production by iNOS decreases myocardial contractility, whereas low, physiological, levels of NO produced by constitutive forms of NOS may improve contractile responses in more physiological situations. In the failing heart, increased production of both reactive oxygen species and NO leads to the formation of peroxynitrite, which causes cellular damage. In addition, NO produced by iNOS is mainly responsible for the cardiodepression in septic shock (Rastaldo et al., 2007).
In this study, we investigated the effects of levosimendan and dextrosimendan (Figure 1) on iNOS expression and NO production and on the production of the inflammatory cytokines IL-6 and TNF-α. The main finding was that the simendans inhibited iNOS expression and NO production by attenuating iNOS promoter activity possibly by downregulating NF-κB-mediated transcription, and these cellular mechanisms may contribute to the beneficial clinical effects of levosimendan.
Figure 1.
The chemical structures of levosimendan and dextrosimendan.
Materials and methods
Cell culture
J774 macrophages (TIB-67; ATCC, Manassas, VA, USA) were cultured at 37 °C in 5% CO2 atmosphere in Dulbecco's modified Eagle's medium with Ultraglutamine 1 (Lonza, Verviers Sprl, Verviers, Belgium) containing 10% heat-inactivated foetal bovine serum (Lonza), 100 U mL−1 penicillin, 100 μg mL−1 streptomycin and 250 ng mL−1 amphotericin B (all from Invitrogen, Paisley, UK).
L929 fibroblasts (CCL-1; ATCC, Manassas, VA, USA) were cultured at 37 °C in 5% CO2 atmosphere in Eagle's minimum essential medium with L-glutamine containing 10% heat-inactivated foetal bovine serum and supplemented with sodium bicarbonate (0.15%), non-essential amino acids (1 mM each), sodium pyruvate (1 mM) (all from Lonza) and 100 U mL−1 penicillin, 100 μg mL−1 streptomycin and 250 ng mL−1 amphotericin B (all from Invitrogen).
J774 macrophages and L929 fibroblasts were seeded on 24-well plates for nitrite measurement, ELISA, reverse transcriptase PCR (RT-PCR) and western blots and on 10-cm dishes for nuclear extract preparation. The cells were then grown to confluence (L929 fibroblasts for 48 h and J774 macrophages for 72 h) before experiments. Before the commencement of the experiments, L929 cells were serum-starved for 16–18 h and the experiments were performed in serum-free media.
Cytotoxicity after treatment with combinations of LPS or cytokine mixture and the tested compounds was ruled out using modified XTT test (Cell Proliferation Kit II, Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions.
Construction of murine iNOS promoter reporter plasmid and stable transfection of murine L929 cell line
The pGL-MNOS II-5′-Luc plasmid (Kleinert et al., 1996) containing 5′-flanking sequence (1171 bp, positions −1570 to +141; promoter and a part of exon 1) of the murine iNOS gene was provided by Professor Hartmut Kleinert (Johannes Gutenberg University, Mainz, Germany). This plasmid was digested with KpnI and HindIII, and the restriction fragment containing murine iNOS promoter and part of exon 1 was then cloned into the KpnI/HindIII site of firefly luciferase reporter plasmid pGL4.17(luc2/neo) (Promega, Madison, WI, USA) generating pGL4(miNOS-prom)neo, in which the luciferase gene is driven by murine iNOS promoter. The plasmid was sequenced to confirm the appropriate size, position and orientation of the insert in the plasmid. To create a stable transfection with murine iNOS promoter-reported plasmid, L929 cells were transfected with pGL4(miNOS-prom)neo and pGL4.17(luc2/neo) as a control using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Transfected cells were selected with G 418 disulphate salt (Sigma-Aldrich Co., St Louis, MO, USA) treatment (800 μg mL−1). After the selection was complete, the survived clones were pooled to give rise to L929-pGL4(miNOS-prom)neo and L929-pGL4(luc)neo cell lines and further cultured in the presence of 400 μg mL−1 of G 418.
Preparation of the stable L929 pNF-κB and pGAS reporter cell lines
The luciferase reporter constructs for NF-κB [pNF-κB(luc)neo] and STAT1 [pGAS(luc)neo] were provided by Professor Hartmut Kleinert (Johannes Gutenberg University, Mainz, Germany). pNF-κB(luc)neo contained five NF-κB-binding sites and pGAS(luc)neo contained four γ-activated sites (GASs) to drive luciferase expression. Both plasmids contained neomycin resistance gene under control of TK promoter for mammalian selection. To create stably transfected cell lines, L929 cells were transfected with pNF-κB(luc)neo or pGAS(luc)neo reporter plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Transfected cells were selected with G 418 disulphate salt (Sigma-Aldrich Co.) treatment (800 μg mL−1). After the selection was complete, the survived clones were pooled to give rise to L929-pNF-κB(luc)neo and L929-pGAS(luc)neo cell lines and further cultured in the presence of 400 μg mL−1 of G 418. The experiments were performed in serum-free media.
Cytokine measurements by ELISA
Tumour necrosis factor-α and IL-6 concentrations in the culture medium were measured by ELISA using reagents from R&D Systems Europe (Abingdon, UK).
Nitrite assays
Cells were incubated with compounds of interest for 24 h. Culture medium was then collected, and nitrite (a stable metabolite of NO in aqueous conditions) levels were measured by the Griess method (Green et al., 1982).
Preparation of cell lysates for western blot
At the indicated time points, cells were rapidly washed with ice-cold phosphate-buffered saline. Cells were solubilized in cold lysis buffer containing 1% Triton-X, 50 mM NaCl, 10 mM Tris-base pH 7.4, 5 mM EDTA, 0.5 mM phenylmethylsulphonylfluoride, 1 mM sodium orthovanadate, 40 μM leupeptin, 50 μg mL−1 aprotinin, 5 mM NaF, 2 mM sodium pyrophosphate and 10 μM N-octyl-β-D-glucopyranoside. After incubation for 15 min on ice, lysates were centrifuged (13 500 g, 5 min). The protein content of the supernatants was measured by the Coomassie blue method (Bradford, 1976). The extracts were boiled in SDS sample buffer and stored at −20 °C.
Western blotting
Protein (20 μg of lysates and nuclear extracts unless otherwise stated) was analysed according to standard western blotting procedure as previously described (Sareila et al., 2006) except that the membrane was incubated with the primary antibodies in the blocking solution at 4 °C overnight. The quantitation of the chemiluminescent signal was carried out with the use of FluorChem (Alpha Innotech Corporation, San Leandro, CA, USA) software version 3.1.
RNA extractions and quantitative RT-PCR
RNA extractions and quantitative real-time RT-PCR were performed as described previously (Sareila et al., 2006). Briefly, total RNA was extracted, treated with DNAse I (Fermentas UAB, Vilnius, Lithuania) and converted to cDNA. iNOS, luciferase and GAPDH mRNA were measured by quantitative RT-PCR. iNOS and luciferase mRNA levels were normalized to GAPDH, which was considered as a housekeeping gene. Each sample was determined in duplicate. iNOS, GAPDH and luciferase primer and probe (6-FAM (6-carboxyfluorescein) as 5′-reporter dye and TAMRA (6-carboxytetramethylrhodamine) as 3′-quencher) sequences are described in Table 1.
Table 1.
Primer and probe sequences
| Gene | Oligonucleotide | Sequence 5′ → 3′ |
|---|---|---|
| Murine iNOS | Forward primer | CCTGGTACGGGCATTGCT |
| Reverse primer | GCTCATGCGGCCTCCTT | |
| Probe | CAGCAGCGGCTCCATGACTCCC | |
| Murine GAPDH | Forward primer | GCATGGCCTTCCGTGTTC |
| Reverse primer | GATGTCATCATACTTGGCAGGTTT | |
| Probe | TCGTGGATCTGACGTGCCGCC | |
| Luciferasea | Forward primer | ACGGCTTCGGCATGTTCA |
| Reverse primer | CTCCTCCTCGAAGCGGTACA | |
| Probe | TTGATCTGCGGCTTTCGGGTCGT | |
| Luciferase2b | Forward primer | AAAAAGTTGCGCGGAGGAG |
| Reverse primer | TTTTTCTTGCGTCGAGTTTTCC | |
| Probe | TGTGTTTGTGGACGAAGTACCGAA AGGTCTTAC |
For pGL4(miNOS-prom)neo-construct.
For pNF-κB(luc)neo and pGAS(luc)neo reporters.
Actinomycin D assay
J774 macrophages were incubated with LPS (10 ng mL−1) in the presence or absence of levosimendan (10 μM) for 6 h and actinomycin D (0.5 μg mL−1) was then added to the cells. L929 fibroblasts were incubated with a mixture of pro-inflammatory cytokines (cytokine mixture (CM); TNF-α, IFN-γ and IL-1β, 10 ng mL−1 each) in the presence or absence of dextrosimendan (10 μM) for 3 h, and actinomycin D (5 μg mL−1) was then added to the cells. After the addition of actinomycin D, cells were further incubated for 2, 4 and 6 h. Cells were then lysed, total cellular RNA was extracted and iNOS mRNA expression was measured by quantitative RT-PCR, and normalized against GAPDH mRNA. iNOS mRNA levels at the time of addition of actinomycin D were set as 100%, and the amount of iNOS mRNA left at the subsequent time points was related to that value.
Preparation of nuclear extracts
Nuclear proteins were extracted as described previously (Sareila et al., 2006). Briefly, the cell membranes were disrupted with a hypotonic buffer, the nuclei were separated by centrifugation after which the nuclear proteins were released and isolated by another centrifugation step. The protein content of the extracts was measured by the Coomassie blue method (Bradford, 1976). For electrophoretic mobility shift assay (EMSA), the extracts were stored at −70 °C. For western blot, the extracts were boiled in SDS sample buffer and stored at −20 °C.
Electrophoretic mobility shift assay
Single-stranded DNA oligonucleotide (5′-CCTTTTCCCCTAACACT-3′) and its complementary DNA oligonucleotide containing STAT1-binding GAS region present in murine iNOS promoter (NOS-GAS) (Gao et al., 1997) (Oligomer, Helsinki, Finland) were annealed in annealing buffer (Ambion, Austin, TX, USA) by heating at 95 °C for 5 min and then slowly cooling down. NOS-GAS oligonucleotides and consensus oligonucleotides for NF-κB (Promega) were 5′-end-labelled with γ-P32-ATP (GE Healthcare Europe GmbH, Munich, Germany) using T4 polynucleotide kinase (Promega). For binding reactions, 5 μg (8 μg for NOS-GAS) of nuclear extracts were incubated in a 20 μL reaction volume containing 10 μg mL−1 (poly)dI–dC, 1 mM dithiotreitol, 10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 40 mM KCl and 10% glycerol for 20 min at room temperature. 32P-labelled oligonucleotide probe (0.2 ng) was added and the reaction mixture was incubated for 10 min. Protein–DNA complexes were separated from DNA probe by electrophoresis on a native 4% polyacrylamide gel. The gel was dried and autoradiographed using intensifying screen at −70 °C.
Statistics
Results are expressed as mean+s.e.mean. When indicated, statistical analysis was carried out by analysis of variances followed by Dunnett multiple comparisons test. Differences were considered significant at P<0.05.
Reagents
Levosimendan [(−)-[[4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl]hydrazono]propanedinitrile] and dextrosimendan [(+)-[[4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazono]propanedinitrile] were kindly provided by Orion Pharma (Espoo, Finland). Simendans were dissolved in dimethylsulphoxide just before the experiments. They were further diluted into culture medium 1:1000 to give the final concentration of 0.1% of dimethylsulphoxide (which was also added to controls). Rabbit anti-iNOS (sc-650), anti-STAT1α p91 (sc-345), anti-NF-κB p65 (sc-7151), anti-actin (sc-1616-R) and anti-lamin A/C (sc-20681) primary antibodies and secondary horseraddish peroxidase-conjugated polyclonal antibody (sc-2004) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Janus kinase inhibitor WHI-P154 [4-[(3-bromo-4-hydroxyphenyl)amino]-6,7-dimethoxyquinazoline] was from Merck Chemicals Ltd (Nottingham, UK), and MG 132 [N-[(phenylmethoxy)carbonyl]-L-leucyl-N-[(1S)-1-formyl-3 -methylbutyl]-L-leucinamide] was from Tocris Bioscience (Ellisville, MO, USA). Recombinant mouse IFN-γ, TNF-α, IL-1β were from R&D systems (Minneapolis, MN, USA). All other reagents were from Sigma-Aldrich Co. unless otherwise stated.
Results
Effects of simendans on LPS-induced production of inflammatory factors TNF-α, IL-6 and NO in J774 macrophages
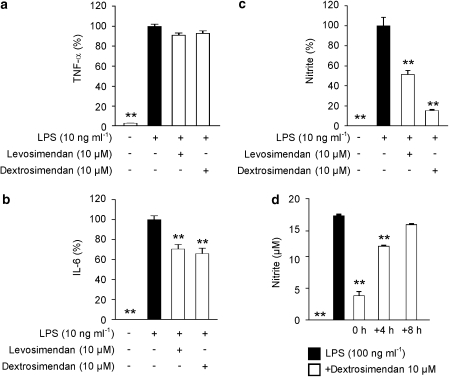
To test the effects of simendans on inflammatory factors produced by LPS-stimulated J774 macrophages, the release of TNF-α, IL-6 and NO into the culture medium was measured. TNF-α, IL-6 and NO production in resting cells was very low, but their release was significantly enhanced in response to LPS (10 ng mL−1). In the presence of levosimendan and dextrosimendan (10 μM), TNF-α levels (4730±70 pg mL−1) were not significantly changed (<10% decrease; Figure 2a). LPS-induced IL-6 production (1070±30 pg mL−1) was inhibited by about 30% by levosimendan and dextrosimendan (Figure 2b). LPS-induced NO production (19.0±0.6 μM) was significantly (by 50–80%) inhibited by both simendans (Figure 2c).
Figure 2.
The effects of simendans on the production of inflammatory cytokines tumour necrosis factor-α (TNF-α) (a) and interleukin-6 (IL-6) (b) and nitric oxide (NO) (c) in lipopolysaccharide (LPS)-stimulated J774 macrophages. Cells were incubated with the indicated combinations of LPS (10 ng mL−1) and simendans (10 μM) for 24 h. TNF-α and IL-6 levels were measured by ELISA. NO production was measured as nitrite accumulated in the culture medium by the Griess reaction. (d) The effect of simendans on LPS-induced NO production in J774 macrophages when the simendans were added simultaneously with LPS or 4 or 8 h after LPS. Cells were incubated with the indicated combinations of LPS (100 ng mL−1) and simendans (10 μM) for 24 h, and NO production was measured as nitrite accumulated in the culture medium by Griess reaction. The results are expressed as mean+s.e.mean (n=4 in a, b and n=6 in c, d). *P<0.05 and **P<0.01 when compared with cells treated with LPS alone.
The time-dependent effect of simendans on NO production was also tested. When dextrosimendan was added into the culture medium 4 or 8 h after LPS (100 ng mL−1), the inhibitory effect on NO production was markedly smaller than when the drug was added at the same time as LPS (Figure 2d). This suggests that simendans do not inhibit the activity of NOS enzyme but they rather inhibit induction of NO production. Also, the higher LPS concentration (100 ng mL−1) did not prevent the effects of simendans on NO production. Cell viability was not affected by simendans or by the combination of LPS and simendans during 24 h incubation as measured by XTT tests.
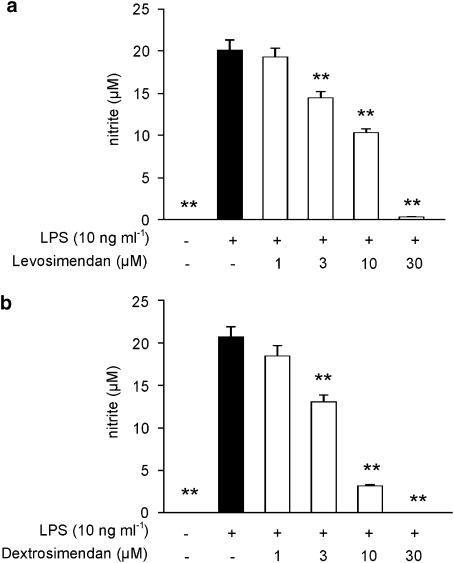
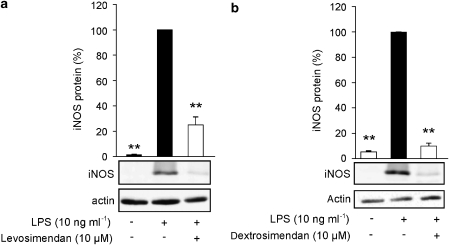
As the simendans inhibited NO production more potently than that of the cytokines, we then studied the effects of increasing concentrations of levosimendan and dextrosimendan on NO production. LPS-induced NO production was inhibited by levosimendan and dextrosimendan in a dose-dependent manner, and 50% inhibition was caused by drug concentrations between 3 and 10 μM (Figure 3). Also, LPS-induced iNOS protein expression was inhibited by both levosimendan and dextrosimendan (Figure 4).
Figure 3.
The concentration-dependent effect of levosimendan (a) and dextrosimendan (b) on lipopolysaccharide (LPS)-induced nitric oxide (NO) production in J774 macrophages. Cells were incubated with LPS (10 ng mL−1) and levosimendan or dextrosimendan (1–30 μM) for 24 h. NO production was measured as nitrite accumulated in the culture medium by Griess reaction. The results are expressed as mean+s.e.mean (n=6). **P<0.01 when compared with cells treated with LPS alone.
Figure 4.
The effects of levosimendan (a) and dextrosimendan (b) on lipopolysaccharide (LPS)-induced iNOS protein expression in J774 macrophages. Cells were incubated with LPS (10 ng mL−1) in the presence or in the absence of levosimendan or dextrosimendan (10 μM) for 24 h, and iNOS expression was detected by western blot. LPS-induced iNOS protein expression was set as 100% and the other values were related to that value. Actin was used as a loading control. The results are expressed as mean+s.e.mean (n=6 in a, n=3–6 in b). **P<0.01 when compared with cells treated with LPS alone.
Effects of simendans on iNOS mRNA expression and stability in J774 macrophages
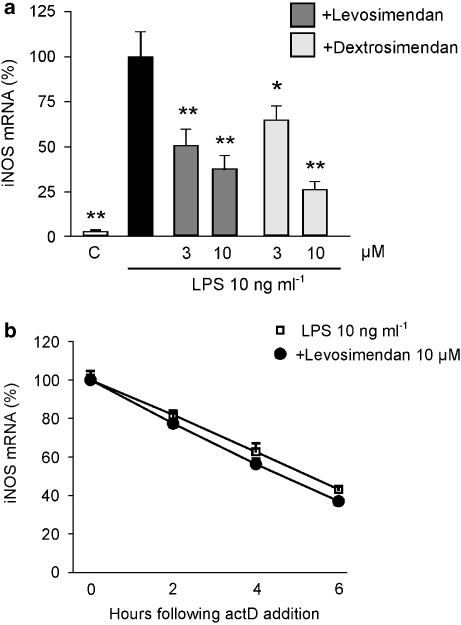
The effects of simendans on iNOS mRNA expression were studied by quantitative RT-PCR. J774 macrophages were stimulated with LPS in the presence or absence of simendans for 3 h, and iNOS mRNA levels were measured. LPS induced iNOS mRNA expression, which was inhibited by levosimendan and dextrosimendan in a dose-dependent manner (Figure 5a).
Figure 5.
The effects of the simendans on levels of iNOS mRNA and its decay in lipopolysaccharide (LPS)-stimulated J774 macrophages. (a) Cells were incubated with LPS (10 ng mL−1) in the presence or absence of levosimendan or dextrosimendan (3 and 10 μM) for 3 h and iNOS mRNA levels were measured by quantitative RT-PCR. LPS-induced iNOS mRNA expression was set as 100% and the other values were related to that value. The results are expressed as mean+s.e.mean (n=3–6). *P<0.05 and **P<0.01 when compared with cells treated with LPS alone. (b) The effects of levosimendan on iNOS mRNA decay in LPS-stimulated J774 macrophages as measured by actinomycin D assay. Cells were incubated with LPS (10 ng mL−1) in the presence or absence of levosimendan (10 μM) for 6 h, and actinomycin D (actD; 0.5 μg mL−1) was then added to stop transcription. Cells were further incubated for 2, 4 or 6 h before RNA was extracted and iNOS mRNA levels were measured by quantitative RT-PCR. iNOS mRNA levels at the time of addition of actinomycin D were set as 100% and the other values were related to that value. The results are expressed as mean+s.e.mean (n=3).
The stabilization of iNOS mRNA, in cells stimulated with LPS or cytokines, has an important function in the regulation of iNOS expression (Kleinert et al., 2004; Korhonen et al., 2005). Therefore, the effect of levosimendan on iNOS mRNA stability was tested by actinomycin D assay. J774 macrophages were stimulated with LPS in the presence or absence of levosimendan. After 6 h incubation, actinomycin D (0.5 μg mL−1) was added into the cell culture to stop transcription, and cells were further incubated for 2, 4 or 6 h. Levosimendan did not affect iNOS mRNA decay (Figure 5b).
Effects of simendans on murine iNOS promoter activity in L929 cells
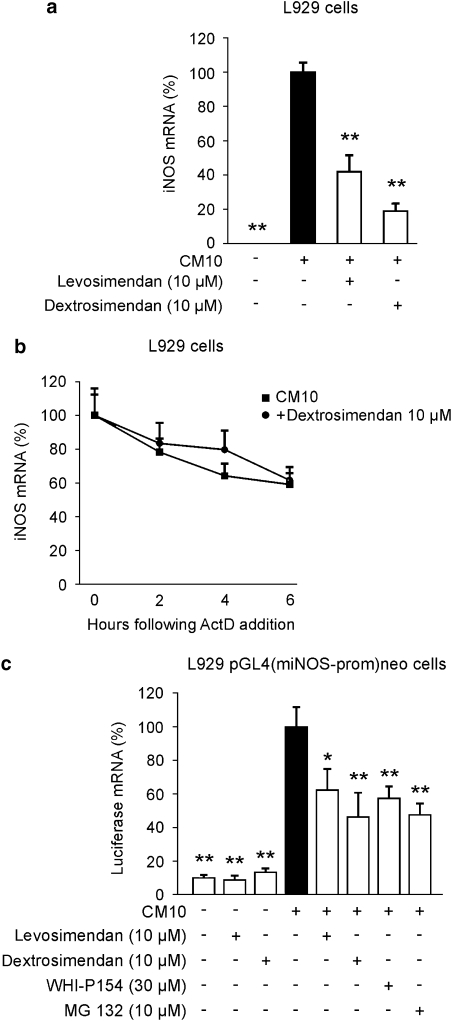
The simendans were found to decrease LPS-induced iNOS mRNA and protein expression in J774 macrophages but iNOS mRNA decay was not altered. Therefore, we investigated the effects of simendans on iNOS promoter activity. We chose to carry out the experiments using murine L929 fibroblasts that expressed iNOS and produced NO in response to a combination of pro-inflammatory cytokines (TNF-α, IFN-γ and IL-1β). Dextro- and levosimendan (1–30 μM) inhibited cytokine-induced NO production (23.6±0.6 μM) in a dose-dependent manner, and 50% inhibition was caused by 3–10 μM drug concentrations (data not shown). Cytokine-induced iNOS mRNA expression was inhibited by simendans (Figure 6a), but dextrosimendan did not affect iNOS mRNA decay (Figure 6b).
Figure 6.
The effects of the simendans on iNOS mRNA expression and decay and on iNOS promoter activity in L929 fibroblasts stimulated with pro-inflammatory cytokines. (a) Cells were incubated with a combination of pro-inflammatory cytokines (cytokine mixture (CM 10), tumour necrosis factor-α, interferon-γ and interleukin-1β, 10 ng mL−1 each) and levo- or dextrosimendan (10 μM) for 6 h, and iNOS mRNA levels were measured by quantitative RT-PCR. Cytokine-induced iNOS mRNA expression was set as 100% and the other values were related to that value. The results are expressed as mean+s.e.mean (n=6). **P<0.01 when compared with cells treated with cytokine mixture alone. (b) Cells were incubated with a combination of pro-inflammatory cytokines (CM 10) in the presence or absence of dextrosimendan (10 μM) for 3 h, and actinomycin D (ActD; 5 μg mL−1) was then added to stop transcription. Cells were further incubated for 2, 4 of 6 h before RNA was extracted, and iNOS mRNA levels were measured by quantitative RT-PCR. iNOS mRNA levels at the time of addition of ActD were set as 100% and the other values were related to that value. The results are expressed as mean+s.e.mean (n=4). (c) The effects of levosimendan and dextrosimendan on iNOS promoter activity in L929 pGL4(miNOS-prom)neo cells. L929 cells stably transfected with pGL4(miNOS-prom)neo were stimulated with a combination of pro-inflammatory cytokines (CM 10) alone or in combination with levosimendan, dextrosimendan, Janus kinase inhibitor WHI-P154 or NF-κB inhibitor MG 132 for 6 h, and luciferase mRNA levels were measured by quantitative RT-PCR. Luciferase mRNA levels in cytokine-treated cells were set as 100% and the other values were related to that value. The results are expressed as mean+s.e.mean (n=4). *P<0.05 and **P<0.01 when compared with luciferase mRNA levels in cells treated with CM alone.
The effect of simendans on iNOS promoter activity was studied in L929 cells stably transfected with pGL4(miNOS-prom)neo, in which luciferase gene is driven by murine iNOS promoter. L929-pGL4(luc)neo was used as a negative control cell line. Stimulation of L929-pGL4(miNOS-prom)neo cells with cytokines enhanced luciferase mRNA expression by about 10-fold as compared with unstimulated cells. Both levosimendan and dextrosimendan inhibited luciferase mRNA expression (Figure 6c). A corresponding inhibition of iNOS promoter activity was caused by an NF-κB inhibitor MG 132 and by the Janus kinase inhibitor WHI-P154. In the control cell line L929-pGL4(luc)neo cells, the basal luciferase mRNA levels were very low (<4% of that of cytokine-stimulated L929-pGL4(miNOS-prom)neo cells), and luciferase mRNA expression was not induced by the cytokine mixture.
Effects of levosimendan and dextrosimendan on NF-κB and STAT1 activation
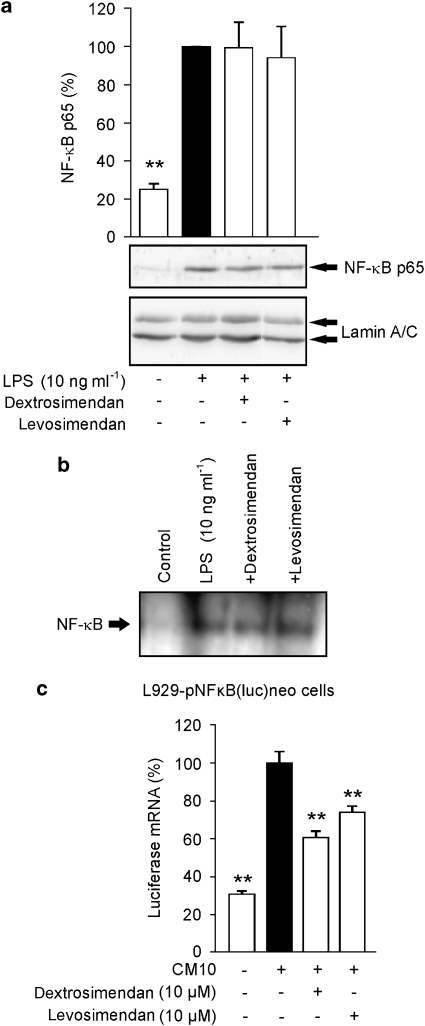
As iNOS promoter activity and iNOS mRNA expression were inhibited by simendans, we studied the effects of levosimendan and dextrosimendan on the activation of NF-κB, which is an important transcription factor in iNOS expression (Xie et al., 1994). When NF-κB is activated, it is translocated into the nucleus where it binds to the NF-κB sites in DNA. J774 macrophages were stimulated with LPS in the presence or absence of simendans, and nuclear proteins were extracted. In LPS-stimulated cells, NF-κB nuclear translocation peaked after 30 min incubation (data not shown) and it was not altered by simendans (Figure 7a). Also, levo- and dextrosimendan did not modify the DNA binding of NF-κB in response to LPS when measured by EMSA (Figure 7b). When the L929-pNF-κB(luc)neo cell line expressing the luciferase reporter gene under the control of NF-κB-responsive promoter was used, levo- and dextrosimendans decreased luciferase mRNA expression stimulated by pro-inflammatory cytokines (Figure 7c).
Figure 7.
The effects of simendans on nuclear translocation, DNA binding and transcriptional activity of nuclear factor-κB (NF-κB). (a) J774 macrophages were incubated with lipopolysaccharide (LPS) (10 ng mL−1), levosimendan (10 μM) or dextrosimendan (3 μM) for 30 min. Nuclear proteins were then extracted and NF-κB p65 was detected by western blot. Lamin A/C was used as a loading control. The nuclear level of NF-κB p65 in LPS-treated cells was set as 100% and the other values were related to that value. The results are expressed as mean+s.e.mean (n=6). **P<0.01 when compared with cells treated with LPS alone. (b) J774 macrophages were treated as in (a), and NF-κB-binding activity in nuclear extracts was analysed by electrophoretic mobility shift assay. One representative blot of three with similar results is shown. (c) L929-pNF-κB(luc)neo cells, that contained NF-κB luciferase reporter construct, were incubated with a combination of pro-inflammatory cytokines (cytokine mixture (CM 10); tumour necrosis factor-α, interferon-γ and interleukin-1β, 10 ng mL−1 each) and dextro- or levosimendan for 6 h, and luciferase mRNA levels were measured by quantitative RT-PCR. Cytokine-induced luciferase expression was set as 100% and the other values were related to that value. The results are expressed as mean+s.e.mean (n=3). **P<0.01 when compared with cells treated with cytokine mixture alone.
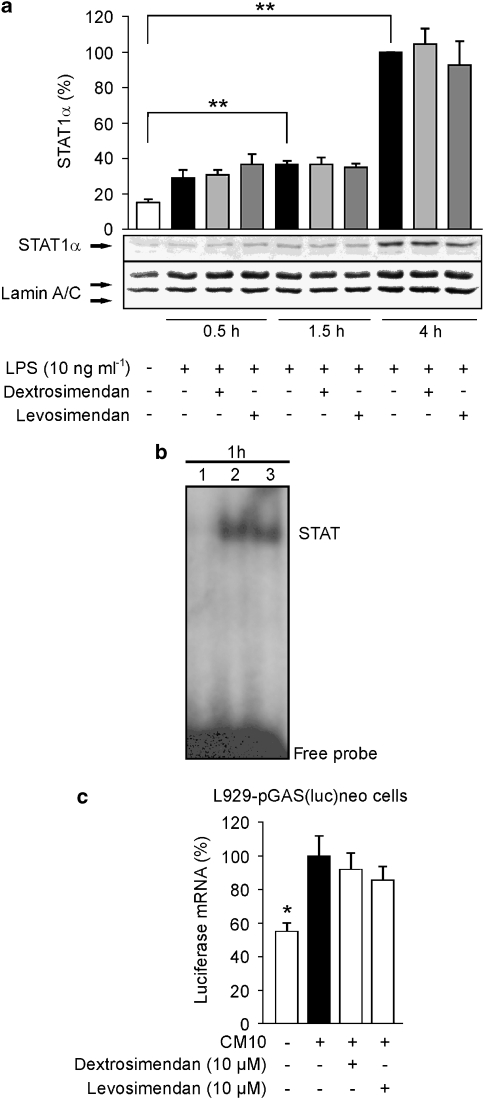
The STAT1 is another important transcription factor involved in the regulation of iNOS promoter activity (Meraz et al., 1996). Phosphorylated STATs dimerize and translocate into the nucleus, where they bind to the GAS sites in DNA (Kisseleva et al., 2002). Therefore, we investigated whether simendans modulate STAT1 activation in LPS-treated macrophages. In J774 macrophages, LPS induced STAT1 phosphorylation, which increased for up to 4 h of incubation (data not shown). The nuclear translocation of STAT1α was observed after 1.5 h of incubation and increased up to 4 h of incubation with LPS, and exposure to the simendans did not affect nuclear translocation of STAT1α (Figure 8a). STAT1 activation was also studied by EMSA using oligonucleotides containing the GAS-binding sequence found in murine iNOS promoter (Gao et al., 1997), and in these experiments, dextrosimendan did not modify STAT1 DNA-binding activity (Figure 8b). In L929-pGAS(luc)neo cell line expressing luciferase reporter gene under the control of GAS promoter, levo- and dextrosimendans did not affect cytokine-induced luciferase mRNA expression (Figure 8c).
Figure 8.
The effects of simendans on nuclear translocation, DNA binding and transcriptional activity of signal transducer and activator of transcription 1 (STAT1). (a) J774 macrophages were incubated with lipopolysaccharide (LPS) and levosimendan or dextrosimendan. Nuclear proteins were extracted at the indicated time points and STAT1α was detected by western blot. Lamin A/C was used as a loading control. The nuclear level of STAT1α in LPS-treated cells was set as 100% and the other values were related to that value. The results are expressed as mean+s.e.mean (n=3). **P<0.01. (b) J774 macrophages were incubated with (1) vehicle, (2) LPS (10 ng mL−1)+interferon-γ (IFN-γ) (5 ng mL−1) or (3) LPS (10 ng mL−1)+IFN-γ (5 ng mL−1)+dextrosimendan (10 μM) for 1 h. Nuclear extracts were then prepared and STAT1α DNA-binding activity was analysed by electrophoretic mobility shift assay. One representative blot of three with similar results is shown. (c) L929-pGAS(luc)neo cells, which contain the GAS-motif (binding site for STAT1)-luciferase reporter construct, were incubated with a combination of pro-inflammatory cytokines (cytokine mixture (CM 10); tumour necrosis factor-α, IFN-γ and interlekin-1β, 10 ng mL−1 each) and dextrosimendan or levosimendan for 6 h and luciferase mRNA levels were measured by quantitative RT-PCR. Cytokine-induced luciferase mRNA expression was set as 100% and the other values were related to that value. The results are expressed as mean+s.e.mean (n=3). *P<0.05 and **P<0.01 when compared with cells treated with cytokine mixture alone.
Discussion and conclusions
Levosimendan is a calcium sensitizer used in the treatment of decompensated heart failure. In this study, we showed that levosimendan and its enantiomer dextrosimendan decreased NO production and IL-6 secretion in response to inflammatory stimuli. The results suggest that the simendans reduced NO production and iNOS expression by inhibiting iNOS promoter activity and NF-κB-dependent transcription, which is a novel finding. The effects of simendans were not cell type specific, as iNOS expression was inhibited both in macrophages and fibroblasts, which produce inflammatory mediators in failing hearts (Brown et al., 2005).
In J774 macrophages, simendans inhibited NO production and iNOS protein and mRNA expression without affecting the activation, nuclear translocation or DNA binding of NF-κB or STAT1. In L929 cells, which are readily transfectable and therefore suitable for reporter gene studies, simendans were found to inhibit iNOS promoter activity and NF-κB-dependent transcription along with their inhibitory action on NO production and iNOS expression. Dextrosimendan was somewhat more potent than levosimendan in inhibiting NO production and iNOS expression in J774 and L929 cells as well as to reduce iNOS promoter activity and NF-κB-mediated transcription in L929 cells. STAT1 activation or STAT1-dependent transcription was not affected by simendans. Simendans did not alter iNOS mRNA decay as measured by actinomycin D assay. Because of methodological reasons, the iNOS promoter and NF-κB reporter gene experiments were carried out in fibroblasts in which the inhibitory effects of simendans on iNOS expression and NO production were very similar to those in J774 macrophages. Taken together, the results suggest that simendans downregulate iNOS expression at transcriptional level by inhibiting NF-κB-dependent transcription. However, it should be pointed out that differences in the signalling events leading to the activation of iNOS transcription between cell types might exist.
Levosimendan has been shown to possess anti-inflammatory properties in vitro and in vivo (Paraskevaidis et al., 2005). It has been shown to reduce circulating levels of pro-inflammatory cytokines, TNF-α and IL-6, TNF-receptors and soluble Fas and Fas ligand in patients with severe heart failure (Parissis et al., 2004; Kyrzopoulos et al., 2005; Trikas et al., 2006). Levosimendan and dextrosimendan reduced swelling with comparable potencies in carrageenan-induced paw oedema model of acute inflammation in the rat (Haikala et al., 2002). Levosimendan has been reported to have beneficial effects in experimental models of septic shock (Oldner et al., 2001; Faivre et al., 2005). In addition, both levosimendan and dextrosimendan were shown to induce apoptosis in isolated human eosinophils, and dextrosimendan reduced lung eosinophilia in ovalbumin-sensitized mice (Kankaanranta et al., 2007). In that study, the effect of levosimendan on the lung eosinophilia was not reported. This study extends the previous data by showing that simendans inhibit NF-κB-dependent transcription and, probably by this mechanism, inhibited also iNOS expression and NO production, because NF-κB is an important transcription factor for iNOS expression (Xie et al., 1994). In addition, simendans (at 10 μM concentrations) had a slight effect on IL-6 production but practically no effect on TNF-α synthesis. These results suggest that the effects of the simendans on inflammatory mediators are focused and regulatory and not non-specific in nature. Similar results have been obtained in patients with decompensated chronic heart failure, when levosimendan treatment was shown to decrease IL-6 but not TNF-α levels (Avgeropoulou et al., 2005).
Chronic heart failure is associated with increased oxidative stress, which contributes to the pathophysiological process (Keith et al., 1998). It has been demonstrated that the negative inotropic effect of cytokines on the heart is mediated, at least partly, by NO (Finkel et al., 1992), and in the heart, overproduction of NO is harmful in the systemic inflammation response syndrome (Ungureanu-Longrois et al., 1995). Excessive NO production by iNOS contributes to the decreased cardiac contractility in relation to decompensated heart failure and cardiodepression in septic shock (Rastaldo et al., 2007). In addition, simultaneous production of superoxide and NO by macrophages may result in the formation of peroxynitrite (ONOO−). Peroxynitrite is a highly reactive molecule that causes cellular damage by oxidation and nitration reactions, which may contribute to the development of heart failure (Pacher et al., 2007; Rastaldo et al., 2007). In patients with exacerbations of chronic heart failure, the levels of nitrotyrosine (a marker of nitrative stress) as well as those of malondialdehyde and protein carbonyls (markers of oxidative stress) were increased in the study group treated with placebo, whereas the plasma levels of all the three markers were not changed in the study group treated with levosimendan (Parissis et al., 2007), suggesting that levosimendan has anti-oxidative properties or has effects on the redox state in vivo. This is of interest because NF-κB is known to be activated by oxidative stress (Ji, 2008) and may, at least partly, explain the observed inhibition of NF-κB-mediated transcription by simendans. However, we were not able to see inhibition of NF-κB activation and/or DNA binding by the simendans, although NF-κB-mediated transcription was reduced. In addition to its use in the treatment of contractile dysfunction in patients with decompensated heart failure, levosimendan was recently reported to ameliorate myocardial depression in a patient with pneumococcal septic shock (Ramaswamykanive et al., 2007) and to have beneficial effects in experimental models of septic shock (Oldner et al., 2001; Faivre et al., 2005). Increased NO production is critically involved in the excessive inflammatory response in sepsis (Kirkebøen and Strand, 1999; Cobb, 2001). Therefore, iNOS inhibition by levosimendan may contribute to the beneficial effects of levosimendan in the treatment of decompensated heart failure and may partly explain the favourable results obtained in the treatment of septic shock.
In general, the activation of NF-κB is considered to be an important step in the induction of the production of various pro-inflammatory mediators, such as TNF-α, IL-6 and iNOS-dependent NO. These results showed, however, that the simendans inhibited iNOS expression along with reduced NF-κB-mediated transcription, whereas TNF-α production remained unaltered. Signalling pathways regulating LPS-induced TNF-α expression in macrophages include Egr-1 and AP-1 in addition to NF-κB (Kishore et al., 2004), and the TNF-α promoter has also been reported to contain binding sites for ETS-1, NFAT, ATF-2/c-jun and SP-1/Egr-1 ((Yao et al., 1997; Becker et al., 1999; Tsai et al., 2000). It is possible that these other pathways compensate for the reduced NF-κB activity in the regulation of TNF-α promoter activity. Dextrosimendan has also been reported to activate c-Jun N-terminal kinase phosphorylation (Kankaanranta et al., 2007), and as c-Jun N-terminal kinase is a direct activator of AP-1, that may overcome the effects of suppressed NF-κB on TNF-α expression. In addition, NF-κB dimer composition and transcriptional activity varies and depends on promoter sequence and transcriptional co-regulators (Doyle and O'Neill, 2006). In this study, the simendans did not affect the activation, nuclear translocation or DNA-binding activity of NF-κB. In contrast, the simendans inhibit iNOS promoter activity along with an inhibitory effect on NF-κB-responsive promoter construct. The iNOS promoter (Lowenstein et al., 1993) and the TNF-α promoter (Doyle and O'Neill, 2006) contain slightly different NF-κB-binding sequences, which may be involved in the different effects of the simendans on the expression of iNOS and TNF-α in this study. A recent article described a significant inhibition of NF-κB activation and iNOS promoter activity by liquiritigenin, but much less inhibition was seen on TNF-α production in LPS-stimulated mouse macrophages (Kim et al., 2008), findings similar to our present results.
In the treatment of cardiac failure, levosimendan is administered intravenously by giving a 10 min bolus of 6–24 μg kg−1 followed by a continuous infusion at the rate of 0.05–2.0 μg kg−1 min−1 (Nieminen et al., 2000). In pharmacokinetic studies in patients with heart failure, the levosimendan plasma concentrations of 120 ng mL−1 (corresponding to 0.45 μM) were reached in the beginning of the infusion (Kivikko et al., 2003), and the drug infusion rate of 0.1 μg kg−1 min−1 resulted in steady-state levosimendan plasma concentration of 34.6±8.5 ng ml−1 (∼125 nM) (Kivikko et al., 2002). In patients with cardiac failure who were treated with levosimendan, according to this treatment protocol, plasma levels of IL-6, soluble Fas and Fas-ligand, and in some cases TNF-α levels, were reduced (Parissis et al., 2004; Avgeropoulou et al., 2005; Kyrzopoulos et al., 2005; Trikas et al., 2006). In this study, 1–10 μM concentrations of the simendans were effective and showed no cytotoxicity. These drug concentrations used in vitro were higher than the steady-state plasma concentrations reached in patients, but the metabolism of the simendans to inactive compounds during the incubations (up to 24 h) was not measured. However, the changes in IL-6 production in vitro by the simendans corresponded to those observed in patients with heart failure. This suggests that the effects on NO and IL-6 production, as well as on iNOS expression, iNOS promoter activity and NF-κB-dependent transcription with the drug concentrations used here, are real pharmacological effects and are not because of cytotoxic actions, and may be obtained in vivo.
Levosimendan has been shown to have about 10-fold greater affinity to myocardial troponin C, than dextrosimendan (Sorsa et al., 2004). Accordingly, levosimendan was found to be a more potent calcium sensitizer than dextrosimendan (potency difference of 76), and the difference was even greater when the inhibitory effects on PDE 3 were studied (levosimendan was 427 times more potent) (Kaheinen et al., 2006). In this study, the potencies of dextrosimendan and levosimendan were about similar when their inhibitory effects on iNOS expression and NO production were studied. Therefore, it is unlikely that binding to troponin C or PDE inhibition may be involved in the mechanism by which the simendans inhibit iNOS expression. Levo- and dextrosimendan have been shown to act also as KATP channel openers at similar concentrations (Haikala et al., 2002). The KATP channel openers diazoxide and iptakalim have been reported to decrease iNOS expression in BV-2 cells (Liu et al., 2005) and in rats exposed to rotenone (Yang et al., 2006). Therefore, it is possible that opening of KATP channels may contribute to the mechanism by which simendans decreased iNOS expression in this study.
In summary, we have shown that levo- and dextrosimendan downregulate iNOS expression and NO production in macrophages and fibroblasts in response to inflammatory stimuli, and that they inhibit iNOS promoter activity and NF-κB-mediated transcription. These results may partly explain the beneficial effects of levosimendan in the treatment of decompensated heart failure and in the models of septic shock. In addition, simendans may have anti-inflammatory effects in conditions and diseases that are complicated by increased NO production through the iNOS pathway.
Acknowledgments
We thank Salla Hietakangas, Eeva Lahtinen, Meiju Kukkonen and Marja-Leena Lampén for excellent technical assistance and Heli Määttä for skilful secretarial help. We acknowledge Orion Pharma (Espoo, Finland) for providing levosimendan and dextrosimendan and Professor Hartmut Kleinert (Johannes Gutenberg University Mainz, Germany) for giving pGL-MNOS II-5′-Luc, pNF-κB(luc)neo and pGAS(luc)neo plasmids. This work was supported by the Competitive Research Funding of The Pirkanmaa Hospital District, Finland, the Satakunta Regional Fund of the Finnish Cultural Foundation and Orion Pharma Research Fund, Finland.
Abbreviations
- CM
cytokine mixture
- EMSA
electrophoretic mobility shift assay
- GAS
γ-activated site
- IFN-γ
interferon-γ
- iNOS
inducible NOS
- IL
interleukin
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- RT-PCR
reverse transcriptase PCR
- STAT
signal transducer and activator of transcription
- TNF-α
tumour necrosis factor-α
Conflict of interest
E Nissinen is an employee of Orion Pharma, which is marketing levosimendan and provided simendans to this study. This study was partly supported by Orion Pharma Research Fund.
References
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgeropoulou C, Andreadou I, Markantonis-Kyroudis S, Demopoulou M, Missovoulos P, Androulakis A, et al. The Ca(2+)-sensitizer levosimendan improves oxidative damage, BNP and pro-inflammatory cytokine levels in patients with advanced decompensated heart failure in comparison to dobutamine. Eur J Heart Fail. 2005;7:882–887. doi: 10.1016/j.ejheart.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Becker C, Barbulescu K, Wirtz S, Meyer zum Büschenfelde KH, Pettersson S, Neurath MF. Constitutive and inducible in vivo protein–DNA interactions at the tumor necrosis factor-alpha promoter in primary human T lymphocytes. Gene Expr. 1999;8:115–127. [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- Cobb JP. Nitric oxide synthase inhibition as therapy for sepsis: a decade of promise. Surg Infect (Larchmt) 2001;2:93–100. doi: 10.1089/109629601750469410. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S. Role of nitric oxide and reactive oxygen species in arthritis. Curr Pharm Des. 2006;12:3551–3570. doi: 10.2174/138161206778343082. [DOI] [PubMed] [Google Scholar]
- Doyle SL, O'Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72:1102–1113. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Faivre V, Kaskos H, Callebert J, Losser MR, Milliez P, Bonnin P, et al. Cardiac and renal effects of levosimendan, arginine vasopressin, and norepinephrine in lipopolysaccharide-treated rabbits. Anesthesiology. 2005;103:514–521. doi: 10.1097/00000542-200509000-00014. [DOI] [PubMed] [Google Scholar]
- Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- Gao J, Morrison DC, Parmely TJ, Russell SW, Murphy WJ. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. J Biol Chem. 1997;272:1226–1230. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Haikala H, Hyttilä-Hopponen M, Nissinen E, Ruotsalainen M, Pippuri A, Lönnberg K. 2002. Inventors. OrionPharma. Assignee. (May 23, 2002) Anti-inflammatory agents. Patent WO 02/40025 A1
- Ji LL. Modulation of skeletal muscle antioxidant defense by exercise: role of redox signaling. Free Radic Biol Med. 2008;44:142–152. doi: 10.1016/j.freeradbiomed.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Kaheinen P, Pollesello P, Hertelendi Z, Borbely A, Szilagyi S, Nissinen E, et al. Positive inotropic effect of levosimendan is correlated to its stereoselective Ca2+-sensitizing effect but not to stereoselective phosphodiesterase inhibition. Basic Clin Pharmacol Toxicol. 2006;98:74–78. doi: 10.1111/j.1742-7843.2006.pto_231.x. [DOI] [PubMed] [Google Scholar]
- Kankaanranta H, Zhang X, Tumelius R, Ruotsalainen M, Haikala H, Nissinen E, et al. Antieosinophilic activity of simendans. J Pharmacol Exp Ther. 2007;323:31–38. doi: 10.1124/jpet.107.124057. [DOI] [PubMed] [Google Scholar]
- Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, et al. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- Kim YW, Zhao RJ, Park SJ, Lee JR, Cho IJ, Yang CH, et al. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-kappaB-dependent iNOS and proinflammatory cytokines production. Br J Pharmacol. 2008;154:165–173. doi: 10.1038/bjp.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkebøen KA, Strand OA. The role of nitric oxide in sepsis—an overview. Acta Anaesthesiol Scand. 1999;43:275–288. doi: 10.1034/j.1399-6576.1999.430307.x. [DOI] [PubMed] [Google Scholar]
- Kishore R, McMullen MR, Cocuzzi E, Nagy LE. Lipopolysaccharide-mediated signal transduction: stabilization of TNF-alpha mRNA contributes to increased lipopolysaccharide-stimulated TNF-alpha production by Kupffer cells after chronic ethanol feeding. Comp Hepatol. 2004;3:S31. doi: 10.1186/1476-5926-2-S1-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- Kivikko M, Antila S, Eha J, Lehtonen L, Pentikainen PJ. Pharmacodynamics and safety of a new calcium sensitizer, levosimendan, and its metabolites during an extended infusion in patients with severe heart failure. J Clin Pharmacol. 2002;42:43–51. doi: 10.1177/0091270002042001005. [DOI] [PubMed] [Google Scholar]
- Kivikko M, Lehtonen L, Colucci WS. Sustained hemodynamic effects of intravenous levosimendan. Circulation. 2003;107:81–86. doi: 10.1161/01.cir.0000043245.00859.11. [DOI] [PubMed] [Google Scholar]
- Kleinert H, Euchenhofer C, Ihrig-Biedert I, Förstermann U. Glucocorticoids inhibit the induction of nitric oxide synthase II by down-regulating cytokine-induced activity of transcription factor nuclear factor-kappa B. Mol Pharmacol. 1996;49:15–21. [PubMed] [Google Scholar]
- Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:471–479. doi: 10.2174/1568010054526359. [DOI] [PubMed] [Google Scholar]
- Kyrzopoulos S, Adamopoulos S, Parissis JT, Rassias J, Kostakis G, Iliodromitis E, et al. Levosimendan reduces plasma B-type natriuretic peptide and interleukin 6, and improves central hemodynamics in severe heart failure patients. Int J Cardiol. 2005;99:409–413. doi: 10.1016/j.ijcard.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu JY, Zhou F, Sun XL, Yao HH, Yang Y, et al. The regulation of rotenone-induced inflammatory factor production by ATP-sensitive potassium channel expressed in BV-2 cells. Neurosci Lett. 2005;394:131–135. doi: 10.1016/j.neulet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, et al. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew L, Katz SD. Calcium sensitising agents in heart failure. Drugs Aging. 1998;12:191–204. doi: 10.2165/00002512-199812030-00003. [DOI] [PubMed] [Google Scholar]
- Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- Moilanen E, Whittle BJ, Moncada S.Nitric oxide as a factor of inflammation Inflammation: Basic Principles and Clinical Correlates 1999Lippincott Williams & Wilkins: Philadelphia; 787–801.In: Gallin JI, Snyderman R (eds). [Google Scholar]
- Nieminen MS, Akkila J, Hasenfuss G, Kleber FX, Lehtonen LA, Mitrovic V, et al. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1903–1912. doi: 10.1016/s0735-1097(00)00961-x. [DOI] [PubMed] [Google Scholar]
- Oldner A, Konrad D, Weitzberg E, Rudehill A, Rossi P, Wanecek M. Effects of levosimendan, a novel inotropic calcium-sensitizing drug, in experimental septic shock. Crit Care Med. 2001;29:2185–2193. doi: 10.1097/00003246-200111000-00022. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevaidis IA, Parissis JT, Kremastinos DT. Anti-inflammatory and anti-apoptotic effects of levosimendan in decompensated heart failure: a novel mechanism of drug-induced improvement in contractile performance of the failing heart. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:243–247. doi: 10.2174/1568016054368232. [DOI] [PubMed] [Google Scholar]
- Parissis JT, Adamopoulos S, Antoniades C, Kostakis G, Rigas A, Kyrzopoulos S, et al. Effects of levosimendan on circulating pro-inflammatory cytokines and soluble apoptosis mediators in patients with decompensated advanced heart failure. Am J Cardiol. 2004;93:1309–1312. doi: 10.1016/j.amjcard.2004.01.073. [DOI] [PubMed] [Google Scholar]
- Parissis JT, Andreadou I, Markantonis SL, Bistola V, Louka A, Pyriochou A, et al. Effects of levosimendan on circulating markers of oxidative and nitrosative stress in patients with advanced heart failure. Atherosclerosis. 2007;195:e210–e215. doi: 10.1016/j.atherosclerosis.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Ramaswamykanive H, Bihari D, Solano TR. Myocardial depression associated with pneumococcal septic shock reversed by levosimendan. Anaesth Intensive Care. 2007;35:409–413. doi: 10.1177/0310057X0703500316. [DOI] [PubMed] [Google Scholar]
- Rastaldo R, Pagliaro P, Cappello S, Penna C, Mancardi D, Westerhof N, et al. Nitric oxide and cardiac function. Life Sci. 2007;81:779–793. doi: 10.1016/j.lfs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- Sareila O, Korhonen R, Kärpänniemi O, Nieminen R, Kankaanranta H, Moilanen E. JAK inhibitors AG-490 and WHI-P154 decrease IFN-gamma-induced iNOS expression and NO production in macrophages. Mediators Inflamm. 2006;2006:16161. doi: 10.1155/MI/2006/16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsa T, Pollesello P, Rosevear PR, Drakenberg T, Kilpelainen I. Stereoselective binding of levosimendan to cardiac troponin C causes Ca2+-sensitization. Eur J Pharmacol. 2004;486:1–8. doi: 10.1016/j.ejphar.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Tinker AC, Wallace AV. Selective inhibitors of inducible nitric oxide synthase: potential agents for the treatment of inflammatory diseases. Curr Top Med Chem. 2006;6:77–92. doi: 10.2174/156802606775270297. [DOI] [PubMed] [Google Scholar]
- Trikas A, Antoniades C, Latsios G, Vasiliadou K, Karamitros I, Tousoulis D, et al. Long-term effects of levosimendan infusion on inflammatory processes and sFas in patients with severe heart failure. Eur J Heart Fail. 2006;8:804–809. doi: 10.1016/j.ejheart.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Tsai EY, Falvo JV, Tsytsykova AV, Barczak AK, Reimold AM, Glimcher LH, et al. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Mol Cell Biol. 2000;20:6084–6094. doi: 10.1128/mcb.20.16.6084-6094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungureanu-Longrois D, Balligand JL, Kelly RA, Smith TW. Myocardial contractile dysfunction in the systemic inflammatory response syndrome: role of a cytokine-inducible nitric oxide synthase in cardiac myocytes. J Mol Cell Cardiol. 1995;27:155–167. doi: 10.1016/s0022-2828(08)80015-6. [DOI] [PubMed] [Google Scholar]
- Vuolteenaho K, Moilanen T, Knowles RG, Moilanen E. The role of nitric oxide in osteoarthritis. Scand J Rheumatol. 2007;36:247–258. doi: 10.1080/03009740701483014. [DOI] [PubMed] [Google Scholar]
- Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- Yang Y, Liu X, Long Y, Wang F, Ding JH, Liu SY, et al. Activation of mitochondrial ATP-sensitive potassium channels improves rotenone-related motor and neurochemical alterations in rats. Int J Neuropsychopharmacol. 2006;9:51–61. doi: 10.1017/S1461145705005547. [DOI] [PubMed] [Google Scholar]
- Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N. The novel calcium sensitizer levosimendan activates the ATP-sensitive K+ channel in rat ventricular cells. J Pharmacol Exp Ther. 1997;283:375–383. [PubMed] [Google Scholar]